Abstract
Purpose
The Gynecologic Oncology Group (GOG) conducted a phase II trial to assess the efficacy and tolerability of the anti-EGFR antibody cetuximab, in persistent or recurrent carcinoma of the cervix.
Patients and Methods
Eligible patients had cervical cancer, measurable disease, and GOG performance status ≤2. Treatment consisted of cetuximab 400 mg/m2 initial dose followed by 250 mg/m2 weekly until disease progression or prohibitive toxicity. The primary endpoints were progression-free survival (PFS) at 6 months and response. The study used a 2-stage group sequential design.
Results
Thirty-eight patients were entered with 3 exclusions, leaving 35 evaluable for analysis. Thirty-one patients (88.6%) received prior radiation as well as either 1 (n = 25, 71.4%) or 2 (n = 10) prior cytotoxic regimens. Twenty-four patients (68.6%) had a squamous cell carcinoma. Grade 3 adverse events possibly related to cetuximab included dermatologic (n = 5), GI (n = 4), anemia (n = 2), constitutional (n = 3), infection (n = 2), vascular (n = 2), pain (n = 2), and pulmonary, neurological, vomiting and metabolic (n = 1 each). No clinical responses were detected. Five patients (14.3%; two-sided 90% CI, 5.8% to 30%) survived without progression for at least 6 months. The median PFS and overall survival (OS) times were 1.97 and 6.7 months, respectively. In this study, all patients with PFS at 6 months harbored tumors with squamous cell histology.
Conclusion
Cetuximab is well tolerated but has limited activity in this population. Cetuximab activity may be limited to patients with squamous cell histology.
Keywords: Cervical neoplasms, cetuximab, EGFR, immunotherapy
INTRODUCTION
Cervical cancer remains the second leading cause of cancer death among women worldwide and remains an important health problem for women especially in underserved and minority groups in the Unites States [1]. Although cervical cancer can be cured by radical surgery or radiotherapy with equal effectiveness, pelvic chemo-radiation represents the standard therapy for the treatment of locally advanced disease [2]. Despite technological advances, however, up to 35% of patients overall will develop persistent/recurrent/metastatic disease. Platinum-based chemotherapy represents the gold standard therapy for the treatment of cervical cancer patients who have not responded to primary therapy or have recurrent disease no longer amenable to control with surgery and/or radiation therapy. Unfortunately, most responses to chemotherapy are partial and of short duration, and the most effective treatment of patients with progressive disease on platinum-based chemotherapy has yet to be defined.
The epidermal growth factor receptor (EGFR; HER1/erbB-1) has recently been identified as a target for cancer therapy in multiple human tumors [3–8]. On endogenous ligand binding, EGFR activation occurs, with receptor homo- or heterodimerization and autophosphorylation of the intracellular tyrosine kinase domain [9,10]. Subsequently, a complex network of signal transduction pathways is induced, which plays a key role in regulating cell proliferation, differentiation, motility, invasion, and angiogenesis [9–13]. EGFR is expressed in a variety of human malignancies and its high level of expression has been previously correlated with poor patient prognosis and resistance to treatment in many tumor entities including cervical carcinoma [13–18].
Cetuximab (C225) is a human/murine chimeric monoclonal antibody formed by cloning the heavy and light chains of the murine antibody (M225) and adapting them for expression with the constant regions of the human immunoglobulin kappa light chain and gamma 1 heavy chain [4,6,19]. Preclinical studies have demonstrated that cetuximab binds to the EGFR with high affinity and is able to compete with EGF and TGFα binding, thereby inhibiting subsequent receptor activation and signaling [20,21]. In addition, binding of cetuximab to EGFR induces receptor dimerization, internalization and receptor down regulation [22,23]. These processes may consequently lead to several effects such as cell-cycle arrest via upregulation of cyclin-dependent kinase inhibitor p27KIP1, and potentiation of apoptosis [24,25]. Other effects include inhibition of tumor production of VEGF leading to reduced tumor microvessel density and inhibition of invasion and metastases by inhibiting matrix metalloproteinases [26,27].
Clinical studies of cetuximab either alone or in combination with cytotoxic agents have demonstrated efficacy in patients with chemotherapy-refractory head and neck or colorectal cancer [28–32]. In patients with irinotecan-refractory advanced colorectal cancer, treatment with cetuximab either as a single agent or in combination with irinotecan resulted in overall remission rates of 10.8% (95% CI, 5.7% to 18.1%) and 22.9% (95% CI, 17.5% to 29.1%), respectively [30–31]. Similar results have been reported for patients with platinum-refractory head and neck cancer or non–small-cell lung cancer with cetuximab in combination with chemotherapy [32].
Cervical cancer patients with metastatic/recurrent disease not amenable to radical local excision or regional radiation and who have progressed on platinum-based chemotherapy have limited therapeutic options. The GOG conducted a phase II trial of single-agent cetuximab in patients with persistent or recurrent squamous or non-squamous cervical carcinoma. The primary endpoints of this study were the frequency of patients with tumor responses or who survived progression-free for at least 6 months (PFS at 6 months). The secondary objectives were to estimate the distribution of overall survival (OS), the distribution of PFS, the duration of objective response, and the frequency and severity of adverse events.
MATERIALS AND METHODS
Eligibility
Eligibility criteria included patients with persistent or recurrent squamous and non-squamous cell carcinoma of the cervix that was measurable by Response Evaluation Criteria in Solid Tumors (RECIST); one or two prior cytotoxic regimens, not including prior cisplatin-based chemotherapy concomitantly administered with primary pelvic radiation; GOG performance status (PS) of 0 or 1, with PS level 2 allowed in patients having received only one prior cytotoxic regimen; and adequate hematologic (absolute neutrophil count ≥1,500/μL and platelets ≥100,000/μL), renal (serum creatinine ≤1.5× the institutional upper limit of normal [ULN]); hepatic (serum bilirubin ≤1.5× ULN, and both AST and alkaline phosphatase ≤2.5× ULN) laboratory values. Histologic documentation of the original primary tumor was required with a pathology report.
Patients with other malignancies evident within 5 years, prior non-cytotoxic therapy with tyrosine kinase inhibitors or another antibody that targets the EGFR pathway for management of recurrent or persistent cervical cancer, infection requiring antibiotics, active bleeding or central nervous system (CNS) disease (craniospinal metastases, uncontrolled seizure disorder) were ineligible. Patients were also excluded for significant cardiovascular disease (uncontrolled hypertension, unstable angina, uncontrolled congestive heart failure, or uncontrolled arrhythmias within 6 months of registration) pregnancy or nursing, or major surgical procedures within 30 days or anticipated while on study. The study received local institutional review board approval at participating institutions and all patients provided authorization permitting the release of personal health information and gave informed consent according to institutional and federal guidelines before enrollment.
Treatment
Patients were treated with an initial dose of 400 mg/m2 of cetuximab followed by 250 mg/m2 weekly until disease progression or prohibitive toxicity. Each 28 day period was considered 1 cycle. Toxicity was monitored with history, physical examination, and laboratory assessment before each treatment cycle, with adverse events defined and graded according to National Cancer Institute Common Terminology Criteria version 3.0. and 4.0 Cetuximab was to be held for grade 3 non-hematologic toxicity for a maximum of 4 weeks to allow resolution to grade 2 or less. Treatment was discontinued for any grade 4 non hematologic toxicity or the 4th occurrence of a grade 3 acneiform rash.
Evaluations
Activity of cetuximab was assessed according to RECIST (1.0 version), either by clinical evaluation, computed tomography or magnetic resonance imaging at baseline, and before every other cycle for the measurement of target lesions, the classification of clinical response, and the determination of disease progression. Therapy was discontinued if there was disease progression, unacceptable toxicity, receipt of other anticancer therapy, or voluntary withdrawal.
Statistical Design
The primary endpoints to evaluate the activity of cetuximab were the frequency of patients who had either a tumor response or survived progression-free for 6 months (PFS at 6 months). A 2-stage group sequential design was employed with a targeted sample size for the first stage accrual of 19 patients but was allowed to deviate for administrative purposes [33]. If 20 patients were accrued, the critical values to proceed to the second stage for the number of patients with responses or PFS at 6 months were 2. The cumulative targeted accrual for the second stage was 34 but was allowed to deviate. If 35 patients were accrued, the critical values for the number of patients with responses or PFS at 6 months were 7 before cetuximab was deemed worthy of further investigation in a phase III trial. The null hypothesis was determined from an examination of a historical control of a similar population treated within GOG studies. Values of uninteresting probabilities of response and PFS at 6 months were determined to be 10% or less. The design had a level of significance of 10% with approximately 90% power to detect an increase in the probability of either outcome by 20%.
The distribution of PFS and OS for patients on this study was described using Kaplan-Meier plots and median estimates. Prognostic factors were evaluated with Cox proportional hazards models using end points for PFS and OS [34]. The factors assessed included performance status of 0 or worse along with squamous versus non-squamous histology. Formal inference could not be done on race since there were only 5 African-Americans on study. These analyses are considered exploratory.
RESULTS
Patient characteristics
From June 2007 through July 2009, 38 patients were enrolled, of whom 3 were excluded (1 for inappropriate prior therapy, 1 because required tests were not performed, and 1 for no documentation of treatment with cetuximab). Thus, the study sample consisted of 35 patients; 26 patients (74.3%) were Caucasian, 5 patients (14.3%) were African-American, 2 patients (5.7%) were Asian and 2 (5.7%) were Hispanic. Patient characteristics of the study group are listed in Table 1.
Table 1.
Patient Characteristics
| Characteristic | Category | No. of | % of |
|---|---|---|---|
| Age | 30–39 | 3 | 8.6 |
| 40–49 | 16 | 45.7 | |
| 50–59 | 10 | 28.6 | |
| 60–69 | 6 | 17.1 | |
| Race | Asian | 2 | 5.7 |
| African American | 5 | 14.3 | |
| Hispanic | 2 | 5.7 | |
| White | 26 | 74.3 | |
| Performance Status | 0 | 24 | 68.6 |
| 1 | 9 | 25.7 | |
| 2 | 2 | 5.7 | |
| Cell Type | Adenocarcinoma, Unsp. | 4 | 11.4 |
| Mixed Epithelial Carcinoma | 1 | 2.9 | |
| Adenosquamous | 6 | 17.1 | |
| Squamous Cell Carcinoma | 24 | 68.6 | |
| Grade | 1: Well differentiated | 6 | 17.1 |
| 2: Moderately differentiated | 16 | 45.7 | |
| 3: Poorly differentiated | 12 | 34.3 | |
| Unknown | 1 | 2.9 | |
| Prior Chemotherapy | 1 Prior Regimen | 25 | 71.4 |
| 2 Prior Regimens | 10 | 28.6 | |
| Prior Radiation | No | 4 | 11.4 |
| Yes | 31 | 88.6 | |
| Prior Surgery | No | 19 | 54.3 |
| Yes | 16 | 45.7 |
A total of 103 cycles of cetuximab were administered, with 18 patients receiving 2 cycles of treatment (51.4%). As of July 2010, all patients have discontinued therapy, 31 patients were taken off of study for disease progression, 3 discontinued therapy for toxicity as permitted by protocol, and one was taken off study protocol for other reasons (i.e., physician decision). Twenty-six patients died of disease progression. Seven patients (20%) are alive; one patient died from treatment and disease and another died from neither treatment nor disease.
Activity of cetuximab
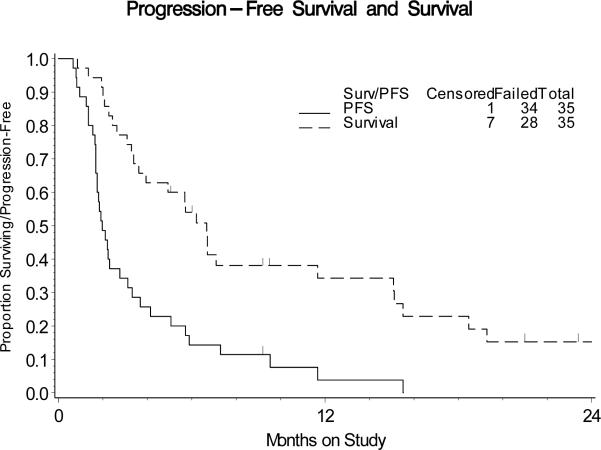
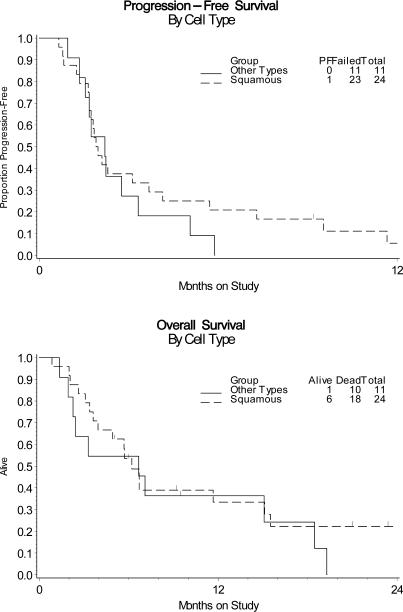
The first stage of the study acquired 20 eligible and evaluable patients. None of these patients had tumor responses but 4 were PFS at 6 months, which was sufficient to open the study to a second stage. The activity of cetuximab was analyzed in a cumulative number of 35 patients. No clinical responses were detected (Table 2). Five patients (14.3%; two-sided 90% CI, 5.8% to 30%) were PFS at 6 months. The median PFS and OS times were 1.97 and 6.7 months, respectively. The graph of the Kaplan-Meier estimates of PFS and OS for the sample is shown in Figure 1. All 5 patients with PFS at 6 months harbored tumors with squamous cell histology, which comprised 69% of the sample. In an exploratory subgroup analysis of these patients, the point estimates for the probability of being progression-free at 6 months was higher among the squamous patients than the whole sample (21% versus 14%, Figure 2). The 95% CI for the odds ratio was 0.59 to infinity.
Table 2.
Patient Response and Progression-Free Survival Status
| Characteristic | Category | No. | % of |
|---|---|---|---|
| Response | Stable disease | 11 | 31.4 |
| Increase disease | 23 | 65.7 | |
| Indeterminate | 1 | 2.9 | |
| PFS > 6 Months | No | 30 | 85.7 |
| Yes | 5 | 14.3 | |
| Cycles of | 1 | 7 | 20.0 |
| 2 | 18 | 51.4 | |
| 3 | 1 | 2.9 | |
| 4 | 4 | 11.4 | |
| 6+ | 5 | 5.7 | |
| Alive | Without progression | 1 | 2.9 |
| With progression | 6 | 17.1 | |
| Dead | From disease | 26 | 74.3 |
| From Rx and disease | 1 | 2.9 | |
| From neither Rx nor | 1 | 2.9 |
The observed proportion responding was 0.0%. The approximate 90% confidence interval for the true response rate is 0 – 6.4%. The observed proportion of patients surviving progression free for at least 6 months was 14.3%. The approximate 2-sided 90% CI for the probability of being PFS at 6 months is 5.8 – 30.0%. The approximate 1-sided 90% CI for the probability of being PFS at 6 months is 0.0– 26.8%. The required number of patients with either responses or PFS at 6 months to declare the regimen interesting was 7 and 7 out of 35 patients (20.0%), respectively.
Figure 1.
Kaplan-Meier estimates of PFS and OS for the entire study population.
Figure 2.
Kaplan-Meier estimates of PFS and OS for the study population of patients harboring squamous versus non-squamous cervical cancer.
Adverse Events
As shown in Table 3, safety of cetuximab in all 35 patients was analyzed descriptively; events listed had been reported as at least possibly related to study drug. There were no grade 4 events. One patient was listed by the GOG Data Safety and Monitoring Board as having died from both treatment and disease (bowel perforation) but the institution did not agree. Grade 3 adverse events at least possibly related to cetuximab included dermatologic events (n = 5), GI (n = 4), anemia (n = 2), constitutional symptoms (n = 3), infection (n = 2), vascular events (n = 2), pain (n = 2), and pulmonary, neurological, vomiting and metabolic events (n = 1 each).
Table 3.
Toxicity Table
| AE Category | 0 | 1 | 2 | 3 | 4 | Total |
|---|---|---|---|---|---|---|
| Leukopenia | 28 | 6 | 1 | 0 | 0 | 35 |
| Thrombocytopenia | 33 | 2 | 0 | 0 | 0 | 35 |
| Neutropenia | 33 | 1 | 1 | 0 | 0 | 35 |
| Anemia | 13 | 9 | 11 | 2 | 0 | 35 |
| Other Hematologic | 31 | 2 | 2 | 0 | 0 | 35 |
| Allergy/Immunology | 34 | 1 | 0 | 0 | 0 | 35 |
| Constitutional | 15 | 12 | 5 | 3 | 0 | 35 |
| Dermatologic | 8 | 9 | 13 | 5 | 0 | 35 |
| Nausea | 25 | 8 | 2 | 0 | 0 | 35 |
| Vomiting | 26 | 7 | 1 | 1 | 0 | 35 |
| Gastrointestinal | 19 | 9 | 3 | 4 | 0 | 35 |
| Hemorrhage | 34 | 1 | 0 | 0 | 0 | 35 |
| Infection | 31 | 0 | 2 | 2 | 0 | 35 |
| Metabolic | 22 | 8 | 4 | 1 | 0 | 35 |
| Musculoskeletal | 33 | 1 | 1 | 0 | 0 | 35 |
| Neurological | 33 | 1 | 0 | 1 | 0 | 35 |
| Ocular/Visual | 33 | 1 | 1 | 0 | 0 | 35 |
| Pain | 25 | 4 | 4 | 2 | 0 | 35 |
| Pulmonary | 30 | 4 | 0 | 1 | 0 | 35 |
| Vascular | 33 | 0 | 0 | 2 | 0 | 35 |
One patient death reported by an institution was listed as due to disease. The DSMB listed her death as due to disease and possibly treatment. She had grade 4 pain with hematuria.
Exploratory Analysis of Patient Characteristics and PFS and OS During Treatment With Cetuximab
Results of the exploratory analyses are reported in Table 4. Performance status may be a prognostic factor associated with OS and possibly PFS. The low number of African Americans included in our study (i.e., a total of 5 patients) prevented the determination of the confidence interval between races. The approximate 95% confidence interval for squamous cell type versus all other cell types ranged from 0.33 – 1.52 for PFS and from 0.33 – 1,57 for OS, respectively, indicating uncertainty of the activity of single agent cetuximab within this subgroup.
Table 4.
Cox Proportional Hazard Ratio Estimates for Various Prognostic Factors
| Progression-Free Survival | ||
|---|---|---|
| Prognostic Factor | Hazard Ratio | 95% Confidence Interval |
| Performance Status1 | 1.77 | 0.82 – 3.82 |
| Race2 | 0.69 | N/A4 |
| Squamous Histology3 | 0.72 | 0.33 – 1.52 |
| Overall Survival | ||
|---|---|---|
| Performance Status1 | 2.42 | 1.01 – 5.78 |
| Race2 | 1.02 | N/A4 |
| Squamous Histology3 | 0.72 | 0.33 – 1.57 |
Performance Status of 1 or 2 versus 0
African American versus all other races
Squamous Cell Type versus all other cell types
The confidence interval could not be determined because there were only 5 African Americans.
DISCUSSION
The management of cervical cancer no longer amenable to control with surgery or radiation therapy has only minimally improved with the advent of modern chemotherapy. The median survival rate for these patients when treated with current systemic cisplatin combination regimens is only 9 to 10 months [35,36]. Thus, the development of innovative, effective therapies against advanced/recurrent/metastatic cervical cancer refractory to standard salvage treatment remains a high priority.
One class of agents with potential activity in this group of patients is represented by cetuximab, a human/murine chimeric monoclonal antibody targeting the epidermal growth factor receptor. Phase I/II studies of cetuximab in locoregionally advanced squamous-cell cancer of the head and neck (SCCHN) showed promising activity in combination with radiotherapy [37,38]. Importantly, in patients with metastatic and recurrent SCCHN who have not responded to platinum-based therapy, cetuximab as a single agent or in combination with chemotherapy has been shown to induce responses. In the study by Baselga et al., patients with metastatic and recurrent SCCHN who had progressed following a platinum-containing regimen were re-challenged with the same regimen with the addition of cetuximab [39]. In 96 evaluable patients, the proportion responding was 14.6% and 39.6% had stable disease. Vermorken et al. evaluated cetuximab monotherapy in 103 patients with metastatic and recurrent SCCHN who had progressed following a platinum-containing regimen [40]. Treatment with cetuximab produced a response rate of 13% and a disease control rate (complete response/partial response/stable disease) of 46%. The current study was designed by the GOG to test the therapeutic benefit of single-agent cetuximab in the setting of treatment failure with 1 or 2 prior regimens for metastatic cervical cancer with squamous and non-squamous histology. In this group of heavily pretreated patients cetuximab showed limited activity, with a response rate of 0% and 14.3% of patients remaining without progression after 6 months on protocol. Because the required number of patients with either responses or PFS at 6 months to declare the regimen interesting was 7 and 7 out of 35 patients (20.0%), respectively, the study was negative for the entire population. A median OS of 6.7 months was found in the current study. It is worth noting, however, that 5 out of 24 of the study patients (21%) who survived progression free for at least 6 months on cetuximab therapy harbored tumors with squamous cell histology. In contrast, no patient survived progression free for at least 6 months in the group of patients treated with cetuximab harboring adenosquamous or adenocarcinoma of the cervix (0 out of 11 patients). Consistent with these results, the point estimates for the probability of being PFS at 6 months when receiving cetuximab therapy was higher among the patients harboring squamous tumors than the whole sample (21% versus 14%). Thus, although the overall level of activity in the current report does not justify a phase III trial of cetuximab in heavily pretreated advanced and recurrent cervical cancer patients, current data cannot rule out potential activity of cetuximab in patients harboring cervical tumors with squamous cell histology.
The safety profile of cetuximab has been extensively studied in previous reports [37–41]. The present study did not identify any new toxicities or an increased frequency of currently reported toxicities of cetuximab in cervical cancer patients with persistent or recurrent disease. Most of the Grade 3 adverse events reported in the study were related to dermatologic toxicity.
In the current trial, patients were not prescreened or preselected on the basis of EGFR expression. It is worth noting, however, that the relation between EGFR protein expression and response to EGFR-target therapies is questionable, as colorectal cancer patients without detectable EGFR protein over-expression may respond to treatment with cetuximab [41]. Moreover, multiple previous studies have shown that EGFR is overexpressed in a large number of cervical cancers, and that it is significantly more expressed in squamous cell carcinoma than in adenocarcinoma and the upregulation is correlated with poor prognosis [42]. Of interest, multiple biomarkers have been found to predict response to treatment with EGFR inhibitors in other human malignancies. For example, in colorectal cancer and non–small cell lung cancer, KRAS mutations are associated with resistance to EGFR inhibitors, whereas specific EGFR mutations and high copy numbers of the EGFR gene predict a better response in non–small cell lung cancer [43–45]. In cervical cancer, however, neither EGFR mutations nor EGFR gene amplification and only a few (0–8%) KRAS mutations have been reported [46–51].
Although multiple mechanisms of action have been attributed to cetuximab including inhibition of tumor proliferation, angiogenesis and metastasis, induction of apoptosis, and/or promotion of cell cycle arrest, strong experimental evidence suggests that engagement of Fc receptors on effector cells (i.e., mainly natural killer (NK) cells) represents the dominant component of the in vivo activity of most antibodies against tumors [52–55]. The efficacy of the anti-EGFR antibody (M225) when used as an F(ab)2 in blocking the growth of tumors in vivo has been previously demonstrated to be only 50% of the activity shown by the intact antibody when it is able to engage the Fc receptors on NK cells [53]. These findings suggest that host factors related to a low number and/or lack of killing activity of effector cells (i.e., NK cells), as previously reported by multiple groups studying the immune-competence of cervical cancer patients heavily pretreated with radiation and chemotherapy, may present a major barrier to the successful clinical development of therapeutic biologic reagents (i.e., IgG1 mAb such as cetuximab) in end-stage cervical cancer patients [56–58]. Prospective evaluation of the immune-competence of such patients therefore seems to be warranted in future studies.
In conclusion, our study indicates that cetuximab has limited activity in patients with persistent or recurrent cervical cancer that has progressed after chemo-radiation and palliative chemotherapy. Cetuximab seems to be well tolerated in this patient population and its activity may be greater in patients with squamous cell histology.
RESEARCH HIGHLIGHTS
The GOG evaluated the efficacy and tolerability of Cetuximab in cervical cancer
Cetuximab is well tolerated but has limited activity in persistent/recurrent disease
Cetuximab activity may be limited to cervical cancer with squamous cell histology
ACKNOWLEDGMENT
We thank Sandra Dascomb, Gynecologic Oncology Group (GOG) Statistical and Data Center (SDC), Buffalo NY, for her support in data abstraction and Kim Blaser for publications management. We also wish to thank BMS/ImClone for their industry support
This study was presented at the Society of Gynecologic Oncology (SGO) Annual Meeting on Women's Cancer, March 6–9, 2011, Orlando, Fl.
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469) and the Gynecologic Oncology Group Statistical Office (CA 37517) and R01 CA122728-01A2 CA Grant to AS from the National Institutes of Health. The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: Roswell Park Cancer Institute, Abington Memorial Hospital, University of Mississippi Medical Center, University of Cincinnati, University of North Carolina School of Medicine, University of California Medical Center at Irvine, Rush-Presbyterian-St. Luke's Medical Center, The Cleveland Clinic Foundation, State University of New York at Stony Brook, Washington University School of Medicine, Memorial Sloan-Kettering Cancer Center, Columbus Cancer Council, MD Anderson Cancer Center, University of Oklahoma, University of Wisconsin Hospital and Community Clinical Oncology Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST Dr. Cecelia H. Boardman is on the Speaker's Bureau for Glaso-Smith-Kline and Merck. All other co-authors have no conflict of interest to declare.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomized study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350:535–40. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- 3.Arteaga CL. Overview of epidermal growth factor receptor biology and its role as a therapeutic target in human neoplasia. Semin Oncol. 2002;29:3–9. doi: 10.1053/sonc.2002.35642. [DOI] [PubMed] [Google Scholar]
- 4.Baselga J. New therapeutic agents targeting the epidermal growth factor receptor. J Clin Oncol. 2000;18:54S–59S. [PubMed] [Google Scholar]
- 5.Raymond E, Faivre S, Armand JP. Epidermal growth factor receptor tyrosine kinase as a target for anticancer therapy. Drugs. 2000;60:15–23. doi: 10.2165/00003495-200060001-00002. discussion, 41–42. [DOI] [PubMed] [Google Scholar]
- 6.Baselga J. Targeting the epidermal growth factor receptor: A clinical reality. J Clin Oncol. 2001;19:41S–44S. [PubMed] [Google Scholar]
- 7.O'Dwyer PJ, Benson AB., III Epidermal growth factor receptor-targeted therapy in colorectal cancer. Semin Oncol. 2002;29(Suppl 14):10–1. doi: 10.1053/sonc.2002.35643. [DOI] [PubMed] [Google Scholar]
- 8.Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–65. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 9.Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110:669–72. doi: 10.1016/s0092-8674(02)00966-2. [DOI] [PubMed] [Google Scholar]
- 10.Sako Y, Minoghchi S, Yanagida T. Single-molecule imaging of EGFR signaling on the surface of living cells. Nat Cell Biol. 2000;2:168–72. doi: 10.1038/35004044. [DOI] [PubMed] [Google Scholar]
- 11.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–25. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 12.Olayioye MA, Beuvink I, Horsch K, Daly JM, Hynes NE. ErbB receptor-induced activation of stat transcription factors is mediated by Src tyrosine kinases. J Biol Chem. 1999;274:17209–18. doi: 10.1074/jbc.274.24.17209. [DOI] [PubMed] [Google Scholar]
- 13.Kim H, Muller WJ. The role of the epidermal growth factor receptor family in mammary tumorigenesis and metastasis. Exp Cell Res. 1999;253:78–87. doi: 10.1006/excr.1999.4706. [DOI] [PubMed] [Google Scholar]
- 14.Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37(suppl 4):S9–S15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- 15.Mendelsohn J, Fan Z. Epidermal growth factor receptor family and chemosensitization. J Natl Cancer Inst. 1997;89:341–3. doi: 10.1093/jnci/89.5.341. [DOI] [PubMed] [Google Scholar]
- 16.Corvo R, Antognoni P, Sanguineti G. Biological predictors of response to radiotherapy in head and neck cancer: Recent advances and emerging perspectives. Tumori. 2001;87:355–63. doi: 10.1177/030089160108700601. [DOI] [PubMed] [Google Scholar]
- 17.Kersemaekers AM, Fleuren GJ, Kenter GG, Van den Broek LJ, Uljee SM, Hermans J, et al. Oncogene alterations in carcinomas of the uterine cervix: overexpression of the epidermal growth factor receptor is associated with poor prognosis. Clin Cancer Res. 1999;5:577–86. [PubMed] [Google Scholar]
- 18.Kim GE, Kim YB, Cho NH, Chung HC, Pyo HR, Lee JD, et al. Synchronous co-expression of epidermal growth factor receptor and cyclooxygenase-2 in carcinomas of the uterine cervix: a potential predictor of poor survival. Clin Cancer Res. 2004;10:1366–74. doi: 10.1158/1078-0432.ccr-0497-03. [DOI] [PubMed] [Google Scholar]
- 19.Baselga J, Pfister D, Cooper MR, Cohen R, Burtness B, Bos M, et al. Phase I studies of anti-epidermal growth factor receptor chimeric antibody C225 alone and in combination with cisplatin. J Clin Oncol. 2000;18:904–4. doi: 10.1200/JCO.2000.18.4.904. [DOI] [PubMed] [Google Scholar]
- 20.Ciardiello F, Damiano V, Bianco R, Bianco C, Fontanini G, De Laurentiis M, et al. Antitumor activity of combined blockade of epidermal growth factor receptor and protein kinase A. J Natl Cancer Inst. 1996;88:1770–6. doi: 10.1093/jnci/88.23.1770. [DOI] [PubMed] [Google Scholar]
- 21.Kawamoto T, Sato JD, Le A, Polikoff J, Sato GH, Mendelsohn J. Growth stimulation of A431 cells by epidermal growth factor: identification of high-affinity receptors for epidermal growth factor by an anti-receptor monoclonal antibody. Proc Natl Acad Sci USA. 1983;80:1337–41. doi: 10.1073/pnas.80.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato JD, Kawamoto T, Le AD, Mendelsohn J, Polikoff J, Sato GH. Biological effects in vitro of monoclonal antibodies to human epidermal growth factor receptors. Mol Biol Med. 1983;1:511–29. [PubMed] [Google Scholar]
- 23.Fan Z, Lu Y, Wu X, Mendelsohn J. Antibody-induced epidermal growth factor receptor dimerization mediates inhibition of autocrine proliferation of A431 squamous carcinoma cells. J Biol Chem. 1994;269:27595–602. [PubMed] [Google Scholar]
- 24.Wu X, Rubin M, Fan Z, DeBlasio T, Soos T, Koff A, et al. Involvement of p27KIP1 in G1 arrest mediated by an anti-epidermal growth factor receptor monoclonal antibody. Oncogene. 1996;12:1397–403. [PubMed] [Google Scholar]
- 25.Mandal M, Adam L, Mendelsohn J, Kumar R. Nuclear targeting of Bax during apoptosis in human colorectal cancer cells. Oncogene. 1998;17:999–1007. doi: 10.1038/sj.onc.1202020. [DOI] [PubMed] [Google Scholar]
- 26.Petit AM, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B, et al. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997;151:1523–30. [PMC free article] [PubMed] [Google Scholar]
- 27.Matsumoto T, Perrotte P, Bar-Eli M, et al. Blockade of EGF-R signalling with anti-EGFR monoclonal antibody (Mb) C225 inhibits matrix metalloproteinase-9 (MMP-9) expression and invasion of human transitional cell carcinoma (TCC) in vitro and in vivo. Proc Am Assoc Cancer Res. 1998;39:83. Abs. [Google Scholar]
- 28.Baselga J, Norton L, Masui H, Pandiella A, Coplan K, Miller WH, Jr., et al. Antitumor effects of doxorubicin in combination with anti-epidermal growth factor receptor monoclonal antibodies. J Natl Cancer Inst. 1993;85:1327–33. doi: 10.1093/jnci/85.16.1327. [DOI] [PubMed] [Google Scholar]
- 29.Milas L, Mason K, Hunter N, Petersen S, Yamakawwa M, Ang K, et al. In vivo enhancement of tumor radio response by C225 antiepidermal growth factor receptor antibody. Clin Cancer Res. 2000;6:701–8. [PubMed] [Google Scholar]
- 30.Prewett MC, Hooper AT, Bassi R, Ellis LM, Waksal HW, Hicklin DJ. Enhanced antitumor activity of anti-epidermal growth factor receptor monoclonal antibody IMC-C225 in combination with irinotecan (CPT-11) against human colorectal tumor xenografts. Clin Cancer Res. 2002;8:994–1003. [PubMed] [Google Scholar]
- 31.Saltz LB, Meropol NJ, Loehrer PJ, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201–8. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 32.Shin DM, Donato NJ, Perez-Soler R, Shin HJ, Wu JY, Zhang P, et al. Epidermal growth factor receptor-targeted therapy with C225 and cisplatin in patients with head and neck cancer. Clin Cancer Res. 2001;7:1204–13. [PubMed] [Google Scholar]
- 33.Sill MW, Yothers G. Technical Report. State University of New York at Buffalo; Buffalo: 2006. A method for utilizing bivariate efficacy outcome measures to screen agents for activity in 2-stage phase II clinical trials. [Google Scholar]
- 34.Cox DR. Regression models and life tables. Journal of the Royal Statistical Society Seriess B. 1972;34:187–220. [Google Scholar]
- 35.Tewari KS, Monk BJ. Gynecologic oncology group trials of chemotherapy for metastatic and recurrent cervical cancer. Curr Oncol Rep. 2005;7:419–34. doi: 10.1007/s11912-005-0007-z. [DOI] [PubMed] [Google Scholar]
- 36.Long HJ, III, Bundy BN, Grendys EC, Benda JA, McMeekin DS, Sorosky J, et al. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: A Gynecologic Oncology Group study. J Clin Oncol. 2005;23:4626–33. doi: 10.1200/JCO.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 37.Robert F, Ezekiel MP, Spencer SA, Meredith RF, Bonner JA, Khazaeli MB, et al. Phase I study of anti-epidermal growth factor receptor antibody cetuximab in combination with radiation therapy in patients with advanced head and neck cancer. J Clin Oncol. 2001;19:3234–43. doi: 10.1200/JCO.2001.19.13.3234. [DOI] [PubMed] [Google Scholar]
- 38.Bonner J, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–78. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 39.Baselga J, Trigo J, Bourhis J, et al. Cetuximab (C225) plus cisplatin/carboplatin is active in patients (pts) with recurrent/metastatic squamous cell carcinoma of the head and neck (SCCHN) progressing on a same dose and schedule platinum-based regimen. Proc Am Soc Clin Oncol. 2002;21:226a. Abstract 900. [Google Scholar]
- 40.Vermorken JB, Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, Rolland F, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol. 2007;25:2171–7. doi: 10.1200/JCO.2006.06.7447. [DOI] [PubMed] [Google Scholar]
- 41.Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23:1803–0. doi: 10.1200/JCO.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 42.Noordhuis MG, Eijsink JJ, Ten Hoor KA, Roossink F, Hollema H, Arts HJ, et al. Expression of epidermal growth factor receptor (EGFR) and activated EGFR predict poor response to (chemo)radiation and survival in cervical cancer. Clin Cancer Res. 2009;15:7389–97. doi: 10.1158/1078-0432.CCR-09-1149. [DOI] [PubMed] [Google Scholar]
- 43.Linardou H, Dahabreh IJ, Kanaloupiti D, Siannis F, Bafaloukos D, Kosmidis P, et al. Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: a systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. Lancet Oncol. 2008;9:962–72. doi: 10.1016/S1470-2045(08)70206-7. [DOI] [PubMed] [Google Scholar]
- 44.Massarelli E, Varella-Garcia M, Tang X, Xavier AC, Ozburn NC, Liu DD, et al. KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Clin Cancer Res. 2007;13:2890–6. doi: 10.1158/1078-0432.CCR-06-3043. [DOI] [PubMed] [Google Scholar]
- 45.Hirsch FR, Herbst RS, Olsen C, Chansky K, Crowley J, Kelly K, et al. Increased EGFR gene copy number detected by fluorescent in situ hybridization predicts outcome in non-small-cell lung cancer patients treated with cetuximab and chemotherapy. J Clin Oncol. 2008;26:3351–7. doi: 10.1200/JCO.2007.14.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arias-Pulido H, Joste N, Chavez A, Muller CY, Dai D, Smith HO, et al. Absence of epidermal growth factor receptor mutations in cervical cancer. Int J Gynecol Cancer. 2008;18:749–54. doi: 10.1111/j.1525-1438.2007.01111.x. [DOI] [PubMed] [Google Scholar]
- 47.Marzano R, Corrado G, Merola R, Sbiroli C, Guadagni F, Vizza E, et al. Analysis of chromosomes 3, 7, X and the EGFR gene in uterine cervical cancer progression. Eur J Cancer. 2004;40:1624–9. doi: 10.1016/j.ejca.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 48.Stenzel A, Semczuk A, Rozynskal K, Jakowicki J, Wojcierowski J. “Low-risk” and “high-risk”HPV-infection and K-ras gene point mutations in human cervical cancer: a study of 31 cases. Pathol Res Pract. 2001;197:597–603. [PubMed] [Google Scholar]
- 49.Pochylski T, Kwasniewska A. Absence of point mutation in codons 12 and 13 of K-RAS oncogenein HPV-associated high grade dysplasia and squamous cell cervical carcinoma. Eur J Obstet Gynecol Reprod Biol. 2003;111:68–73. doi: 10.1016/s0301-2115(03)00205-7. [DOI] [PubMed] [Google Scholar]
- 50.Kang S, Kim HS, Seo SS, Park SY, Sidransky D, Dong SM. Inverse correlation between RASSF1A hypermethylation, KRAS and BRAF mutations in cervical adenocarcinoma. Gynecol Oncol. 2007;105:662–6. doi: 10.1016/j.ygyno.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 51.Pappa KI, Choleza M, Markaki S, Giannikaki E, Kyroudi A, Vlachos G, et al. Consistent absence of BRAF mutations in cervical and endometrial cancer despite KRAS mutation status. Gynecol Oncol. 2006;100:596–600. doi: 10.1016/j.ygyno.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 52.Farley JH, Oliver KE. Cisplatin plus Cetuximab in the Treatment of Recurrent and Persistent Cancers of the Cervix US Oncological Review. 2010;6:45–49. [Google Scholar]
- 53.Fan Z, Masui H, Altas I, Mendelsohn J. Blockade of epidermal growth factor receptor function by bivalent and monovalent fragments of 225 anti-epidermal growth factor receptor monoclonal antibodies. Cancer Res. 1993;53:4322–8. [PubMed] [Google Scholar]
- 54.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 55.Bellone S, Frera G, Landolfi G, Romani C, Bandiera E, Tognon G, et al. Overexpression of epidermal growth factor type-1 receptor (EGF-R1) in cervical cancer: implications for Cetuximab-mediated therapy in recurrent/metastatic disease. Gynecol Oncol. 2007;106:513–20. doi: 10.1016/j.ygyno.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 56.Fiander A, Adams M, Evans AS, Bennett AJ, Borysiewicz LK. Immunocompetent for immunotherapy? A study of the immunocompetence of cervical cancer patients. Int J Gynecol Oncol. 1995;5:438–42. doi: 10.1046/j.1525-1438.1995.05060438.x. [DOI] [PubMed] [Google Scholar]
- 57.Santin AD, Hermonat PL, Ravaggi A, Bellone S, Roman J, Pecorelli S, et al. Effects of concurrent cisplatinum administration during radiotherapy vs. radiotherapy alone on the immune function of patients with cancer of the uterine cervix. Int J Rad Oncol Biol Phys. 2000;48:997–1006. doi: 10.1016/s0360-3016(00)00769-0. [DOI] [PubMed] [Google Scholar]
- 58.Santin AD, Bellone S, Palmieri M, Bossini B, Dunn D, Roman JJ, et al. Effect of blood transfusion during radiotherapy on the immune function of patients with cancer of the uterine cervix: role of interleukin-10. Int J Rad Oncol Biol Phys. 2002;54:1345–55. doi: 10.1016/s0360-3016(02)03757-4. [DOI] [PubMed] [Google Scholar]