Abstract
Estrogen deficiency results in accelerated bone turnover with a net increase in bone resorption. Subcutaneous administration of leptin attenuates bone loss in ovariectomized (ovx) rats by reducing bone resorption. However, in addition to its direct beneficial effects, leptin has been reported to have indirect (central nervous system-mediated) antiosteogenic effects on bone, which may limit the efficacy of elevated serum leptin to prevent estrogen deficiency-associated bone loss. The present study evaluated the long-term effects of increased hypothalamic leptin transgene expression, using recombinant adeno-associated virus-leptin (rAAV-Lep) gene therapy, on bone mass, architecture, and cellular endpoints in sexually mature ovx Sprague-Dawley rats. Ovx rats were implanted with cannulae in the 3rd ventricle of the hypothalamus and injected with either rAAV-Lep or rAAV-GFP (control vector encoding green fluorescent protein) and maintained for 10 weeks. Additional controls consisted of ovary-intact rats and ovx rats pair-fed to rAAV-Lep rats. Lumbar vertebrae were analyzed by micro-computed tomography and tibiae by histomorphometry. Cancellous bone volume was lower and osteoclast perimeter, osteoblast perimeter, and bone marrow adipocyte density were greater in ovx rats compared to ovary-intact controls. In contrast, differences among ovx groups were not detected for any endpoint evaluated. In conclusion, whereas estrogen deficiency resulted in marked cancellous osteopenia, increased bone turnover and marrow adiposity, increasing hypothalamic leptin transgene expression in ovx rats had neither detrimental nor beneficial effects on bone mass, architecture, or cellular endpoints. These findings demonstrate that the antiresorptive effects of subcutaneous leptin administration in ovx rats are mediated through leptin targets in the periphery.
Keywords: Bone marrow adiposity, White adipose tissue, μCT, Histomorphometry, Osteoclasts, Osteoblasts
1. Introduction
Estrogen is an important physiological regulator of bone metabolism [48,49]. The decrease in circulating estrogen levels following menopause leads to accelerated bone turnover with a net increase in bone resorption, resulting in bone loss and increased risk for osteoporotic fractures. The magnitude of bone loss is influenced by genetic and lifestyle factors. A low body weight is associated with a greatly increased risk for postmenopausal osteoporosis, whereas high body weight confers partial protection [7,14,16,38]. There is evidence to suggest that the adipocyte-derived hormone leptin may contribute to the protective effect of higher body weight to slow bone loss following menopause [20,46,53].
Leptin is produced primarily by adipocytes and acts on multiple tissues, including bone [15,18,30,34,42,43,45]. Normal bone growth is reduced and bone maturation is disrupted in leptin-deficient ob/ob mice [43], and leptin receptor-deficient db/db mice [30,52] and fa/fa (Zucker) rats [45]. Leptin receptor-deficient fa/fa rats, in addition to having reduced bone size, have decreased cancellous bone volume. The cancellous osteopenia in the fa/fa rats is associated with an increase in osteoclast-lined bone perimeter. The inhibitory effect of leptin on osteoclast number could be mediated by the hormone’s direct actions to suppress osteoclast differentiation [21]. However, osteoclast differentiation and survival is regulated by locally produced factors such as receptor activator of nuclear factor kappa B ligand (RANKL) which is required for osteoclast differentiation and by osteoprotegerin (OPG) which is a decoy receptor for RANKL that attenuates osteoclast differentiation by sequestering RANKL. Leptin has been reported to increase the ratio of OPG/RANKL produced by cultured osteoblasts and peripheral blood mononuclear cells [6,21,27,29], suggesting an indirect mechanism by which serum leptin could inhibit osteoclastogenesis and bone resorption.
Ovariectomy (ovx) results in weight gain, hyperleptinemia, and skeletal changes in rats similar to those following menopause in women [5,23,37,47,49]. Because of the notable similarities, the ovx rat is the gold standard as a preclinical model for post-menopausal bone loss. Ovx-induced bone loss was attenuated following continuous subcutaneous administration of leptin [5]. Thus, high circulating levels of leptin may partially protect the skeleton against the detrimental effects of low estrogen levels by suppressing bone resorption. However, in addition to its systemic (peripheral) actions, leptin has also been reported to suppress bone formation and increase bone resorption in mice through a hypothalamic relay [20]. The putative antiosteogenic effects of central leptin may limit the efficacy of increased serum leptin to prevent bone loss. Therefore, we evaluated the effect of increased hypothalamic leptin transgene expression to impact bone loss in sexually mature ovx rats.
2. Methods
2.1. Animals
Female Sprague-Dawley rats weighing 230–250 g were obtained from Harlan (Indianapolis, IN). The rats were maintained in accordance with the NIH Guide for the Care and Use of Laboratory Animals, and the experimental protocol was approved by the Institutional Animal Care and Use Committee at the University of Florida (Gainesville, FL) where the study was conducted. The rats were housed individually in a temperature (22–25 °C) and light (lights on 5 am–7 pm) controlled room at the McKnight Brain Institute under specific pathogen-free conditions.
2.2. Construction and packaging of rAAV vectors
To enhance leptin transgene expression in the hypothalamus, a non-pathogenic and non-immunogenic recombinant adeno-associated virus encoding either leptin (rAAV-Lep) or the control vector (green fluorescent protein, rAAV-GFP) was packaged, purified, concentrated, and titered as described [47].
2.3. Surgery
The rats were bilaterally ovx and then stereotaxically implanted with a permanent steel cannula in the third cerebroventricle of the hypothalamus under ketamine–xylazine (100 mg/kg and 15 mg/kg body weight) anesthesia as described [47]. After one week of recovery, the rats were weight-matched and injected intracerebroventricularly (icv) with either rAAV-Lep (5 μL, 4.6 × 1013 infectious particles/mL, n = 7) or rAAV-GFP (5 μL, 4.3 × 1012 infectious particles/mL, n = 6). Control groups included unoperated intact rats (n = 6) and ovx rats (n = 6) pair-fed to consume the same amount of food as the rAAV-Lep rats. The animals were monitored for 10 weeks and then sacrificed by decapitation. Second lumbar vertebrae and tibiae were excised and placed in 70% ethanol for micro-computed tomography (μCT) and histomorphometric assessment.
2.4. μCT
μCT was used for nondestructive three-dimensional evaluation of cancellous bone mass and architecture. Second lumbar vertebrae were scanned using a Scanco μCT40 scanner (Scanco Medical AG, Basserdorf, Switzerland) at a voxel size of 16 μm × 16 μm × 16 μm. The threshold for analysis was determined empirically and set at 245 (scale 0–1000). Analysis of the vertebral body included the region of secondary spongiosa between the cranial and caudal growth plates. Cancellous bone measurements included cancellous bone volume/tissue volume (bone volume, %), trabecular thickness (μm), trabecular number (mm−1), and trabecular separation (μm).
2.5. Histomorphometry
For histomorphometric evaluation of cancellous bone, proximal tibiae were dehydrated in a graded series of ethanol and xylene, and embedded undecalcified in modified methyl methacrylate as described [24]. Longitudinal sections (4 μm thick) were cut with a vertical bed microtome (Leica 2065) and affixed to gelatin-coated slides. One section/animal was stained according to the Von Kossa method with a tetrachrome counter stain (Poly-sciences, Warrington, PA) and used for assessing bone area and cell-based measurements. Measurements were performed in a standard metaphyseal sampling site, 0.5 mm distal to the growth plate, using the OsteoMeasure System (OsteoMetrics, Inc., Atlanta, GA). Measurements included cancellous bone area/tissue area (bone area, %) and the derived architectural indices of trabecular thickness (μm), trabecular number (mm−1) and trabecular separation (μm). Osteoblast and osteoclast perimeters were measured and expressed as % total cancellous bone perimeter. Adipocyte area/tissue area (adipocyte area, %), adipocyte density (#/mm2), and adipocyte size (μm2) were determined as described [33]. Morphologically, adipocytes were identified as large circular or oval shaped cells, bordered by a prominent cell membrane and deficient in cytoplasmic staining due to alcohol extraction of intracellular lipids during processing. All measured and derived data were generated according to standardized methods, derivations, and nomenclature [36].
2.6. Statistical analysis
A one-way ANOVA followed by a Bonferroni post hoc test was used to evaluate differences among treatment groups (SPSS 17.0, SPSS Inc., Chicago, IL). If ANOVA assumptions of homogeneity of variance were not met, a Kruskal–Wallis followed by a Tamhane post hoc test was used. Differences were considered significant at P < 0.05. All data are expressed as mean ± SE.
3. Results
The effects of ovx and central rAAV-Lep gene therapy on body weight and food intake over the 10-week duration of treatment and on hypothalamic leptin mRNA levels, abdominal white adipose tissue weight, and serum leptin levels at necropsy from this study are described in detail elsewhere [47]. In brief, the increase in hypothalamic leptin expression attenuated ovx-induced hyperphagia and weight gain and resulted in lower abdominal white adipose tissue mass and serum leptin levels. The unreported effects on bone architecture and other skeletal endpoints in these animals are presented below.
3.1. Lumbar vertebra (μCT)
The effects of ovx and hypothalamic rAAV-Lep gene therapy on cancellous bone mass and architecture in the 2nd lumbar vertebra are shown in Table 1. Cancellous bone volume and trabecular number were lower while trabecular separation was higher in ovx rats compared to intact controls, irrespective of rAAV treatment. Trabecular thickness was lower in ovx rAAV-Lep and rAAV-GFP rats compared to intact controls. Significant differences in trabecular thickness were not detected between pair-fed ovx rats and intact control rats. Significant differences between rAAV-Lep and rAAV-GFP rats were not detected for any of the endpoints evaluated.
Table 1.
Effects of ovariectomy and increased hypothalamic rAAV-lep gene expression on cancellous bone mass and architecture in the 2nd lumbar vertebra.
| Endpoint | Treatment
|
ANOVA P< | |||
|---|---|---|---|---|---|
| Intact control | Ovx + pair-fed | Ovx + rAAV-GFP | Ovx + rAAV-Lep | ||
| Bone volume/tissue volume (%) | 38.6 ± 1.0 | 29.2 ± 2.4a | 26.4 ± 1.0a | 23.3 ± 1.3a | 0.001 |
| Trabecular number (mm−1) | 4.5 ± 0.0 | 3.7 ± 0.2a | 3.6 ± 0.1a | 3.2 ± 0.1a | 0.001 |
| Trabecular thickness (μm) | 82 ± 2 | 79 ± 2 | 74 ± 1a | 74 ± 1a | 0.003 |
| Trabecular separation (μm) | 203 ± 1 | 262 ± 16* | 264 ± 8a | 304 ± 15a | 0.001 |
Mean ± SE.
Different from intact control, P < 0.05.
P < 0.1.
3.2. Tibial metaphysis (histomorphometry)
The effects of ovx and hypothalamic rAAV-Lep gene therapy on cancellous bone mass and architecture in the proximal tibial metaphysis are shown in Table 2. Cancellous bone area and trabecular number were lower while trabecular separation was higher in ovx rats compared to intact control rats, irrespective of rAAV treatment. Significant differences among treatment groups were not detected for trabecular thickness.
Table 2.
Effects of ovariectomy and increased hypothalamic rAAV-lep gene expression on cancellous bone mass and architecture in the proximal tibial metaphysis.
| Endpoint | Treatment
|
ANOVA P< | |||
|---|---|---|---|---|---|
| Intact control | Ovx + pair-fed | Ovx + rAAV-GFP | Ovx + rAAV-Lep | ||
| Bone area/tissue area (%) | 30.4 ± 3.3 | 12.4 ± 2.7a | 12.5 ± 1.7a | 9.5 ± 0.9a | 0.001 |
| Trabecular number (mm−1) | 5.1 ± 0.4 | 2.5 ± 0.3a | 2.4 ± 0.3a | 1.9 ± 0.2a | 0.001 |
| Trabecular thickness (μm) | 60 ± 3 | 49 ± 6 | 52 ± 2 | 50 ± 2 | 0.171 |
| Trabecular separation (μm) | 143 ± 15 | 390 ± 59a | 398 ± 52a | 508 ± 59a | 0.001 |
Mean ± SE.
Different from intact control, P < 0.05.
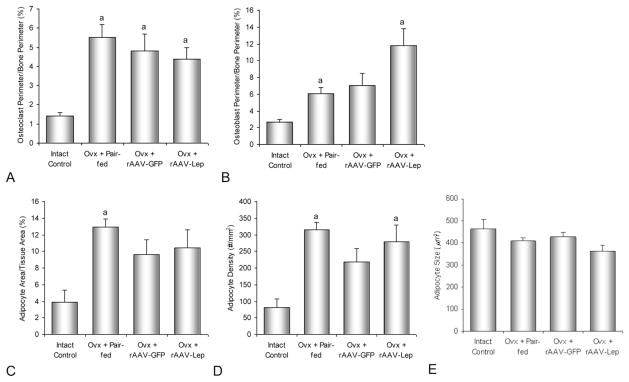
The effects of ovx and hypothalamic rAAV-Lep gene therapy on cellular endpoints in the proximal tibial metaphysis are shown in Figs. 1 and 2. Osteoclast perimeter, an index of bone resorption, was higher in ovx rats compared to ovary-intact controls. Also, osteoblast perimeter, an index of bone formation, was higher in ovx pair-fed rats and rAAV-Lep rats compared to ovary-intact controls. However, significant differences in osteoclast perimeter or osteoblast perimeter were not detected among ovx treatment groups. Bone marrow adipocyte density was higher in rAAV-Lep and pair-fed ovx rats compared to intact controls. Significant differences in adipocyte size were not detected with treatment. Significant differences between ovx rAAV-Lep and AAV-GFP rats were not detected for any of the cellular endpoints evaluated.
Fig. 1.
Effects of ovariectomy and increased hypothalamic rAAV-lep gene expression on osteoclast perimeter/bone perimeter (A), osteoblast perimeter/bone perimeter (B), adipocyte area/tissue area (C), adipocyte density (D), and adipocyte size (E) in the proximal tibial metaphysis. aDifferent from ovary-intact control, P < 0.05.
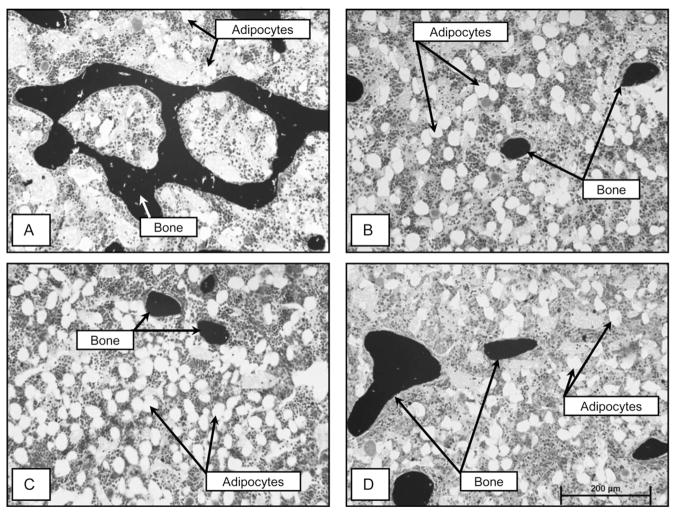
Fig. 2.
Photomicrographs showing representative histology in proximal tibia in an ovary-intact control rat (A), an ovx + pair-fed rat (B), an ovx rAAV-GFP-treated rat (C), and an ovx rAAV-Lep-treated rat (D). Please note the low bone mass and high adiposity in the three ovx treatment groups compared to the ovary-intact group. See Tables 1 and 2 for quantitative changes in bone mass and architecture and Fig. 1 for changes in cellular endpoints.
4. Discussion
Ovx resulted in anticipated changes in bone mass, architecture, and turnover at the axial (lumbar vertebra) and appendicular (proximal tibial metaphysis) sites evaluated: ovx animals had reduced cancellous bone volume, reduced trabecular number, increased trabecular separation, increased osteoclast and osteoblast perimeters, and increased bone marrow adiposity. Despite significantly elevated hypothalamic leptin transgene expression and attenuation of weight gain in rAAV-Lep-treated ovx rats [47], differences among ovx pair-fed, rAAV-GFP-treated, and rAAV-Lep-treated rats were not detected for any bone measurements evaluated.
We have shown previously that hypothalamic leptin gene therapy increases the hypothalamic availability of leptin without increasing leptin transgene expression in extrahypothalamic tissues or the overflow of transduced leptin into cerebrospinal fluid or systemic circulation [9,25,50]. As a consequence, this method provides a unique technology to unambiguously separate central from peripheral actions of the hormone on bone and energy metabolism. This contrasts with systemic administration of leptin which has been shown to rapidly increase not only systemic but also central levels of the hormone. Similarly, icv administration of leptin was found to raise the hormone rapidly in systemic circulation [32].
As reported in detail elsewhere [47], hypothalamic leptin gene expression was increased while food intake, weight gain, abdominal white adipose tissue mass and serum leptin levels were decreased in ovx rAAV-Lep compared to ovx rAAV-GFP rats. These findings are consistent with numerous other studies documenting the efficacy of central leptin gene therapy in attenuating weight gain in various paradigms in rats and mice [2,3,11,22,26]. Hypothalamic leptin gene therapy also modified the expression of hypothalamic genes that regulate body weight, such as NPY, POMC and CART, and systemic levels of hormones and adipokines, such as leptin, insulin, IGF-I, adiponectin, and IL-6 [2,10,12,22,28,47], factors shown to influence bone metabolism [31,46]. Although not assessed in this study, based on the current results it is tempting to speculate that these potential modifications had little or no impact on the skeletal response to ovx.
Our results raise a pertinent question on the significance of these findings. The bone loss following ovx in rodents and following menopause in women is due to increased bone resorption [48,49]. Peripheral leptin administration attenuated weight gain and bone loss in ovx animals without increasing bone formation [5]. Dose-dependent increases in OPG and decreases in RANKL in the presence of leptin were observed in cultured osteoblasts, consistent with an osteoblast-mediated action of leptin to suppress bone resorption [5]. In contrast to peripheral administration of leptin, which results in an increase in serum leptin, hypothalamic leptin gene therapy results in decreased serum leptin levels [47]. The failure of hypothalamic leptin gene therapy to attenuate cancellous bone loss following ovx observed in this study strongly suggests that beneficial bone sparing effects of peripherally administered leptin in ovx rats are solely mediated through targets in the periphery. This is the only study to date to affirm that (1) the ovx-induced detrimental skeletal effects greatly exceed any additional actions attributable to increased hypothalamic leptin; and (2) the bone sparing effects following peripheral administration of leptin in ovx rats is not due to hypothalamic actions of the hormone. In addition, our findings add to an increasing body of evidence that central leptin is not antiosteogenic, as currently believed by many researchers in the field [20].
The absence of a significant effect of increased hypothalamic leptin gene expression on bone mass and architecture contrasts with the dramatic bone loss following ovx in this and numerous prior studies [23,48]. In this context, it is important to consider that the rate of bone loss in the ovx rat model is modifiable by a variety of nutritional, hormonal, and environmental factors. For example, increases in serum leptin levels or physical activity slow ovx-induced bone loss whereas inflammation or skeletal disuse accelerates it [5,8,51]. Thus, modifiable factors including peripheral leptin can partially compensate for reduced estrogen levels and slow bone loss following menopause.
There are two other new implications of this study. First, weight loss and excessive weight gain are each associated with increased bone marrow adiposity [4,13]. Postmenopausal osteoporotic patients also exhibit an increase in bone marrow fat [1,35], a finding replicated in estrogen-deficient rats in this and earlier studies [40]. There are multiple mechanisms by which excessive bone marrow adiposity could negatively impact bone metabolism, which include adipocyte differentiation at the expense of osteoblast differentiation, and/or excessive production of adipokines that antagonize bone formation and/or enhance bone resorption [39,44]. In the present study, the increase in adipocyte density was associated with ovx-induced weight gain but did not occur at the expense of reduced osteoblast number as both osteoblast perimeter and adipocyte density were increased in rats following ovx. Other studies examining cell differentiation indicate that in vivo differentiation of osteoblasts or adipocytes is not mutually exclusive [33]. For example, highly osteoporotic senescent rats, in spite of increased bone marrow adiposity, retain the capacity to respond to the potent bone anabolic agent prostaglandin E2 [41]. Also, aged animals become more responsive to the bone anabolic actions of parathyroid hormone, which can dramatically increase bone formation without reducing bone marrow adiposity [17].
Second, Hamrick et al. [19] showed a rapid reduction in adipocyte number and increased adipocyte apoptosis in bone marrow following twice daily administration of leptin into the hypothalamus. In contrast, we did not observe an effect of hypothalamic rAAV-Lep on bone marrow adiposity. Possible explanations for the inter-study differences in marrow fat following leptin treatment include effects of leptin leakage into the periphery after central injection, differences in exposure to the hormone (intermittent versus continuous), gonadal status (normal versus ovx), and duration of treatment (<1 week versus 10 weeks).
In summary, despite attenuation of weight gain and reduced abdominal fat in ovx rats [47], central leptin gene therapy produced no significant impact on the magnitude of bone loss in vertebra or tibia following ovx. Therefore, it is highly conceivable that the protective effects of increased body weight in reducing the risk of postmenopausal osteoporosis may be mediated peripherally, in part, by elevated serum leptin levels.
Acknowledgments
This work was supported by grants from the National Institute of Health to U.T. Iwaniec (AR 054609) and S.P. Kalra (DK 37273 and 27372) and the Center for Healthy Aging Research Life Scholar Award, Oregon State University to M.A. Jackson.
Footnotes
Conflict of interest
None of the authors have a conflict of interest.
Individual contribution to article
M.A. Jackson: μCT data collection and manuscript preparation.
U.T. Iwaniec: Data analysis and manuscript preparation.
R.T. Turner: Histomorphometric data collection and manuscript preparation.
T.J. Wronski: Preparation of bone specimens for histology and manuscript preparation.
S.P. Kalra: Experimental design, study execution, and manuscript preparation.
References
- 1.Astudillo P, Rios S, Pastenes L, Pino AM, Rodriguez JP. Increased adipogenesis of osteoporotic human-mesenchymal stem cells (MSCs) characterizes by impaired leptin action. J Cell Biochem. 2008;103:1054–65. doi: 10.1002/jcb.21516. [DOI] [PubMed] [Google Scholar]
- 2.Beretta E, Dube MG, Kalra PS, Kalra SP. Long-term suppression of weight gain, adiposity, and serum insulin by central leptin gene therapy in prepubertal rats: effects on serum ghrelin and appetite-regulating genes. Pediatr Res. 2002;52:189–98. doi: 10.1203/00006450-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Boghossian S, Lecklin A, Torto R, Kalra PS, Kalra SP. Suppression of fat deposition for the life time with gene therapy. Peptides. 2005;26:1512–9. doi: 10.1016/j.peptides.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 4.Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, et al. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring) 2010 doi: 10.1038/oby.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burguera B, Hofbauer LC, Thomas T, Gori F, Evans GL, Khosla S, et al. Leptin reduces ovariectomy-induced bone loss in rats. Endocrinology. 2001;142:3546–53. doi: 10.1210/endo.142.8.8346. [DOI] [PubMed] [Google Scholar]
- 6.Cornish J, Callon KE, Bava U, Lin C, Naot D, Hill BL, et al. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol. 2002;175:405–15. doi: 10.1677/joe.0.1750405. [DOI] [PubMed] [Google Scholar]
- 7.De Laet C, Kanis JA, Oden A, Johanson H, Johnell O, Delmas P, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16:1330–8. doi: 10.1007/s00198-005-1863-y. [DOI] [PubMed] [Google Scholar]
- 8.Desimone DP, Greene VS, Hannon KS, Turner RT, Bell NH. Prostaglandin E2 administered by subcutaneous pellets causes local inflammation and systemic bone loss: a model for inflammation-induced bone disease. J Bone Miner Res. 1993;8:625–34. doi: 10.1002/jbmr.5650080514. [DOI] [PubMed] [Google Scholar]
- 9.Dhillon H. Effects of recombinant adeno-associated virus encoding leptin on body weight regulation and energy homeostasis. Gainesville: University of Florida; 2000. [Google Scholar]
- 10.Dhillon H, Kalra SP, Kalra PS. Dose-dependent effects of central leptin gene therapy on genes that regulate body weight and appetite in the hypothalamus. Mol Ther. 2001;4:139–45. doi: 10.1006/mthe.2001.0427. [DOI] [PubMed] [Google Scholar]
- 11.Dhillon H, Kalra SP, Prima V, Zolotukhin S, Scarpace PJ, Moldawer LL, et al. Central leptin gene therapy suppresses body weight gain, adiposity and serum insulin without affecting food consumption in normal rats: a long-term study. Regul Pept. 2001;99:69–77. doi: 10.1016/s0167-0115(01)00237-3. [DOI] [PubMed] [Google Scholar]
- 12.Dube MG, Torto R, Kalra SP. Increased leptin expression selectively in the hypothalamus suppresses inflammatory markers CRP and IL-6 in leptin-deficient diabetic obese mice. Peptides. 2008;29:593–8. doi: 10.1016/j.peptides.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ecklund K, Vajapeyam S, Feldman HA, Buzney CD, Mulkern RV, Kleinman PK, et al. Bone marrow changes in adolescent girls with anorexia nervosa. J Bone Miner Res. 2010;25:298–304. doi: 10.1359/jbmr.090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Hage R, Jacob C, Moussa E, Benhamou CL, Jaffre C. Total body, lumbar spine and hip bone mineral density in overweight adolescent girls: decreased or increased? J Bone Miner Metab. 2009;27:629–33. doi: 10.1007/s00774-009-0074-6. [DOI] [PubMed] [Google Scholar]
- 15.Farooqi IS, O’Rahilly S. Leptin: a pivotal regulator of human energy homeostasis. Am J Clin Nutr. 2009;89:980S–4S. doi: 10.3945/ajcn.2008.26788C. [DOI] [PubMed] [Google Scholar]
- 16.Fogelholm GM, Sievanen HT, Kukkonen-Harjula TK, Pasanen ME. Bone mineral density during reduction, maintenance and regain of body weight in premenopausal, obese women. Osteoporos Int. 2001;12:199–206. doi: 10.1007/s001980170130. [DOI] [PubMed] [Google Scholar]
- 17.Friedl G, Turner RT, Evans GL, Dobnig H. Intermittent parathyroid hormone (PTH) treatment and age-dependent effects on rat cancellous bone and mineral metabolism. J Orthop Res. 2007;25:1454–64. doi: 10.1002/jor.20433. [DOI] [PubMed] [Google Scholar]
- 18.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–70. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 19.Hamrick MW, Della Fera MA, Choi YH, Hartzell D, Pennington C, Baile CA. Injections of leptin into rat ventromedial hypothalamus increase adipocyte apoptosis in peripheral fat and in bone marrow. Cell Tissue Res. 2007;327:133–41. doi: 10.1007/s00441-006-0312-3. [DOI] [PubMed] [Google Scholar]
- 20.Hamrick MW, Ferrari SL. Leptin and the sympathetic connection of fat to bone. Osteoporos Int. 2008;19:905–12. doi: 10.1007/s00198-007-0487-9. [DOI] [PubMed] [Google Scholar]
- 21.Holloway WR, Collier FM, Aitken CJ, Myers DE, Hodge JM, Malakellis M, et al. Leptin inhibits osteoclast generation. J Bone Miner Res. 2002;17:200–9. doi: 10.1359/jbmr.2002.17.2.200. [DOI] [PubMed] [Google Scholar]
- 22.Iwaniec UT, Boghossian S, Trevisiol CH, Wronski TJ, Turner RT, Kalra SP. Hypothalamic leptin gene therapy prevents weight gain without long-term detrimental effects on bone in growing and skeletally mature female rats. J Bone Miner Res. doi: 10.1002/jbmr.365. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwaniec UT, Turner RT. Animal models of osteoporosis. In: Marcus R, Feldman D, Nelson DA, Rosen C, editors. Osteoporosis. 3. Amsterdam: Elsevier; 2008. pp. 985–1110. [Google Scholar]
- 24.Iwaniec UT, Wronski TJ, Turner RT. Histological analysis of bone. Methods Mol Biol. 2008;447:325–41. doi: 10.1007/978-1-59745-242-7_21. [DOI] [PubMed] [Google Scholar]
- 25.Kalra SP. Global life-long health benefits of repression of hypothalamic NPY system by central leptin gene therapy. Curr Top Med Chem. 2007;7:1675–81. doi: 10.2174/156802607782340993. [DOI] [PubMed] [Google Scholar]
- 26.Kalra SP, Kalra PS. Neuroendocrine control of energy homeostasis: update on new insights. Prog Brain Res. 2010;181:17–33. doi: 10.1016/S0079-6123(08)81002-3. [DOI] [PubMed] [Google Scholar]
- 27.Lamghari M, Tavares L, Camboa N, Barbosa MA. Leptin effect on RANKL and OPG expression in MC3T3-E1 osteoblasts. J Cell Biochem. 2006;98:1123–9. doi: 10.1002/jcb.20853. [DOI] [PubMed] [Google Scholar]
- 28.Lecklin A, Dube MG, Torto RN, Kalra PS, Kalra SP. Perigestational suppression of weight gain with central leptin gene therapy results in lower weight F1 generation. Peptides. 2005;26:1176–87. doi: 10.1016/j.peptides.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 29.Lee YJ, Park JH, Ju SK, You KH, Ko JS, Kim HM. Leptin receptor isoform expression in rat osteoblasts and their functional analysis. FEBS Lett. 2002;528:43–7. doi: 10.1016/s0014-5793(02)02889-2. [DOI] [PubMed] [Google Scholar]
- 30.Lorentzon R, Alehagen U, Boquist L. Osteopenia in mice with genetic diabetes. Diabetes Res Clin Pract. 1986;2:157–63. doi: 10.1016/s0168-8227(86)80017-1. [DOI] [PubMed] [Google Scholar]
- 31.Magni P, Dozio E, Galliera E, Ruscica M, Corsi MM. Molecular aspects of adipokine–bone interactions. Curr Mol Med. 2010;10:522–32. doi: 10.2174/1566524011009060522. [DOI] [PubMed] [Google Scholar]
- 32.Maness LM, Kastin AJ, Farrell CL, Banks WA. Fate of leptin after intracerebroventricular injection into the mouse brain. Endocrinology. 1998;139:4556–62. doi: 10.1210/endo.139.11.6319. [DOI] [PubMed] [Google Scholar]
- 33.Menagh PJ, Turner RT, Jump DB, Wong CP, Lowry MB, Yakar S, et al. Growth hormone regulates the balance between bone formation and bone marrow adiposity. J Bone Miner Res. 2010;25:757–68. doi: 10.1359/jbmr.091015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–8. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 35.Nuttall ME, Gimble JM. Controlling the balance between osteoblastogenesis and adipogenesis and the consequent therapeutic implications. Curr Opin Pharmacol. 2004;4:290–4. doi: 10.1016/j.coph.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, et al. Bone histomorphometry: standardization of nomenclature, symbols, and units, Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 37.Rachon D, Teede H. Ovarian function and obesity – interrelationship, impact on women’s reproductive lifespan and treatment options. Mol Cell Endocrinol. 2010;316:172–9. doi: 10.1016/j.mce.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 38.Reid IR. Fat and bone. Arch Biochem Biophys. 2010;503:22–7. doi: 10.1016/j.abb.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 39.Rosen CJ, Klibanski A. Bone, fat, and body composition: evolving concepts in the pathogenesis of osteoporosis. Am J Med. 2009;122:409–14. doi: 10.1016/j.amjmed.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 40.Sharp JC, Copps JC, Liu Q, Ryner LN, Sebastian RA, Zeng GQ, et al. Analysis of ovariectomy and estrogen effects on body composition in rats by X-ray and magnetic resonance imaging techniques. J Bone Miner Res. 2000;15:138–46. doi: 10.1359/jbmr.2000.15.1.138. [DOI] [PubMed] [Google Scholar]
- 41.Sibonga JD, Zhang M, Ritman EL, Turner RT. Restoration of bone mass in the severely osteopenic senescent rat. J Gerontol A: Biol Sci Med Sci. 2000;55:B71–8. doi: 10.1093/gerona/55.2.b71. discussion B9–84. [DOI] [PubMed] [Google Scholar]
- 42.Smith CK, Romsos DR. Cold acclimation of obese (ob/ob) mice: effects of energy balance. Metabolism. 1984;33:853–7. doi: 10.1016/0026-0495(84)90114-8. [DOI] [PubMed] [Google Scholar]
- 43.Steppan CM, Crawford DT, Chidsey-Frink KL, Ke H, Swick AG. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept. 2000;92:73–8. doi: 10.1016/s0167-0115(00)00152-x. [DOI] [PubMed] [Google Scholar]
- 44.Takada I, Kouzmenko AP, Kato S. Molecular switching of osteoblastogenesis versus adipogenesis: implications for targeted therapies. Expert Opin Ther Targets. 2009;13:593–603. doi: 10.1517/14728220902915310. [DOI] [PubMed] [Google Scholar]
- 45.Tamasi JA, Arey BJ, Bertolini DR, Feyen JH. Characterization of bone structure in leptin receptor-deficient Zucker (fa/fa) rats. J Bone Miner Res. 2003;18:1605–11. doi: 10.1359/jbmr.2003.18.9.1605. [DOI] [PubMed] [Google Scholar]
- 46.Thomas T, Burguera B, Melton LJ, 3rd, Atkinson EJ, O’Fallon WM, Riggs BL, et al. Role of serum leptin, insulin, and estrogen levels as potential mediators of the relationship between fat mass and bone mineral density in men versus women. Bone. 2001;29:114–20. doi: 10.1016/s8756-3282(01)00487-2. [DOI] [PubMed] [Google Scholar]
- 47.Torto R, Boghossian S, Dube MG, Kalra PS, Kalra SP. Central leptin gene therapy blocks ovariectomy-induced adiposity. Obesity (Silver Spring) 2006;14:1312–9. doi: 10.1038/oby.2006.149. [DOI] [PubMed] [Google Scholar]
- 48.Turner RT, Rickard DJ, Iwaniec UT, Spelsberg TC. Estrogens and progestins. In: Bilezikian JP, Raisz LG, Martin J, editors. Principles of bone biology. 3. San Diego: Academic Press; 2008. pp. 847–77. [Google Scholar]
- 49.Turner RT, Riggs BL, Spelsberg TC. Skeletal effects of estrogen. Endocr Rev. 1994;15:275–300. doi: 10.1210/edrv-15-3-275. [DOI] [PubMed] [Google Scholar]
- 50.Ueno N, Dube MG, Inui A, Kalra PS, Kalra SP. Leptin modulates orexigenic effects of ghrelin and attenuates adiponectin and insulin levels and selectively the dark-phase feeding as revealed by central leptin gene therapy. Endocrinology. 2004;145:4176–84. doi: 10.1210/en.2004-0262. [DOI] [PubMed] [Google Scholar]
- 51.Westerlind KC, Wronski TJ, Ritman EL, Luo ZP, An KN, Bell NH, et al. Estrogen regulates the rate of bone turnover but bone balance in ovariectomized rats is modulated by prevailing mechanical strain. Proc Natl Acad Sci U S A. 1997;94:4100–204. doi: 10.1073/pnas.94.8.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams GA, Callon KE, Watson M, Costa JL, Ding Y, Dickinson M, et al. Skeletal phenotype of the leptin receptor-deficient db/db mouse. J Bone Miner Res. doi: 10.1002/jbmr.367. in press. [DOI] [PubMed] [Google Scholar]
- 53.Yamauchi M, Sugimoto T, Yamaguchi T, Nakaoka D, Kanzawa M, Yano S, et al. Plasma leptin concentrations are associated with bone mineral density and the presence of vertebral fractures in postmenopausal women. Clin Endocrinol (Oxf) 2001;55:341–7. doi: 10.1046/j.1365-2265.2001.01361.x. [DOI] [PubMed] [Google Scholar]