Abstract
GABA (γ-Aminobutyric acid) is the major inhibitory neurotransmitter in the brain. The GABAergic system is indispensable for maintaining the balance between excitation and inhibition (E/I balance) required for normal neural circuit function. E/I imbalances that result from perturbations in the development of this system, ranging from the generation of inhibitory neurons to the formation of their synaptic connections, have been implicated in several neurodevelopmental disorders. In this review, we discuss how impairments at different stages in GABAergic development can lead to disease states. We also highlight recent studies which show that modulation of the GABAergic system can successfully reverse cognitive deficits in disease models and suggest that therapeutic strategies targeting the GABAergic system could be effective in treating neurodevelopmental disorders.
The GABAergic system and neurodevelopmental disorders
The basic wiring of our nervous system is established during the early developmental period. Key events during this period - birth and migration of neurons, synapse formation and maturation, and experience-dependent refinement of circuit connections - create functional neural circuits with well balanced excitatory and inhibitory components. Perturbation of these early events can lead to life-long cognitive and emotional disabilities, including epilepsy, autism and schizophrenia, which, for the most part, lack effective treatments. Basic research into early development has promoted advancements in clinical diagnosis. As a result, disorders such as schizophrenia that were traditionally thought to have late onsets are now being linked to deficits during early development [1]. Recently, it was proposed that a disrupted excitatory/inhibitory (E/I) balance in key circuits might underlie many neurodevelopmental disorders [2]. This hypothesis is particular appealing because it provides a theoretical framework within which clinical observations and detailed neural circuit analysis can be integrated. However, although E/I imbalance is often observed in disease conditions such as epilepsy, autism and schizophrenia [1–3], evidence directly implicating E/I imbalance as a causal factor in these diseases has just begun to emerge with the use of disease mouse models (Table 1). Although E/I balance can be upset by disrupting either excitation or inhibition within a neural circuit, we focus here on the relationship between dysfunctional inhibition and neurodevelopmental disorders.
Table 1.
Summary of neurodevelopmental disorders and mouse models with alterations in the GABAergic system
| Disease | Neurological Phenotype in Human Patients | Identified or Hypothesized Human Genetic Mutation | Mouse Model | Mouse Model Behavioral/Plasticity Phenotypes | Inhibitory Neuron/Synapse Phenotype | Refs |
|---|---|---|---|---|---|---|
| Autism | Repetitive stereotyped behavior, impaired social interaction | Neuroligin 3 Arg451 → Cys substitution in NLGN3 |
Arg451 → Cys substitution in NLGN3 | Gain-of-function mutation. Impaired social interaction, enhanced spatial learning | Increase in inhibitory synapse strength and spontaneous inhibitory transmission, no change in excitation | [48] |
| Neuroligin 4 Multiple mutations in NLGN4 have been identified. |
Nlgn4 KOa | Loss-of-function mutation. Impaired social interaction and social memory. Reduced ultrasonic vocalizations. | R87W mutant abolishes NLGN4-induced suppression of excitatory synaptic transmission | [49] [89] |
||
| Neurexin 1 Multiple mutations in the NRXN1 have been identified. |
Nrxn1A KO | Loss-of-function mutation. Decreased prepulse inhibition, impaired nest building, increased grooming. No change in spatial learning or social interaction. | Hypothesized alterations in E/I balance. Decreased excitation/no change in inhibition. | [90] | ||
| Rett Syndrome | Autism, learning impairments, speech/motor impairments, epilepsy |
MECP2 Majority of patients are female. MECP2 mutations are found in 95% of Rett Syndrome patients. |
Mecp2 KO | Loss-of-function mutation. Seizures, motor impairments, learning impairments, breathing abnormalities, decreased anxiety. Phenotypes in female mice are milder and have a later onset in comparison to hemizygous males. | GABAergic neuron specific deletions of Mecp2 (Viaat-Mecp2 KO and Dlx5/6-Mecp2 KO) recapitulate many of the phenotypes observed in the global KO. Loss of Mecp2 in these neurons results in decreased levels of GAD1, GAD2, and GABA. This results in a reduction of mISPC quantal size, EEG abnormalities, and LTP impairments. | [91–93] |
| Mecp2308/Y | Loss-of-function mutation. Seizures, motor impairments, learning impairments, increased anxiety. Phenotypes in female mice are milder and have a later onset in comparison to hemizygous males. | [93, 94] | ||||
| Mecp2 OE | Overexpression mutation. Seizures, motor impairments, increased anxiety. Both male and female mice show similar phenotypes. | [95], [93] | ||||
| Viaatb-Cre+/−:: Mecp2flox/+ | Loss-of-function mutation. Seizures, motor impairments, learning impairments, breathing abnormalities. Phenotypes characterized in hemizygous males. | [96] | ||||
| Dlx5/6-Cre+/−:: Mecp2flox/+ | Loss-of-function mutation. Seizures, motor impairments, learning impairments. Phenotypes characterized in hemizygous males. | |||||
| Neurofibromatosis type I | Learning impairments, attention deficits, visuospatial impairments | Heterozygous NF1 mutation |
Nf1+/− Synapsin-Cre+/−:: Nf1flox/+ Dlx5/6-Cre+/−:: Nf1flox/+ |
Loss-of-function mutation. Spatial learning deficits and LTP impairments. Working memory deficits. | Dlx5/6-Cre+/−x Nf1flox/+ recapitulates the learning deficits observed in the Nf1+/− and Synapsin-Cre KO. The learning and plasticity deficits are thought to be the results of increased inhibition (increased mIPSC frequency under conditions of high activity). Treatment of these animals with picrotoxin rescues the learning deficit | [68, 77, 97] |
| Down Syndrome | Learning impairments, epilepsy | Trisomy 21 | Ts65Dn | Learning impairments (object recognition, spatial learning, spontaneous alternation), epilepsy. | Increased frequency of mIPSCs resulting in impaired LTP in dentate gyrus. Suppressing inhibition by applying picrotoxin restores normal LTP. Learning and LTP deficits can be rescued by treating the animals with picrotoxin. Two-week treatment with picrotoxin leads to long-term (2 months) recovery of learning and synaptic plasticity. | [67, 70, 74] |
| Schizophrenia | Delusions, hallucinations, anhedonia, working memory deficits | Hypothesized: NR1 | Nr1 hypomorph | Loss-of-function mutation Hyperactivity, increased stereotypy, impaired social and sexual interaction. | Postmortem analyses of schizophrenic patients exhibit decreases in GABA, GAD65, GAD67, as well as decreases in NMDA receptor expression on PV neurons. Inhibitory neuron specific knockout of Nr1 or Erbb4 produces schizophrenia-like phenotypes seen in global knockouts. Inhibitory neuron knockout of Nr1 results in decreased expression of GAD67, reduced inhibition and reduced neuronal synchrony. PV specific knockout of Erbb4 results in impaired GABA release and a decreased in inhibitory drive. | [98] |
| Cortical interneuron KO of Nr1: Ppp1r2c-Cre+/− :: Nr1flox/flox | Loss-of-function mutation Hyperactivity, increased stereotypy, impaired social and sexual interaction. Impaired spatial working memory and prepulse inhibition. Anhedonia/anxiety behaviors. Early postnatal deletion of Nr1 results in these phenotypes. No abnormalities are detected when Nr1 is deleted post-adolescence. | [81] | ||||
| NRG1; ERBB4 |
Erbb4 KO Nrg1 KO PV- Cre+/− :: Erbb4 flox/flox |
Loss-of-function mutation Hyperactivity, impaired working memory, contextual fear conditioning, and prepulse inhibition. | [82, 83] | |||
| DISC1d, others | Various Disc1 mutants. | Hyperactivity, impaired social interaction, impaired spatial memory. | [99, 100] | |||
| Tourette Syndrome | Involuntary motor and vocal tics that produce stereotyped movements or sounds | Unknown | Unknown | Unknown | Postmortem analysis of TS patients exhibit decreased numbers of PV positive GABAergic interneurons in striatum and increased in the globus pallidus. Possible migration deficit. | [5, 6] |
| Epilepsy | Recurrent seizures. Most commonly studied forms are absence epilepsy and temporal lobe epilepsy | Alteration in GABA receptor subunit composition. Mutations in GAD65, KCNQe, GABAA. α1, s3, γ2 | Pharmacologically induced seizures. Gad65, GABAA. α1, β3, γ2 KO mice | Recurrent seizures. | Modulations in GABAergic system seem to be a major factor in seizure disorders. | [101] |
| Fragile X | Learning impairments, autism, epilepsy | Expansion of trinucleotide sequence leading to a failure in the expression of FMR1 | Fmr1 KO | Loss-of-function mutation Learning impairments, seizures (audiogenic), OD plasticity. These phenotypes can be rescued by decreasing mGlUR5 activity. | Possible E/I imbalance. Increased excitatory synaptic transmission. Decreased mRNA and protein expression of GABAA receptor subunits. Decreased excitatory drive onto inhibitory neurons and decreased number of GABAergic interneurons. | [102–106] |
Knock-out
vesicular inhibitory amino acid transporter
protein phosphatase 1, regulatory (inhibitor) subunit 2
disrupted in schizophrenia 1
potassium voltage-gated channel, KQT-like subfamily member
The major inhibitory neurotransmitter in mammalian brains is GABA (γ-Aminobutyric acid), which is produced by GABAergic neurons and released at GABAergic synapses formed between GABAergic neurons and their targets. The level of inhibition, generally measured by synaptic GABA release, is modulated by the synaptic drive (both excitatory and inhibitory) received by GABAergic neurons. The development of the GABAergic system coincides with the onset of many neurodevelopmental disorders, pointing to a critical role for inhibition in neural circuit development and function. As an example, the autism spectrum disorder (ASD) Rett syndrome is caused by mutations in the transcriptional regulator MeCP2 (methyl-CpG-binding protein 2), which result in predominantly neurological symptoms despite MeCP2’s ubiquitous expression. Disrupting MeCP2 in GABAergic neurons recapitulates most Rett phenotypes, indicating an important role for the GABAergic system in the etiology of Rett syndrome, and probably also for at least a subset of ASDs [2]. This study highlights one of many mouse disease models that not only provide support for the E/I balance hypothesis (Table 1), but should also provide insight into the etiology of neurodevelopmental disorders.
Limited by space, we focus here on three major aspects of the GABAergic system: generation and migration of GABAergic neurons, development of GABAergic synapses, and the impact of the GABAergic system on neural circuit function. Our knowledge of the development of the GABAergic system has grown rapidly in recent years thanks to newly developed genetic tools, giving us an unprecedented opportunity to examine the process of GABAergic development in the context of neurodevelopmental disorders and, hopefully, to reevaluate and even redesign therapeutic strategies.
Generation and migration of GABAergic neurons
The generation and migration of GABAergic neurons, the first stage of GABAergic system development, is responsible for producing the appropriate numbers and kinds of GABAergic neurons in the correct locations. This stage probably has the greatest impact on the final neural circuits. Notably, alterations in specific subtypes of GABAergic neurons have been observed in several neurodevelopmental disorders [4–8]. For instance, in Tourette’s syndrome the number of parvalbumin (PV)-positive neurons is increased in the striatum, but decreased in the globus pallidus [5].
With a few exceptions in humans and non-human primates [9], GABAergic neurons are derived from the ventral part of the telecephalon (subpallium) [10]. The subpallium is subdivided into lateral, medial and caudal ganglionic eminences (LGEs, MGEs and CGEs, respectively), as well as the anterior entopeduncula and preoptic area (POA), which are structurally less well defined. Whereas progenitors from LGE give rise to GABAergic neurons in the olfactory bulb and striatum, cortical GABAergic neurons (often called cortical interneurons) are mainly derived from MGEs and CGEs, and perhaps the POA [11, 12].
GABAergic neuronal fate is determined by MASH1 (mammalian achaete-scute homolog 1) and DLX (distal-less homeobox) family members, transcription factors whose expression is restricted to the subpallium [13, 14]. Regional expression of transcription factors in ganglionic eminences further specifies the subgroup of GABAergic neurons generated in those areas [15]. For example, dorsal LGE expresses Pax6 (paired box gene 6) and produces olfactory bulb interneurons, whereas ventral LGE expresses Gsh2 (glutathione synthase 2) and low levels of Pax6 and generates mainly striatal neurons, including most striatal projection neurons [16]. Cortical GABAergic neurons derived from MGE are specified by Nkx2.1 (NK2 homeobox 1) [17]. Molecular markers for GABAergic progenitors in CGE are currently unknown.
A striking feature of GABAergic neurons is their diversity. Although no single parameter can unequivocally differentiate all subtypes of GABAergic neurons, they can be classified by morphology (basket, chandelier, bipolar, double bouquet cells, etc.), physiology (fast-spiking, regular firing, bursting, stuttering etc.), and by the molecular markers they express [18]. Molecular markers, in particular, have proven useful for examining postmortem tissues from patients. The most commonly used include calcium-binding proteins such as PV, calretinin (CR) and calbindin (CB) and neuropeptides such as somatostatin (SST), vasoactive intestinal peptide (VIP), neuropeptide Y (NPY) and cholecystokinin (CCK).
The molecular mechanism which specifies the subtype of GABAergic neurons is not fully understood. Gene profiling of cortical interneuron precursors suggested that GABAergic subtypes are already determined at the progenitor stage, long before they are functionally integrated into neural circuits [19]. Genetic fate-mapping studies suggest that subtype specification is regulated by both spatial and temporal factors. For example, neurons from MGE preferentially express PV and SST/CB, whereas CGE-derived neurons tend to be CR/VIP or NPY positive [15]. Temporally, SST neurons are born before PV neurons, which are followed by VIP neurons [20]. This is consistent with the fact that neurogenesis in MGE (producing SST and PV neurons) happens earlier than in CGE (producing VIP neurons) [21]. The next challenge will be to identify the genetic programs that determine the fates of various GABAergic subtypes.
Newly born cortical GABAergic neurons first migrate tangentially away from the ganglionic eminence, following two migratory streams into regions above and below the developing cortical plate [22]. Once there, they adopt various radial migration modes to settle into specific cortical layers in an inside-out fashion according to their birth order, with older cells taking the deeper layers and younger cells occupying the superficial layers [23].
Many factors regulate the tangential migration of GABAergic neurons. These include a set of attractant and repellent guidance molecules similar to those used by glutamatergic neurons, growth factors and their receptors, and neurotransmitters [24]. Dysregulation of several of these factors has been implicated in neurological disorders [25]. Neuregulin 1 (NRG1), the product of a known schizophrenia susceptibility gene, and its receptor ErbB4 are worth special mention [26]. NRG1 secreted in cortical regions can serve as a potent chemoattactant, guiding ErbB4-expressing GABAergic neurons to migrate tangentially into the cortex [27]. Impaired GABAergic neuron migration resulting from defects in NRG1-ErbB4 signaling might contribute to the pathology of schizophrenia, at least in a subset of patients [28].
Many transcription factors that specify the fate of GABAergic neurons are also involved in their migration, with some even interacting directly with guidance cues [29–33]. For example, Nkx2.1 down-regulates the semaphorin receptor neuropilin 2 (NRP2) [33]. Because striatum expresses the repellent ligands of NRP2, SEMA3A and SEMA3F, neurons expressing low levels of Nkx2.1 avoid the striatum and migrate to the cortex, whereas neurons with high levels of Nkx2.1 migrate into the striatum. Further investigation of migration mechanisms for different subtypes of GABAergic neurons is necessary, given that they could lead to altered distributions of certain subtypes in neurodevelopmental disorders such as Tourette’s syndrome [5].
Whereas tangential migration distributes GABAergic neurons longitudinally around the cortical plate, radial migration sorts GABAergic neurons into the correct cortical layers, where they are connected to glutamatergic projection neurons within each layer and can directly influence the E/I balance of the cortical circuits. In contrast to tangential migration, very little is known about regulation of radial migration. The only signaling pathway implicated in this process to date involves the chemokine CXCL12 and its receptors CXCR4 and CXCR7, which affect the timing of switching from tangential to radial migration [34–36]. In humans, severe defects in cortical radial migration of glutamatergic neurons result in lissencephaly, a disorder associated with mental retardation and seizures [37]. It is not known whether radial migration of GABAergic neurons is also involved in this condition. Abnormal laminar positioning of GABAergic neurons alone seems unlikely to cause gross anatomical changes similar to those seen in lissencephaly, but would still have a profound impact on the function of cortical circuits. Closer examination of the laminar distribution of GABAergic neurons in neurodevelopmental disorders that do not involve gross anatomical abnormalities could generate new insights into their etiology.
Development of GABAergic synapses
Whereas the generation of GABAergic neurons occurs during the mid-embryonic stage, development of inhibitory synapses occurs mostly postnatally. Correct development of GABAergic synapses is critical to achieve an optimal E/I balance within a neural circuit, given that impairments in this process are associated with a wide spectrum of neurodevelopmental disorders [2]. Although we focus here on the inhibitory role of GABAergic synapses, there is an important developmental stage during which these synapses are excitatory, and GABA’s role as an excitatory neurotransmitter has been implicated in various seizure conditions in juveniles and adults [38]. Interested readers are referred to several excellent reviews covering this important topic [39, 40].
GABAergic synapse development is regulated by both neuronal activity-independent and -dependent mechanisms, but these processes are interdependent and should not be considered in isolation [41]. Similar to glutamatergic synapses, the initial formation of GABAergic synapses is thought to be independent of neuronal activity, occurring through elaborate cell–cell recognition processes mediated by transmembrane cell adhesion molecules such as neurexin (NRXN) and neuroligin (NLGN) family members [42]. NRXNs are thought to be predominantly presynaptic proteins mediating the formation of specializations that contribute to neurotransmitter release; NLGNs are postsynaptic NRXN ligands. Together they form complexes, with specific isoforms localizing to excitatory or inhibitory synapses [42]. Complexes containing NLGN2 are mainly found at GABAergic synapses [43] and mutations in Nlgn2 cause defective inhibitory synapse development and aberrant inhibitory transmission [42, 44].
Several mutations in NRXN1, NLGN3, and NLGN4 have been linked to autism [45–47]. Importantly, mouse models mimicking the human mutations in NLGN3 and NLGN4 produce autism-like phenotypes such as impaired social interaction and communication [48, 49]. It is particularly interesting to note that knock-in mice with an Arg451 to Cys substitution in NLGN3, identified in two brothers with ASDs [45], have increased inhibitory synaptic transmission with no obvious changes in excitatory transmission [48]. There are conflicting reports regarding whether these mutant mice exhibit autistic behaviors similar to human patients [48, 50]; nevertheless, it is clear that these mice display at least some degree of developmental and behavioral abnormality, which might result from a GABAergic system impairment.
In addition to the NRXN–NLGN pair, NRG1 and its receptor ErbB4 were recently shown to be important regulators of GABAergic synapse development and, as mentioned earlier, have been repeatedly identified as risk genes for schizophrenia [51]. ErbB4 expression is enriched in PV-positive basket and chandelier cells and contributes to the formation of inhibitory synapses on pyramidal neurons, as well as excitatory synapses on PV inhibitory neurons [52, 53]. These findings suggest that the NRG1–ErbB4 complex has an important role in regulating inhibitory drive, the alteration of which is a defining hallmark of schizophrenia [54]. This might indicate a critical role for the GABAergic system in schizophrenia etiology.
FGF7, a member of the fibroblast growth factor family, and its receptor FGFR2, were recently added to the limited number of receptor–ligand complexes known to function selectively at GABAergic synapses [55]. Expressed in CA3 pyramidal neurons, FGF7 regulates GABAergic synapse development in this region of the hippocampus by selectively promoting the organization of GABAergic presynaptic terminals. Fgf7-deficient mice are prone to epileptic seizure, presumably as a result of reduced inhibition [55]. Whether the FGF signaling pathway is affected in epileptic patients has not been examined.
Although the receptor–ligand complexes we have discussed play an important role in creating GABAergic synapses, neuronal activity is critical for their further maturation [41]. For example, postnatal development of inhibitory synapses in brain regions such as the primary sensory cortex is modified by neuronal activity and sensory experience [56, 57]. This positions inhibition to mediate neural circuit development based on experience, but the molecular and cellular mechanisms underlying this process are largely unknown. A recent report showed that the transcription factor neuronal PAS domain protein 4 (NPAS4) plays a critical role in the activity-dependent regulation of inhibitory synapse formation [58]. NPAS4 is rapidly induced by excitatory synaptic activity and regulates a genetic program that triggers the formation and/or maintenance of inhibitory synapses on excitatory neurons. NPAS4 itself has not been associated with any diseases, but one of its potential transcriptional targets, the (Na+, K+)/H+ exchanger NHE9, is deleted in some autistic patients [59]. Although it is not clear whether NHE9 mutations result in reduced levels of inhibition, NHE9 deficiency often leads to co-morbid epilepsy, suggesting that the genetic program downstream of NPAS4 might contribute to E/I balance.
A genome-wide screen for NPAS4 targets identified several genes with known roles in inhibitory synapse formation, including brain-derived neurotrophic factor (BDNF) [58]. Although the role of BDNF in central nervous system (CNS) function seems to be quite broad [60], its function as a mediator of inhibitory synapse formation and maintenance is well documented [57, 61–63]. BDNF is highly regulated by activity and is considered to be a major player in activity-dependent development of GABAergic synapses. Supporting this hypothesis, recent work showed that genetically abolishing the function of one of BDNF’s activity-dependent promoters, promoter IV, leads to an impairment of GABAergic synapses, but no change in excitatory synapses, in cortical areas [51, 52].
Impaired BDNF function has been implicated in several neurodevelopment disorders [64]. Remarkably, knock-in mice expressing a mutant version that harbors a common single-nucleotide polymorphism (SNP), Val66Met, recapitulated anxiety-related behavioral phenotypes seen in humans who have the same SNP [65], making this one of the most exciting mouse models of human neurological disorders. However, the mechanism by which the polymorphism gives rise to the disease remains unknown; it would be interesting to examine whether the GABAergic system is affected in this mouse model.
Impact of the GABAergic system on neural circuit function
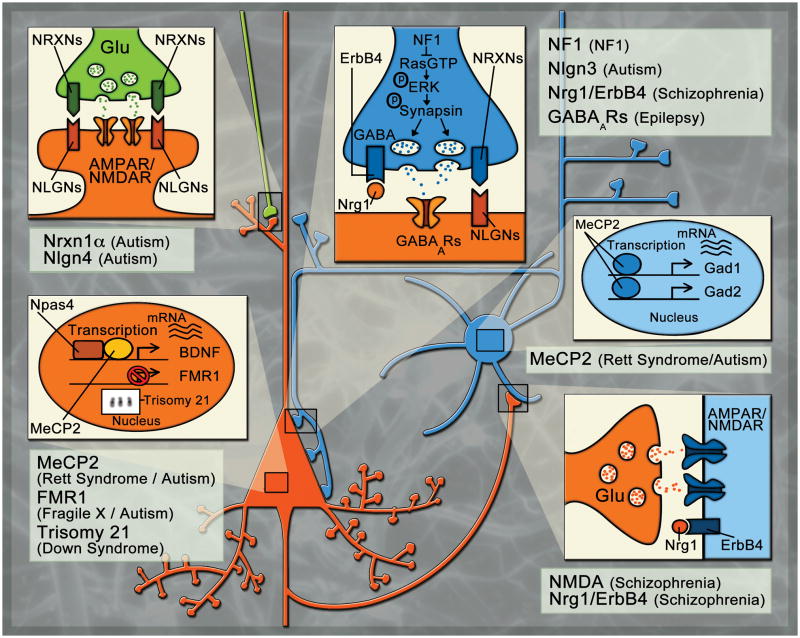
The ability of neural circuits to modify their connections based on changing external inputs is thought to underlie core processes such as learning and memory [66]. This modification is usually measured by changes in excitatory synaptic transmission, which can exhibit plasticity such as long-term potentiation (LTP) and depression (LTD) [66]. Until recently, the contribution of inhibition to these processes was relatively unknown, but it is becoming evident that altered inhibitory function can profoundly impair excitatory synaptic transmission and subsequent circuit plasticity [67–70]. For example, the onset of visual cortex plasticity can be accelerated or delayed by respectively increasing or decreasing inhibitory transmission [71]. In this section we will highlight several diseases that have recently been shown to involve aberrant inhibitory transmission to show how alterations in the GABAergic system can profoundly affect neural circuit function (Fig 1).
Figure 1. Sites of action of molecules implicated in neurodevelopment disorders.
The figure shows a pair of interconnected pyramidal (orange) and GABAergic (blue) neurons. The pyramidal neuron also receives an excitatory input from another excitatory neuron (green). Details of the nuclei of the neurons and synapses formed between them are shown, according to their color codes, in expanded boxes. Depending on their subcellular localizations, disease-relevant molecules and pathways are depicted in the boxes and related disorders indicated next to them. (i) Excitatory synapse formed on a pyramidal neuron. Mutations in NRNX1A and NLGN4 have been reported in autistic patients; mutant mice exhibit similar phenotypes[42, 89, 90]. (ii) Nucleus of a pyramidal neuron. Suppression of FMR1 expression leads to Fragile X disease, whereas triplication of chromosome 21 leads to Down Syndrome. MeCP2 mutations cause alterations in gene expression (e.g. BDNF) contributing to Rett Syndrome, an autism spectrum disorder. [73, 93, 107]. (iii): Excitatory synapse formed on an inhibitory neuron. Alterations in NRG1–ErbB4 complexes, as well as NMDA receptor function, have been reported in schizophrenic patients and produce schizophrenia-like phenotypes in mutant mice[52, 81, 108]. (iv): Nucleus of an inhibitory neuron. Mouse models with selective impairment in Mecp2 function in inhibitory neurons produces Rett syndrome-like phenotypes and a decrease in GABA transmission[96]. (v): Inhibitory synapse on a pyramidal neuron. Selective deletion of Nf1 from GABAergic neurons results in hyperactive GABA release. Mutations in NLGN3 have been reported in autistic patients, and mutant mice exhibit enhanced inhibitory transmission and autistism-like phenotypes. Deletion of ErbB4 in inhibitory neurons results in a decrease in the number of GABAergic synapse on pyramidal neurons and produces schizophrenia-like phenotypes in mouse models. Impairments in the GABAergic system seem to be a major factor in several seizure disorders[48, 52, 68, 101].
Down syndrome, a triplication of chromosome 21 that results in an extra copy of approximately 300 genes, is the most common aneuploid disorder leading to mental retardation [72]. It is not known which genes in the duplicated region are responsible for the disease pathology. There are several mouse models of Down syndrome, the best characterized being the Ts65Dn line which is trisomic for a large region of chromosome 16 (homologous to human chromosome 21) [73]. These mice exhibit a variety of behavioral deficits that are consistent with learning and memory impairment [70, 73, 74] (Table 1). In addition to structural abnormalities at synapses [75], electrophysiological recordings from the dentate gyrus (DG) of Ts65Dn mice show increased inhibitory transmission and enhanced feedback inhibition, with no change in excitation. Furthermore, these mice exhibit impaired DG LTP [67, 70]. These findings suggest that the plasticity and behavioral impairments seen in the Ts65Dn line could result from an altered E/I balance due to enhanced inhibition.
Neurofibromatosis type 1, caused by mutations in NF1, is characterized by similar increases in inhibition that potentially contribute to plasticity and behavioral impairments [68]. In humans, NF1 mutations in lead to deficits in learning and memory, attention, and visuospatial tasks [76]. These phenotypes have been successfully recapitulated in a mouse model of neurofibromatosis in which one copy of Nf1 is deleted (Table 1) [68, 77]. At the synaptic level, Nf1 mutant mice exhibit an overall increase in inhibitory synaptic transmission and no change in excitatory synaptic transmission. Possibly as a consequence of heightened inhibition, these mice exhibit attenuated excitatory synaptic plasticity as measured by hippocampal LTP [68, 77]. Remarkably, deleting one copy of Nf1 from inhibitory neurons, but not excitatory neurons or glial cells, is sufficient to reproduce the inhibitory transmission, LTP, and behavioral phenotypes seen in the global Nf1 mutant, suggesting that dysfunction of the GABAergic system is responsible for the cognitive phenotypes seen in neurofibromatosis [68]. It is believed that the loss of NF1 leads to hyperactive Ras-extracellular signal-regulated kinase (ERK) signaling, which causes an increase in GABA release and a suppression of the plasticity required for cognitive function (Fig 1) [68]. Consistent with this model, reducing Ras activity, either genetically or pharmacologically, leads to amelioration of the behavioral and plasticity impairments in Nf1 mutant mice [77].
Down syndrome and neurofibromatosis mouse models provide examples of disease states that feature excessive levels of inhibition. Decreases in inhibition are also associated with neurodevelopmental disorders. One of the most consistent observations in schizophrenia is a decreased inhibitory drive on to glutamatergic neurons [54]. Postmortem studies of schizophrenia patients show decreases in NMDA (N-Methyl-D-aspartic) receptor expression on PV neurons [78, 79], which would result in a decreased inhibitory drive, decreases in overall GABA levels, as well decreases in levels of GABA-producing enzymes GAD65 and GAD67 (65 kDa and 67 kDa isoforms of gluatmic acid decarboxylase), as well as other presynaptic components of the GABAergic system [80].
Several behavioral and molecular (decreased GAD67 and PV expression) phenotypes similar to those seen in schizophrenic patients have been reported in mice in which NMDA receptor subunit 1 (Nr1) is deleted in GABAergic neurons, resulting in decreased inhibition in the cortex and hippocampus (Table 1) [81]. Similarly, the deletion of ErbB4 from hippocampal PV-positive GABAergic neurons (PV-ErbB4), which results in impaired inhibitory synapse formation [52], produced schizophrenia-like phenotypes and impaired GABAergic modulation of hippocampal LTP [82, 83]. Treatment of the PV-ErbB4 mutant mice with diazepam, a GABA agonist, ameliorated some of the behavioral deficits, suggesting that impairment of inhibition might contribute to the behavioral phenotypes [83].
Despite the range of cognitive and behavioral phenotypes seen in Down syndrome, neurofibromatosis, and schizophrenia, the studies described here strongly support the idea that deficits in the GABAergic system could be a common underlying factor contributing to the etiology of these diseases. However, these observations are mostly limited to mouse models and further investigation is required to establish these links in human patients. Additionally, before applying these findings in the clinical setting, it will be necessary to determine whether changes in GABAergic system are the primary pathological cause of the disease or merely a secondary outcome of the disease process.
Concluding remarks and future perspectives
It is clear from the studies we have reviewed here that altering the level of inhibition can have profound effects on circuit function. Although the various disease states we have considered are different in their causes and phenotypes, a common theme is the improper regulation of inhibitory function. Any perturbation in the development of the GABAergic system, from the generation and migration of these neurons to the formation and refinement of their synaptic connections, can lead to severe neurological impairment. Furthermore, selectively modulating the function of susceptibility genes such as MeCP2, NF1, and ErbB4 in GABAergic neurons can be sufficient to cause deficits previously seen in global knockouts. As a consequence of having a basic understanding of the general mechanisms mediating GABAergic development, there is potential for generating targeted and specific therapeutic interventions.
Several recent studies have used the transplantation of GABAergic neuron precursors (from embryonic MGEs) as a strategy to increase inhibition [84–87]. MGE precursors transplanted into the postnatal mouse brain mature into inhibitory neurons that effectively increase inhibition within preexisting circuits [84]. This procedure is sufficient to induce ocular dominance plasticity after the closing of the critical period, even at ages when pharmacological augmentation of inhibition is insufficient to induce this type of plasticity.[71, 87]. These findings suggest that the developmental program of inhibitory neurons can be exploited to regulate and induce plasticity in visual cortex. Furthermore, transplantation of MGE precursors into the postnatal brain has shown promise in suppressing seizures in mouse models of epilepsy [85, 88] and improving motor impairment in rodent models of Parkinson’s disease [86]. Although it is still in its early days, transplantation of inhibitory neuron precursors may provide a novel therapeutic intervention to treat neural circuit impairment by modulating levels of inhibition.
Pharmacological modulation of GABAergic synapses has also been shown to restore circuit plasticity [68, 70, 74]. Several groups have shown a possible causal relationship between the regulation of inhibition and impaired neural circuit function. For example, treatment of the Nf1 mutant mouse with a subthreshold dose of a GABAA antagonist rescues both the behavioral and plasticity deficits [68]. This finding is particularly significant because NF1 is a developmental disorder, yet the cognitive impairments can be reversed in adult mice with acute modulation of the GABAergic system. Similarly, in the mouse model of Down syndrome, chronic treatment with GABAA antagonists improved both plasticity and memory [67, 70, 74]. These studies show that pharmacological modulation of the GABAergic system could be a useful treatment for a number of neurodevelopmental disorders. Studying how specific GABAergic regulators (Figure 1) modulate inhibition should help to identify targets for specific therapeutic interventions.
In conclusion, we note that our understanding of the GABAergic system is still in its infancy. The advent of new molecular tools will provide the ability to further examine and modulate the GABAergic system with exquisite specificity. This will surely lead to a greater understanding of the GABAergic system, thereby providing key insights into disease states and their potential treatments.
Acknowledgments
We thank C. M. Fletcher for critical reading of the manuscript. Y.L. acknowledges the generous support of the McGovern Institute for Brain Research at MIT. Supported by the MIT Presidential Marcus Fellowship to Honor Norman B. Leventhal (K.R.), and Whitehall Foundation research grant, Anonymous Foundation research grant, John Merck Scholar Program, James H. Ferry Fund, MIT Simons Initiative on Autism and the Brain, and NIH grant MH091220-01 (Y.L.).
Glossary
- BDNF (brain-derived neurotrophic factor)
neurotrophic factor implicated in several neuronal processes. Highly regulated by activity, BDNF is thought to be an important regulator of activity-dependent GABAergic synapse development. Many of its actions are mediated through the TrkB receptor
- CB (calbindin)
a calcium-binding protein often used to label a subset of GABAergic neurons
- CCK (cholecystokinin)
a neuropeptide often used to label a subset of GABAergic neurons
- CR (calreticulin)
a calcium-binding protein often used to label a subset of GABAergic neurons
- DLX (distal-less homeobox)
certain members of this family of transcription factors are thought to play an important role in the generation and development of GABAergic neurons. The DLX5/6 promoter is commonly used to target GABAergic neurons
- Down syndrome
a triplication of chromosome 21 that results in an extra copy of approximately 300 genes. This syndrome often results in mental retardation and learning impairments
- ErbB4 (epidermal growth factor receptor (EGFR) 4)
Member of a family of receptor tyrosine kinases. Polymorphisms in ERBB4 have been identified in schizophrenic patients
- FGF/FGFR (fibroblast growth factor/receptor)
a family of growth factors involved in several cellular processes. Recent data suggest that FGF7 might regulate GABAergic synapse development
- GABA (γ-Aminobutyric acid)
the major inhibitory neurotransmitter in the brain
- GABAergic system
the major inhibitory system in the brain including inhibitory neurons, synapses, and circuits
- Ganglionic eminence
the majority of GABAergic neurons are derived from this structure located in the ventral portion of the telecephalon. These can be divided into three groups (CGEs, LGEs, MGEs) based on their location
- Glutamatergic neurons
neurons that produce glutamate as their neurotransmitter. Glutamate is the primary excitatory neurotransmitter in the brain
- LTD (long-term depression)
long-lasting depression of a synaptic connection
- LTP (long-term potentiation)
long-lasting potentiation of a synaptic connection
- MASH1 (mammalian achaete-scute homolog 1)
a transcription factor thought to play an important role in the generation and development of GABAergic neurons
- MeCP2 (methyl-CpG-binding protein 2)
a prominent and ubiquitously expressed transcriptional regulator. Mutations in this gene are associated with Rett syndrome
- Neurodevelopmental disorders
alterations in central nervous system development that result in impaired brain function, including sensory development, emotion, learning, and memory
- NF1(neurofibromatosis type I)
in humans, heterozygous mutations result in learning, attentional, and visuopatial impairments
- NHE9 ((Na+
K+)/H+ exchanger 9), this gene was recently found to be mutated in autistic patients
- Nkx2.1(NK2 homeobox 1)
a transcription factor implicated in proper migration of GABAergic neurons
- NLGN (neuroligin)
type I membrane proteins enriched at postsynaptic densities; NRXN ligands
- NMDA (N-Methyl-D-aspartic) receptor
an ionotropic glutamate receptor critical for several forms of synaptic plasticity
- NPAS4 (neuronal PAS domain protein 4)
an activity-regulated transcription factor implicated in GABAergic synapse development
- NPY (neuropeptide Y)
a neuropeptide often used to label a subset of GABAergic neurons
- NR1
an essential subunit of the NMDA receptor
- NRG1(neuregulin 1)
a trophic factor that interacts with ErbB family receptors. NRG1 polymorphisms have been identified in schizophrenic patients
- NRP2 (neuropilin 2)
a semaphorin receptor implicated in proper migration of GABAergic neurons
- NRXN (neurexin)
a type I membrane protein found on presynaptic terminals
- PV (parvalbumim)
a calcium binding protein. PV-positive neurons are a subset of GABAergic neurons, often characterized as fast-spiking with perisomatic innervation of their postsynaptic targets
- SST (somatostatin)
a neuropeptide often used to label a subset of GABAergic neurons
- Tourette’s syndrome
a neurodevelopmental disorder that usually results in involuntary motor and vocal tics that produce stereotyped movements or sounds
- VIP (vasoactive intestinal peptide)
a neuropeptide often used to label a subset of GABAergic neurons
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–32. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- 2.Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–67. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner RP. EEG in convulsive and nonconvulsive status epilepticus. J Clin Neurophysiol. 2004;21:319–31. [PubMed] [Google Scholar]
- 4.Casanova MF, et al. Minicolumnar pathology in autism. Neurology. 2002;58:428–32. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- 5.Kalanithi PS, et al. Altered parvalbumin-positive neuron distribution in basal ganglia of individuals with Tourette syndrome. Proc Natl Acad Sci U S A. 2005;102:13307–12. doi: 10.1073/pnas.0502624102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kataoka Y, et al. Decreased number of parvalbumin and cholinergic interneurons in the striatum of individuals with Tourette syndrome. J Comp Neurol. 2010;518:277–91. doi: 10.1002/cne.22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levitt P, et al. Regulation of neocortical interneuron development and the implications for neurodevelopmental disorders. Trends Neurosci. 2004;27:400–6. doi: 10.1016/j.tins.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Lewis DA, et al. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–24. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 9.Letinic K, et al. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417:645–9. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- 10.Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–96. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- 11.Xu Q, et al. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–22. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gelman DM, et al. The embryonic preoptic area is a novel source of cortical GABAergic interneurons. J Neurosci. 2009;29:9380–9. doi: 10.1523/JNEUROSCI.0604-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson SA, et al. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 1997;19:27–37. doi: 10.1016/s0896-6273(00)80345-1. [DOI] [PubMed] [Google Scholar]
- 14.Fode C, et al. A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes Dev. 2000;14:67–80. [PMC free article] [PubMed] [Google Scholar]
- 15.Batista-Brito R, Fishell G. The developmental integration of cortical interneurons into a functional network. Curr Top Dev Biol. 2009;87:81–118. doi: 10.1016/S0070-2153(09)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yun K, et al. Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development. 2001;128:193–205. doi: 10.1242/dev.128.2.193. [DOI] [PubMed] [Google Scholar]
- 17.Sussel L, et al. Loss of Nkx2.1 homeobox gene function results in a ventral to dorsal molecular respecification within the basal telencephalon: evidence for a transformation of the pallidum into the striatum. Development. 1999;126:3359–70. doi: 10.1242/dev.126.15.3359. [DOI] [PubMed] [Google Scholar]
- 18.Markram H, et al. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 19.Batista-Brito R, et al. Gene expression in cortical interneuron precursors is prescient of their mature function. Cereb Cortex. 2008;18:2306–17. doi: 10.1093/cercor/bhm258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyoshi G, et al. Physiologically distinct temporal cohorts of cortical interneurons arise from telencephalic Olig2-expressing precursors. J Neurosci. 2007;27:7786–98. doi: 10.1523/JNEUROSCI.1807-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butt SJ, et al. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 22.Lavdas AA, et al. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J Neurosci. 1999;19:7881–8. doi: 10.1523/JNEUROSCI.19-18-07881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson SA, et al. Distinct origins of neocortical projection neurons and interneurons in vivo. Cereb Cortex. 2002;12:702–9. doi: 10.1093/cercor/12.7.702. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez-Miranda LR, et al. Molecules and mechanisms involved in the generation and migration of cortical interneurons. ASN Neuro. 2010;2:e00031. doi: 10.1042/AN20090053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinowich K, et al. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–93. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- 26.Stefansson H, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–92. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flames N, et al. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44:251–61. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 28.Corfas G, et al. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci. 2004;7:575–80. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- 29.Anderson SA, et al. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–6. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- 30.Alifragis P, et al. Lhx6 regulates the migration of cortical interneurons from the ventral telencephalon but does not specify their GABA phenotype. J Neurosci. 2004;24:5643–8. doi: 10.1523/JNEUROSCI.1245-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azim E, et al. SOX6 controls dorsal progenitor identity and interneuron diversity during neocortical development. Nat Neurosci. 2009;12:1238–47. doi: 10.1038/nn.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colasante G, et al. Arx is a direct target of Dlx2 and thereby contributes to the tangential migration of GABAergic interneurons. J Neurosci. 2008;28:10674–86. doi: 10.1523/JNEUROSCI.1283-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nobrega-Pereira S, et al. Postmitotic Nkx2-1 controls the migration of telencephalic interneurons by direct repression of guidance receptors. Neuron. 2008;59:733–45. doi: 10.1016/j.neuron.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez-Bendito G, et al. Chemokine signaling controls intracortical migration and final distribution of GABAergic interneurons. J Neurosci. 2008;28:1613–24. doi: 10.1523/JNEUROSCI.4651-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li G, et al. Regional distribution of cortical interneurons and development of inhibitory tone are regulated by Cxcl12/Cxcr4 signaling. J Neurosci. 2008;28:1085–98. doi: 10.1523/JNEUROSCI.4602-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, et al. CXCR4 and CXCR7 have distinct functions in regulating interneuron migration. Neuron. 2011;69:61–76. doi: 10.1016/j.neuron.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olson EC, Walsh CA. Smooth, rough and upside-down neocortical development. Curr Opin Genet Dev. 2002;12:320–7. doi: 10.1016/s0959-437x(02)00305-2. [DOI] [PubMed] [Google Scholar]
- 38.Ben-Ari Y. Seizures beget seizures: the quest for GABA as a key player. Crit Rev Neurobiol. 2006;18:135–44. doi: 10.1615/critrevneurobiol.v18.i1-2.140. [DOI] [PubMed] [Google Scholar]
- 39.Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–39. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 40.Ben-Ari Y, et al. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–84. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- 41.Lu B, et al. Molecular mechanisms underlying neural circuit formation. Curr Opin Neurobiol. 2009;19:162–7. doi: 10.1016/j.conb.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–11. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varoqueaux F, et al. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004;83:449–56. doi: 10.1078/0171-9335-00410. [DOI] [PubMed] [Google Scholar]
- 44.Chubykin AA, et al. Activity-dependent validation of excitatory versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron. 2007;54:919–31. doi: 10.1016/j.neuron.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jamain S, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–9. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim HG, et al. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet. 2008;82:199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yan J, et al. Neurexin 1alpha structural variants associated with autism. Neurosci Lett. 2008;438:368–70. doi: 10.1016/j.neulet.2008.04.074. [DOI] [PubMed] [Google Scholar]
- 48.Tabuchi K, et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–6. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jamain S, et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc Natl Acad Sci U S A. 2008;105:1710–5. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chadman KK, et al. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008;1:147–58. doi: 10.1002/aur.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–52. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fazzari P, et al. Control of cortical GABA circuitry development by Nrg1 and ErbB4 signalling. Nature. 2010;464:1376–80. doi: 10.1038/nature08928. [DOI] [PubMed] [Google Scholar]
- 53.Ting AK, et al. Neuregulin 1 promotes excitatory synapse development and function in GABAergic interneurons. J Neurosci. 2011;31:15–25. doi: 10.1523/JNEUROSCI.2538-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karam CS, et al. Signaling pathways in schizophrenia: emerging targets and therapeutic strategies. Trends Pharmacol Sci. 2010;31:381–90. doi: 10.1016/j.tips.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Terauchi A, et al. Distinct FGFs promote differentiation of excitatory and inhibitory synapses. Nature. 2010;465:783–7. doi: 10.1038/nature09041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Knott GW, et al. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron. 2002;34:265–73. doi: 10.1016/s0896-6273(02)00663-3. [DOI] [PubMed] [Google Scholar]
- 57.Chattopadhyaya B, et al. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24:9598–611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin Y, et al. Activity-dependent regulation of inhibitory synapse development by Npas4. Nature. 2008;455:1198–204. doi: 10.1038/nature07319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morrow EM, et al. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321:218–23. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bolton MM, et al. Brain-derived neurotrophic factor differentially regulates excitatory and inhibitory synaptic transmission in hippocampal cultures. J Neurosci. 2000;20:3221–32. doi: 10.1523/JNEUROSCI.20-09-03221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang ZJ, et al. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–55. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- 63.Marty S, et al. Neuronal activity and brain-derived neurotrophic factor regulate the density of inhibitory synapses in organotypic slice cultures of postnatal hippocampus. J Neurosci. 2000;20:8087–95. doi: 10.1523/JNEUROSCI.20-21-08087.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hong EJ, et al. Transcriptional control of cognitive development. Curr Opin Neurobiol. 2005;15:21–8. doi: 10.1016/j.conb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 65.Soliman F, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 327:863–6. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Neves G, et al. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- 67.Kleschevnikov AM, et al. Hippocampal long-term potentiation suppressed by increased inhibition in the Ts65Dn mouse, a genetic model of Down syndrome. J Neurosci. 2004;24:8153–60. doi: 10.1523/JNEUROSCI.1766-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cui Y, et al. Neurofibromin regulation of ERK signaling modulates GABA release and learning. Cell. 2008;135:549–60. doi: 10.1016/j.cell.2008.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fernandez F, Garner CC. Over-inhibition: a model for developmental intellectual disability. Trends Neurosci. 2007;30:497–503. doi: 10.1016/j.tins.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 70.Fernandez F, et al. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat Neurosci. 2007;10:411–3. doi: 10.1038/nn1860. [DOI] [PubMed] [Google Scholar]
- 71.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–88. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 72.Gardiner K, et al. Down syndrome: from understanding the neurobiology to therapy. J Neurosci. 2010;30:14943–5. doi: 10.1523/JNEUROSCI.3728-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reeves RH, et al. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat Genet. 1995;11:177–84. doi: 10.1038/ng1095-177. [DOI] [PubMed] [Google Scholar]
- 74.Rueda N, et al. Chronic pentylenetetrazole but not donepezil treatment rescues spatial cognition in Ts65Dn mice, a model for Down syndrome. Neurosci Lett. 2008;433:22–7. doi: 10.1016/j.neulet.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 75.Belichenko PV, et al. Synaptic structural abnormalities in the Ts65Dn mouse model of Down Syndrome. J Comp Neurol. 2004;480:281–98. doi: 10.1002/cne.20337. [DOI] [PubMed] [Google Scholar]
- 76.Costa RM, Silva AJ. Mouse models of neurofibromatosis type I: bridging the GAP. Trends Mol Med. 2003;9:19–23. doi: 10.1016/s1471-4914(02)00008-4. [DOI] [PubMed] [Google Scholar]
- 77.Costa RM, et al. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature. 2002;415:526–30. doi: 10.1038/nature711. [DOI] [PubMed] [Google Scholar]
- 78.Bitanihirwe BK, et al. Glutamatergic deficits and parvalbumin-containing inhibitory neurons in the prefrontal cortex in schizophrenia. BMC Psychiatry. 2009;9:71. doi: 10.1186/1471-244X-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Woo TU, et al. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Arch Gen Psychiatry. 2004;61:649–57. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- 80.Torrey EF, et al. Neurochemical markers for schizophrenia, bipolar disorder, and major depression in postmortem brains. Biol Psychiatry. 2005;57:252–60. doi: 10.1016/j.biopsych.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 81.Belforte JE, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nat Neurosci. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen YJ, et al. ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1010669107. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wen L, et al. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2010;107:1211–6. doi: 10.1073/pnas.0910302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alvarez-Dolado M, et al. Cortical inhibition modified by embryonic neural precursors grafted into the postnatal brain. J Neurosci. 2006;26:7380–9. doi: 10.1523/JNEUROSCI.1540-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baraban SC, et al. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proc Natl Acad Sci U S A. 2009;106:15472–7. doi: 10.1073/pnas.0900141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martinez-Cerdeno V, et al. Embryonic MGE precursor cells grafted into adult rat striatum integrate and ameliorate motor symptoms in 6-OHDA-lesioned rats. Cell Stem Cell. 2010;6:238–50. doi: 10.1016/j.stem.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Southwell DG, et al. Cortical plasticity induced by inhibitory neuron transplantation. Science. 2010;327:1145–8. doi: 10.1126/science.1183962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sebe JY, Baraban SC. The promise of an interneuron-based cell therapy for epilepsy. Dev Neurobiol. 2010;71:107–17. doi: 10.1002/dneu.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang C, et al. A neuroligin-4 missense mutation associated with autism impairs neuroligin-4 folding and endoplasmic reticulum export. J Neurosci. 2009;29:10843–54. doi: 10.1523/JNEUROSCI.1248-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Etherton MR, et al. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc Natl Acad Sci U S A. 2009;106:17998–8003. doi: 10.1073/pnas.0910297106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen RZ, et al. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–31. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- 92.Guy J, et al. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–6. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- 93.Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–37. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 94.Shahbazian M, et al. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–54. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- 95.Collins AL, et al. Mild overexpression of MeCP2 causes a progressive neurological disorder in mice. Hum Mol Genet. 2004;13:2679–89. doi: 10.1093/hmg/ddh282. [DOI] [PubMed] [Google Scholar]
- 96.Chao HT, et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–9. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shilyansky C, et al. Neurofibromin regulates corticostriatal inhibitory networks during working memory performance. Proc Natl Acad Sci U S A. 107:13141–6. doi: 10.1073/pnas.1004829107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mohn AR, et al. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–36. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- 99.Pletnikov MV, et al. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry. 2008;13:173–86. 115. doi: 10.1038/sj.mp.4002079. [DOI] [PubMed] [Google Scholar]
- 100.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–9. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Noebels JL. The biology of epilepsy genes. Annu Rev Neurosci. 2003;26:599–625. doi: 10.1146/annurev.neuro.26.010302.081210. [DOI] [PubMed] [Google Scholar]
- 102.D’Antuono M, et al. Involvement of cholinergic and gabaergic systems in the fragile X knockout mice. Neuroscience. 2003;119:9–13. doi: 10.1016/s0306-4522(03)00103-9. [DOI] [PubMed] [Google Scholar]
- 103.Dolen G, et al. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–62. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Selby L, et al. Major defects in neocortical GABAergic inhibitory circuits in mice lacking the fragile X mental retardation protein. Neurosci Lett. 2007;412:227–32. doi: 10.1016/j.neulet.2006.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gibson JR, et al. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J Neurophysiol. 2008;100:2615–26. doi: 10.1152/jn.90752.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Curia G, et al. Downregulation of tonic GABAergic inhibition in a mouse model of fragile X syndrome. Cereb Cortex. 2009;19:1515–20. doi: 10.1093/cercor/bhn159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fmr1 knockout mice: a model to study fragile X mental retardation. The Dutch-Belgian Fragile X Consortium. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- 108.Hall J, et al. A neuregulin 1 variant associated with abnormal cortical function and psychotic symptoms. Nat Neurosci. 2006;9:1477–8. doi: 10.1038/nn1795. [DOI] [PubMed] [Google Scholar]