Abstract
Mercury is a toxic metal that can exist in multiple chemical species. Humans are commonly exposed to methylmercury and mercury vapor, which is converted to mercuric mercury in the body. Despite years of research, there is a paucity of information on the similarity and differences in the mechanisms of mercury toxicity. The relative toxicity of mercuric chloride (HgCl2) and methylmercury chloride (MeHgCl) in C. elegans was determined using assays that measured growth, feeding, reproduction, and locomotion. The effect of HgCl2 and MeHgCl on the expression of several archetypal stress-response genes was also determined. There was no significant difference between the EC50s of the two mercurials on C. elegans growth. However, MeHgCl was more toxic to C. elegans than HgCl2 when assessing feeding, movement and reproduction, all of which require proper neuromuscular activity. Methylmercury chloride exposure resulted in increased steady-state levels of the stress response genes at lower concentrations than HgCl2. In general, MeHgCl was more toxic to C. elegans than HgCl2, particularly when assaying behaviors that require neuromuscular function.
Keywords: C. elegans, Mercury, Methylmercury, Stress-response, Metal toxicity
Introduction
Methylmercury and inorganic mercury are two mercurial species of great concern. Approximately one third of global mercury emissions are natural in origin, while anthropogenic sources account for the remaining two thirds [1]. Anthropogenic emissions in 2050 are projected to as much as double 2006 emission levels [2]. Mercury undergoes complex environmental cycling and can exist in multiple chemical forms. Atmospheric mercury can be oxidized, then dissolve in water and return to the earth's surface in rain [3]. In the aquatic environment, microbes can methylate divalent mercury [4]. The methylated mercury can undergo biomagnification, so that top predators (e.g., tuna) have methylmercury levels that are several orders of magnitude higher than those found in the environment [5]. Humans are exposed to methylmercury through the consumption of fish, where approximately 95% of the mercurial can be absorbed in the gastrointestinal tract [6]. Humans are also exposed to various forms of inorganic mercury from dental amalgams, chlor-alkali plants, fluorescent lamp factories, and artisanal gold mining [7, 8]. Mercury vapor may not be toxic to cells, but it can be rapidly oxidized to divalent mercury, which is believed to be the proximate toxic form [9]. Humans are also exposed to inorganic mercury through their diet, although less than 10% of ingested inorganic mercury is absorbed in the gastrointestinal tract [10].
The toxicity of methylmercury became evident after the mass poisonings in the region surrounding Minamata Bay, Japan in the 1950s and 1960s, and in Iraq in the early 1970s [9]. Affected adults presented various neurological deficits. In addition, the sensitivity in developing humans was demonstrated by the severe cognitive and physical congenital defects observed in children whose mothers were asymptomatic [11]. Inorganic mercury is a well-known renal toxicant, and occupational exposure to this metal has been associated with neurological and renal defects [7, 12, 13].
Despite years of research, only a fragmented understanding of the mechanisms of mercurial toxicity exists. In addition, there is a paucity of information regarding the molecular mechanisms of toxicity for inorganic and methylmercury. The mechanistic data that are available are primarily based on in vitro studies using cultured cells. In cultured cells, exposure to either mercurial results in oxidative stress, microtubule disruption, and alterations in intracellular calcium levels [14–19]. Little is known about the molecular mechanisms of mercurial toxicity in vivo.
There is a need for a whole organism model that can be used to investigate the mechanisms of toxicity of different mercurials. Caenorhabditis elegans (C. elegans) has many attributes that make it a good toxicological animal model [20]. The short life cycle (~70 h), small size (~1 mm) and transparency of C. elegans facilitate their use in medium and high-throughput toxicity assays. Using these assays, it is possible to assess the effects of toxicants on large numbers of animals using various endpoints [21]. While the evolutionary distance between C. elegans and humans is considerable, a high level of conservation in regulatory signal transduction pathways and a high degree of genetic homology exist between the two species. Homologs to 60–80% of human genes have been identified in C. elegans [22]. An impressive array of genetic tools coupled with the ability to study whole organism effects makes C. elegans a useful model organism in which to study the toxicity of mercurials.
In the present study, the toxicity of mercuric chloride (HgCl2) and methylmercury chloride (MeHgCl) in C. elegans were compared. C. elegans growth, reproduction, feeding, movement, and expression of stress-response genes were monitored as toxicological endpoints. In all of the assays, MeHgCl was more toxic to C. elegans than HgCl2. The relative toxicity between the mercurials however, differed by endpoint. These results provide insight into the mechanisms of mercurial toxicity and establish a framework in which the molecular toxicity of mercurials can be further examined.
Materials AND Methods
Maintenance of C. elegans
The Bristol N2 strain of C. elegans was used in all experiments with the Escherichia coli OP50 strain as a food source. C. elegans were maintained on K-agar plates at 20°C unless otherwise noted [23]. C. elegans were age synchronized by treating gravid adult nematodes with sodium hypochlorite/sodium hydroxide and allowing the embryos to hatch in the abscemce of food [24].
Growth assay
The effect of HgCl2 and MeHgCl on C. elegans growth was determined as previously described [24]. Briefly, 50 age-synchronized L1 larvae were dispensed into 96-well plates containing a range of concentrations of either HgCl2 or MeHgCl in complete K medium plus OP50 [25]. Nematodes were incubated at 20°C for 48 h. Under these conditions, untreated L1 larvae will develop to the L4 larval stage. At the end of 48 h, the size of each nematode, as determined by its optical density (EXT), was assessed using the COPAS Biosort (Union Biometrica). Twelve conditions (eleven concentrations plus untreated control) were assayed for both mercurials. The concentrations examined for HgCl2 were: 1.5, 2.1, 3, 4.3, 6.1, 8.7, 12, 18, 25, 35, and 50 μM and those for MeHgCl were: 1, 1.3, 1.8, 2.5, 3.3, 4.5, 6, 8.1, 11, 15 and 20 μM. Each condition was assayed in six wells per 96-well plate (~300 nematodes/condition/plate), and three replicates were performed. Growth was calculated by subtracting the mean EXT of the starting L1 larvae from the EXT measurement of each nematode at the end of the 48 h exposure. The 50% effective concentration (EC50) was estimated by fitting growth data to the Hill function [26].
Reproduction assay
A reproduction assay that uses the COPAS Biosort to directly count the number of offspring was used to examine the effect of mercurials as previously described [27]. In this assay, five age-synchronized L4 larvae were dispensed into each well of a 96-well plate containing varying concentrations of either HgCl2 or MeHgCl in K-medium with OP50. Six wells were used for each concentration (total of 30 L4s for each concentration). Twelve conditions (eleven concentrations plus untreated control) were tested for HgCl2, while seventeen conditions (sixteen concentrations plus untreated control) were tested for MeHgCl. The concentrations assayed for HgCl2 were: 0.3, 0.48, 0.75, 1.2, 1.9, 3, 4.8, 7.5, 12, 19, and 30 μM and those for MeHgCl were: 0.03, 0.048, 0.075, 0.12, 0.19, 0.3, 0.48, 0.75, 1.2, 1.9, 3, 4.8, 7.5, 12, 19, and 30 μM. After 48 h incubation at 20°C, the number of L1 C. elegans was determined. Each concentration had three to eight replicates. Fifty percent effective concentrations were estimated by fitting the data to the Hill function.
Feeding assay
The effect of mercurial exposure on feeding in C. elegans was determined as previously described [28]. In brief, 25 three-day-old age-synchronized adult nematodes were dispensed into each well of a 96-well plate containing various concentrations of HgCl2 or MeHgCl in K-medium with OP50. Six wells (total of 150 adult nematodes) were used for each concentration (eleven concentrations plus untreated control). The concentrations for HgCl2 were: 2, 3.2, 5, 8, 13, 20, 32, 50, 80, 130, and 200 μM and for MeHgCl: 0.5, 0.8, 1.3, 2, 3.2, 5, 8, 13, 20, 32, and 50 μM. Nematodes were incubated for 5 h at 20°C. At the end of the incubation, red fluorescent microspheres (0.5 μm diameter) were added to each well, and nematodes were allowed to feed for 15 min. Nematodes were then anesthetized with sodium azide, and the COPAS Biosort was used to measure the amount of red fluorescence (RFP) in each nematode. Feeding was determined by subtracting the RFP value post-feeding from the mean background RFP of nematodes at the beginning of the experiment. Three replicates were performed and EC50s were estimated by fitting to the Hill function.
Motion tracking
The effects of mercurial exposure on locomotion was determined as previously described [21]. This assay utilized a myo-2∷GFP strain of C. elegans (CB5584), which expressed high levels of green fluorescent protein in pharyngeal muscles [29]. Motion tracking was performed using an inverted fluorescence microscope (Leica) equipped with a camera (Hamamatsu), motorized stage, and temperature/humidity controlled incubator (Okolab). Approximately 40 age-synchronized L4 larval C. elegans were exposed five concentrations of HgCl2 or MeHgCl in K-medium for 4 h. The HgCl2 concentrations used were: 1, 3, 10, 30, and 100 μM and for MeHgCl were: 0.3, 1, 3, 10, and 30 μM. Exposed nematodes were then transferred to agar pads (1% agar, 51mM NaCl) and nematode movement was then monitored at low magnification for ~35 sec (3.5 frames/sec). The motion tracking software (Hamamatsu) generated Computer Assisted Sperm Analysis-like statistics for each animal. Three parameters were reported: curvilinear distance, which is the total distance traveled by the nematode; curvilinear velocity, which is the speed of the nematode; and mean amplitude of the head (ALH mean), which is the average amplitude of the sinusoidal movement of the nematode. The Kruskal-Wallis test was used to find significant differences between treated and untreated samples. Three replicates were performed and the criterion for statistical significance was set at p < 0.01.
Quantitative reverse transcription-real time PCR
Quantitative reverse transcription-real time-PCR (qRT-PCR) was used to measure the effect of the mercurials on steady-state mRNA levels of the C. elegans genes: gcs-1 (γ-glutamylcysteine synthetase), gst-38 (glutathione S-transferase), hsp-16.2, hsp-70 (heat shock protein). Mixed stage populations of C. elegans in complete S-medium liquid culture [30] were then exposed to HgCl2 (1, 2.5, 4, 8, 10, 16 or 25 μM) or MeHgCl (0.1, 0.25, 0.6, 1.6, 4, 10 or 16 μM) for 24 h at 20°C with constant agitation.
Mixed stage populations were used for qRT-PCR due to the higher concentrations used in this study. These concentrations may delay nematode development; therefore, mixed-stage populations were used to help ensure that differences in gene expression were due to mercurial exposure, and not differences in the developmental stages of different treatment groups.
After exposure, C. elegans were collected and stored at −80°C until RNA purification, as previously described [31]. Total RNA was isolated using RNeasy Mini kit (Qiagen) according to manufacturer's instructions. cDNAs were generated using the SuperScript® First-Strand Synthesis System for RT-PCR from 2 μg total RNA according to manufacturer's instructions (Invitrogen). cDNAs were subsequently used in qRT-PCR using Power SYBR Green RT-PCR kits according to manufacturer's instructions (Applied Biosystems). Quantitative PCR was performed in an ABI 7900 HT Fast Real-Time System and fold-changes in mRNA levels were calculated using the ΔΔCt method [32] using mlc-2 (myosin light chain) as reference mRNA. Primers (Table 1) were designed using the open source Primer3 program [33]. Results were presented as the mean ± standard error of the mean. The significance of mean differences was determined by 1-way ANOVA of log-transformed data followed by Dunnett's multiple comparison test to find differences in treated and control samples. Three replicates were performed and the criterion for statistical significance was set at p < 0.05.
Table 1.
Primers used in qRT-PCR reactions
| Gene | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| mlc-2 | TTGACAGGAACTGACCCAGAGG | ATAGCCTTGACCTCATCCTCG |
| gcs-1 | CCACCAGATGCTCCAGAAAT | TGCATTTTCAAAGTCGGTCA |
| gst-38 | AAGATAACAGACTTACCGATGAGGA | GAAGCTGGTTGTATGGGGTTT |
| hsp-16.2 | TGCAGAATCTCTCCATCTGAGT | TGGTTTAAACTGTGAGACGTTGA |
| hsp-70 | CGGTATTTATCAAAATGGAAAGGTT | TACGAGCGGCTTGATCTTTT |
Results
Effect of mercurials on C. elegans growth
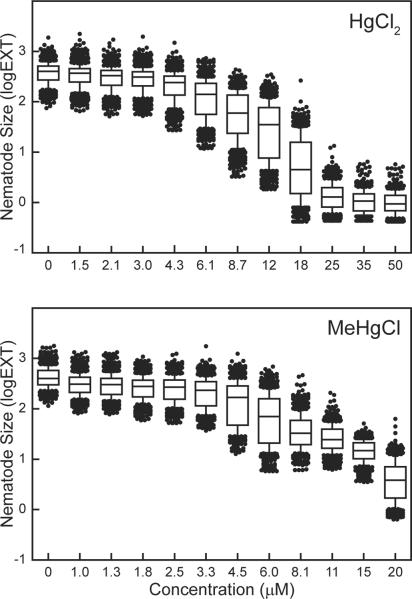
C. elegans increase in size as they develop from the L1 larval stage to reproductive adult [34]. Thus, while the growth assay measures the change in nematode size, a deficit in growth indicates delayed or arrested development. The effect of HgCl2 and MeHgCl on the growth of C. elegans, relative to untreated nematodes, was determined following a 48 h exposure to eleven concentrations of each mercurial (Fig. 1). The EC50s, the concentration of a mercurial that inhibits growth by 50%, were not significantly different between the two mercurials. The EC50 for HgCl2 was 11.8 μM, while the EC50 for MeHgCl was 11.3 μM (Table 2). However, differences in lethality, defined as nematodes that were immobile and smaller than L1s, were observed between the mercurials at higher concentrations. Nematodes exposed to HgCl2 at concentrations greater than 25 μM appeared to arrest in the L1 stage, while the same MeHgCl concentrations resulted in nematode death. Exposure to MeHgCl concentrations higher than 20 μM was 100% lethal (data not shown), while nematodes tolerated HgCl2 concentrations as high as 50 μM (Fig. 1).
Figure. 1.
Effect of mercurials on C. elegans growth. Nematode size is expressed as the log of the absorbance (EXT) of individual nematodes at the end of 48 h exposure to HgCl2 or MeHgCl minus the mean absorbance of all nematodes at the beginning of exposure. Lines in box plots indicate mean, boxes include 25th–75th percentile, whiskers include 10th–90th percentile. Data represent combined results of three independent experiments. Each box plot represents 500 to 900 nematodes.
Table 2.
Levels of mercurial toxicity in C. elegans
| Assay | Mercury chloride | Methylmercury chloride | ||
|---|---|---|---|---|
| EC50 (μM) | 95% Confidence Interval (μM) | EC50 (μM) | 95% Confidence Interval (μM) | |
| Growth | 11.8 | 11.2 – 12.4 | 11.3 | 10.4 – 12.2 |
| Feeding | 34.8 | 29.9 – 39.7 | 11.3 | 10.8 – 11.9 |
| Reproduction | 11.3 | 7.1 – 15.5 | 0.81 | 0.68 – 0.95 |
EC50, 50% effective concentration
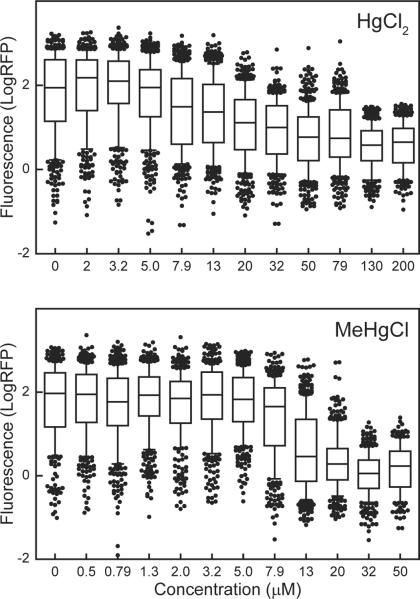
Effect of mercurials on C. elegans feeding
Feeding in C. elegans is accomplished by the coordinated pumping of three sections of the pharynx, which simultaneously push bacteria into the intestine and expel excess liquid out of the mouth. The neuromuscular activity of feeding is regulated by a self-contained nervous system [35]. The effect of mercurials on feeding was assessed by exposing adult nematodes to eleven concentrations of HgCl2 and MeHgCl for 5 h (Fig. 2). The feeding EC50 for HgCl2 was 34.8 μM and for MeHgCl it was 11.3 μM (Table 2). Feeding was completely inhibited after exposure to 32 μM MeHgCl. In contrast, exposure to 200 μM HgCl2 did not completely inhibit feeding (Fig. 2). The difference between the EC50s for the two mercurials suggested that MeHgCl was more neurotoxic than HgCl2.
Figure. 2.
Effect of mercurials on C. elegans feeding. The level of feeding (LogRFP) was measured in individual nematodes following a 5 h exposure to HgCl2 or MeHgCl followed by a 15 min incubation with fluorescent beads. LogRFP values are the measured fluorescence values following exposure minus mean background fluorescence of all nematodes at the beginning of mercurial exposure [28]. Lines in box plots indicate mean, boxes include 25th to 75th percentile, whiskers include 10th to 90th percentile. Data represent combined results of 3 independent experiments. Each box plot represents 200 to 400 nematodes.
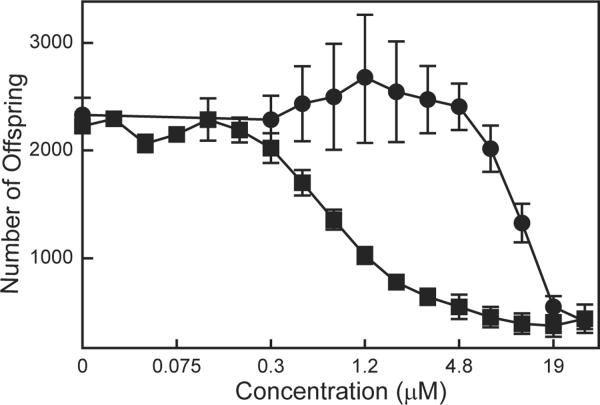
Effect of mercurials on C. elegans reproduction
Reproduction in C. elegans involves the integration of several processes: gamete formation, embryonic development and egg laying, which is a neuromuscular activity. This makes reproduction a sensitive endpoint in measuring effects of toxicants. The effect of mercurials on C. elegans reproduction was assessed by determining the number of progeny produced after 48 h exposure to different concentrations of either HgCl2 or MeHgCl (Fig. 3). There was a 14-fold difference between the effective concentrations of HgCl2 and MeHgCl: EC50 for HgCl2 was 11.3 μM, while the EC50 for MeHgCl was 0.81 μM (Table 2). The difference between the HgCl2 and MeHgCl on the reproduction EC50s was the greatest of the endpoints examined. In addition, the EC50 for MeHgCl reproduction was 14-fold lower than that calculated for the growth assay. As both the growth and reproduction endpoints assess the effects of a 48 h exposure, these results indicate that C. elegans reproduction is particularly sensitive to MeHgCl exposure.
Figure. 3.
Effect of mercurials on C. elegans reproduction. Nematodes in final larval stage were exposed to HgCl2 (●) or MeHgCl (∎) for 48 h. The number of offspring from treated nematodes was then measured [27]. Data presented are mean ± standard mean error of 3 to 8 independent experiments.
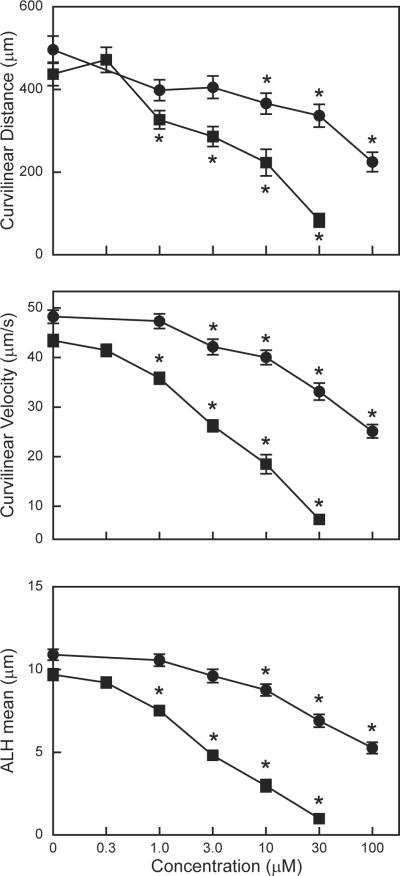
Effect of mercurials on C. elegans locomotion
C. elegans move forward or backward in a sinusoidal motion across solid substrates. To determine the effect of mercurials on locomotion, C. elegans movement was measured following a 4 h exposure to HgCl2 or MeHgCl. The distance (curvilinear distance), speed (curvilinear velocity) and amplitude of the nematodes sinusoidal motion (ALH mean) all decreased in a exposure-dependent manner in response to exposure to either mercurial (Fig. 4). Exposure to 1 μM MeHgCl significantly affected C. elegans by all three motion measurements. Conversely, exposure to 3 μM HgCl2 was required to significantly decrease curvilinear velocity and exposure to 10 μM HgCl2 was required to significantly decrease curvilinear distance and ALH mean. Supplemental Figure 1 shows representative computer-generated tracks for untreated nematodes and those exposed to 10 μM of HgCl2 and MeHgCl. The effect of MeHgCl on the length and amplitude of the tracks of nematodes is particularly evident.
Figure. 4.
Effect of mercurials on C. elegans locomotion. Movement of L4 larvae was tracked after a 4 h exposure to various concentrations of HgCl2 or MeHgCl. Three motion parameters were then calculated: curvilinear distance (upper panel), curvilinear velocity (middle panel), and mean amplitude of the head (ALH mean) (lower panel). Data presented are mean ± standard mean error of three independent experiments. Significant differences relative to untreated are indicated with asterisks.
Effect of mercurials on stress-response gene expression
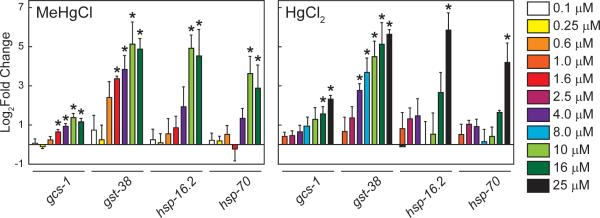
To determine the effect of mercurials on C. elegans at a molecular level, the exposure-response of HgCl2 and MeHgCl on steady-state mRNA levels of four stress-responsive genes: gcs-1, gst-38, hsp-16.2, and hsp-70; were determined. γ-glutamyl-cysteine synthetase (gcs-1) catalyzes the rate-limiting first step in the synthesis of glutathione and is involved in the oxidative stress pathway [36] Glutathione S-transferase-38 is up-regulated in response to multiple stressors [37, 38]. The genes that encode the C. elegans heat shock proteins, hsp-16.2 and hsp-70 are up-regulated by heat shock and other environmental stressors [39, 40]. Steady-state mRNA levels of these genes were measured following a 24 h exposure of a mixed-stage population of C. elegans to HgCl2 or MeHgCl (Fig. 5).
Figure. 5.
Effect of mercurials on stress-response gene expression. Relative mRNA levels of four C. elegans stress-responsive genes, gcs-1, gst-38, hsp-16.2 and hsp-70, were measured from mixed-stage C. elegans populations following a 24 h exposure to HgCl2 or MeHgCl. Data represent mean ± standard mean error of 3 to 4 independent experiments. Significant differences relative to untreated are indicated with asterisks. Values for the fold change in stress-response gene expression can be found in Supplemental Table 1
There were exposure-dependent increases in mRNA levels after exposure to either mercurial. There was a significant increase in mRNA levels of gcs-1 and gst-38 in nematodes exposed to 1.6 μM MeHgCl. Conversely, exposure to 16 μM HgCl2 was required to significantly increase gcs-1 levels, and exposure to 48 μM HgCl2 was required to significantly increase gst-38 mRNA levels. A lower concentration of MeHgCl was also required for induction of heat shock protein mRNAs. Exposure to 10 μM MeHgCl significantly increased mRNA levels of both hsp-16.2 and hsp-70, while a 25 μM HgCl2 exposure was required to significantly increase mRNA levels of the two heat shock proteins.
Discussion
Mercury is an environmental toxicant of great public health concern. In the present study, the toxicity of an inorganic (HgCl2) and organic (MeHgCl) mercury in C. elegans was compared. When examining the effect of mercurials on growth, there was not a significant difference between the EC50 values (Table 2). MeHgCl concentrations greater than 20 μM, however, were lethal to L1 larvae. In contrast, exposure to HgCl2 at concentrations greater than 20 μM resulted in viable, but growth arrested L1 larvae. For feeding, the HgCl2 EC50 was approximately three-fold higher than that for MeHgCl. In addition, exposure to 32 μM MeHgCl completely inhibited feeding, while C. elegans exposed to HgCl2 concentrations as high as 200 μM did not completely abrogate feeding. For the three measurements of locomotion, MeHgCl had significant inhibitory effects at concentrations lower than those observed following HgCl2 exposure. The largest difference in toxicity between HgCl2 and MeHgCl was observed in reproduction. The HgCl2 EC50 was ~14-fold higher than that for MeHgCl. Finally, MeHgCl exposure induced significant increases in stress-response gene expression at lower concentrations than HgCl2. While the relative toxicity between HgCl2 and MeHgCl differed by end-point, MeHgCl was consistently more toxic to nematodes than HgCl2 (Table 2).
Previous studies have used different methods to investigate the toxicity of HgCl2 or MeHgCl in C. elegans. The 24 h LC50 of HgCl2 in adult C. elegans exposed on agar plates or liquid culture were 500 μM and 90 μM, respectively [23, 41]. The difference in LC50s may be due to a greater bioavailability of HgCl2 in liquid. In agreement with the data presented here, a study that examined the effect of continuous MeHgCl exposure on C. elegans brood size found that C. elegans cultured on agar containing 10 μM MeHgCl had a 90% decrease in brood size and a 40% increase in time to adulthood [42]. However, a study of the effects of a 15 h MeHgCl exposure (100 μM – 400 μM) in C. elegans found no effect on life span or brood size, a temporary deficit in feeding and delayed growth [43]. It is therefore possible that nematodes can recover from MeHgCl toxicity after cessation of MeHgCl exposure. The present study is the first to directly compare the toxicity of HgCl2 and MeHgCl in C. elegans.
Methylmercury was generally more toxic than HgCl2 in C. elegans. This observation is consistent with experiments using cultured cells. Among three human neural cell lines, SH-SY5Y (neuroblastoma), U373MG (glioblastoma) and D407 (retinal pigment epithelial), the effects of 24 h and 48 h exposures to HgCl2 and MeHgCl on mitochondrial enzyme activity were compared [44]. As was observed in the present study, MeHgCl exposure was more toxic than HgCl2, though the relative toxicity of the mercurials varied by cell line and time of exposure. For example, the 24 h EC50s of HgCl2 and MeHgCl in D407 cells were not significantly different. In contrast, the 24 h HgCl2 EC50 was eight-fold greater than the MeHgCl EC50 in SH-SY5Y cells. Similarly, the 24 h HgCl2 EC50 was five-fold greater than that for MeHgCl in differentiated rat pheochromocytoma cells (PC12) [45].
Human exposure to mercury vapor can produce tremors and loss of coordination, while exposure to methylmercury can result in ataxia [11, 46]. Similarly, a disruption of neuromuscular activity was observed in C. elegans after mercurial exposure. Neuromuscular function was more sensitive to MeHgCl than HgCl2. For feeding, the MeHgCl EC50 was 3-fold lower than that for HgCl2. Methylmercury exposure also inhibited locomotion at lower concentrations than HgCl2. There was not a significant difference between the mercurial on C. elegans growth, which is not a sensitive measure of neurotoxicity. This suggests that MeHgCl is a more potent neurotoxicant than HgCl2 in C. elegans.
In utero methylmercury exposure results in congenital cognitive and anatomical defects in humans in the absence of overt maternal toxicity [47]. Methylmercury also causes a dose-dependent decrease in mouse litter size [48]. Little is known about in utero inorganic mercury exposures, but the progeny of pregnant Long-Evans rats exposed to mercury vapor exhibited developmental toxicity. This only occurred, however, at concentrations that caused significant maternal toxicity [49]. C. elegans early life stages were highly susceptible to MeHgCl, but not HgCl2, toxicity. For reproduction, the MeHgCl EC50 was 14-fold lower than the HgCl2 EC50 (Table 2). Exposure to 0.81 μM was not toxic to post-embryonic nematodes. In contrast, the HgCl2 EC50s for reproduction and growth were not significantly different. These data suggest that the phenomenon of exquisite susceptiblilty of early life stages to methlymercury, but not inorganic mercury, is conserved across species.
Both HgCl2 and MeHgCl exposure resulted in an increase in the expression of stress response genes, though the induction of these genes varied by mercurial and target gene. Induction of heat shock proteins required higher concentrations of either HgCl2 or MeHgCl than did gcs-1 or gst-38. The induction of glutathione-S-transferase and γ-glutamylcysteine synthetase transcription is used as a marker of toxicant induced intracellular oxidative stress [50]. Glutathione is critical in the oxidative stress response, as supplementation with a glutathione precursor protects against both MeHgCl and HgCl2 toxicity [51, 52]. Thus both mercurials induce oxidative stress in C. elegans. In addition, MeHgCl induces oxidative stress at lower concentrations than HgCl2. Methylmercury exposure induced the glutathione-response genes (gcs-1 and gst-38) at 5–10-fold lower concentrations than HgCl2 exposure. In contrast, MeHgCl exposure induced expression of heat shock genes at concentrations only two to three-fold lower than HgCl2. The heat shock response may be a secondary response following mercurial- or reactive oxygen species-mediated protein damage, which occurs when the oxidative stress defense mechanisms are depleted. In all genes tested, lower concentrations of MeHgCl were required to induce significant increases in expression relative to HgCl2. This suggests that MeHgCl is a particularly potent inducer of intracellular damage and this damage may be a consequence of mercurial-induced oxidative stress.
Inorganic and methylmercury remain toxicants of great concern to human health. The extent to which inorganic and methylmercury have similar or divergent mechanisms of toxicity is a question that requires further inquiry. In the present study, a detailed comparison of the toxicity of inorganic and methylmercury in C. elegans was performed. The C. elegans model has many traits that make it an appealing model in which to study molecular mechanisms of toxicity. The present study establishes the framework in which comparisons in the molecular mechanisms of mercurial toxicity can be studied in C. elegans.
Supplementary Material
Acknowledgement
We would like to thank Windy Boyd, Julie Rice, Marjolein Smith, and Daniel Snyder for their assistance in experiments using the COPAS Biosort. This work was supported (in part) by the Intramural Research Program of the NIH, and NIEHS (Z01ES102045).
References
- [1].Pacyna EG, Pacyna JM, Steenhuisen F, Wilson S. Global anthropogenic mercury emission inventory for 2000. Atmos Environ. 2006;40:4048–4063. [Google Scholar]
- [2].Streets DG, Zhang Q, Wu Y. Projections of global mercury emissions in 2050. Environ Sci Technol. 2009;43:2983–2988. doi: 10.1021/es802474j. [DOI] [PubMed] [Google Scholar]
- [3].Lin CJ, Pehkonen SO. The chemistry of atmospheric mercury: A review. Atmos Environ. 1999;33:2067–2079. [Google Scholar]
- [4].Ullrich SM, Tanton TW, Abdrashitova SA. Mercury in the aquatic environment: A review of factors affecting methylation. Crit Rev Environ Sci Technol. 2001;31:241–293. [Google Scholar]
- [5].Downs SG, Macleod CL, Lester JN. Mercury in precipitation and its relation to bioaccumulation in fish: A literature review. Water Air Soil Pollut. 1998;108:149–187. [Google Scholar]
- [6].World Health Organization . Environmental Health Criteria 101: Methylmercury. World Health Organization; Geneva: 1990. 92 4 157101 2. [Google Scholar]
- [7].National Toxicology Program . Toxicology and carcinogenesis studies of mercuric chloride in F344 rats and B6C3F1 mice. National Toxicology Program; Research Triangle Park, NC: 1993. 93-3139. [PubMed] [Google Scholar]
- [8].Malm O. Gold mining as a source of mercury exposure in the Brazilian Amazon. Environ Res. 1998;77:73–78. doi: 10.1006/enrs.1998.3828. [DOI] [PubMed] [Google Scholar]
- [9].Clarkson TW, Magos L. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol. 2006;36:609–662. doi: 10.1080/10408440600845619. [DOI] [PubMed] [Google Scholar]
- [10].Rahola T, Hattula T, Korolainen A, Miettinen JK. Elimination of free and protein bound ionic mercury (203Hg2+) in man. Ann Clin Res. 1973;5:214–219. [PubMed] [Google Scholar]
- [11].Ekino S, Susa M, Ninomiya T, Imamura K, Kitamura T. Minamata disease revisited: An update on the acute and chronic manifestations of methyl mercury poisoning. J Neurol Sci. 2007;262:131–144. doi: 10.1016/j.jns.2007.06.036. [DOI] [PubMed] [Google Scholar]
- [12].Risher JF. Elemental mercury and inorganic mercury compounds: Human health aspects. World Health Organization; Geneva, Switzerland: 2003. [Google Scholar]
- [13].Corbett CEP, El Khouri M, Costa AN, Gyuricza JV, Corbett JF, Frizzarini R, Andrade D, Cordeiro Q, Stravogiannis A, Chassot CA, Vieira JLF, Pinheiro MD. Health evaluation of gold miners living in a mercury-contaminated village in Serra Pelada, Para, Brazil. Arch Environ Occup Health. 2007;62:121–128. doi: 10.3200/AEOH.62.3.121-128. [DOI] [PubMed] [Google Scholar]
- [14].Monroe RK, Halvorsen SW. Mercury abolishes neurotrophic factor-stimulated Jak-STAT signaling in nerve cells by oxidative stress. Toxicol Sci. 2006;94:129–138. doi: 10.1093/toxsci/kfl073. [DOI] [PubMed] [Google Scholar]
- [15].Yin Z, Milatovic D, Aschner JL, Syversen T, Rocha JBT, Souza DO, Sidoryk M, Albrecht J, Aschner M. Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes. Brain Res. 2007;1131:1–10. doi: 10.1016/j.brainres.2006.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bonacker D, Stoiber T, Wang M, Bohm KJ, Prots I, Unger E, Thier R, Bolt HM, Degen GH. Genotoxicity of inorganic mercury salts based on disturbed microtubule function. Arch Toxicol. 2004;78:575–583. doi: 10.1007/s00204-004-0578-8. [DOI] [PubMed] [Google Scholar]
- [17].Lawton M, Iqbal M, Kontovraki M, Lloyd Mills C, Hargreaves AJ. Reduced tubulin tyrosination as an early marker of mercury toxicity in differentiating N2a cells. Toxicol In Vitro. 2007;21:1258–1261. doi: 10.1016/j.tiv.2007.03.018. [DOI] [PubMed] [Google Scholar]
- [18].Burlando B, Bonomo M, Fabbri E, Dondero F, Viarengo A. Hg2+ signaling in trout hepatoma (RTH-149) cells: Involvement of Ca2+-induced Ca2+ release. Cell Calcium. 2003;34:285–293. doi: 10.1016/s0143-4160(03)00123-4. [DOI] [PubMed] [Google Scholar]
- [19].Hare MF, Atchison WD. Methylmercury mobilizes Ca++ from intracellular stores sensitive to inositol 1,4,5-trisphosphate in NG108-15 cells. J Pharmacol Exp Ther. 1995;272:1016–1023. [PubMed] [Google Scholar]
- [20].Leung MC, Williams PL, Benedetto A, Au C, Helmcke KJ, Aschner M, Meyer JN. Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicol Sci. 2008;106:5–28. doi: 10.1093/toxsci/kfn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Boyd WA, Smith MV, Kissling GE, Freedman JH. Medium- and high-throughput screening of neurotoxicants using C. elegans. Neurotoxicol Teratol. 2010;32:68–73. doi: 10.1016/j.ntt.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kaletta T, Hengartner MO. Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Discov. 2006;5:387–398. doi: 10.1038/nrd2031. [DOI] [PubMed] [Google Scholar]
- [23].Williams PL, Dusenbery DB. Using the nematode Caenorhabditis elegans to predict mammalian acute lethality to metallic salts. Toxicol Ind Health. 1988;4:469–478. doi: 10.1177/074823378800400406. [DOI] [PubMed] [Google Scholar]
- [24].Boyd WA, Smith MV, Kissling GE, Rice JR, Snyder DW, Portier CJ, Freedman JH. Application of a mathematical model to describe the effects of chlorpyrifos on Caenorhabditis elegans development. PLoS One. 2009;4:e7024. doi: 10.1371/journal.pone.0007024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Williams PL, Dusenbery D, B Aquatic toxicity testing using the nematode, Caenorhabditis elegans. Environ Toxicol Chem. 1990;9:1285–1290. [Google Scholar]
- [26].Hill AV. The combinations of haemoglobin with oxygen and with carbon monoxide. Biochem J. 1913;7:471–480. doi: 10.1042/bj0070471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Boyd WA, McBride SJ, Rice JR, Snyder DW, Freedman JH. A high-throughput method for assessing chemical toxicity using a Caenorhabditis elegans reproduction assay. Toxicol Appl Pharmacol. 2010;245:153–159. doi: 10.1016/j.taap.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Boyd WA, McBride SJ, Freedman JH. Effects of genetic mutations and chemical exposures on Caenorhabditis elegans feeding: evaluation of a novel, high-throughput screening assay. PLoS ONE. 2007;2:e1259. doi: 10.1371/journal.pone.0001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Graves AL, Boyd WA, Williams PL. Using transgenic Caenorhabditis elegans in soil toxicity testing. Arch Environ Contam Toxicol. 2005;48:490–494. doi: 10.1007/s00244-004-0031-2. [DOI] [PubMed] [Google Scholar]
- [30].Sulston J, Hodgkin J. Methods. In: Wood WB, editor. The Nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1988. pp. 587–606. [Google Scholar]
- [31].Liao VH, Freedman JH. Cadmium-regulated genes from the nematode Caenorhabditis elegans. Identification and cloning of new cadmium-responsive genes by differential display. J Biol Chem. 1998;273:31962–31970. doi: 10.1074/jbc.273.48.31962. [DOI] [PubMed] [Google Scholar]
- [32].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods (Duluth) 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- [33].Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- [34].Hirsh D, Oppenheim D, Klass M. Development of the reproductive system of Caenorhabditis elegans. Dev Biol. 1976;49:200–219. doi: 10.1016/0012-1606(76)90267-0. [DOI] [PubMed] [Google Scholar]
- [35].Albertson DG, Thomson JN. The pharynx of Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1976;275:299–325. doi: 10.1098/rstb.1976.0085. [DOI] [PubMed] [Google Scholar]
- [36].Liao VHC, Yu CW. Caenorhabditis elegans gcs-1 confers resistance to arsenic-induced oxidative stress. Biometals. 2005;18:519–528. doi: 10.1007/s10534-005-2996-3. [DOI] [PubMed] [Google Scholar]
- [37].Cui Y, McBride SJ, Boyd WA, Alper S, Freedman JH. Toxicogenomic analysis of Caenorhabditis elegans reveals novel genes and pathways involved in the resistance to cadmium toxicity. Genome Biol. 2007;8:R122. doi: 10.1186/gb-2007-8-6-r122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hasegawa K, Miwa S, Isomura K, Tsutsumiuchi K, Taniguchi H, Miwa J. Acrylamide-responsive genes in the nematode Caenorhabditis elegans. Toxicol Sci. 2008;101:215–225. doi: 10.1093/toxsci/kfm276. [DOI] [PubMed] [Google Scholar]
- [39].Candido EP. The small heat shock proteins of the nematode Caenorhabditis elegans: structure, regulation and biology. Prog Mol Subcell Biol. 2002;28:61–78. doi: 10.1007/978-3-642-56348-5_4. [DOI] [PubMed] [Google Scholar]
- [40].Mukhopadhyay I, Nazir A, Saxena DK, Kar Chowdhuri D. Heat shock response: hsp70 in environmental monitoring. J Biochem Mol Toxicol. 2003;17:249–254. doi: 10.1002/jbt.10086. [DOI] [PubMed] [Google Scholar]
- [41].Jones D, Candido EP. Feeding is inhibited by sublethal concentrations of toxicants and by heat stress in the nematode Caenorhabditis elegans: relationship to the cellular stress response. J Exp Zool. 1999;284:147–157. doi: 10.1002/(sici)1097-010x(19990701)284:2<147::aid-jez4>3.3.co;2-q. [DOI] [PubMed] [Google Scholar]
- [42].VanDuyn N, Settivari R, Wong G, Nass R. SKN-1/Nrf2 inhibits dopamine neuron degeneration in a Caenorhabditis elegans model of methylmercury toxicity. Toxicol Sci. 2010;118:613–624. doi: 10.1093/toxsci/kfq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Helmcke KJ, Syversen T, Miller DM, Aschner M. Characterization of the effects of methylmercury on Caenorhabditis elegans. Toxicol Appl Pharmacol. 2009;240:265–272. doi: 10.1016/j.taap.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Toimela T, Tahti H. Mitochondrial viability and apoptosis induced by aluminum, mercuric mercury and methylmercury in cell lines of neural origin. Arch Toxicol. 2004;78:565–574. doi: 10.1007/s00204-004-0575-y. [DOI] [PubMed] [Google Scholar]
- [45].Parran DK, Mundy WR, Barone S., Jr Effects of methylmercury and mercuric chloride on differentiation and cell viability in PC12 cells. Toxicol Sci. 2001;59:278–290. doi: 10.1093/toxsci/59.2.278. [DOI] [PubMed] [Google Scholar]
- [46].Magos L, Clarkson TW. Overview of the clinical toxicity of mercury. Ann Clin Biochem. 2006;43:257–268. doi: 10.1258/000456306777695654. [DOI] [PubMed] [Google Scholar]
- [47].Masazumi H. Congenital Minamata disease: Intrauterine methylmercury poisoning. Teratology. 1978;18:285–288. doi: 10.1002/tera.1420180216. [DOI] [PubMed] [Google Scholar]
- [48].Hughes JA, Annau Z. Postnatal behavioral effects in mice after prenatal exposure to methylmercury. Pharmacol Biochem Behav. 1976;4:385–391. doi: 10.1016/0091-3057(76)90052-6. [DOI] [PubMed] [Google Scholar]
- [49].Morgan DL, Chanda SM, Price HC, Fernando R, Liu J, Brambila E, O'Connor RW, Beliles RP, Barone S., Jr Disposition of inhaled mercury vapor in pregnant rats: Maternal toxicity and effects on developmental outcome. Toxicol Sci. 2002;66:261–273. doi: 10.1093/toxsci/66.2.261. [DOI] [PubMed] [Google Scholar]
- [50].Shukla GS, Shukla A, Potts RJ, Osier M, Hart BA, Chiu JF. Cadmium-mediated oxidative stress in alveolar epithelial cells induces the expression of gamma-glutamylcysteine synthetase catalytic subunit and glutathione S-transferase alpha and pi isoforms: potential role of activator protein-1. Cell Biol Toxicol. 2000;16:347–362. doi: 10.1023/a:1007696610186. [DOI] [PubMed] [Google Scholar]
- [51].Becker A, Soliman KFA. The role of intracellular glutathione in inorganic mercury-induced toxicity in neuroblastoma cells. Neurochem Res. 2009;34:1677–1684. doi: 10.1007/s11064-009-9962-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kaur P, Aschner M, Syversen T. Glutathione modulation influences methyl mercury induced neurotoxicity in primary cell cultures of neurons and astrocytes. Neurotoxicology. 2006;27:492–500. doi: 10.1016/j.neuro.2006.01.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.