Abstract
Objective
Neurovascular coupling may be involved in compensatory mechanisms responsible for preservation of gait speed in elderly people with cerebrovascular disease. Our study examines the association between neurovascular coupling in the middle cerebral artery and gait speed in elderly individuals with impaired cerebral vasoreactivity.
Methods
Twenty-two fast and 20 slow walkers in the lowest quartile of cerebral vasoreactivity were recruited from the MOBILIZE Boston Study. Neurovascular coupling was assessed in bilateral middle cerebral arteries by measuring cerebral blood flow during the N-Back Task. Cerebral white matter hyperintensities were measured for each group using magnetic resonance imaging.
Results
Neurovascular coupling was attenuated in slow compared to fast walkers (2.8% [CI95%: −0.9–6.6] vs. 8.2% [CI95%: 4.7–11.8]; p=0.02). The odds of being a slow walker were 6.4 (CI95%: 1.7–24.9, p=0.007) if there was a high burden of white matter hyperintensity, however, this risk increased to 14.5 (CI95%: 2.3–91.1, p=0.004) if neurovascular coupling was also attenuated.
Interpretation
Our results suggest that intact neurovascular coupling may help preserve mobility in elderly people with cerebral microvascular disease.
INTRODUCTION
Gait speed, a reliable marker of mobility1, tends to decline with age. Slow gait speed is strongly associated with cerebrovascular disease2 and cerebral white matter hyperintensities (WMH)3–9. Despite the strong associations between cerebral microvascular brain damage and gait speed, not all individuals with WMH have slow gait10, 11. Some individuals seem to maintain a normal gait speed despite the presence of WMH on their MRIs12, 13. It is unclear how these high functioning individuals compensate for WMH and maintain a normal gait speed.
We have recently shown that impaired cerebral vasoreactivity, a nitric oxide mediated vasodilatory response to CO2 in cerebral arterioles14–17, is associated with slow gait speed and the development of falls in elderly people.10, 18 However, the relationship between metabolic cerebral blood flow regulation and gait speed is unknown. Because neuronal activity requires the delivery of adequate oxygen and glucose to specific regions of the brain, cerebral blood flow and cerebral metabolic rate are normally coupled. In other words, an increase in metabolic demand will lead to an increase in blood flow. This link between regional synaptic activity and regional cerebral blood flow is termed, neurovascular coupling19, 20 and may be involved in compensatory mechanisms responsible for the preservation of gait speed in elderly people.
Our study was designed to examine the relationship between neurovascular coupling and gait speed in elderly individuals with impaired cerebral vasoreactivity as an indicator of cerebrovascular dysregulation. Neurovascular coupling was gauged by changes in bilateral middle cerebral artery blood flow velocities during the N-Back Task. We hypothesized that neurovascular coupling would be attenuated in slow walkers. Since gait speed and cerebral vasoreactivity are known to be associated with white matter hyperintensities on magnetic resonance imaging10, we also examined the relationship between gait speed, neurovascular coupling and white matter hyperintensities. We also hypothesized that impaired neurovascular coupling would be associated with white matter hyperintensities.
METHODS
The study sample consisted of 42 individuals from the MOBILIZE Boston Study (MBS). The MBS is a prospective cohort study of a unique set of risk factors for falls in community-dwelling seniors living in the Boston area. The design and methodology for this study have been previously described in detail.21, 22 In brief, 765 persons aged 70 and older were enrolled using door-to-door population-based recruitment. To be included, individuals had to be >70 years of age (or age >65 if living with a spouse of a participant), able to understand and communicate in English, able to walk 20 feet without personal assistance (walking aids permitted), and expected to be in the Boston area for 2 years. Exclusion criteria included terminal disease, severe vision or hearing deficits and cognitive impairment (Mini-Mental State Examination <18).
The sample was recruited from the MBS cohort on the basis of their gait speed and cerebral vasoreactivity (VR). The cohort was divided into quartiles of VR. Individuals in the lowest quartile of vasoreactivity were then divided into quartiles of gait speed and those in the highest and lowest quartiles of gait speed were recruited for this study. Study subjects were characterized as slow (<0.67m/s) or fast (≥ 0.67 m/s) walkers on the basis of the shortest time to complete a 4 meter walk at their usual pace during 2 trials, starting from a standing position23. All individuals were assessed with a home interview and a detailed clinic examination, which included measures of lower extremity mobility performance (Short Physical Performance Battery (SPPB)23), executive function (Trail Making Tests A and B)24, and memory function (mini mental status exam, MMSE)25. In addition, they had transcranial Doppler ultrasound (TCD) measurements of cerebral blood flow and magnetic resonance imaging of the brain (MRI, 1.5 Tesla or 3.0 Tesla) as part of this protocol. This study was approved by the Institutional Review Boards of Hebrew SeniorLife and the Brigham and Women's Hospital. All subjects provided written informed consent.
Transcranial Doppler ultrasound
Instrumentation
Subjects reported to the Cerebrovascular Laboratory at the Hebrew SeniorLife Institute for Aging Research and were instrumented for heart rate (HR), electrocardiogram (ECG) and beat-to-beat arterial pressure monitoring (ABP, Finapres, Ohmeda Monitoring Systems, Englewood, CO) as previously described26. End-tidal CO2 was measured using a Vacumed CO2 Analyzer (Ventura, CA) attached to a nasal cannula.
TCD ultrasonography (MultiDop X4, DWL-Transcranial Doppler Systems Inc., Sterling, VA) was used to measure middle cerebral artery (MCA) mean blood flow velocity (BFV) at rest and in response to: 1) changes in end-tidal CO226 and 2) the N-Back cognitive task (see description below). The MCA signal was identified according to the criteria of Aaslid et al.27 and recorded at a depth of 50 to 60 mm. A Mueller-Moll probe fixation device was used to stabilize the Doppler probe at the temporal bone window for the duration of the study. The envelope of the velocity waveform, derived from a fast-Fourier analysis of the Doppler frequency signal, was digitized at 500 Hz, displayed simultaneously with the ABP, ECG, and end-tidal CO2 signals, and stored for later off-line analysis. Previous studies using a variety of techniques (133Xe, SPECT, MRI) have confirmed that relative changes in BFV are representative of changes in cerebral blood flow.28, 29
CO2 Vasoreactivity Protocol
BFV in the MCA was measured continuously while subjects inspired a gas mixture of 8% CO2, 21% O2, and balance nitrogen for 2 minutes and then mildly hyperventilated to an end-tidal CO2 of approximately 25 mmHg for 2 minutes. To determine cerebral vasoreactivity (VR) using this technique, MCA BFV was plotted against end tidal CO2 while breathing room air, 5% CO2 or hyperventilating. VR was measured as the slope of this relationship and expressed as change in cerebral blood flow per mmHg change in end-tidal CO218, 30, 31.
Neurovascular Coupling Protocol
While subjects were resting in the supine position, they were asked to watch a computer display projected onto the ceiling and complete the following three tasks:
-
1)
Identify X – This is the control condition, from which changes in blood flow during the 1-back and 2-back tasks (described below) were calculated. A series of single letters appeared in succession on the screen. Each letter was displayed for 4 seconds. A 5 second delay between flashing letters, indicated by a blank screen, reduced the occurrence of “tracers”. Subjects were asked to click the right button on a custom-built keypad each time they saw the letter X and to click the left button for all other cases.
-
2)
1-Back or 2-Back – A series of single letters appeared in succession on the screen. Subjects were asked to click the right button each time they saw a letter repeated (1-back) or each time they saw a letter repeated every other letter (2-back).
The sequence of testing for each N-back test (1-back and 2-back) was as follows: 2 minutes of identify the letter X followed by 2 minutes of N-back. The 1-back task was always given before the 2-back task. Each individual's performance during the N-back task was recorded and scored as the percent correct of the total. The “Identify the letter X” task was inserted to standardize cognitive activity during baseline measurements. Blood flow activation was normalized to control task (identify the letter X) to minimize any effects from differences in resting baseline cerebral blood flow velocities that are known to decrease with aging.
To allow BFV measures to stabilize after changing tasks, mean values were extracted from the middle 100 second time window for each 120-second task block. The mean percent change for each MCA was calculated as a ratio of the percent difference between the BFV during the N-back (BFVNB) and its corresponding “Identify the letter X” control period (BFVIDX) divided by BFV during Identify X (BFVIDX) and multiplied by 100 ([(BFVNB -BFVIDX)/ (BFVIDX)] *100). We report the percent change in BFV during the N-back task for each MCA individually (right or left MCA blood flow changes) as well as combine the values for the right and left MCA to report a total change in BFV during the N-Back task in bilateral MCA territories (total blood flow changes).
MRI Protocol
Brain MR imaging and analysis was performed on a GE Signa 1.5 Tesla or a 3 Tesla (General Electrics, Milwaukee, WI) MRI system at the Brigham and Women's Hospital. Images were acquired on a 1.5 Tesla scanner with a dual-echo spin-echo imaging pulse sequence, proton density weighted (PDW) and T2-weighted (T2W), using the following scan parameters: TR 3000 milliseconds (ms), TE 30–80 ms, slice thickness 3 millimeters (mm), 54 axial slices, matrix size 256×192, and field of view (FOV) 24×24 square-centimeters (cm2), total scan time was 11 minutes and 36 seconds. Quantitative assessment of the white matter hyper-intensity (WMH) burden on these 1.5-Tesla images was done with the method previously described32.
Brain imaging on a 3-Tesla scanner was also performed and the following three series were acquired: 1) Spoiled Gradient-Recalled Acquisition (SPGR), TR=7.80 ms, TE=2.98 ms, TI=450 ms, flip angle=15, slice thickness= 1 mm, matrix=256×256, FOV=25.6×25.6 cm2; 2) T2, TR=3000 ms, TE=71.00 ms, flip angle=90, slice thickness= 1.2 mm, matrix=256×256, FOV=30.7×30.7 cm2; and 3) fluid attenuated inversion recovery (FLAIR), TR=6200 ms, TE=200 ms, flip angle=90, slice thickness= 1.2 mm, matrix=256×256, FOV=30.7×30.7 cm2. The method described in33 was used to assess WMH burden in these 3 Tesla high-resolution images.
For both scans, brain volumes were expressed as percentage of intracranial cavity volume (ICC) in order to produce standardized indices suitable for group analysis. The brain parenchymal fraction (BPF) was the sum of total white matter and total gray matter volumes divided by the ICC.
Statistical Analysis
The study sample was characterized using means and standard deviations for continuous variables, and frequencies for categorical variables. T-tests, Wilcoxon rank-sum tests and chi-square tests were performed to determine if key variables differed by gait speed (fast or slow) status. Because of the two MRI magnet strengths (i.e., 1.5 Tesla and 3 Tesla), WMH volumes were categorized as low or high by dichotomizing below and above the median of the distribution for each technique. Then groups of subjects with low or high burden of WMH were combined for the analysis. Chi-square tests and logistic regression were used to examine the association between WMH groups (predictor) and gait speed (response). Unadjusted and adjusted odds ratios and 95% confidence intervals were calculated. Analysis of variance was performed to examine the association between gait speed and key neurovascular coupling variables for both 1-back and 2-back tests. Logistic regression was used to examine the association between percent change in blood flow velocity and gait speed (response). Linear regression was used to examine the association between percent change in blood flow velocity and percent correct during 2-back. An alpha level of 0.05 was used to determine statistical significance. All analyses were conducted using SAS version 9.2 (SAS Institute).
RESULTS
Subject Characteristics
Table 1 summarizes the demographic and clinical characteristics of the MBS cohort who participated in both the MRI and neurovascular coupling studies, stratified by gait speed. Individuals with a slower gait speed were significantly older, had a higher prevalence of diabetes and performed more poorly on their SPPB, MMSE, Trails A and B tests. However, there were no significant differences between slow and fast walkers with respect to their cerebrovascular hemodynamic variables. Cerebral vasoreactivity was low in both groups, as this was the basis for their selection for this study.
Table 1.
Subject Characteristics stratified by gait speed
| VARIABLES | FAST (N =22) | SLOW (N = 20) | p-Value |
|---|---|---|---|
| Age | 76 ± 5 | 82. ± 6 | 0.001 |
| Female % (N) | 50 (11) | 60 (12) | 0.52 |
| White % (N) | 100 (22) | 85 (17) | 0.06 |
| Education, years | 16 ± 2 | 14 ± 3 | 0.09 |
| Hypertension, % With Hx | 45 (10) | 75 (15/20) | 0.05 |
| Diabetes, % With Hx | 9 (2) | 35 (7) | 0.04 |
| Hyperlipidemia, % With Hx | 68 (15) | 50 (10) | 0.23 |
| Stroke, % with Hx | 5 (1) | 25 (5) | 0.06 |
| Gait Speed (meters/second) | 1.15 ± 0.19 | 0.57 ± 0.08 | 0.0001 |
| Trails A (sec) | 39.9 ± 16 | 64.6 ± 23 | 0.0003 |
| Trails B (sec) | 87.4 ± 28 | 173 ± 90 | 0.0001 |
| Adjusted Trails B (sec) | 48 ± 24 | 113 ± 79 | 0.003 |
| MMSE | 28.9 ± 1.2 | 26.1 ± 2.9 | 0.0006 |
| SPPB | 11.8 ± 1.2 | 7.7 ± 2.0 | 0.0001 |
| Hemodynamic Variables | |||
| Right VR | 0.7 ± 0.2 | 0.6 ± 0.4 | 0.69 |
| Left VR | 0.7 ± 0.2 | 0.6 ± 0.4 | 0.70 |
| Right MCA BFV | 30.6 ± 9.2 | 26.7 ± 12.3 | 0.25 |
| Left MCA BFV | 30.2 ± 10.5 | 26.5 ± 12.2 | 0.3 |
Mean (SD).
MMSE= Mini Mental Status Exam; SPPB= Short Physical Performance Battery;
VR= Vasoreactivity, MCA= Middle Cerebral Artery; BFV= Blood Flow Velocity
Neurovascular Coupling and Gait Speed
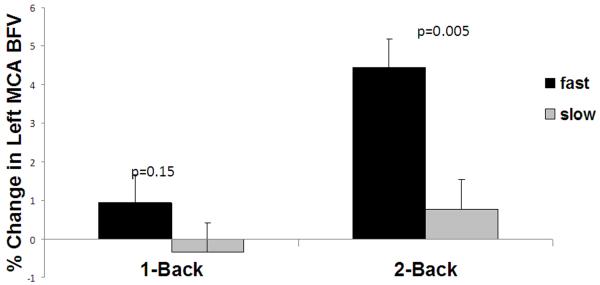
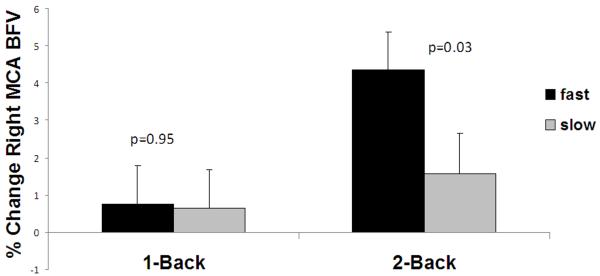
Figure 1 shows the association between blood flow activation during the N-back task (neurovascular coupling) and gait speed. Fast walkers had significantly more blood flow activation than the slow walkers in both the left and right MCAs during the 2-back task. In the left MCA, cerebral blood flow velocities increased by 4.1% [CI95%: 2.3– 6.0] in the fast and 1.0% [CI95%:−1.0–3.0] in the slow walkers (p=0.01). In the right MCA, cerebral blood flow velocities increased by 4.0% [CI95%: 2.2– 6.0] in the fast and 1.8% [CI95%:−0.1–3.8] in the slow walkers (p=0.07). The total blood flow change across the two MCA territories during the 2-back task was 8.2% [CI95%: 4.7–11.8] for the fast walkers and 2.8% [CI95%: −0.9–6.6] for the slow walkers (p=0.02). There were no significant differences in blood flow velocities in the MCAs during the 1-back task. Therefore, only blood flow velocities in the MCAs during the 2-back task (NVC during 2-back) were included in the subsequent analyses.
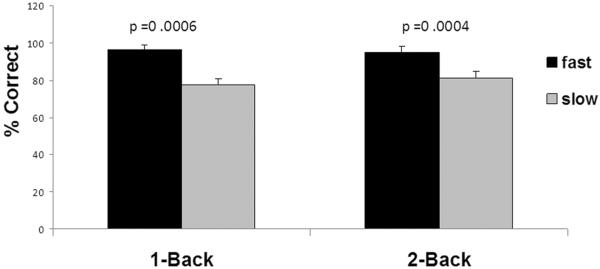
Figure 1.
Neurovascular Coupling and Gait Speed. The relationships between neurovascular coupling (a,b) or performance during N-back (c) and gait speed. 1a) Percent change in flood blow velocity during N-back in the left MCA; 1b) percent change in blood flow velocity during N-back in the right MCA; and 1c) percent correct during N-back (neurovascular coupling) in slow and fast walkers.
Since the slow walkers were significantly older and had a higher incidence of hypertension and cognitive dysfunction, we also examined the relationship between these variables and neurovascular coupling. We found a significant inverse relationship only between age and the left MCA blood flow changes during the 2-back task (p=0.03). None of the other variables were related to neurovascular coupling. After adjusting for age, the relationship between gait speed and left MCA blood flow changes during 2-back remained significant (p=0.03).
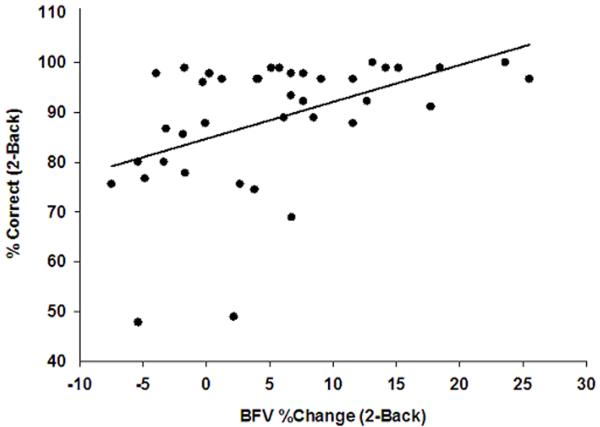
Overall, the fast walkers also performed significantly better than slow walkers in both N-Back tasks. Figure 2 shows the relationship between blood flow activation (neurovascular coupling) and performance during the 2-back task in all the subjects. In a regression of percent correct on the 2-back task on the percent change in blood flow velocity, the slope was 0.74 (CI95%: 0.31– 1.18; p=0.0014). Greater blood flow activation was associated with better performance.
Figure 2.
Neurovascular Coupling and Performance. Scatter plot of the percent change in blood blow velocity and the percent correct during the 2-back task. The line represents the relationship estimated by linear regression (slope estimate=0.74, standard error=0.22, p-value=.001).
White Matter Hyperintensities and Gait Speed
Table 2 summarizes the relationship between MRI measures and gait speed. Slow walkers had a higher burden of WMH as compared to fast walkers, but the differences were only significant in those individuals who underwent the 3Tesla MRI evaluation. There was no significant difference in total WM between the 2 gait speed groups, but there was a trend toward a smaller brain parenchymal fraction in the slow walkers.
Table 2.
Imaging Characteristics of the Slow and Fast Walkers for the 3.0 TESLA and 1.5 TESLA MRI groups (n=42).
| VARIABLES† |
||||||
|---|---|---|---|---|---|---|
| GAIT SPEED | WMH | Total WMH | WMH%IC C | ICC | WMH%BPF | BPF |
| 3.0T | ||||||
| FAST (N=17) | 3.14 (0.72–27.7) | 457.9 (366.7–624.5) | 0.2 (0.05–1.94) | 1425.9 (1219.0–1649.0) | 0.26 (0.07–2.50) | 1128.9 (982.0–1344.5) |
| Slow (N=12) | 25.01 (3.13–70.7) | 422.2 (361.3–516.2) | 1.66 (0.22–5.13) | 1430.2 (1298.0–1562.6) | 2.33 (0.29–6.43) | 1093.4 (973.6–1215.8) |
| p-Value | 0.002 | 0.39 | 0.001 | 0.71 | 0.001 | 0.15 |
| 1.5T | ||||||
| FAST (N=5) | 1.95 (1.13–7.51) | 474.8 (222.9–569.4) | 0.15 (0.08–0.42) | 1356.9 (1177.5–1777.7) | 0.18 (0.10–0.57) | 1140.2 (837.8–1320.7) |
| Slow (N=8) | 3.92 (0.47–33.0) | 340.7 (280.5–488.1) | 0.28 (0.04–2.41) | 1359.2 (1087.2–1630.4) | 0.35 (0.05–3.18) | 1019.3 (854.4–1181.9) |
| p-Value | 0.21 | 0.34 | 0.21 | 0.83 | 0.21 | 0.21 |
= Median values (variable range)
Volumes are expressed in milliliters or percent of ICC or BPF;
MRI= Magnetic resonance imaging, WMH= white matter hyperintensity;
WM=white matter; ICC= intracranial cavity volume; %ICC= percent of intracranial
cavity; BPF= brain parenchymal fraction
To further examine the relationship between WMH and gait speed, we divided WMH in each of the MRI groups (1.5 TESLA and 3 TESLA) at the median and classified individuals as those with high and low WMH burden. Table 3 summarizes the results of this analysis and shows that 68% of the subjects with high WMH had slow gait while only 25% of subjects with low WMH had slow gait (P=0.005). In a logistic regression model, the odds of being a slow walker with a high burden of white matter hyperintensity was 6.4 (CI95%: 1.7–24.9; p=0.007) compared to subjects with low burden of white matter hyperintensity.
Table 3.
Association between white matter hyperintensity (WMH) and gait speed (N=42).
| Low WMH | High WMH | Total | |
|---|---|---|---|
| Fast Gait Speed | 15 | 7 | 22 |
| Slow Gait Speed | 5 | 15 | 20 |
| Total | 20 | 22 | 42 |
68.2% of subjects with High WMH have slow gait.
25.0% of subjects with Low WMH have slow gait.
Chi-sq value (7.8); p-value=.005
White Matter Hyperintensities, Neurovascular Coupling and Gait Speed
Table 4 shows the extent of blood flow activation (neurovascular coupling during 2-back) for each gait speed group as a function of WMH burden. Unexpectedly, in both the slow and fast walkers, neurovascular coupling was higher in those with higher WMH burden. However, the odds of being a slow walker with a high burden of white matter hyperintensity was significantly higher if neurovascular coupling was also impaired (14.5 [CI95%: 2.3–91.1; p=0.004]). The test for interaction between WMH and NVC was not significant (p=0.97).
Table 4.
Neurovascular Coupling in subject groups stratified by White Matter Hyperintensity (WMH) and Gait Speed
| GAIT SPEED | WMH |
|
|---|---|---|
| Low | High | |
| Fast | 7.81% (7.99) | 11.25% (8.71) |
| Slow | −1.27% (8.48) | 3.47% (5.72) |
Mean (sd) values for neurovascular coupling by gait speed and white matter hyperintensity (N=40; fast/high=6, fast/low=15, slow/high=14, slow/low=5).
DISCUSSION
This study shows that impaired neurovascular coupling is associated with slow gait in elderly people with WMH. While those individuals with a high burden of WMH were more likely to be slow walkers, the likelihood of being a slow walker was more than twice as high if neurovascular coupling was also impaired.
Given that both impaired cerebral vasoreactivity and slow gait speed are associated with WMH, which likely results from long standing cerebral vasculopathy and ischemic injury, we initially hypothesized that WMH would also be associated with impaired neurovascular coupling. However, we did not see this relationship. Individuals who could activate their blood flow effectively were able to maintain a fast speed despite a high burden of WMH. Therefore, neurovascular coupling may be a compensatory mechanism that serves to maintain functional performance in the face of structural brain lesions.
Very few studies of neurovascular coupling have been conducted in humans. In one study, impaired neurovascular coupling was associated with untreated hypertension. The effect of hypertension was studied in 37 untreated hypertensive subjects and 59 normotensive control subjects during the performance of two memory and one sensorimotor tasks. Global and regional cerebral blood flow was assessed with positive emission tomography (PET). Regional cerebral blood flow responses to the tasks were blunted in the hypertensives, particularly in parietal cortex34. However, we did not find a significant relationship between treated hypertension and neurovascular coupling.
The effect of aging on neurovascular coupling is less clear. Despite age related changes in vascular structure, cerebral blood flow regulation has not been shown to be altered in human aging.35, 36 In one study of 20 healthy volunteers 10–60 years old without vascular disease, blood flow activation during a visual stimulation paradigm was similar across all age groups37. However, in another study, prefrontal neurovascular coupling was attenuated in older individuals.38 We have previously shown a tendency for elderly subjects to have greater and more generalized increases in anterior and posterior cerebral artery blood flow velocities than young subjects during word-stem completion and visual tasks.39 In animal studies, aging has been shown to be associated with impaired neurovascular coupling.40 In our study, across a limited, older age range, age was associated with impaired neurovascular coupling only in the left MCA.
The lack of a stronger relationship between neurovascular coupling and hypertension or age in our cohort may be related to at least two issues. The first may be a power issue. All prior studies of neurovascular coupling, including our own study, included a relatively small sample size. A second critical factor to consider is cerebral vasoreactivity. Since hypertension and aging are both associated with reduced cerebral vasoreactivity36 and this variable was not controlled for in all the other studies of neurovascular coupling, the confounding effect of vasoreactivity on the findings from prior studies can not be excluded. Vasoreactivity was impaired to the same extent for all the participants in our study. This characteristic of our cohort could have reduced the effects of age and hypertension on neurovascular coupling. Future longitudinal studies designed to explore these mechanistic pathways are needed.
We chose an executive function task for assessing the relation between neurovascular coupling and gait speed, rather than a motor task, because executive function is strongly related to gait speed41, and we could not obtain reliable TCD recordings during motor activity. Future studies should determine if blood flow changes are similar across different tasks and vascular territories or if they are task and territory specific.
In summary, we showed that neurovascular coupling was attenuated in subjects with slow gait speed. Intact neurovascular regulatory mechanisms were associated with fast gait speed even in individuals with a high burden of WMH. Neurovascular coupling may be involved in compensatory mechanisms responsible for preservation of gait speed in elderly people with vascular disease and as such may prove to be a valuable therapeutic target in cerebrovascular disorders.
AKNOWLEDGEMENTS
The authors thank the participants in the MBS for their contribution of time and information to this study. They also thank programmers Christopher Rocket and Margaret Bryan for efforts in developing and analyzing the MBS dataset. This work was supported by grants K23-AG030967 (FAS), P01-AG004390 (LAL), R37-AG25037 (LAL) and P50 AG005134 (LAL) from the National Institute on Aging, Bethesda, MD. There was no industry or corporate sponsorship. The authors do not have any other financial disclosures to report.
REFERENCES
- 1.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011 Jan 5;305(1):50–8. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGinn AP, Kaplan RC, Verghese J, et al. Walking speed and risk of incident ischemic stroke among postmenopausal women. Stroke. 2008 Apr;39(4):1233–9. doi: 10.1161/STROKEAHA.107.500850. [DOI] [PubMed] [Google Scholar]
- 3.Briley DP, Haroon S, Sergent SM, Thomas S. Does leukoaraiosis predict morbidity and mortality? Neurology. 2000;54(1):90–4. doi: 10.1212/wnl.54.1.90. [DOI] [PubMed] [Google Scholar]
- 4.Briley DP, Wasay M, Sergent S, Thomas S. Cerebral white matter changes (leukoaraiosis), stroke, and gait disturbance. J Am Geriatr Soc. 1997;45(12):1434–8. doi: 10.1111/j.1532-5415.1997.tb03192.x. [DOI] [PubMed] [Google Scholar]
- 5.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995 Mar 2;332(9):556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett DA, Beckett LA, Murray AM, et al. Prevalence of parkinsonian signs and associated mortality in a community population of older people. N Engl J Med. 1996 Jan 11;334(2):71–6. doi: 10.1056/NEJM199601113340202. [DOI] [PubMed] [Google Scholar]
- 7.Baloh RW, Yue Q, Socotch TM, Jacobson KM. White matter lesions and disequilibrium in older people. I. Case- control comparison. Arch Neurol. 1995;52(10):970–4. doi: 10.1001/archneur.1995.00540340062013. [DOI] [PubMed] [Google Scholar]
- 8.Kerber KA, Enrietto JA, Jacobson KM, Baloh RW. Disequilibrium in older people: a prospective study. Neurology. 1998 Aug;51(2):574–80. doi: 10.1212/wnl.51.2.574. [DOI] [PubMed] [Google Scholar]
- 9.Guttmann CR, Benson R, Warfield SK, et al. White matter abnormalities in mobility-impaired older persons. Neurology. 2000 Mar 28;54(6):1277–83. doi: 10.1212/wnl.54.6.1277. [DOI] [PubMed] [Google Scholar]
- 10.Baezner H, Blahak C, Poggesi A, et al. Association of gait and balance disorders with age-related white matter changes: the LADIS study. Neurology. 2008 Mar 18;70(12):935–42. doi: 10.1212/01.wnl.0000305959.46197.e6. [DOI] [PubMed] [Google Scholar]
- 11.Gootjes L, Teipel SJ, Zebuhr Y, et al. Regional distribution of white matter hyperintensities in vascular dementia, Alzheimer's disease and healthy aging. Dement Geriatr Cogn Disord. 2004;18(2):180–8. doi: 10.1159/000079199. [DOI] [PubMed] [Google Scholar]
- 12.Bazner H, Oster M, Daffertshofer M, Hennerici M. Assessment of gait in subcortical vascular encephalopathy by computerized analysis: a cross-sectional and longitudinal study. J Neurol. 2000 Nov;247(11):841–9. doi: 10.1007/s004150070070. [DOI] [PubMed] [Google Scholar]
- 13.de Laat KF, van Norden AG, Gons RA, et al. Gait in elderly with cerebral small vessel disease. Stroke. 2010 Aug;41(8):1652–8. doi: 10.1161/STROKEAHA.110.583229. [DOI] [PubMed] [Google Scholar]
- 14.Akopov S, Sercombe R, Seylaz J. Cerebrovascular reactivity: role of endothelium/platelet/leukocyte interactions. Cerebrovasc Brain Metab Rev. 1996 Spring;8(1):11–94. [PubMed] [Google Scholar]
- 15.Lavi S, Egbarya R, Lavi R, Jacob G. Role of nitric oxide in the regulation of cerebral blood flow in humans: chemoregulation versus mechanoregulation. Circulation. 2003 Apr 15;107(14):1901–5. doi: 10.1161/01.CIR.0000057973.99140.5A. [DOI] [PubMed] [Google Scholar]
- 16.Lavi S, Gaitini D, Milloul V, Jacob G. Impaired cerebral CO2 vasoreactivity: association with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2006 Oct;291(4):H1856–61. doi: 10.1152/ajpheart.00014.2006. [DOI] [PubMed] [Google Scholar]
- 17.Zimmermann C, Haberl RL. L-arginine improves diminished cerebral CO2 reactivity in patients. Stroke. 2003 Mar;34(3):643–7. doi: 10.1161/01.STR.0000056526.35630.47. [DOI] [PubMed] [Google Scholar]
- 18.Sorond FA, Galica A, Serrador JM, et al. Cerebrovascular hemodynamics, gait, and falls in an elderly population: MOBILIZE Boston Study. Neurology. 2010 May 18;74(20):1627–33. doi: 10.1212/WNL.0b013e3181df0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007 Nov;10(11):1369–76. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 20.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006 Jan;100(1):328–35. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- 21.Leveille SG, Kiel DP, Jones RN, et al. The MOBILIZE Boston Study: design and methods of a prospective cohort study of novel risk factors for falls in an older population. BMC Geriatr. 2008;8:16. doi: 10.1186/1471-2318-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samelson EJ, Kelsey JL, Kiel DP, et al. Issues in conducting epidemiologic research among elders: lessons from the MOBILIZE Boston Study. Am J Epidemiol. 2008 Dec 15;168(12):1444–51. doi: 10.1093/aje/kwn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994 Mar;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 24.DWDAGsO, editor. Trailmaking Tests A and B. Washington; 1944. [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975 Nov;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Sorond FA, Khavari R, Serrador JM, Lipsitz LA. Regional cerebral autoregulation during orthostatic stress: age-related differences. J Gerontol A Biol Sci Med Sci. 2005 Nov;60(11):1484–7. doi: 10.1093/gerona/60.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982 Dec;57(6):769–74. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- 28.Clark JM, Skolnick BE, Gelfand R, et al. Relationship of 133Xe cerebral blood flow to middle cerebral arterial flow velocity in men at rest. J Cereb Blood Flow Metab. 1996 Nov;16(6):1255–62. doi: 10.1097/00004647-199611000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Dahl A, Russell D, Nyberg-Hansen R, Rootwelt K. A comparison of regional cerebral blood flow and middle cerebral artery blood flow velocities: simultaneous measurements in healthy subjects. J Cereb Blood Flow Metab. 1992 Nov;12(6):1049–54. doi: 10.1038/jcbfm.1992.142. [DOI] [PubMed] [Google Scholar]
- 30.Serrador JM, Sorond FA, Vyas M, Gagnon M, Iloputaife ID, Lipsitz LA. Cerebral pressure-flow relations in hypertensive elderly humans: transfer gain in different frequency domains. J Appl Physiol. 2005 Jan;98(1):151–9. doi: 10.1152/japplphysiol.00471.2004. [DOI] [PubMed] [Google Scholar]
- 31.Sorond FA, Serrador JM, Jones RN, Shaffer ML, Lipsitz LA. The Sit-to-Stand Technique for the Measurement of Dynamic Cerebral Autoregulation. Ultrasound Med Biol. 2008 Sep 30; doi: 10.1016/j.ultrasmedbio.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei X, Warfield SK, Zou KH, et al. Quantitative analysis of MRI signal abnormalities of brain white matter with high reproducibility and accuracy. J Magn Reson Imaging. 2002 Feb;15(2):203–9. doi: 10.1002/jmri.10053. [DOI] [PubMed] [Google Scholar]
- 33.Moscufo N, Guttmann CR, Meier D, et al. Brain regional lesion burden and impaired mobility in the elderly. Neurobiol Aging. 2009 May 8; doi: 10.1016/j.neurobiolaging.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jennings JR, Muldoon MF, Ryan C, et al. Reduced cerebral blood flow response and compensation among patients with untreated hypertension. Neurology. 2005 Apr 26;64(8):1358–65. doi: 10.1212/01.WNL.0000158283.28251.3C. [DOI] [PubMed] [Google Scholar]
- 35.Carey BJ, Eames PJ, Blake MJ, Panerai RB, Potter JF. Dynamic cerebral autoregulation is unaffected by aging. Stroke. 2000 Dec;31(12):2895–900. doi: 10.1161/01.str.31.12.2895. [DOI] [PubMed] [Google Scholar]
- 36.Lipsitz LA, Mukai S, Hamner J, Gagnon M, Babikian V. Dynamic regulation of middle cerebral artery blood flow velocity in aging and hypertension. Stroke. 2000 Aug;31(8):1897–903. doi: 10.1161/01.str.31.8.1897. [DOI] [PubMed] [Google Scholar]
- 37.Zonta M, Angulo MC, Gobbo S, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003 Jan;6(1):43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- 38.Solbakk AK, Fuhrmann Alpert G, Furst AJ, et al. Altered prefrontal function with aging: insights into age-associated performance decline. Brain Res. 2008 Sep 26;1232:30–47. doi: 10.1016/j.brainres.2008.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sorond FA, Schnyer DM, Serrador JM, Milberg WP, Lipsitz LA. Cerebral blood flow regulation during cognitive tasks: Effects of healthy aging. Cortex. 2008 Feb;44(2):179–84. doi: 10.1016/j.cortex.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iadecola C, Park L, Capone C. Threats to the mind: aging, amyloid, and hypertension. Stroke. 2009 Mar;40(3 Suppl):S40–4. doi: 10.1161/STROKEAHA.108.533638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herman T, Mirelman A, Giladi N, Schweiger A, Hausdorff JM. Executive control deficits as a prodrome to falls in healthy older adults: a prospective study linking thinking, walking, and falling. J Gerontol A Biol Sci Med Sci. 2010 Oct;65(10):1086–92. doi: 10.1093/gerona/glq077. [DOI] [PMC free article] [PubMed] [Google Scholar]