Abstract
Loss of spiral ganglion neurons is a major cause of age-related hearing loss (presbycusis). Despite being the third most prevalent condition afflicting elderly persons, there are no known medications to prevent presbycusis. Because calcium signaling has long been implicated in age-related neuronal death, we investigated T-type calcium channels. This family is comprised of three members (Cav3.1, Cav3.2, and Cav3.3), based on their respective main pore-forming alpha subunits: α1G, α1H, and α1I. In the present study, we report a significant delay of age-related loss of cochlear function and preservation of spiral ganglion neurons in α1H null and heterozygous mice, clearly demonstrating an important role for Cav3.2 in age-related neuronal loss. Furthermore, we show that anticonvulsant drugs from a family of T-type calcium channel blockers can significantly preserve spiral ganglion neurons during aging. To our knowledge, this is the first report of drugs capable of diminishing age-related loss of spiral ganglion neurons.
Keywords: Presbycusis, Spiral ganglion neuron, Hair cell, Anti-epileptic drug, Aging
1. Introduction
Functional decline of the nervous system is a cardinal feature of normal aging. In the central nervous system, loss of neuronal connections, rather than loss of neurons, may be the major cause of age-related functional decline (Morrison and Hof, 1997). In the peripheral nervous system, however, age-related loss of neurons significantly contributes to functional decline (Bao and Ohlemiller, 2010; Rattner and Nathans, 2006; Thrasivoulou et al., 2006). The mammalian cochlea typically displays progressive age-related hearing loss (presbycusis), starting in the high frequencies. This loss primarily reflects the loss of hair cell receptors and their post-synaptic targets, the spiral ganglion neurons (SGNs), in the basal region of the cochlea (Ohlemiller and Frisina, 2008; Schacht and Hawkins, 2005). Currently, there is no effective medication to prevent or treat presbycusis, which affects about half of the population over 75 years old (Gates and Mills, 2005).
Disturbance of neuronal calcium homeostasis has long been suggested to be an important factor underlying age-related impairment of neuronal function (Bao et al., 2005; Buchholz et al., 2007; Toescuet et al., 2004; Zettel et al., 1997; Zettel et al., 2003). Human population studies have linked use of calcium channel blockers to better hearing thresholds in females during aging (Mills et al., 1999), suggesting that calcium dysregulation contributes to presbycusis. In both hair cells and SGNs, intracellular calcium levels are regulated, in part, by several types of calcium channels, including voltage-gated calcium channels (VGCCs) (Lee et al., 2007; Lopez et al., 2003; Nie et al., 2008; Shen et al., 2007). These VGCCs can be divided into two groups: high-voltage activated and low-voltage activated calcium channels (Errington et al., 2005). The family of low-voltage activated, or T-type, calcium channels, is comprised of three members (Cav3.1, Cav3.2, and Cav3.3), based on their respective main pore-forming alpha subunits: α1G, α1H, and α1I (Perez-Reyes et al., 1998). It has been shown that expression of the α1G, α1H, or α1I subunit alone can reproduce characteristics of T-type calcium channels (Lacinova, 2004; Perez-Reyes, 2003; Yunker and McEnery, 2003). With regard to distribution, T-type calcium channels are found throughout the nervous system (Talley et al., 1999). In the organ of Corti, where hair cell receptorss are located, α1G and α1I subunits are weakly expressed in both outer and inner hair cells (OHCs and IHCs), while robust α1H expression and moderate expression of the other two subunits are found in SGNs (Shen et al., 2007). One recent study implicated the role of α1G gene in a hypoxia-like neuronal death (Kim et al., 2011). To date, no studies have explored possible functions of T-type calcium channels in age-related neuronal loss in vivo.
To investigate how T-type calcium channels may affect age-related loss of spiral ganglion neurons, we examined mice homozygous or heterozygous for a null allele of the α1H calcium channel subunit on a C57BL/6 (B6) genetic background. The aging B6 mouse cochlea reproduces several characteristics of the aging human inner ear and is commonly used to study processes that underlie presbycusis (Ohlemiller, 2006). In the present study, we demonstrate that B6 mice homozygous or heterozygous for a null allele of the α1H calcium channel subunit show significant delay of age-related increase in auditory brainstem response (ABR) thresholds, and that this delay is associated with a dramatic decrease in age-related SGN loss. In addition, we show that members of a family of anti-epileptic drugs that block T-type calcium channels can delay age-related SGN loss and preserve ABR thresholds in B6 mice. Our findings implicate T-type calcium channels in age-related SGN loss and demonstrate a possible therapy for presbycusis using FDA-approved drugs that regulate these channels.
2. Materials and methods
2.1. Animals and treatments
B6 congeninc α1H−/+ and α1H−/− mice were housed five per cage, with food and water B6 congenic α1H−/+ and α1H−/− mice had been backcrossed to B6 over 10 generations. Mice were maintained in a noise-controlled environment on a 12-hour light/dark cycle, with light onset at 6:00 a.m. T-type channel blockers trimethadione and ethosuximide were obtained from Sigma Chemical Co. (St. Louis, MO). To determine the appropriate dosage of these drugs for mice, with guidance from previous studies (Barton et al., 2001; Shen et al., 2007; Zhang et al., 1996), we treated four groups (10 mice per group) with trimethadione or ethosuximide at 0, 40, 200, and 400 mg/kg body weight in drinking water for two months. Gross and histopathological examination of the liver and kidney showed no differences between control and drug-treated groups at the three dosages. Thus, drug-treated groups were administered trimethadione or ethosuximide (200 mg/kg body weight) and 1% sucrose in drinking water. The drug solution was adjusted to body weight, maintained in colored bottles, and changed once every three days. Control mice were given drinking water containing 1% sucrose only. The body weight and general health of all mice were monitored daily. All procedures were approved by the Animal Studies Committee at Washington University in St. Louis.
2.2. Functional assays
ABR testing was performed in a foam-lined, double-walled soundproof room. Animals were anesthetized (80 mg/kg ketamine, 15 mg/kg xylazine, i.p.) and stabilized in a custom head holder. Core temperature was maintained at 37 °C using a thermostatically controlled heating pad in conjunction with a rectal probe. Platinum needle electrodes were inserted subcutaneously just behind the right ear (active), at the vertex (reference), and in the back (ground). Electrodes were led to a Grass P15 differential amplifier (100 Hz-10 kHz, X100), then to a custom broadband amplifier (0.1 Hz-10 kHz, X1000), and digitized at 30 kHz using a Cambridge Electronic Design Micro1401 data acquisition unit in conjunction with SIGNAL and custom signal averaging software operating on a 120 MHz Pentium PC. Sine wave stimuli generated by a Wavetek Model 148 oscillator were shaped by a custom electronic switch to 5 ms total duration, including 1ms rise/fall times. The stimulus was amplified by a Crown D150A power amplifier with output to a KSN1020A piezo ceramic speaker located 7 cm directly lateral to the right ear. Stimuli were presented freefield and calibrated using a ¼ inch microphone placed where the pinna would normally be. Toneburst stimuli at each frequency and level were presented 1000 times at 20/s. The minimum sound pressure level required for a response (short-latency negative wave) was determined at 5, 10, 14.2, 20, 28.3, 40, and 56.6 kHz, using a 5 dB minimum step size. The experimenter was blind to the experimental conditions.
2.3. Real-time RT-PCR and immunostaining assays
Total RNAs from individual mice (two cochleae) at each age group were extracted using RNAqueous (Ambion, Austin, TX). To avoid any DNA contamination during RNA extraction, at the final step of extraction, one microliter of DNase I was added to 49 μl elution buffer to extract total RNA from an RNA extraction column. The solution was incubated at 37°C for 15 minutes and heated to 100°C for 5 minutes to kill the DNase I. Then the RNA was quantified with RiboGreen RNA quantitation reagent (Molecular Probes, Eugene, OR). Prior to reverse-transcription to generate cDNA, the quality of RNA was determined by checking ribosomal RNA integrity on a 3% denatured agarose gel. Ten microliters of a total of 50 μl RNA were reverse transcribed in total 20 μl using random hexamers and Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA). Standard curves for GAPDH and three α1 subunits were made using their respective cDNA plasmids at five dilutions. Primers used for real-time RT-PCR of these mouse genes were: GAPDH: 5-CCTGGCCAAGGTCATCCATGACAAC-3 and 5-TGTCATACCAGGAAATGAGCTTGAC-3; α1G Subunit: 5-AATGGCAAGTCGGCTTCAGG-3 and 5-TGTCAGAGACCATGGACACCAG-3; α1H Subunit: 5-ATGTTCCGGCCCTGTGAGGA-3 and 5-CCATGACGTAGTACATGATGTCC-3; α1I Subunit: 5-ATCTGCTCCCTGTCGG-3 and 5-GAGAACTGGGTCGCTATG-3; The change of each α1 subunit during aging was measured using the LightCycler System 1.5 (Roche, Mannheim, Germany). PCR protocols of the LightCycler System for GAPDH and each α1 subunit were optimized with the LightCycler FastStart DNA MasterPLUS SYBR Green I kit (Roche, Mannheim, Germany). Two microliters of each 20 μl cDNA was added to a 20 μl PCR reaction mixture containing 1X PCR buffer, 0.5 μM of each primer, and Master Mix from the kit. The number of PCR cycles until the fluorescence intensity exceeded a predetermined threshold was measured automatically during PCR. Quantification of the initial amount of template molecules was achieved by calculating this number of PCR cycles with the number from the standard samples. The difference in the initial amount of total RNA among the samples was normalized using the housekeeping gene, GAPDH. Besides using melting curve analysis to ensure the right PCR product, we also examined each PCR product on a 3% agarose gel after each experiment.
Immunocytochemisty was performed as described previously (Bao et al., 2004). Cochleae were removed under a dissection scope, and were fixed overnight at 4°C and decalcified for 2 days in 0.1 M sodium phosphate solution (PBS, pH 7.4) containing 4% paraformaldehyde and 0.25M EDTA. The cochleae were cryoprotected in 20% glycerol in PBS at −20°C. The tissue was then embedded in Tissue-Tek Optimal Cutting Temperature (OCT) compound (Sakura, USA) on dry ice. Frozen sections were cut vertically to the axis of the cochlea. Sections were rinsed in PBS and permeabilized in 0.3% Triton X-100 for 20 minutes. Next, tissues were incubated in the primary antibody for α1 subunits (1:100, Santa Cruz) overnight at 4°C. The tissues were then washed in PBS three times for 10 minutes each, and incubated with secondary antibodies of Alexa Fluor 660-conjugated anti-rabbit IgG, Alexa Fluor 660-conjugated anti-goat IgG, or Alexa Fluor 488-conjugated anti-sheep IgG (both from Molecular Probes) at a dilution of 1:200 for 1 hour. All sections were washed and coverslipped for observation. Images were captured using a Nikon microscope. Control experiments were performed to verify that specific immunostaining does not occur in the absence of primary antibodies.
2.4. Quantification of SGNs, IHCs, and OHCs
Cochleae were sectioned parallel to the modiolar axis at a thickness of 50 μm. A Nikon microscope was equipped with a motorized stage controlled by the Stereo Investigator software for precise, well-defined movements along the x, y, and z axes. High-resolution images and a thin focal plane were obtained using a 60x oil immersion objective lens from Nikon with a numerical aperture of 1.40. The optical fractionator method was used to estimate the total number of SGNs in each spiral ganglion by the optical dissector. A clear nucleolus, a large nucleus, and a clearly defined oval body were used to distinguish SGNs from glial cells. The specific feature chosen for counting was the nucleolus of each SGN. For quantification of missing IHCs and OHCs, the whole length of mouse cochlea basilar membrane was measured using Stereo Investigator software by focusing on the pillar heads between the IHCs and OHCs. All hair cells throughout the cochlea were examined with the 60x oil immersion objective lens. The percent loss of IHCs and OHCs throughout the whole cochlea was then divided into five segments that represented 20% of the organ of Corti length.
2.5. Statistical analyses
Statistical significance was evaluated using two-way ANOVA, one-way ANOVA, or Student’s t-tests, as appropriate by data set.
3. Results
3.1. Age-related changes in the expression of T-type calcium channels
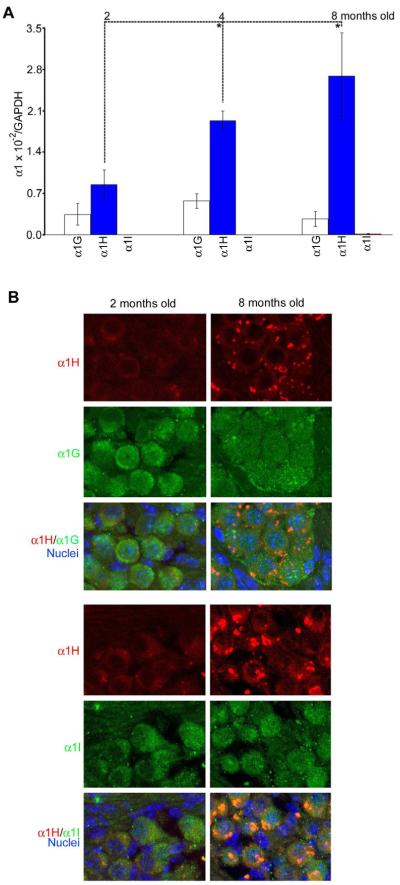
Having previously shown that all three T-type calcium channel subunits are present in SGNs, while only α1G and α1I subunits are present in the organ of Corti (Shen et al., 2007), we next sought to determine whether there are age-related changes in the expression of these subunits. Real-time RT-PCR was used to quantify and compare mRNA expression levels of specific α1 subunits of T-type calcium channels in B6 mouse cochleae during aging. The ratio between the expression level of each subunit and GAPDH for α1G, α1H, and α1I was 0.25 × 10−2, 0.86 × 10−2, and 2.0 × 10−5 at 2 months old; 0.57 × 10−2, 1.93 × 10−2, and 1.59 × 10−5 at 4 months old; 0.20 × 10−2, 2.63 × 10−2, and 14.9 × 10−5 at 8 months old. Thus the expression level of the α1H subunit was the highest of the three subunits, and was significantly higher in older mice (Fig. 1A). Immunostaining supported the conclusion that the α1H subunit is co-localized with the other two subunits in SGNs, yet is the mostly highly expressed of these (Fig. 1B).
Fig. 1.
Age-related changes in expression of the α1 subunits in the cochlea during aging. (A) Mean (+SD) expression of the α1G, α1H and α1I subunits as determined by real-time RT-PCR quantification in female mouse cochleae at 2, 4, and 8 months of age (n=6 per group), and a significant difference was found between adjacent ages for the α1H gene (t-test, *p < 0.01). The expression level for each subunit was determined by the ratio between each α1 subunit and GAPDH to eliminate possible differences in the amount of total RNAs from each sample. (B) Immunocytochemistry showing co-localization of the α1H subunit with the other two subunits in SGNs at 2 and 8 months of age.
3.2. Delay of age-related increase of ABR thresholds in α1H null or α1H heterozygous mice
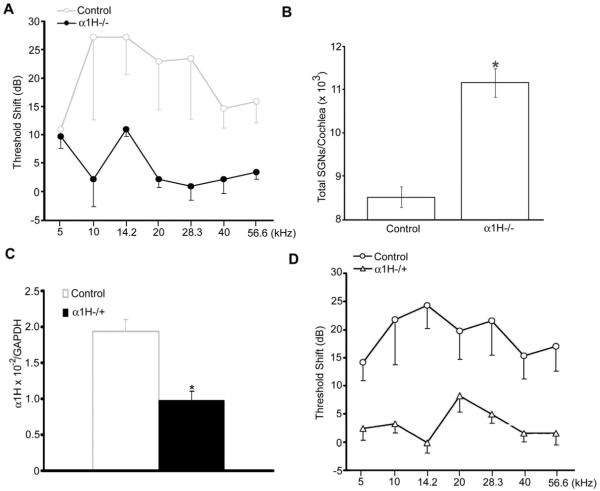
The age-related increase in ABR thresholds in B6 mice accelerates around 8-9 months of age, particularly above 12 kHz (Ison et al., 2007; Spongr et al., 1997). We therefore measured ABR threshold shifts in α1H−/− mice over the interval 9-11 months of age, and found significant preservation of ABR thresholds in these mice compared to B6 controls (Fig. 2A). More SGNs were also found in the α1H−/− mice than in the control mice (Fig. 2B). A potential caveat to this finding, however, was posed by reports of cardiac pathology in α1H knockouts (Chen et al., 2003), although cardiovascular disease should actually exacerbate hearing loss (Gates et al., 1993) . To rule out this confound, we examined α1H−/+ mice, in which the heart is healthy (Chen et al., 2003). Real-time RT-PCR showed that the α1H expression level in the cochleae of heterozygous mice was significantly lower than in controls (Fig. 2C). We next compared ABR threshold shifts over the interval between 9-11 months of age in B6 WT and α1H−/+ mice. Threshold sensitivity in α1H−/+ mice appeared significantly preserved, compared to age-matched controls (Fig. 2D). These data implicate the α1H subunit of T-type calcium channels in age-related increase of ABR thresholds in B6 inbred mice.
Fig. 2.
Delay of age-related threshold shifts in α1H null and heterozygous mice. (A) ABR threshold shifts in female B6 WT and α1H−/− occurring between 9 and 11 months (n=4 per group; two-way ANOVA, F = 23.916, p < 0.001). (B) Comparison of total SGN number per cochlea between the same control and α1H−/− mice (t-test, *p< 0.01). (C) Real-time RT-PCR quantification of the α1H subunit in cochleae from female B6 WT and α1H/+ mice at 4 months of age (n=6 per group; t-test, *p< 0.01). (D) ABR threshold shifts in female B6 WT and α1H+/− between 9 and 11 months (n=4 per group; two-way ANOVA, F = 52.155, p < 0.001).
3.3. Delay of age-related increase of ABR thresholds by blockers for T-type calcium channels
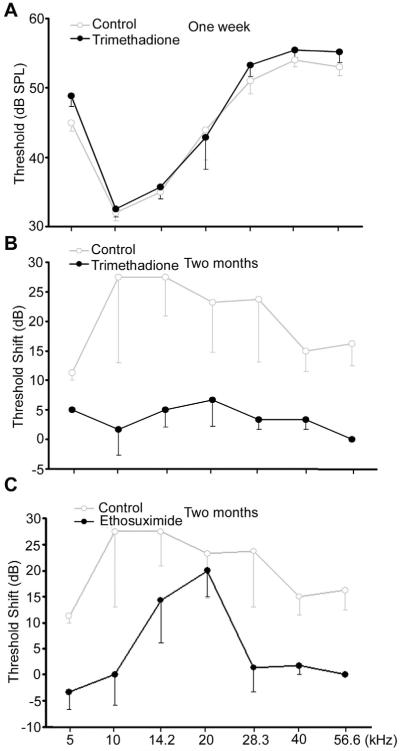
We next tested whether T-type calcium channel blockers trimethadione and ethosuximide can delay age-related increase of ABR thresholds. One week after administration of trimethadione in drinking water, ABR showed no difference in hearing thresholds between treated and untreated mice at 8 months old (Fig. 3A), demonstrating that this drug has no acute effect on hearing thresholds. However, after administration of trimethadione to 10-month-old mice for two months, significant reduction of age-related ABR threshold shifts was observed compared to controls (Fig. 3B). The average difference across seven frequencies tested was 14.9 ± 6.5 dB; while the drug treatment had no effect on body weight or food uptake (Fig. 4). Treatment with ethosuximide demonstrated a similar level of protection (Fig.3C). The average difference across seven frequencies tested was 15.8 ± 7.7 dB between control and treated mice. These data support the contention that T-type calcium channel blockers can delay age-related increase of ABR thresholds.
Fig. 3.
Mean (−SD) ABR thresholds showing delay of age-related hearing loss by anti-epileptic drugs. (A) Thresholds for controls (n=5, three males and two females) and mice administered trimethadione in drinking water for one week (n=6, three of each sex; all 8-month-old B6 WT mice; two-way ANOVA, F = 2.012, p = 0.162). (B) Threshold shifts occurring between 10-12 months of age for female B6 controls and mice treated with trimethadione for two months (n=5 per group, started at 10 months; two-way ANOVA, F = 89.868, p < 0.001). (C) ABR threshold shifts occurring between 10-12 months of age for female B6 controls and mice treated with ethosuximide for two months (n=3 per group, started at 10 months; 3Ctwo-way ANOVA, F = 15.906, p < 0.001).
Fig. 4.
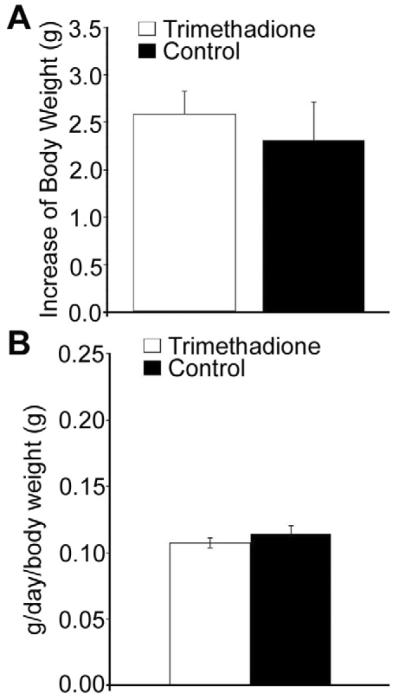
Comparison of body weight and food uptake between B6 control mice and mice receiving trimethadione at 200 mg/kg body weight. (A) No difference in gain of body weight between control (n = 10, five of each sex) and trimethadione treated (n = 10, five of each sex) after two months. (B) No difference in food uptake between the same groups of animals. Food uptake was monitored every five days for two months.
3.4. Delay of age-related SGN loss by blockers of T-type calcium channels
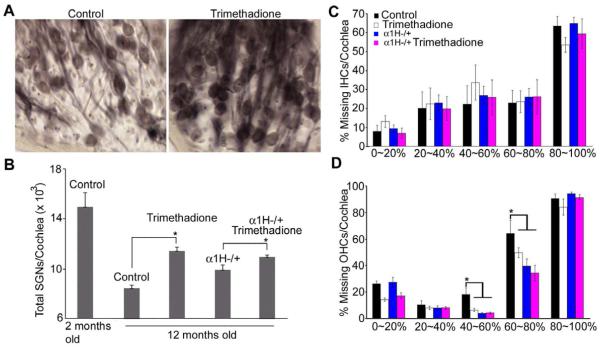
Because the α1H subunit is highly expressed in SGNs (Fig. 1), we hypothesized that preservation of ABR thresholds by down-regulating the α1H subunit or by blocking T-type channels reflected improved SGN survival with age. Consistent with this hypothesis, more SGNs were observed in 12-month-old B6 mice treated with trimethadione (Fig. 5A). We further quantified the number of SGNs, IHCs, and OHCs across four groups: B6 controls, B6 mice treated with trimethadione, α1H heterozygous mice, and α1H heterozygous mice treated with trimethadione. Age-related SGN loss was dramatically reduced in the cochleae of B6 mice treated with trimethadione compared to untreated mice (Fig. 5B; t-test, p < 0.01). Preservation of SGNs during aging was also found in the cochleae of α1H−/+ mice (one-way ANOVA, F = 16.476, p < 0.001). Moreover, additional SGN preservation was observed in α1H−/+ mice after trimethadione treatment (Fig. 5B; t-test, p < 0.01). Consistent with the expression pattern of T-type calcium channels in the cochlea, from the apex (0%) to the base end (100%) of the cochlea, there was no difference in age-related loss of IHCs among the same four groups of mice (Fig. 5C). However, significant preservation was found in the three experimental groups for OHCs located 40% to 80% from the apex (Fig.5D). Because α1H expression was not detected in OHCs (Shen et al., 2007), the preservation of OHCs could be due to other reasons such as reciprocal trophic support between hair cells and SGNs (Fritzsch et al., 2004; Rubel and Fritzsch, 2002). However, no direct relation between the survival of SGNs and OHCs was observed. That is, OHC preservation was not clearly greater in regions that retained more SGNs (Fig. 6).
Fig. 5.
Cellular targets for anticonvulsants in delaying age-related threshold shifts. (A) Age-related SGN loss in mid-modiolar cochlear sections (close to the 34% region) from 12-month-old B6 mice (left) and mice treated with trimethadione in drinking water for two months (right). (B) Quantitative comparison of total SGN number among B6 WT mice at 2 months and 12 months old, with or without trimethadione in drinking water (n=8, four of each sex), and α1H/+ mice at 12 months with or without trimethadione, 9-11 months of age (n=6 per each group, three of each sex). (C) Comparison of missing IHCs by apical-basal cochlear location in the same 12-month-old mice (one-way ANOVA, F = 0.944, p = 0.442, 0.986, 0.815, 0.978, and 0.461 at 0-20%, 20-40%, 40-60%, 60-80%, and 80-100%, respectively). (D) Comparison of missing OHCs by apical-basal cochlear location in the same 12-month-old mice (one-way ANOVA, F = 6.314, p = 0.05, 0.743, 0.003, 0.027, and 0.290 at 0-20%, 20-40%, 40-60%, 60-80%, and 80-100%, respectively).
Fig. 6.
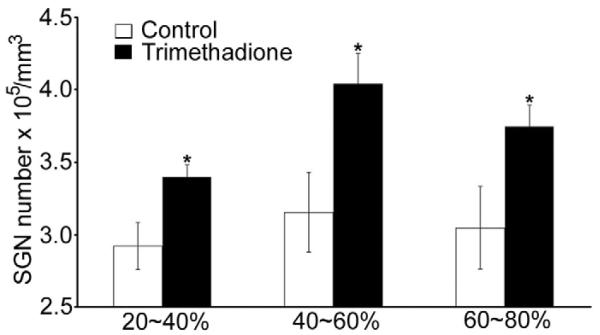
Comparison of SGN density in B6 WT control mice and mice receiving trimethadione at cochlear locations 20-40%, 40-60%, and 60-80% from the apex (5 female mice in each group; t-test between the control and trimethadione-treated groups, *p < 0.01)
4. Discussion
We show here that blockers of T-type calcium channels, specifically, anti-epileptic drugs, can significantly slow age-related ABR threshold shifts in mice, most likely through effects on the α1H T-type calcium channel subunit. At the cellular level, slowing of age-related SGN loss, possibly along with OHC loss, appears to represent the principal action. Both effects could help account for the threshold improvements we observed. Our findings suggest a new use of these anticonvulsants, and provide a molecular target for prevention of age-related hearing and SGN loss. Notably, all current anticonvulsant drugs produce side-effects such as dizziness. Nevertheless, because we have now identified the molecular target, it should be possible to modify these drugs to reduce side-effects while preserving their therapeutic effects for hearing.
One unresolved issue pertains to the cause, of the preservation of OHCs observed in both older α1H−/+ mice and WT mice treated with trimethadione. Reciprocal trophic support between OHCs and SGNs seems unlikely given that, in mice as in all mammals examined, only about 7% of SGNs (the type II or spiral afferents) innervate OHCs, while 93% (the type I or radial afferents) innervate IHCs (Ehret, 1979). Another possibility is that interference with T-type calcium currents extends vitality and lifespan, thereby broadly preserving the survival of neuronal subpopulations (Evason et al., 2005). Were that the mechanism, however, IHCs also should have been preserved, which was not the case. It is also possible that the α1H subunit is expressed in OHCs, but its expression is too weak to be detected by immunocytochemistry (Shen et al., 2007). Yet another possibility is non-specificity of our blockers for the α1H subnunit. In vitro tests of the neuroprotective effects of T-type blocker on cortical and hippocampal neurons revealed protection even for neurons from α1H null mice (Wildburger et al., 2009). Because these neurons also express α1G and α1I subunits (Talley et al., 1999), it is possible that the drugs protect neurons by blocking other α1 subunits. Also of note from the in vitro tests was the finding that blockers of L-type calcium channels conferred protection upon neurons in long-term cultures (15 days), while the same T-type blockers did not. For reasons not presently clear, the utility of drug screens in long-term neuron cultures in an effort to identify therapeutic agents against neurodegnerative diseases such as presbycusis may be limited.
Currently, there are no known effective medications against age-related cochlear neuronal degeneration. Clinical studies have correlated use of L-type calcium channel blockers with lower hearing thresholds in females during aging (Mills et al., 1999), suggesting that altered calcium regulation contributes to presbycusis. This would fit well with the “calcium hypothesis of neuronal aging,” which attributes age-related neuronal loss to excess calcium influx via L-type voltage-gated calcium channels, and impaired calcium intracellular buffering (Foster, 2007; Thibault et al., 2007). Few studies, however, have explored the role of T-type voltage-gated calcium channels in similar processes. Our findings suggest that Cav3.2 contributes to age-related SGN loss, and support significant delays in in the manifestation of hearing loss by drugs that block T-type calcium channels.
Acknowledgements
We thank R. Chole, K. Evason, and K. Kornfeld for helpful discussions. We are also grateful to B. Bohne, R. Davis, D. Dickman, and D. Whitlon for providing comments on the manuscript. This work was supported by grants from the National Organization for Hearing Research Foundation, NIH NIA (R01AG024250), and NIH NIDCD (R21DC010489) to J.B., and core grants from NIH NIDCD (P30DC004665) and NIH NINDS (P30NS057105).
Abbreviations
- SGN
spiral ganglion neuron
- VGCC
voltage-gated calcium channel
- OHC
outer hair cell
- IHC
inner hair cell
- ABR
auditory brainstem response
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bao J, Lei D, Du Y, Ohlemiller KK, Beaudet AL, Role LW. Requirement of nicotinic acetylcholine receptor subunit beta2 in the maintenance of spiral ganglion neurons during aging. J. Neurosci. 2005;25:3041–3045. doi: 10.1523/JNEUROSCI.5277-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J, Lin H, Ouyang Y, Lei D, Osman A, Kim TW, Mei L, Dai P, Ohlemiller KK, Ambron RT. Activity-dependent transcription regulation of PSD-95 by neuregulin-1 and Eos. Nat Neurosci. 2004;7:1250–1258. doi: 10.1038/nn1342. [DOI] [PubMed] [Google Scholar]
- Bao J, Ohlemiller KK. Age-related loss of spiral ganglion neurons. Hear Res. 2010;264:93–97. doi: 10.1016/j.heares.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton ME, Klein BD, Wolf HH, White HS. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res. 2001;47:217–227. doi: 10.1016/s0920-1211(01)00302-3. [DOI] [PubMed] [Google Scholar]
- Buchholz JN, Behringer EJ, Pottorf WJ, Pearce WJ, Vanterpool CK. Age-dependent changes in Ca2+ homeostasis in peripheral neurons: implications for changes in function. Aging Cell. 2007;6:285–296. doi: 10.1111/j.1474-9726.2007.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Lamping KG, Nuno DW, Barresi R, Prouty SJ, Lavoie JL, Cribbs LL, England SK, Sigmund CD, Weiss RM, Williamson RA, Hill JA, Campbell KP. Abnormal coronary function in mice deficient in alpha1H T-type Ca2+ channels. Science. 2003;302:1416–1418. doi: 10.1126/science.1089268. [DOI] [PubMed] [Google Scholar]
- Ehret G. Quantitative analysis of nerve fibre densities in the cochlea of the house mouse (Mus musculus) J. Comp. Neurol. 1979;183:73–88. doi: 10.1002/cne.901830107. [DOI] [PubMed] [Google Scholar]
- Errington AC, Stohr T, Lees G. Voltage gated ion channels: targets for anticonvulsant drugs. Curr. Top. Med. Chem. 2005;5:15–30. doi: 10.2174/1568026053386872. [DOI] [PubMed] [Google Scholar]
- Evason K, Huang C, Yamben I, Covey DF, Kornfeld K. Anticonvulsant medications extend worm life-span. Science. 2005;307:258–262. doi: 10.1126/science.1105299. [DOI] [PubMed] [Google Scholar]
- Foster TC. Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging Cell. 2007;6:319–325. doi: 10.1111/j.1474-9726.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog. Brain Res. 2004;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- Gates GA, Cobb JL, D’Agostino RB, Wolf PA. The relation of hearing in the elderly to the presence of cardiovascular disease and cardiovascular risk factors. Arch. Otolaryngol. Head Neck Surg. 1993;119:156–161. doi: 10.1001/archotol.1993.01880140038006. [DOI] [PubMed] [Google Scholar]
- Gates GA, Mills JH. Presbycusis. Lancet. 2005;366:1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- Kim J, Woo J, Park YG, Chae S, Jo S, Choi JW, Jun HY, Yeom YI, Park SH, Kim KH, Shin HS, Kim D. Thalamic T-type Ca Channels mediate frontal lobe dysfunctions caused by a hypoxia-like damage in the prefrontal cortex. J. Neurosci. 2011;31:4063–4073. doi: 10.1523/JNEUROSCI.4493-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison JR, Allen PD, O’Neill WE. Age-realted hearing loss in C57BL/6J mice has both frequency-specific and non-frequency-specific components that produce a hyperacusis-like exaggeration of the acoustic startle reflex. JARO. 2007;8:539–550. doi: 10.1007/s10162-007-0098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacinova L. Pharmacology of recombinant low-voltage activated calcium channels. Curr. Drug Targets CNS Neurol. Disord. 2004;3:105–111. doi: 10.2174/1568007043482543. [DOI] [PubMed] [Google Scholar]
- Lee S, Briklin O, Hiel H, Fuchs P. Calcium-dependent inactivation of calcium channels in cochlear hair cells of the chicken. J. Physiol. 2007;583:909–922. doi: 10.1113/jphysiol.2007.135582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez I, Ishiyama G, Acuna D, Ishiyama A, Baloh RW. Immunolocalization of voltage-gated calcium channel alpha1 subunits in the chinchilla cochlea. Cell Tissue Res. 2003;313:177–186. doi: 10.1007/s00441-003-0759-4. [DOI] [PubMed] [Google Scholar]
- Mills JH, Matthews LJ, Lee FS, Dubno JR, Schulte BA, Weber PC. Gender-specific effects of drugs on hearing levels of older persons. Ann. NY Acad. Sci. 1999;884:381–388. doi: 10.1111/j.1749-6632.1999.tb08656.x. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Nie L, Zhu J, Gratton MA, Liao A, Mu KJ, Nonner W, Richardson GP, Yamoah EN. Molecular identity and functional properties of a novel T-type Ca2+ channel cloned from the sensory epithelia of the mouse inner ear. J Neurophysiol. 2008;100:2287–2299. doi: 10.1152/jn.90707.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK. Contributions of mouse models to understanding of age- and noise-related hearing loss. Brain Res. 2006;1091:89–102. doi: 10.1016/j.brainres.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Frisina RD. Age-related hearing loss and its cellular and molecular bases. In: Schacht J, Popper AN, Fay RR, editors. Auditory Trauma, Protection, and Repair. Springer; New York: 2008. pp. 145–194. [Google Scholar]
- Perez-Reyes E. Molecular physiology of low-voltage-activated T-type calcium channels. Physiol. Res. 2003;83:117–161. doi: 10.1152/physrev.00018.2002. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E, Cribbs LL, Daud A, Lacerda AE, Barclay J, Williamson MP, Fox M, Rees M, Lee JH. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature. 1998;391:896–900. doi: 10.1038/36110. [DOI] [PubMed] [Google Scholar]
- Rattner A, Nathans J. Macular degeneration: recent advances and therapeutic opportunities. Nat. Rev. Neurosci. 2006;7:860–872. doi: 10.1038/nrn2007. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu. Rev. Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Schacht J, Hawkins JE. Sketches of otohistory. Part 9: presby[a]cusis. Audiol. Neurootol. 2005;10:243–247. doi: 10.1159/000086524. [DOI] [PubMed] [Google Scholar]
- Shen H, Zhang B, Shin J, Lei D, Du Y, Gao X, Wang Q, Ohlemiller KK, Piccirillo J, Bao J. Prophylactic and therapeutic functions of T-type calcium blockers against noise-induced hearing loss. Hear. Res. 2007;226:52–60. doi: 10.1016/j.heares.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spongr VP, Flood DG, Frisina RD, Salvi RJ. Quantitative measures of hair cell loss in CBA and C57B1/6 mice throughout their life spans. J. Acoustical Society of America. 1997;101:3546–3553. doi: 10.1121/1.418315. [DOI] [PubMed] [Google Scholar]
- Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J. Neurosci. 1999;19:1895–1911. doi: 10.1523/JNEUROSCI.19-06-01895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer’s disease: minding the store. Aging Cell. 2007;6:307–317. doi: 10.1111/j.1474-9726.2007.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrasivoulou C, Soubeyre V, Ridha H,, Giuliani D, Giaroni C, Michael GJ, Saffrey MJ, Cowen T. Reactive oxygen species, dietary restriction and neurotrophic factors in age-related loss of myenteric neurons. Aging Cell. 2006;5:247–257. doi: 10.1111/j.1474-9726.2006.00214.x. [DOI] [PubMed] [Google Scholar]
- Toescu EC, Verkhratsky A, Landfield PW. Ca2+ regulation and gene expression in normal brain aging. Trends Neurosci. 2004;27:614–620. doi: 10.1016/j.tins.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Wildburger NC, Lin-Ye A, Baird MA, Lei D, Bao J. Neuroprotective effects of blockers for T-type calcium channels. Mol. Neurodegeneration. 2009;4:44. doi: 10.1186/1750-1326-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunker AM, McEnery MW. Low-voltage-activated (“T-Type”) calcium channels in review. J. Bioenerg. Biomembr. 2003;35:533–575. doi: 10.1023/b:jobb.0000008024.77488.48. [DOI] [PubMed] [Google Scholar]
- Zettel ML, Frisina RD, Haider S, O’Neill WE. Age-related changes in the immunoreactivity of calbindin D28K and calretinin in the inferior colliculus of the CBA/J and C57/6J mouse. J. Comparative Neurology. 1997;386:92–110. doi: 10.1002/(sici)1096-9861(19970915)386:1<92::aid-cne9>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Zettel ML, Trang TT, O’Neill WE, Frisina RD. Activity-dependent age-related regulation of calcium-binding proteins in the mouse dorsal cochlear nucleus. Hearing Res. 2003;183:57–66. doi: 10.1016/s0378-5955(03)00216-8. [DOI] [PubMed] [Google Scholar]
- Zhang YF, Gibbs JW, III, Coulter DA. Anticonvulsant drugs effects on spontaneous thalamocortical rhythms in vitro: ethosuximide, trimethadione, and dimethadione. Epilepsy Res. 1996;23:15–36. doi: 10.1016/0920-1211(95)00079-8. [DOI] [PubMed] [Google Scholar]