Summary
BMP signaling is broadly implicated in dorsoventral (DV) patterning of bilaterally symmetric animals [1–3], and its role in axial patterning apparently predates the birth of Bilateria [4–7]. In fly and vertebrate embryos, BMPs and their antagonists (primarily Sog/chordin) diffuse and interact to generate signaling gradients that pattern fields of cells [8–10]. Work in other species reveals diversity in essential facets of this ancient patterning process, however. Here, we report that BMP signaling patterns the DV axis of segmental ectoderm in the leech Helobdella, a clitellate annelid (Super-phylum Lophotrochozoa) featuring stereotyped developmental cell lineages; but the detailed mechanisms of DV patterning in Helobdella differ markedly from fly and vertebrates. In Helobdella, BMP2/4s are expressed broadly, rather than in dorsal territory, while a dorsally expressed BMP5-8 specifies dorsal fate by short-range signaling. A BMP antagonist, gremlin, is up-regulated by BMP5-8 in dorsolateral territory, rather than ventral, and yet the BMP-antagonizing activity of gremlin is required for normal ventral cell fates. Gremlin promotes ventral fates without disrupting dorsal fates by selectively inhibiting BMP2/4s, not BMP5-8. Thus, DV patterning in the development of the leech revealed unexpected evolutionary plasticity of the ‘conserved’ BMP patterning system, presumably reflecting its adaptation to different modes of embryogenesis.
Results and Discussion
DV patterning in segmental ectoderm of the leech: the O/P equivalence group
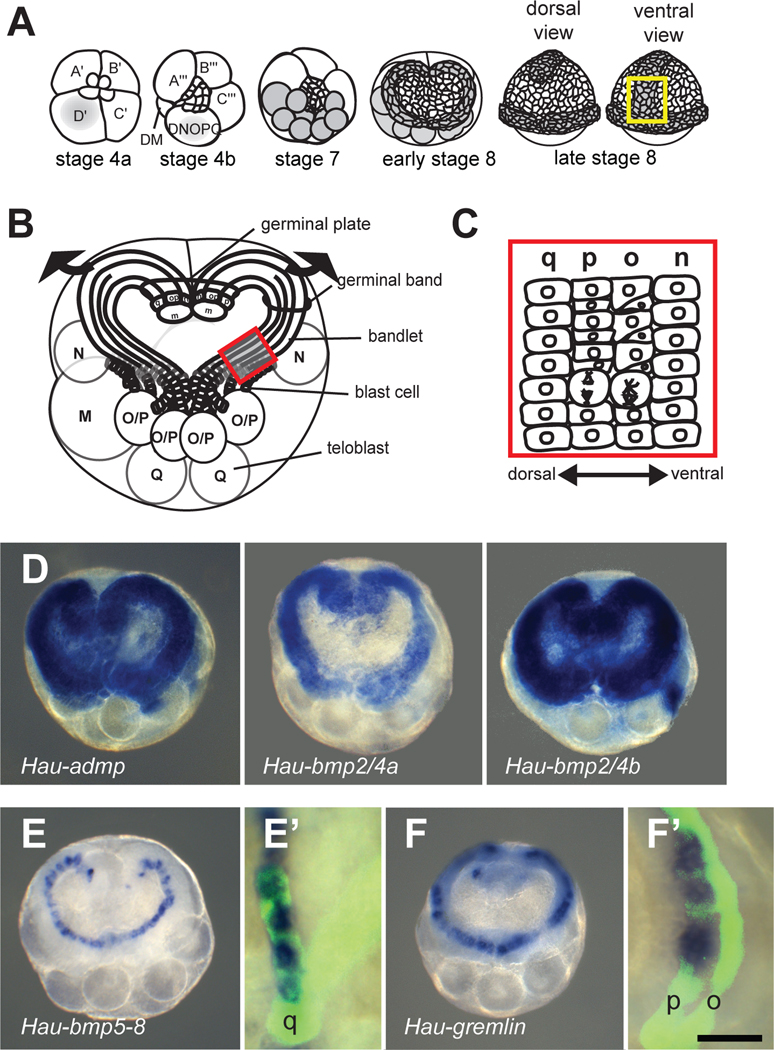
Cell lineage plays a predominant role in leech embryogenesis. Segmental ectoderm and mesoderm arise from a posterior growth zone consisting of five bilateral pairs of lineage-restricted segmentation stem cells (M, N, O/P, O/P and Q teloblasts). Each teloblast divides repeated and asymmetrically, giving rise to a column (bandlet) of segmental founder cells (primary blast cells). Near the surface in prospective dorsoposterior territory, the ipsilateral bandlets converge into parallel arrays, forming left and right germinal bands (Figures 1A and 1B). As the teloblasts continue adding blast cells posteriorly, the germinal bands move ventrally/vegetally, zippering together in anteroposterior progression along the prospective ventral midline to form the germinal plate. Segmental ectoderm arises from the four ectodermal lineages (N, O, P and Q), each of which contributes segmentally iterated patterns of specific neural and epidermal cells [11]. Consistent with their definitive fates [12], the four ectodermal bandlets (designated by lower case letters q, p, o, n, respectively) form a DV array within each germinal band (Figure 1C).
Figure 1.
BMP and antagonist expression during DV patterning of segmental ectoderm. In this and all subsequent figures, images depict dorsal views unless noted.
(A) Second axis specification in the Helobdella embryo originates with segregation of teloplasm (grey) into the D quadrant blastomeres (stages 1–6) and thence into five bilateral pairs of teloblasts (stage 7). Teloblasts give rise to the left and right germinal bands, covered by a micromere-derived epithelium (early stage 8). Germinal bands coalesce to form germinal plate (late stage 8); yellow box indicates the portion of the germinal plate illustrated in Figures 2I–2K, and 3A–3D.
(B) Early stage 8 embryo with epithelium removed, showing the DV array of ectodermal bandlets in each germinal band. Arrows indicate germinal band movements in germinal plate formation; red box indicates the portion of the germinal band illustrated Figures 1C, 1E’, 1F’ and 3E–3G.
(C) Primary o and p blast cells divide with differing degrees of asymmetry (see text for details).
(D) Hau-admp, Hau-bmp2/4a, and Hau-bmp2/4b were expressed throughout the germinal bands.
(E) Hau-bmp5-8 is specifically expressed in a single bandlet. Combined WMISH and lineage tracing revealed that the bandlet expressing Hau-bmp5-8 is the q bandlet (E’).
(F) Hau-gremlin is specifically up-regulated in one bandlet, revealed by combined WMISH and lineage tracing as the p bandlet (F’); fluorescent lineage tracer was injected into the OP proteloblast to label o and p bandlets (O/P teloblasts not visible). Scale bar, D, E, and F: 150 µm; E’ and F’: 30 µm.
Despite the overall determinacy of Helobdella development, the primary o and p blast cells in the lateral ectoderm constitute a developmental equivalence group. They are initially equipotent, and assume distinct O (ventrolateral) or P (dorsolateral) fates based on their positions in the germinal band [13–18]. Previous work has revealed that contact with the dorsalmost, q bandlet specifies the adjacent O/P-derived blast cells to assume the dorsolateral, P fate [13, 16]. By analogy to DV patterning in other protostome embryos, we hypothesized that BMP may provide the Q-derived, DV patterning signal in the leech O/P equivalence group.
Short-range BMP5-8 signaling is necessary and sufficient to specify the P fate
To test this hypothesis, we first surveyed BMPs and their antagonists in Helobdella (Figure S1; Table S1); four BMPs (Hau-admp, Hau-bmp2/4a, Hau-bmp2/4b, Hau-bmp5-8) and one antagonist (Hau-gremlin) are expressed during stage 7/8, when patterning of the O/P equivalence group occurs (Figure S1). Whole-mount in situ hybridization (WMISH) revealed broad expression of Hau-admp, Hau-bmp2/4a, and Hau-bmp2/4b throughout the germinal bands, with no evident differences across the germinal band (Figure 1D). In contrast, Hau-bmp5-8 was expressed specifically in the dorsalmost (q) bandlet (Figures 1E). These results raised the possibility that BMP5-8 is the principle ligand in DV patterning of leech segmental ectoderm; this would be a stark contrast to most other species, where BMP2/4s are the principle ligands. Furthermore, in vertebrates and in planarian flatworms, ADMP and BMP2/4 are expressed at poles of the DV axis and form an autoregulatory loop to maintain the polarity of gene expression [19–21]; in Helobdella germinal bands, the expression of these genes was completely overlapping, implying that this regulatory loop does not operate in leech.
Also unexpectedly, Hau-gremlin was expressed at markedly higher levels in the p bandlet, immediately adjacent to the dorsalmost, q bandlet, rather than in ventral territory (Figures 1F). Gremlin has not been shown to play a role in primary body axis patterning for other species. In vertebrates, its expression is first detected in neural crest cells [22] and it plays critical roles in later development [23–27].
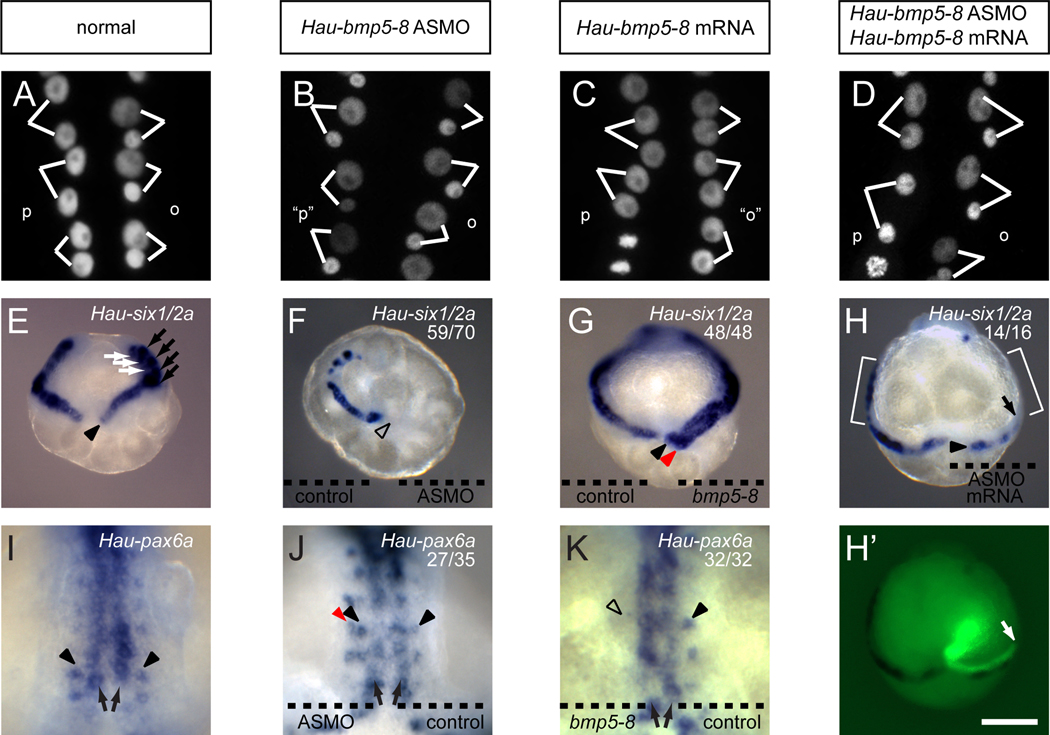
To see if Hau-BMP5-8 is necessary for patterning the O/P equivalence group, we knocked down Hau-BMP5-8 expression by injecting the Q teloblast or a progenitor (proteloblast OPQ or NOPQ) with Hau-bmp5-8 antisense morpholino oligomer (ASMO). In normal embryos, the o blast cell divides with marked asymmetry, giving rise to a larger anterior daughter and a smaller posterior daughter, while the p blast cell divides nearly equally, giving rise to two similarly sized daughters (Figures 2A, Table S2, and Movie S1). In Hau-bmp5-8 morphant embryos, however, primary blast cells in both the nominal o and p bandlets underwent the O-like asymmetric divisions (Figure 2B, Table S2, and Movie S2). Furthermore, the nominal p bandlet ceased to express the P fate marker Hau-six1/2a (Figures 2E and 2F)[28] and expressed Hau-pax6a, a gene normally expressed in O and N sub-lineages (Figures 2I and 2J) [29]. These results indicated that knockdown of Hau-BMP5-8 results in a P-to-O fate change. No such fate change was observed when a control morpholino oligomer was injected (Table S2), and a subsequent injection of mutated ASMO-resistant Hau-bmp5-8 mRNA into the Q teloblast rescued the morphant phenotype (i.e, restored Hau-six1/2a expression and nearly equal blast cell divisions to the nominal p blast cells; Figures 2D, 2H and Table S2).
Figure 2.
Hau-BMP5-8 regulates morphological and molecular markers of O and P fates. (A–D) blast cell division patterns in right germinal band revealed by histone 2B:GFP fluorescence; (E–H) Hau-six1/2a expression in germinal bands at early stage 8 (dorsal view); (I–K) Hau-pax6a expression in germinal plate at late stage 8 (ventral view; see Figure 1A).
(A) Lineage-specific o and p blast cell divisions in normal embryo.
(B) Nominal p blast cells (“p”) made O-like divisions in embryos injected with Hau-bmp5-8 ASMO.
(C) Nominal o blast cells (“o”) made P-like divisions in embryos whose N teloblast was injected with Hau-bmp5-8 mRNA.
(D) Normal blast cell divisions in embryo co-injected with Hau-bmp5-8 ASMO and an ASMO-resistant Hau-bmp5-8 mRNA.
(E) Normal expression of Hau-six1/2a in the primary p blast cells (arrowhead) and also in OP (rostral segments, black arrows) and Q (white arrows) sub-lineages within older blast cell clones.
(F) Hau-six1/2a expression was not detected in the right germinal band (open arrowhead), containing Hau-bmp5-8 ASMO, but was normal in the left germinal band, containing control MO.
(G) Misexpressing Hau-BMP5-8 in the n bandlet induced ectopic Hau-six1/2a expression in the adjacent nominal o bandlet (red arrowhead); control mRNA in the left n bandlet had no effect.
(H and H’) Hau-six1/2a expression in the p bandlet was rescued (arrowhead) by introducing ASMO-resistant Hau-bmp5-8 mRNA into QR 48 hours after injection of Hau-bmp5-8 ASMO into proteloblast NOPQR. Rescuing mRNA was co-injected with fluorescent lineage tracer (H’). The anterior boundary of the clone inheriting rescuing mRNA (arrows) aligned with the anteriormost Hau-six1/2a expressing cells in the p bandlet. Hau-six1/2a expression was absent from cells anterior to this boundary (compare brackets on the left and right sides).
(I) Normal expression of Hau-pax6a within the n (arrows) and o (arrowheads) bandlets.
(J) Hau-pax6a was normally expressed in the n (arrows) and o bandlets (black arrowheads) on both sides and was ectopically expressed in the nominal p bandlet (red arrowhead) of the right side, containing Hau-bmp5-8 ASMO. The left side received a control MO.
(K) Misexpressing Hau-BMP5-8 in the right n bandlet and Hau-myostatin in the left n bandlet; Hau-pax6a expression was blocked in the right o bandlet (open arrowhead) but was normal in n bandlets (arrows) and left o bandlet (black arrowhead). Scale bar, A–D: 15 µm; E–H and H’: 120 µm; I–K: 50 µm.
We next determined the effects of expressing Hau-BMP5-8 ectopically in the n bandlet. In embryos injected with Hau-bmp5-8 mRNA in an N teloblast, primary blast cells in both of the ipsilateral o/p bandlets underwent P-like division patterns (Figure 2C, Table S2, and Movie S3); Hau-six1/2a was up-regulated in nominal o blast cells (Figure 2G), and Hau-pax6a expression disappeared from the nominal o bandlet (Figure 2K). Interestingly, expression of Hau-pax6a in the n bandlet itself was not affected, suggesting that Hau-pax6a expression in the ventral, n bandlet is insensitive to BMP signaling. This is similar to Platynereis, a polychaete annelid, where expression of ventral midline genes is not as sensitive to ectopic BMP as the lateral neuroectoderm genes [30].
Thus, we have shown that BMP5-8 signaling is both necessary and sufficient to direct O/P-derived blast cells to assume the P fate. Moreover, three aspects of our results suggest that the spatial range of BMP signaling within the germinal band is restricted to one cell diameter or less: 1) each of the o/p bandlets responds to only to BMP5-8 expressed in the adjacent n or q bandlet; 2) when ASMO-induced knockdown of BMP5-8 expression in the q bandlet was rescued by subsequent mRNA injection, the rescued expression of Hau-six1/2a in the p lineage was restricted to cells immediately adjacent to the q blast cells inheriting the ASMO-resistant Hau-bmp5-8 mRNA; 3) similarly, when BMP5-8 was expressed ectopically in the n bandlet by mRNA injection, Hau-six1/2a expression was detected only in the o blast cells immediately adjacent to n blast cells containing Hau-bmp5-8 mRNA (Figure S2). The spatially restricted nature of BMP5-8 signaling also suggested that it should be possible to invert the normal O and P fates within the germinal band by combining ASMO injection in the Q teloblast with Hau-bmp5-8 mRNA injection in the N teloblast, and this was indeed the case (Figure S2; Table S2).
Gremlin expression is up-regulated by BMP signaling
Similarities in the expression patterns of Hau-gremlin and Hau-six1/2a, a target of BMP signaling, suggest that Hau-gremlin is up-regulated by BMP signaling. To test this hypothesis, we assayed Hau-gremlin expression by WMISH in the Hau-BMP5-8 misexpression and knockdown experiments. As with Hau-six1/2a, Hau-gremlin expression was down-regulated in the nominal p bandlet when the Q teloblast or its progenitor had been injected with Hau-bmp5-8 ASMO (Figure S3A) and up-regulated in the nominal o bandlet when the N teloblast had been injected with Hau-bmp5-8 mRNA (Figure S3B).
Gremlin expression in P lineage is required for the O fate
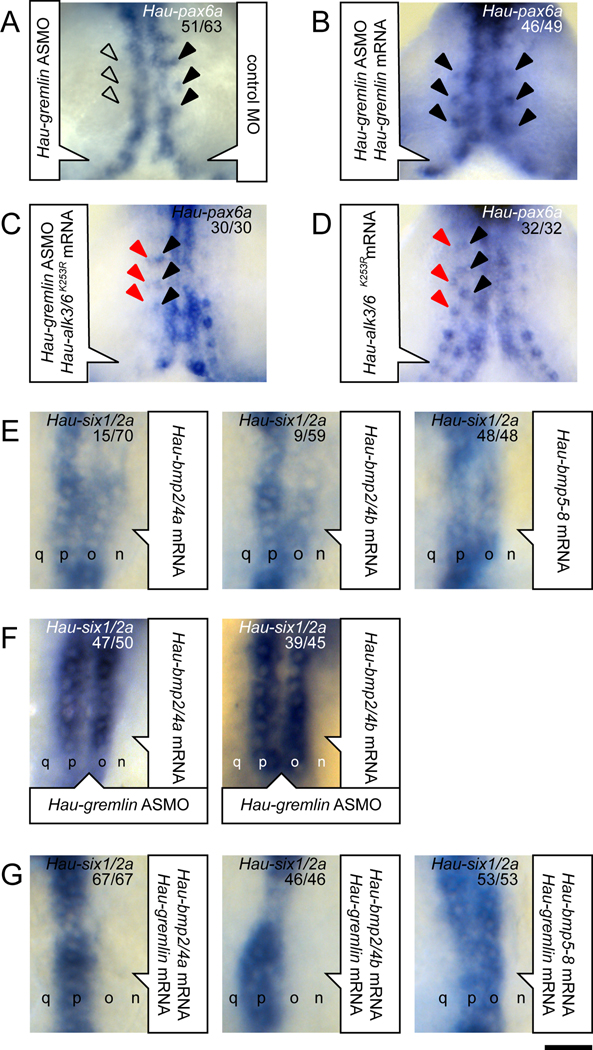
To determine the roles of gremlin in specifying the O and P fates, we knocked down Hau-gremlin expression with ASMO. In Hau-gremlin morphant embryos, the nominal o blast cells exhibited an intermediate fate: they still divided in the asymmetric O manner (Table S2), but failed to express the O fate marker Hau-pax6a (Figure 3A). Hau-pax6a expression in the o bandlet was rescued by co-injecting ASMO-resistant Hau-gremlin mRNA along with the Hau-gremlin ASMO (Figure 3B), suggesting the loss of Hau-pax6a expression in morphant embryos was not due to non-specific toxicity of the ASMO. Moreover, Hau-pax6a expression in morphant embryos was also rescued by injecting the proteloblast OP with mRNA encoding a dominant negative (DN) type I BMP receptor kinase (Hau-alk3/6K253R) (Figure 3C). The same pattern of Hau-pax6a expression was observed in embryos expressing DN receptor alone (Figure 3D). Thus, the disruption of Hau-pax6a expression in the o bandlet in the Hau-gremlin knockdown was caused by hyperactive BMP signaling, consistent with Hau-gremlin functioning as a BMP antagonist.
Figure 3.
Hau-gremlin is required for normal O fate in the O–P equivalence group and is a BMP2/4-specific antagonist. Panels A–D are ventral views of the germinal plate; panels E–G are dorsal views of the right germinal band.
(A) Hau-pax6a expression in the o bandlet was blocked in the right side, injected with Hau-gremlin ASMO (open arrowheads) but is normal in the left side (black arrowheads), injected with a control MO.
(B) Hau-pax6a expression in the o bandlet is rescued (compare arrowheads on left and right) by co-injecting ASMO-resistant Hau-gremlin mRNA with Hau-gremlin ASMO.
(C) Hau-pax6a expression was detected in the nominal o bandlet (black arrowheads) and the nominal p bandlet (red arrowheads) on the right side, co-injected with Hau-alk3/6K253R mRNA (encoding a dominant negative receptor) and Hau-gremlin ASMO.
(D) Injection of Hau-alk3/6K253R mRNA alone similarly induces ectopic Hau-pax6a expression in the p bandlet (red arrowheads) in addition to normal Hau-pax6a expression in the nominal o bandlet (black arrowheads).
(E) Misexpressing Hau-BMP2/4a, Hau-BMP2/4b or Hau-BMP5-8 in the n bandlet induced ectopic Hau-six1/2a expression in the adjacent nominal o bandlet. The differing efficacies indicate that Hau-BMP2/4a and Hau-BMP2/4b are not as efficient as Hau-BMP5-8 in inducing Hau-six1/2a expression.
(F) Ectopic Hau-six1/2a expression induced by misexpressing Hau-BMP2/4a or Hau-BMP2/4b in the n bandlet was enhanced in germinal bands containing Hau-gremlin ASMO.
(G) Injecting N teloblasts with equimolar mixtures of Hau-gremlin plus Hau-bmp2/4a or Hau-bmp2/4b failed to induce ectopic Hau-six1/2a expression in the adjacent o bandlet, but equimolar mixtures of Hau-gremlin plus Hau-bmp5-8 induced Hau-six1/2a expression in the nominal o bandlet. Scale bar, A–D: 50 µm; E–G: 30 µm.
To test the sufficiency of Hau-gremlin for determining the O fate, Hau-gremlin mRNA was injected into the OP proteloblast. In embryos overexpressing Hau-gremlin, primary blast cell division patterns in both the nominal o and p bandlets exhibited the O-like division pattern (Figure S3C and Table S2), but Hau-gremlin overexpression did not lead to a complete or permanent P-to-O transformation. In these embryos, Hau-six1/2a expression in the p bandlet was delayed but not abolished (Figure S3D). Thus, overexpressing Hau-gremlin only delayed expression of the P fate and was not sufficient to specify an ectopic O fate; the definitive P fate was presumably restored by a gremlin-insensitive mechanism or after ectopic gremlin had decayed. These results are consistent with previous work showing that the O lineage can be re-specified to the P fate even after the blast cell has undergone the O-specific asymmetric division [14, 31]. Together, our results suggested that the cells in the O–P equivalence group can assume fates that are intermediate between the normal O and normal P fates, and that the BMP antagonist Hau-gremlin is required for specifying the normal O.
Gremlin preferentially antagonizes BMP2/4a and BMP2/4b, but not BMP5-8
Based on its expression and the knockdown/overexpression phenotypes, we postulated that Hau-gremlin may participate in patterning the O and P fates by antagonizing the broadly expressed BMPs, but not the dorsally localized BMP5-8. To test this hypothesis, we first asked if any of the broadly expressed BMPs could induce ectopic P fate when over-expressed in the n bandlet. Hau-ADMP over-expression failed to affect normal O and P fates in the germinal band (data not shown). In contrast, both Hau-BMP2/4a and Hau-BMP2/4b efficiently induced an O-to-P change in blast cell division patterns (Table S2). But, ectopic Hau-six1/2a expression was detected in only 15–20% of embryos mis-expressing BMP2/4s (Figure 3E and Table S2), and in those embryos, the level of ectopic Hau-six1/2a expression in the nominal o bandlet was significantly lower than that induced by Hau-BMP5-8, and lower than in the normal p bandlet (Figure 3E). Thus, Hau-BMP2/4a and Hau-BMP2/4b are weaker than Hau-BMP5-8 at inducing the P fate, and Hau-ADMP cannot induce P fate at all.
In embryos misexpressing Hau-bmp2/4a or Hau-bmp2/4b in the n bandlet, Hau-gremlin knockdown resulted in increasing levels and frequencies of ectopic Hau-six1/2a expression in the nominal o bandlet (Figure 3F), suggesting that endogenous Hau-gremlin normally inhibits signaling activity of BMP2/4s. To further test interaction between BMP2/4s and gremlin, we injected equimolar mixtures of mRNAs encoding Hau-gremlin and one of these BMPs into the N teloblast, then scored cell division patterns and Hau-six1/2a expression patterns in the adjacent, nominal o bandlet. As expected, Hau-gremlin effectively blocked P-inducing activity of Hau-BMP2/4a and Hau-BMP2/4b (Figure 3G and Table S2). In contrast, Hau-gremlin did not block P-inducing activity of Hau-BMP5-8 (Figure 3G and Table S2), indicating that Hau-gremlin specifically antagonizes Hau-BMP2/4a and Hau-BMP2/4b, but not Hau-BMP5-8.
Baseline-level BMP signaling is required for maintaining teloblast stem cells
The expression patterns of the BMP2/4s and their receptors suggested that low level BMP signaling operates in all four ectodermal bandlets. To determine the role of baseline-level BMP signaling provided by the broadly expressed BMP2/4s, we sought to indiscriminately inhibit BMP signaling in ectodermal bandlets by expressing high levels of a DN receptor. Because expression of DN receptor by mRNA injection had failed to completely prevent nuclear phosphoSMAD accumulation (data not shown), we used a transient transgene system [32] to achieve higher sustained levels of expression and thus a stronger DN effect. When proteloblast OP was co-injected with tracer and the plasmid containing the DN receptor construct, Hau-ALK3/6K253R, blast cell production by teloblasts was disrupted; this was evidenced by the truncation of bandlets and irregularly shaped ‘teloblasts’ (Figure S4A). When the wildtype Hau-ALK3/6 construct was injected at similar dosage, the bandlets remained intact and the teloblast morphology appeared normal (Figure S4B). Hence, in addition to DV patterning, BMP signaling is also required for the stem cell activity in the posterior growth zone.
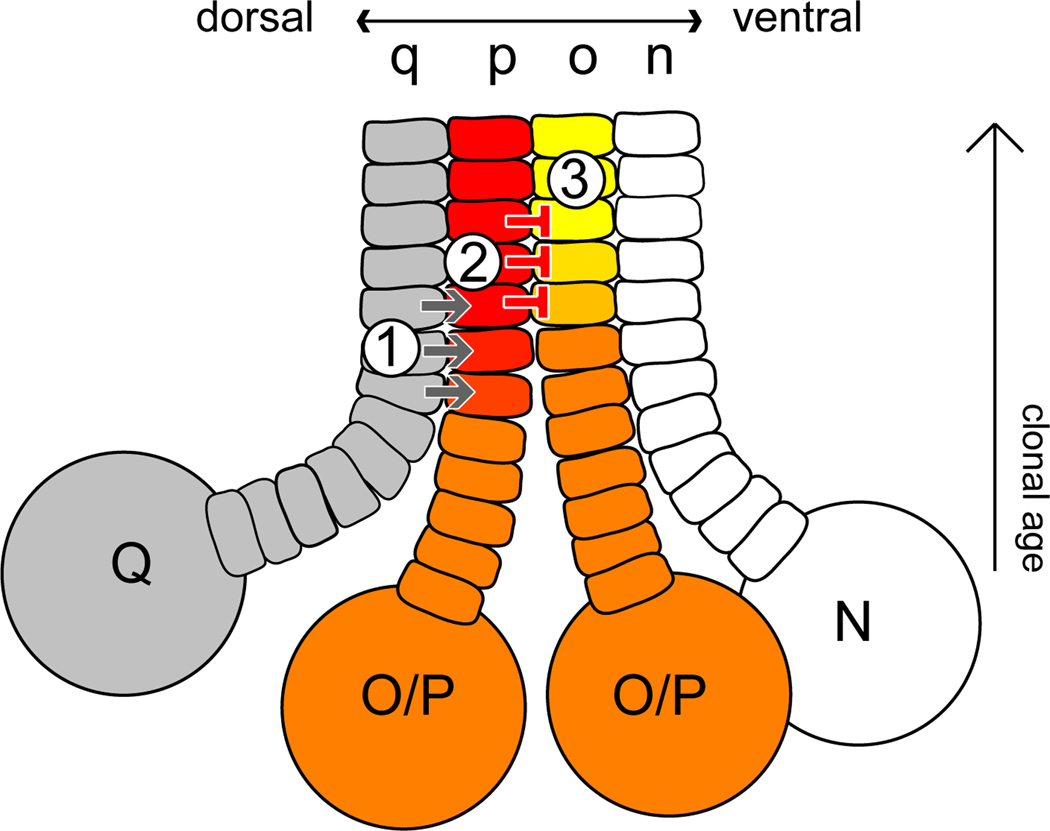
A feed-forward mechanism for BMP-mediate DV patterning in Helobdella
On the basis of this work, we propose the following model for patterning the Helobdella O/P equivalence group (Figure 4): 1) short-range Hau-BMP5-8 signaling from the dorsalmost q bandlet elevates BMP signal activity specifically in the adjacent dorsolateral p bandlet and not in the ventrolateral o bandlet; 2) elevated levels of BMP signaling in the p bandlet activate transcription of genes that specify the P fate, such as Hau-six1/2a, and also up-regulate Hau-gremlin; 3) up-regulation of gremlin in the p bandlet further dampens BMP signal activity in the adjacent o bandlet to permit normal O fate development (e.g., pax6a expression), while signaling in the p bandlet remains high due to the gremlin-insensitive Hau-BMP5-8 signal from the q bandlet. Since Hau-gremlin expression is positively regulated by BMP signaling, it is possible that gremlin and BMP2/4s form homeostatic feedback to maintain low-level BMP signaling activity throughout the germinal band. The functional significance of such low-level BMP signaling in the DV patterning is not entirely clear, because knocking out BMP signaling entirely interferes with the blast cell-forming divisions of the teloblasts.
Figure 4.
A three-step model for BMP signaling in the Helobdella O–P equivalence group (orange): 1) short-range signaling from q bandlet to p bandlet mediated by BMP5-8; 2) BMP5-8 signaling specifies the P fate (red), including up-regulation of gremlin; 3) gremlin signaling specifies O fate (yellow) in the neighboring bandlet by inhibiting broadly occurring BMP2/4 signaling (see text for further details).
We have shown that the BMP2/4-specific antagonist gremlin is the principle BMP antagonist in DV patterning of leech ectoderm. Unlike gremlin, chordin - the principle antagonist in DV patterning of other species - does not discriminate different classes of BMPs [33]. Henceforth, deploying chordin in the same context as gremlin in leech ectoderm would be expected to block both BMP2/4 and BMP5-8, as in sea urchin embryos, where cells co-expressing chordin and BMPs sit at the low end of the signaling gradient [34]. The use of gremlin in leech DV patterning, however, allows gremlin-insensitive BMP5-8 signaling in the very cells expressing the highest levels of gremlin. Thus, specificity of ligand-antagonist interactions is critical for using this unusual combination of ligand and antagonist in the DV patterning of leech segmental ectoderm.
Evolutionary significance
BMP-mediated DV patterning in Helobdella is consistent with the overall conservation of BMP signaling in bilaterian DV patterning but shows significant differences from that in other systems: 1) BMP5-8, rather than BMP2/4, exhibits dorsally localized expression and is the principle ligand in Helobdella DV patterning; 2) gremlin, rather than chordin, is principle BMP antagonist and is expressed dorsolaterally, rather than ventrally; 3) critical signaling interactions requires immediate cell contact. These differences in specific patterning mechanisms among species may reflect the requirements for patterning different types of embryos (indeterminate lineages with large fields of equipotent cells as opposed to fixed lineages with limited sets of equipotent cells).
We note that Capitella teleta, the only other annelid for which whole genome sequence is available, also lacks a chordin homolog, whereas both gremlin and chordin are present in the mollusc Lottia gigantea (Table S1). Thus, pending the sequencing of other annelid genomes, we speculate that the loss of chordin may be an annelid synapomorphy. Since the role of cell lineage in Capitella DV patterning remains to be determined, we cannot rule out the possibility that the loss of chordin preceded the evolution of lineage-driven embryogenesis, in the branch leading to leeches. In any event, given that BMP signaling in DV patterning appears to have been lost completely in C. elegans [35] and in ascidians [36], representing the convergent evolution of lineage-driven embryogenesis in Ecdysozoa and Deuterostomia respectively, the leech provides a unique opportunity for exploring the evolutionary plasticity of BMP signaling in patterning the DV axis.
Experimental Procedures
Molecular cloning was performed following standard procedures, and details are given in Supplemental Information. Procedures for WMISH are described elsewhere [37]. Cell lineage tracing was carried out by injecting identified blastomere(s) with fluorescent dextrans (Molecular Probes). Synthetic mRNA [38], plasmid constructs [32] and custom morpholino oligomers (Gene Tools) were used to perform misexpression and knockdown experiments. Capped RNA was transcribed from linearized DNA template using mESSAGE mACHINE kit (Ambion). Wild-type Hau-myostatin1 and a mutant form lacking the mature TGFβ cysteine knot domain (Hau-myostatin1ΔC) were used as negative control for BMP misexpression experiments; both yielded similar results. h2b:gfp mRNA was used to label cell nuclei to reveal the size ratios of the daughters of primary blast cells [32]. Transfection-grade plasmid DNA was prepared following standard procedure. Unless otherwise indicated, each mRNA was injected at 1 µM in the micropipette. Plasmid DNA was injected at 0.2 µg/µL in the micropipette. Morpholino oligomer was injected at 1 mM in the micropipette. Teloblasts or proteloblasts were injected with a volume corresponding to ~0.5% of the cell volume.
Highlights.
BMP patterns ‘O-P equivalence group’ of leech segmental ectoderm.
Dorsally expressed BMP5-8 upregulates gremlin expression in P lineage.
Gremlin tunes down activity of broadly expressed BMP2/4 and is required for O fate.
BMP5-8, not sensitive to gremlin, produces high signaling activity in P lineage.
Supplementary Material
Time-lapse video showing H2B:GFP-labeled cells in the O and P lineages of a normal embryo. In this movie, two (roughly equal) blast cell divisions are seen in the p bandlet (left) with the posterior cell dividing very soon after the anterior cell. In the o bandlet, parts of three (distinctly unequal) mitoses are evident, but occur slightly out of order, i.e., the most anterior cell to divide does so after its posterior neighbor.
Time-lapse video showing H2B:GFP-labeled cells in the O and P lineages of an embryo in which the ipsilateral Q lineage was treated with Hau-bmp5-8 ASMO. Cells in both bandlets undergo unequal, O-like mitoses.
Time-lapse video showing H2B:GFP-labeled cells in the O and P lineages of an embryo in which the ipsilateral N lineage was forced to express Hau-bmp5-8 by mRNA injection. Cells in both bandlets undergo roughly equal, P-like mitoses.
Acknowledgements
We thank Ian Quigley and Marty Shankland for providing Hau-six1/2a and Hau-pax6a plasmids, Mike Levine and members of his laboratory for making their confocal microscope available to us, John Gerhart for his critical comments on this manuscript, and Rebecca Heald for many helpful discussions. This work was supported by NIH RO1 grant GM 074619 to DAW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferguson EL. Conservation of dorsal-ventral patterning in arthropods and chordates. Curr. Opin. Genet. Dev. 1996;6:424–431. doi: 10.1016/s0959-437x(96)80063-3. [DOI] [PubMed] [Google Scholar]
- 2.De Robertis EM, Sasai Y. A common plan for dorsoventral patterning in Bilateria. Nature. 1996;380:37–40. doi: 10.1038/380037a0. [DOI] [PubMed] [Google Scholar]
- 3.Mizutani CM, Bier E. EvoD/Vo: the origins of BMP signalling in the neuroectoderm. Nat. Rev. Genet. 2008;9:663–677. doi: 10.1038/nrg2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finnerty JR, Pang K, Burton P, Paulson D, Martindale MQ. Origins of bilateral symmetry: Hox and Dpp expression in a sea anemone. Science. 2004;304:1335–1337. doi: 10.1126/science.1091946. [DOI] [PubMed] [Google Scholar]
- 5.Matus DQ, Thomsen GH, Martindale MQ. Dorso/ventral genes are asymmetrically expressed and involved in germ-layer demarcation during cnidarian gastrulation. Curr. Biol. 2006;16:499–505. doi: 10.1016/j.cub.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 6.Matus DQ, Pang K, Marlow H, Dunn CW, Thomsen GH, Martindale MQ. Molecular evidence for deep evolutionary roots of bilaterality in animal development. Proc. Natl. Acad. Sci. USA. 2006;103:11195–11200. doi: 10.1073/pnas.0601257103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saina M, Genikhovich G, Renfer E, Technau U. BMPs and Chordin regulate patterning of the directive axis in a sea anemone. Proc. Natl. Acad. Sci. USA. 2009;106:18592–18597. doi: 10.1073/pnas.0900151106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Ann. Rev. Cell Dev. Biol. 2004;20:285–308. doi: 10.1146/annurev.cellbio.20.011403.154124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schier AF, Talbot WS. Molecular genetics of axis formation in zebrafish. Ann. Rev. Genet. 2005;39:561–613. doi: 10.1146/annurev.genet.37.110801.143752. [DOI] [PubMed] [Google Scholar]
- 10.O'Connor MB, Umulis D, Othmer HG, Blair SS. Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development. 2006;133:183–193. doi: 10.1242/dev.02214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weisblat DA, Shankland M. Cell lineage and segmentation in the leech. Philos. Trans. R. Soc. Lond. B. 1985;312:39–56. doi: 10.1098/rstb.1985.0176. [DOI] [PubMed] [Google Scholar]
- 12.Weisblat DA, Kim SY, Stent GS. Embryonic origins of cells in the leech Helobdella triserialis. Dev. Biol. 1984;104:65–85. doi: 10.1016/0012-1606(84)90037-x. [DOI] [PubMed] [Google Scholar]
- 13.Huang FZ, Weisblat DA. Cell fate determination in an annelid equivalence group. Development. 1996;122:1839–1847. doi: 10.1242/dev.122.6.1839. [DOI] [PubMed] [Google Scholar]
- 14.Shankland M, Weisblat DA. Stepwise commitment of blast cell fates during the positional specification of the O and P cell lines in the leech embryo. Dev. Biol. 1984;106:326–342. doi: 10.1016/0012-1606(84)90231-8. [DOI] [PubMed] [Google Scholar]
- 15.Weisblat DA, Blair SS. Developmental interderterminacy in embryos of the leech Helobdella triserialis. Dev. Biol. 1984;101:326–335. doi: 10.1016/0012-1606(84)90146-5. [DOI] [PubMed] [Google Scholar]
- 16.Kuo D-H, Shankland M. Evolutionary diversification of specification mechanisms within the O/P equivalence group of the leech genus Helobdella. Development. 2004;131:5859–5869. doi: 10.1242/dev.01452. [DOI] [PubMed] [Google Scholar]
- 17.Ho RK, Weisblat DA. A provisional epithelium in leech embryo: cellular origins and influence on a developmental equivalence group. Dev. Biol. 1987;120:520–534. doi: 10.1016/0012-1606(87)90255-7. [DOI] [PubMed] [Google Scholar]
- 18.Zackson SL. Cell lineage, cell-cell interaction, and segment formation in the ectoderm of a glossiphoniid leech embryo. Dev. Biol. 1984;104:143–160. doi: 10.1016/0012-1606(84)90044-7. [DOI] [PubMed] [Google Scholar]
- 19.Reversade B, De Robertis EM. Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell. 2005;123:1147–1160. doi: 10.1016/j.cell.2005.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaviño MA, Reddien PW. A bmp/admp regulatory circuit controls maintenance and regeneration of dorsal-ventral polarity in planarians. Curr. Biol. 2011;21:294–299. doi: 10.1016/j.cub.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molina MD, Neto A, Maeso I, Gómez-Skarmeta JL, Saló E, Cebrià F. Noggin and noggin-like genes control dorsoventral axis regeneration in planarians. Curr. Biol. 2011;21:300–305. doi: 10.1016/j.cub.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Hsu DR, Economides AN, Wang X, Eimon PM, Harland RM. The Xenopus dorsalizing factor Gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol. Cell. 1998;1:673–683. doi: 10.1016/s1097-2765(00)80067-2. [DOI] [PubMed] [Google Scholar]
- 23.Capdevila J, Tsukui T, Rodríquez Esteban C, Zappavigna V, C IBJ. Control of vertebrate limb outgrowth by the proximal factor Meis2 and distal antagonism of BMPs by Gremlin. Mol. Cell. 1999;4:839–849. doi: 10.1016/s1097-2765(00)80393-7. [DOI] [PubMed] [Google Scholar]
- 24.Zúñiga A, Haramis A-PG, McMahon AP, Zeller R. Signal relay by BMP antagonism controls the SHH/FGF4 feedback loop in vertebrate limb buds. Nature. 1999;401:598–602. doi: 10.1038/44157. [DOI] [PubMed] [Google Scholar]
- 25.Khokha MK, Hsu D, Brunet LJ, Dionne MS, Harland RM. Gremlin is the BMP antagonist required for maintenance of Shh and Fgf signals during limb patterning. Nat. Genet. 2003;34:303–307. doi: 10.1038/ng1178. [DOI] [PubMed] [Google Scholar]
- 26.Michos O, Panman L, Vintersten K, Beier K, Zeller R, Zuniga A. Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development. 2004;131:3401–3410. doi: 10.1242/dev.01251. [DOI] [PubMed] [Google Scholar]
- 27.Verheyden JM, Sun X. An Fgf/Gremlin inhibitory feedback loop triggers termination of limb bud outgrowth. Nature. 2008;454:638–641. doi: 10.1038/nature07085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quigley IK, Schmerer MW, Shankland M. A member of the Six gene family promotes the specification of P cell fates in the O/P equivalence group of the leech Helobdella. Dev. Biol. 2010;344:319–330. doi: 10.1016/j.ydbio.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 29.Quigley IK, Xie X, Shankland M. Hau-Pax6A expression in the central nervous system of the leech embryo. Dev. Genes Evol. 2007;217:459–468. doi: 10.1007/s00427-007-0156-1. [DOI] [PubMed] [Google Scholar]
- 30.Denes AS, Jékely G, Steinmetz PRH, Raible F, Snyman H, Prud'homme B, Ferrier DEK, Balavoine G, Arendt D. Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in Bilateria. Cell. 2007;129:277–288. doi: 10.1016/j.cell.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 31.Shankland M. Determination of cleavage pattern in embryonic blast cells of the leech. Dev. Biol. 1987;120:494–498. doi: 10.1016/0012-1606(87)90252-1. [DOI] [PubMed] [Google Scholar]
- 32.Gline SE, Kuo D-H, Stolfi A, Weisblat DA. High resolution cell lineage tracing reveals developmental variability in leech. Dev. Dyn. 2009;238:3139–3151. doi: 10.1002/dvdy.22158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lapraz F, Besnardeau L, Lepage T. Patterning of the dorsal-ventral axis in echinoderms: insights into the evolution of the BMP-Chordin signaling network. PLoS Biol. 2009;7:e1000248. doi: 10.1371/journal.pbio.1000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patterson GI, Padgett RW. TGFβ-related pathways: roles in Caenorhabditis elegans development. Trends Genet. 2000;16:27–33. doi: 10.1016/s0168-9525(99)01916-2. [DOI] [PubMed] [Google Scholar]
- 36.Lemaire P, Smith WC, Nishida H. Ascidians and the plasticity of the chordate developmental program. Curr. Biol. 2008;18:R620–R631. doi: 10.1016/j.cub.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weisblat DA, Kuo D-H. Emerging Model Organisms: a Laboratory Manual. Cold Spring Harbor, NY: CSHL Press; 2009. Helobdella (Leech): a model for developmental studies; pp. 245–267. [DOI] [PubMed] [Google Scholar]
- 38.Zhang SO, Weisblat DA. Applications of mRNA injections for analyzing cell lineage and asymmetric cell divisions during segmentation in the leech Helobdella robusta. Development. 2005;132:2103–2113. doi: 10.1242/dev.01802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Time-lapse video showing H2B:GFP-labeled cells in the O and P lineages of a normal embryo. In this movie, two (roughly equal) blast cell divisions are seen in the p bandlet (left) with the posterior cell dividing very soon after the anterior cell. In the o bandlet, parts of three (distinctly unequal) mitoses are evident, but occur slightly out of order, i.e., the most anterior cell to divide does so after its posterior neighbor.
Time-lapse video showing H2B:GFP-labeled cells in the O and P lineages of an embryo in which the ipsilateral Q lineage was treated with Hau-bmp5-8 ASMO. Cells in both bandlets undergo unequal, O-like mitoses.
Time-lapse video showing H2B:GFP-labeled cells in the O and P lineages of an embryo in which the ipsilateral N lineage was forced to express Hau-bmp5-8 by mRNA injection. Cells in both bandlets undergo roughly equal, P-like mitoses.