Abstract
Background
Recent evidence suggests that G protein coupled receptors, especially those linked to Gαi, contribute to the mechanisms of anesthetic action. Regulator of G protein signaling (RGS) proteins bind to activated Gαi and inhibit its signal transduction. Genomic knock-in mice with an RGS-insensitive Gαi2 G184S (Gαi2 GS) allele exhibit enhanced Gαi2 signaling and provide a novel approach for investigating the role of Gαi2 signaling and RGS proteins in general anesthesia.
Methods
Homozygous Gαi2 GS/GS and wild type (WT) mice were anesthetized with isoflurane and time (s) to loss and resumption of righting response was quantified. During recovery from isoflurane anesthesia breathing was quantified in a plethysmography chamber for both lines of mice.
Results
Gαi2 GS/GS mice required significantly less time for loss of righting and significantly more time for resumption of righting than WT mice. During recovery from isoflurane anesthesia, Gαi2 GS/GS mice exhibited significantly greater respiratory depression. Poincaré analyses show that GS/GS mice have diminished respiratory variability compared to WT mice.
Conclusion
Modulation of Gαi2 signaling by RGS proteins alters loss and resumption of wakefulness, and state-dependent changes in breathing.
Introduction
Volatile anesthetics are thought to produce a loss of consciousness by numerous mechanisms that include activating inhibitory ionotropic gamma-aminobutyric acid type A (GABAA) receptors, inhibiting excitatory ionotropic glutamate receptors, and direct effects on ion channels such as the two-pore potassium channels (1–5). Less is known about the contribution of metabotropic receptor signaling to the generation of anesthetic states (6–8).
There is evidence that G protein coupled receptors (GPCRs) are altered by anesthetics (8–11), and GPCRs may contribute to anesthesia by mimicking signaling that occurs during normal states of sleep and wakefulness (6,7,12–14). GPCRs activate heterotrimeric G proteins by inducing the binding of GTP, and activated G proteins are subsequently turned off by hydrolysis of GTP to GDP to restore the inactive state (15). There are four families of G proteins, referred to as Gs, Gi, Gq, and G12. Receptors that activate Gi lead to inhibition of neural activity by multiple mechanisms (15,16). Gi linked pathways in the brain are known to modulate states of behavioral arousal. For example, M2 and M4 muscarinic cholinergic receptors contribute to the regulation of wakefulness (17) and ketamine inhibits muscarinic receptors (18) as well as acetylcholine release (19). Volatile anesthetics act on GPCRs (6,20), including cholinergic (21) and hypocretinergic (12) receptors that promote cortical and behavioral arousal.
Regulators of G protein signaling (RGS) proteins inhibit G protein signaling by speeding the rate of GTP hydrolysis (22–24), which allows RGS proteins to tightly control G protein activity and subsequent downstream signaling. Knock-in mice containing an RGS-insensitive Gαi2 G184S allele recently have been described (25,26). These mice lose the negative regulatory activity of RGS proteins such that receptor-stimulated Gαi2 proteins remain active for a longer period of time. As a result, the Gαi2 RGS-insensitive knock-in mice (either homozygous Gαi2 GS/GS or heterozygous Gαi2 GS/+) exhibit enhanced Gαi2 signaling compared to wild type (WT) mice (25,26). Some (25,26) but not all (27) physiological responses are enhanced by the Gαi2 G184S mutation, while other physiological responses are selectively enhanced by a similar Gαo G184S mutation (27). The prolonged inhibitory signaling makes the Gαi2 GS/GS mice a novel tool for examining the role of Gαi2 signaling and RGS proteins in anesthetic action.
This study tested the hypothesis that compared to WT mice, increased Gαi2 signaling in Gαi2 GS/GS mice alters the loss of consciousness caused by isoflurane anesthesia and breathing. The results provide an initial phenotyping of the effects of isoflurane in Gαi2 GS/GS mice and suggest that RGS proteins contribute to isoflurane anesthesia. Portions of these data have been presented as abstracts (28,29).
Methods
Animals
The University of Michigan Committee on Use and Care of Animals reviewed and approved all procedures. Experiments were conducted in accordance with the National Institutes of Health Policy on Humane Care and Use of Laboratory Animals (NIH publication 80-23). Adult male homozygous Gαi2 GS/GS knock-in (n=16) and WT littermate control mice (n=10) derived from the C57BL/6J strain were obtained from an internal colony. The development of the knock-in mice and the genotyping protocol have been described in detail (25). Mice were housed in a temperature and humidity controlled environment with ad libitum access to food and water, and were kept on a 24h light cycle to minimize circadian influences. For data collection, experimenters were blinded to mouse genotype.
Quantifying loss of righting response
Gαi2 GS/GS and WT mice were used to quantify the time to become immobile after a single exposure to isoflurane (Hospira Inc, Lake Forest, IL). Mice (n=8 per genotype) were conditioned to handling and to being placed in an acrylic chamber for one week prior to experiments. On the experiment day, mice were placed in the acrylic chamber (25 cm × 11 cm × 13 cm) and administered 5% isoflurane in 100% oxygen at a flow rate of 1 L/min. Loss of righting response was operationally defined as the isoflurane-induced loss of a weight-bearing posture and the inability to resume a normal weight-bearing posture after being placed in dorsal recumbency. The time (s) from onset of isoflurane administration until the loss of righting response was recorded manually. Each mouse was used for one trial.
Quantifying resumption of righting response
Gαi2 GS/GS and WT (n=5 for each group) were used to quantify time for resumption of righting response after isoflurane anesthesia. Mice were conditioned to handling as described above. On the day of the experiment, mice were placed in the acrylic chamber and administered 3% isoflurane in 100% oxygen until they exhibited loss of righting. Mice were then transferred to a Kopf Model 923-B mouse anesthesia mask (David Kopf Instruments Tujunga, CA) and anesthesia was maintained with 1.3% isoflurane in 100% oxygen (30). Administered isoflurane concentration was monitored continuously using a Datex-Ohmeda Cardiocap 5 (Madison, WI), and body temperature was held at 37°C throughout the entire experiment using a water-filled heating pad (Gaymar Industries, Orchard Park, NY). Isoflurane delivery was discontinued 30 min later and mice were placed in dorsal recumbency under a heating lamp. The time (s) until mice resumed a normal, upright posture was recorded. Each mouse was used for 6 experiments with one week between experiments.
Quantifying breathing during recovery from isoflurane anesthesia
Gαi2 GS/GS and WT littermate controls were used to quantify isoflurane-induced alterations in breathing. Mice were conditioned to handling as described above and to being placed in a PLY 3211 Buxco plethysmography chamber (Wilmington, NC). The same anesthesia protocol used for the resumption of righting response experiments was followed. After 30 min of isoflurane anesthesia, mice were placed in the plethysmography chamber for 1 h and exposed to room air while breathing was recorded. Temperature in the plethysmography chambers was maintained between 26–28°C. The plethysmography system allowed for the quantification of respiratory rate, tidal volume, minute ventilation, inspiratory time, expiratory time, total respiratory cycle time, inspiratory flow, and inspiratory effort. All respiratory measurements containing volume units were expressed as per g body weight to normalize for weight differences between mice. Each mouse was used for 4 trials with one week between experiments. The number of mice used in the present experiments is comparable to previous studies with statistical power adequate for phenotyping strain-specific aspects of ventilatory control (31).
Data Analysis
Descriptive statistics, Student’s t-test, and Wilcoxon Rank Sum test were used to determine significant differences between genotypes. Respiratory rate during recovery from isoflurane anesthesia was also plotted using Poincaré analyses in which the mean respiratory rate for 5 min segments was plotted against the mean respiratory rate for the subsequent 5 min segment. As described in detail elsewhere (32,33), Poincaré analysis provides unique insights into the temporal organization of periodic phenomena. Data are reported as mean ± standard error of the mean (SEM). A p value less than 0.05 was interpreted to indicate a statistically significant difference.
Results
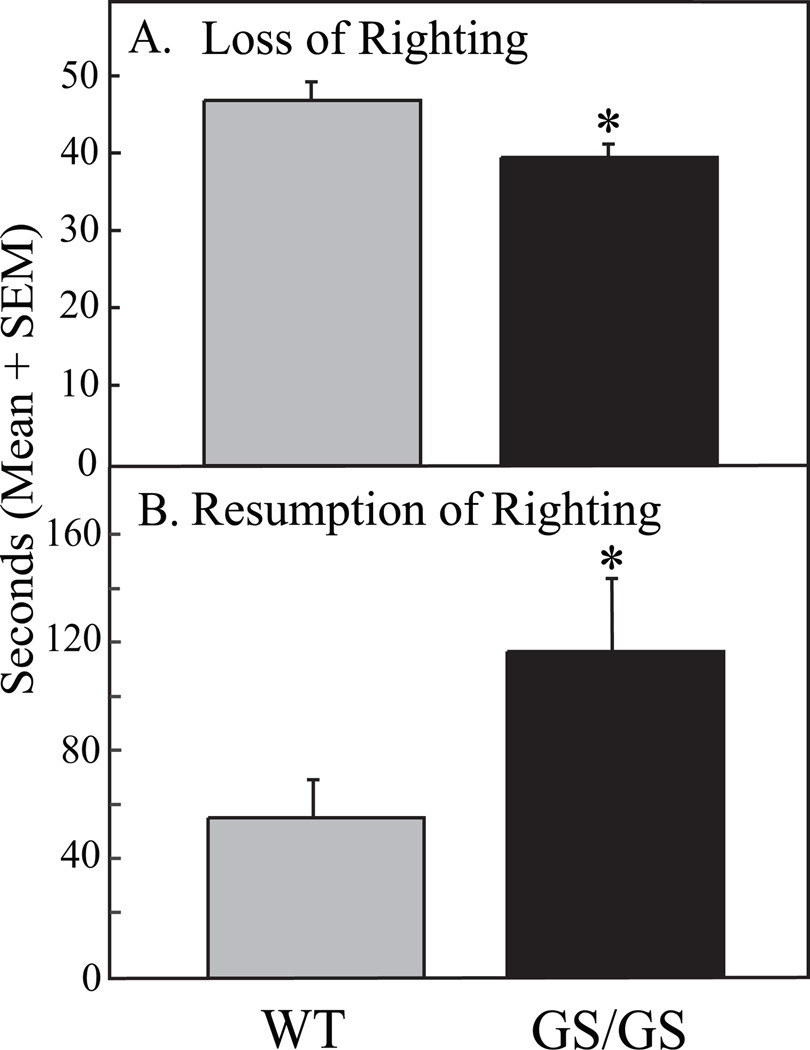
Figure 1A shows that Gαi2 GS/GS mice exhibited a significant decrease in time to loss of righting response compared to WT mice. To rule out changes in kinetics of isoflurane distribution, recovery from anesthesia also was assessed. Figure 1B illustrates that time required for resumption of righting after cessation of isoflurane was significantly increased in Gαi2 GS/GS mice compared to WT mice.
Figure 1.
Gαi2 GS/GS mice show a more rapid loss of righting caused by isoflurane than WT controls. A. Time for loss of righting response was significantly shorter (*, p = 0.01) in Gαi2 GS/GS mice compared to WT control mice. B. Time to resumption of righting response in Gαi2 GS/GS mice was significantly longer (*, p = 0.02) compared to WT control.
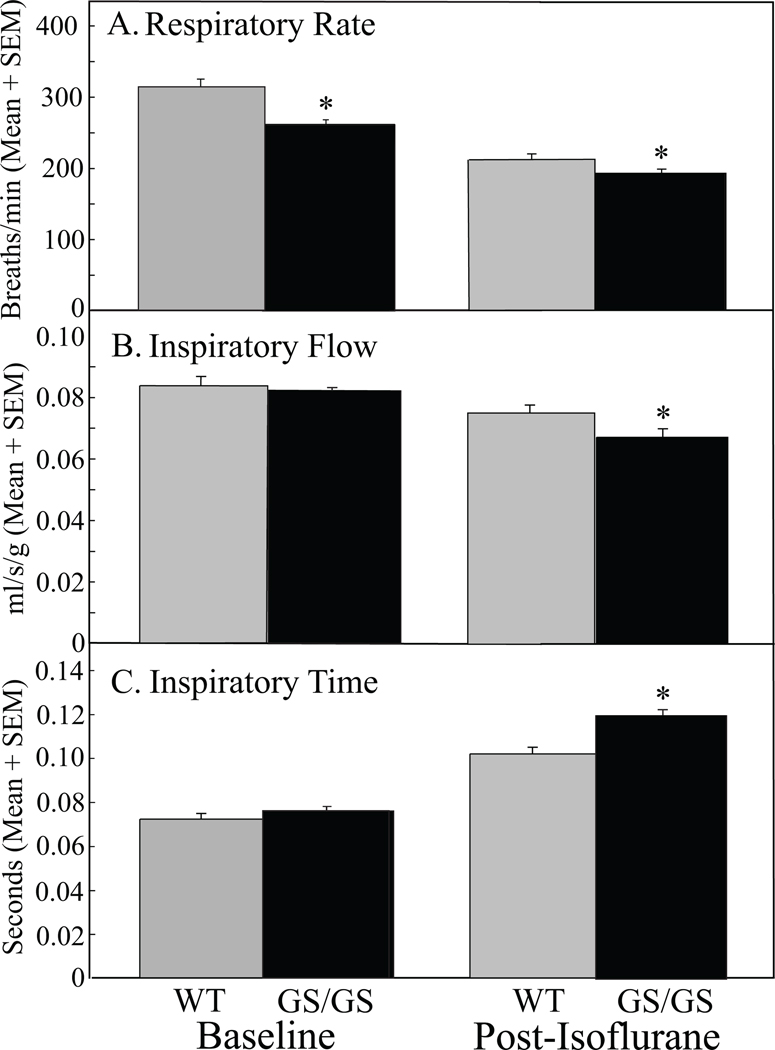
All measures of breathing were analyzed and expressed as per g body weight. Phenotyping of baseline ventilatory behavior was provided by pre-anesthesia measures of respiratory rate, inspiratory flow, and inspiratory time (Figs. 2A, B, and C; left histograms). The GS/GS mice revealed a significantly slower rate of breathing than WT before isoflurane anesthesia (Fig. 2A). In contrast to baseline, all respiratory measures were significantly different between WT and GS/GS mice after isoflurane anesthesia (Figs. 2A, B, and C; right histograms). Table 1 summarizes the percent change in breathing caused by isoflurane anesthesia. The greatest change in breathing was the increased duration of inspiration in GS/GS mice (Fig. 2C, Post-Isoflurane).
Figure 2.
Gαi2 GS/GS mice exhibited greater respiratory depression during the initial 20 min post-isoflurane anesthesia recovery period compared to WT mice. Each bar summarizes data from 3 mice randomly chosen from the two genotypes. The left pair of histograms in frames A, B, and C plots baseline measures prior to isoflurane exposure. A. At baseline, respiratory rate in Gαi2 GS/GS mice was significantly less than in WT mice. B. Inspiratory flow and C. inspiratory time were not different between Gαi2 GS/GS and WT mice during baseline recordings. The right pair of histograms illustrates breathing measures in Gαi2 GS/GS and WT mice after isoflurane. A. After isoflurane, respiratory rate in Gαi2 GS/GS mice was significantly (*, p =0.01) lower than WT mice. B. Inspiratory flow was significantly (*, p = 0.008) decreased compared to WT mice by isoflurane. C. Inspiratory time was significantly (*, p < 0.0001) increased in Gαi2 GS/GS mice compared to WT mice during recovery from isoflurane anesthesia.
Table 1.
Percent Change in Breathing Caused by Isoflurane
| Respiratory Rate | Inspiratory Flow | Inspiratory Time | |
|---|---|---|---|
| WT | −32 | −11 | 39 |
| GS/GS | −26 | −18 | 58 |
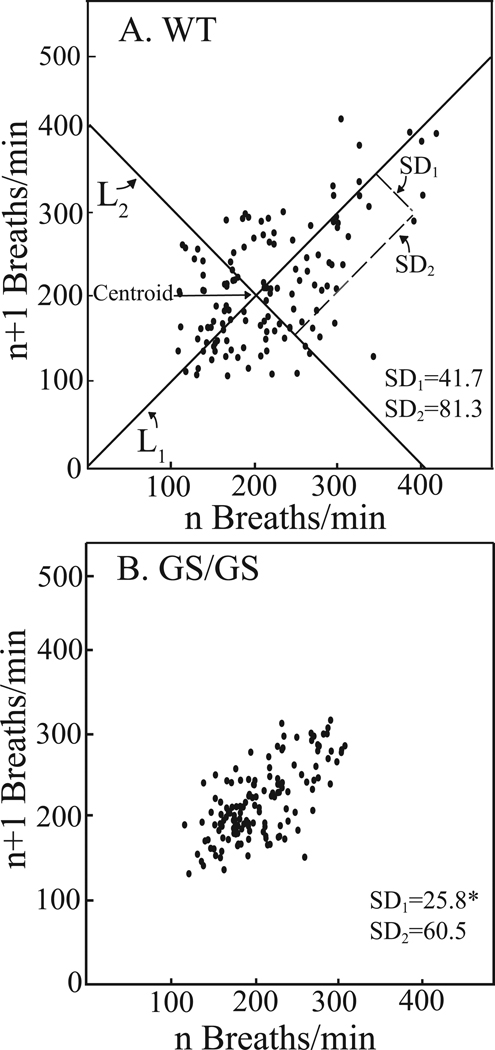
The Poincaré plots illustrate and quantify the variability in respiratory rate characteristic of WT (Fig. 3A) and Gαi2 GS/GS (Fig. 3B) mice. For one h following cessation of isoflurane delivery, WT mice exhibited a wider range of respiratory rate variability than Gαi2 GS/GS mice. The diminished variability in rate of breathing by the Gαi2 GS/GS mice can be visualized by contrasting the distribution of points in Figs. 3A and 3B. Poincaré analyses quantify the differences in variability in two ways. The first standard deviation (SD1) represents breath-to-breath (short-term) variability with respect to the line of identity (y = x). The second standard deviation (SD2) represents the amount by which the data points varied from a line orthogonal to and intersecting the line of identity at the mean. SD2 reflects the overall (long-term) variability in respiratory cycle length throughout the one h post-isoflurane recovery period. Gαi2 GS/GS mice exhibited significantly less breath-to-breath variation than WT mice.
Figure 3.
Poincaré plots illustrating the range of respiratory rates in WT (A) and Gαi2 GS/GS (B) mice during the 1 h recovery period following cessation of isoflurane delivery. Each point represents the mean respiratory rate (n = 3 mice per genotype) of a 5 min segment plotted against the next 5 min segment. The plot was analyzed by determining two standard deviation values: SD1 and SD2 for each data point. SD1 is defined as the dispersion of points perpendicular to the line-of-identity (L1). SD1 is used to analyze short-term variation. For these analyses SD1 corresponds to breath-to-breath variation of the respiratory cycle. The centroid is the point representing the overall mean respiratory rate. SD2 is defined as the dispersion of points along the line-of-identity passing through the centroid. SD2 is also referred to as long-term variation and for these data SD2 characterizes variability in respiratory rate due to genotype. Breath-to-breath respiratory rate of Gαi2 GS/GS mice was significantly (*, p < 0.001) less variable (smaller SD1) than WT mice.
Discussion
The results show decreased time to loss of righting response with exposure to isoflurane and increased time for resumption of righting following cessation of isoflurane in GS/GS knock-in mice (Fig. 1). These findings support the interpretation that RGS proteins can modulate, in part via Gαi2 signaling, both the loss and the resumption of wakefulness. The Fig. 1 data could be due to a direct action of isoflurane on a GPCRs (10–12) or to the effect of isoflurane on signaling molecules downstream of the primary site of anesthetic action (6,7). This new finding concerning RGS proteins is consistent with recent data regarding the role of GPCRs in arousal state control. For example, the hypothalamic peptide hypocretin/orexin promotes wakefulness via two G protein coupled receptors (34). Wake-active hypocretin neurons are inhibited by isoflurane, and genetic deletion of hypocretin/orexin delays the resumption of righting following isoflurane anesthesia (12). Adenosine promotes sleep via four GPCRs and recovery from isoflurane anesthesia can be significantly delayed or prolonged by cortical delivery of an adenosine A1 receptor agonist or antagonist, respectively (11).
Arousal state-dependent alterations in breathing can be modulated by Gαi coupled-receptors, including muscarinic M2 and M4, adenosine A1, and adrenergic α2 (7,21,35–38). Gαi2 GS/GS mice exhibited greater isoflurane-induced respiratory depression compared to WT littermate controls (Figs. 2 and 3). The significantly decreased inspiratory flow and increased inspiratory time in the Gαi2 GS/GS mice show that enhanced Gαi2 signaling also contributes to respiratory rate depression during recovery from isoflurane anesthesia.
Poincaré analyses provide a novel tool for quantifying and visualizing breath-to-breath variability in respiratory rate (39). GPCRs amplify transmembrane signaling in the time domain and previous studies demonstrate that RGS proteins alter cardiovascular control (25,26). In view of the relevance of cardio-respiratory control for anesthesiology, a logical extension of these previous findings was for the present study to quantify the extent to which RGS proteins might alter respiratory rhythm generation. A waxing and waning of respiratory rate is one characteristic of disordered breathing associated with the loss of wakefulness (40). Poincaré analyses can uniquely phenotype the temporal organization of ventilatory behavior (31,41). Furthermore, variability in breathing pattern has been shown to be clinically useful as a weaning predictor for post-anesthesia patients (32), for diagnosis of sleep apnea (42), and for elucidating which components of the central respiratory rhythm generator are most altered by opioids (43).
The present Poincaré analyses show (Fig. 3) that compared to WT mice, the Gαi2 GS/GS mice have a less variable respiratory rate during recovery from isoflurane anesthesia. A highly variable rate of breathing (Fig. 3A) observed among WT mice is characteristic of a ventilatory control system that can rapidly respond to rapidly changing metabolic and environmental demands. In the GS/GS mice (Fig. 3B) a clustering of points along the line of identity indicates that long duration breaths were followed by similarly timed breaths. In contrast to WT mice that have normal RGS proteins, the Gαi2 GS/GS mice have prolonged inhibitory signaling (24). Thus, the phenotype of ventilatory timing revealed by Poincaré analysis fits with differences in RGS protein dynamics between WT and GS/GS mice. Compared to WT control mice, isoflurane caused a rapid loss of consciousness and a delayed resumption of wakefulness in the GS/GS mice (Fig. 1). The isoflurane effects on breathing may also be due to the loss of a wakefulness stimulus on breathing (44).
Respiratory depression and decreased breathing rate variability may also be a consequence of the enhanced Gαi2 signaling outside the brain. For example, a decrease in upper airway and respiratory pump muscle activity due to lowered Ca2+ levels in the respiratory accessory and pump muscle cells (45,46) may also contribute to decreased variability in breathing. Some anesthetics inhibit upper airway muscles (47) and decrease diaphragm contractility (48). The greater respiratory rate depression in the Gαi2 GS/GS mice may reflect a synergistic effect of enhanced Gαi2 signaling on central and peripheral control mechanisms.
A general limitation of the approach used in this study is that transgenic animals can develop compensatory modulation by other genes and gene products (49,50). Any study of genetically modified animals has the potential to be confounded by compensatory changes that occur during development. Indeed, Gαi2 knock-out mice show that Gαi3 is upregulated (51). While such secondary changes could cause or contribute to the observed changes in anesthetic responses, the evidence for Gi-coupled receptor signaling in arousal state control suggests that the simple model of enhanced Gαi2 signaling as the mechanism is reasonable. Further study with other Gi/o protein mutants (either RGS-insensitive or knockout) would provide additional support for this interpretation. Future studies designed to identify the receptor and signaling pathways involved in the presently observed changes in arousal state and respiratory effects of isoflurane can provide additional important information.
The present study is also limited by lack of data regarding relevant isoflurane minimum alveolar concentration (MAC). It is not yet known how MAC values in these knock-in Gαi2 GS/GS mice compare to MAC of the WT mice. The present study was undertaken in part to determine if phenotype differences justify the effort and cost of creating enough GS/GS mice and heterozygote mice for a study designed to quantify MAC (30). The present finding of statistically significant differences, even with the relatively small number of WT and GS/GS mice studied, supports the interpretation of a robust effect of RGS proteins on breathing as well as loss and recovery of righting. These results encourage development of a transgenic colony large enough to enable studies of receptor systems and neural networks through which RGS proteins modulate anesthesia and breathing phenotypes.
In conclusion, compared to WT controls, homozygous knock-in Gαi2 GS/GS mice with enhanced Gαi2 signaling exhibited shorter time for loss of righting caused by isoflurane and increased time for recovery of righting after isoflurane anesthesia. Gαi2 GS/GS mice also revealed decreased breathing rate and inspiratory flow, increased inspiratory time, and a less variable respiratory rate during recovery from isoflurane anesthesia. The results are consistent with the interpretation that Gαi2 signaling contributes to the loss of consciousness and respiratory depression caused by isoflurane. Finally, these data suggest a link between RGS proteins and isoflurane modulation of wakefulness and breathing, and encourage future studies to determine whether RGS proteins contribute to the mechanisms of anesthetic action.
Acknowledgements
Financial Support: National Institutes of Health Grants HL65272, GM39561, HL57120, HL40881, MH45361, and the Department of Anesthesiology
For expert assistance we thank Mary A. Norat and Aaron Muncey. We also thank Raelene Charbeneau for genotyping and mouse colony management. Statistical analyses were conducted in consultation with Kathy Welch of the University of Michigan Center for Statistical Consultation and Research.
Footnotes
Conflict of Interest: None
References
- 1.Hemmings HC, Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26:503–510. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Mashour GA, Forman SA, Campagna JA. Mechanisms of general anesthesia: from molecules to mind. Best Pract Res Clin Anaesthesiol. 2005;19:349–364. doi: 10.1016/j.bpa.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Orser BA. Extrasynaptic GABAA receptors are critical targets for sedative-hypnotic drugs. J Clin Sleep Med. 2006;2:S12–S18. [PubMed] [Google Scholar]
- 4.Vanini G, Watson CJ, Lydic R, Baghdoyan HA. Gamma-aminobutyric acid-mediated neurotransmission in the pontine reticular formation modulates hypnosis, immobility, and breathing during isoflurane anesthesia. Anesthesiology. 2008;109:978–988. doi: 10.1097/ALN.0b013e31818e3b1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zecharia AY, Nelson LE, Gent TC, Schumacher M, Jurd R, Rudolph U, Brickley SG, Franks NP. The involvement of hypothalamic sleep pathways in general anesthesia: testing the hypothesis using the GABA-A receptor β3 N265M knock-in mouse. J Neurosci. 2009;29:2177–2187. doi: 10.1523/JNEUROSCI.4997-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 7.Lydic R, Baghdoyan HA. Sleep, anesthesiology, and the neurobiology of arousal state control. Anesthesiology. 2005;103:1268–1295. doi: 10.1097/00000542-200512000-00024. [DOI] [PubMed] [Google Scholar]
- 8.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–720. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 9.Hollmann MW, Strumper D, Herroeder S, Durieux ME. Receptors, G proteins, and their interactions. Anesthesiology. 2005;103:1066–1078. doi: 10.1097/00000542-200511000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Ishizawa Y, Pidikiti R, Liebman PA, Eckenhoff RG. G protein-coupled receptors as direct targets of inhaled anesthetics. Mol Pharmacol. 2002;61:945–952. doi: 10.1124/mol.61.5.945. [DOI] [PubMed] [Google Scholar]
- 11.Van Dort CJ, Baghdoyan HA, Lydic R. Adenosine A1 and A2A receptors in mouse prefrontal cortex modulate acetylcholine release and behavioral arousal. J Neurosci. 2009;29:871–881. doi: 10.1523/JNEUROSCI.4111-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelz MB, Sun Y, Chen J, Cheng Meng Q, Moore JT, Veasey SC, Dixon S, Thornton M, Funato H, Yanagisawa M. An essential role for orexins in emergence from general anesthesia. Proc Nat Acad Sci USA. 2008;105:1309–1314. doi: 10.1073/pnas.0707146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clement EA, Richard A, Thwaites M, Ailon J, Peters S, Dickson CT. Cyclic and sleep-lke spontaneous alternations of brain state under urethane anaesthesia. PLoS ONE. 2008;3 doi: 10.1371/journal.pone.0002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Dort CJ, Baghdoyan HA, Lydic R. Neurochemical modulators of sleep and anesthetic states. In: Mashour GA, editor. Int Anesthesiol Clin. Philadelphia: Lippencott Williams & Wilkins; 2008. pp. 75–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. 2005;85:1159–1204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- 16.Tas PW, Eisemann C, Roewer N. Indirect activation of adenosine A1 receptors in cultured rat hippocampal neurons by volatile anaesthetics. Eur J Anaesthesiol. 2005;22:694–702. doi: 10.1017/s0265021505001158. [DOI] [PubMed] [Google Scholar]
- 17.Baghdoyan HA, Lydic R. M2 muscarinic receptor subtype in the feline medial pontine reticular formation modulates the amount of rapid eye movement sleep. Sleep. 1999;22:835–847. doi: 10.1093/sleep/22.7.835. [DOI] [PubMed] [Google Scholar]
- 18.Durieux ME. Inhibition by ketamine of muscarinic acetylcholine receptor function. Anesth Analg. 1995;81:57–62. doi: 10.1097/00000539-199507000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Lydic R, Baghdoyan HA. Ketamine and MK-801 decrease ACh release in the pontine reticular formation, slow breathing, and disrupt sleep. Sleep. 2002;25:617–622. [PubMed] [Google Scholar]
- 20.Nakayama T, Penheiter AR, Penheiter SG, Chini EN, Thompson M, Warner DO, Jones KA. Differential effects of volatile anesthetics on M3 muscarinic receptor coupling to the G alpha q heterotrimeric G protein. Anesthesiology. 2006;105:313–324. doi: 10.1097/00000542-200608000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Dong HL, Fukuda S, Murata E, Higuchi T. Excitatory and inhibitory actions of isoflurane on the cholinergic ascending arousal system of the rat. Anesthesiology. 2006;104:122–133. doi: 10.1097/00000542-200601000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Hepler JR. RGS protein and G protein interactions: a little help from their friends. Mol Pharmacol. 2003;64:547–549. doi: 10.1124/mol.64.3.547. [DOI] [PubMed] [Google Scholar]
- 23.Neubig RR. Regulators of G protein signaling (RGS proteins): novel central nervous system drug targets. J Pept Res. 2002;60:312–316. doi: 10.1034/j.1399-3011.2002.21064.x. [DOI] [PubMed] [Google Scholar]
- 24.Neubig RR, Siderovski DP. Regulators of G-protein signalling as new central nervous system drug targets. Nat Rev Drug Discov. 2002;1:187–197. doi: 10.1038/nrd747. [DOI] [PubMed] [Google Scholar]
- 25.Huang X, Fu Y, Charbeneau RA, Saunders TL, Taylor DK, Hankenson KD, Russell MW, D'Alecy LG, Neubig RR. Pleiotropic phenotype of a genomic knock-in of an RGS-insensitive G184S Gnαi2 allele. Mol Cell Biol. 2006;26:6870–6879. doi: 10.1128/MCB.00314-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu Y, Huang X, Zhong H, Mortensen RM, D'Alecy LG, Neubig RR. Endogenous RGS proteins and Gα subtypes differentially control muscarinic and adenosine-mediated chronotropic effects. Circ Res. 2006;98:659–666. doi: 10.1161/01.RES.0000207497.50477.60. [DOI] [PubMed] [Google Scholar]
- 27.Goldenstein BL, Nelson BW, Xu K, Luger EJ, Pribula JA, Wald JM, O'Shea LA, Weinshenker D, Charbeneau RA, Huang X, Neubig RR, Doze VA. Regulator of G protein signaling protein suppression of Gαo protein-mediated α2A adrenergic receptor inhibition of mouse hippocampal CA3 epileptiform activity. Mol Pharm. 2009;75:1222–1230. doi: 10.1124/mol.108.054296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Icaza EE, Fu Y, Huang X, Neubig RR, Baghdoyan HA, Lydic R. Role of an inhibitory G protein (Gαi2) and regulators of G protein signaling (RGS proteins) in isoflurane anesthesia. Society for Neuroscience Abstract Viewer/Itinerary Planner Online. 2006 Program No. 795.3. [Google Scholar]
- 29.Icaza EE, Huang X, Neubig RR, Baghdoyan HA, Lydic R. Genomic knock-in mice with enhanced Gαi2 signaling exhibit altered breathing during recovery from isoflurane anesthesia compared to wild type mice. Sleep. 2007;30 Abstr Suppl:0024. [Google Scholar]
- 30.Sonner JM, Gong D, Li J, Eger EI, 2nd, Laster MJ. Mouse strain modestly influences minimum alveolar anesthetic concentration and convulsivity of inhaled compounds. Anesth Analg. 1999;89:1030–1034. doi: 10.1097/00000539-199910000-00039. [DOI] [PubMed] [Google Scholar]
- 31.Yamauchi M, Dostal J, Kimura H, Strohl KP. Effects of buspirone on posthypoxic ventilatory behavior in C57BL/6J and A/J mouse strains. J Appl Physiol. 2008;105:518–526. doi: 10.1152/japplphysiol.00069.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bien M-Y, Shu-Shya H, Benjamin I-TK, Yu-Ting L, Jia-Horng W, Yu RK. Breathing pattern variability: a weaning predictor in postoperative patients recovering from systemic inflammatory response syndrome. Intensive Care Med. 2004;30:241–247. doi: 10.1007/s00134-003-2073-8. [DOI] [PubMed] [Google Scholar]
- 33.Woo MA, Stevenson WG, Moser DK, Trelease RB, Harper RM. Patterns of beat-to-beat heart rate variability in advanced heart failure. Am Heart J. 1992;123:704–710. doi: 10.1016/0002-8703(92)90510-3. [DOI] [PubMed] [Google Scholar]
- 34.Nishino S, Sakurai T. The Orexin/Hypocretin System. In: Lydic R, Baghdoyan HA, editors. Contemporary Clinical Neuroscience. Humana Press; 2006. pp. 1–397. [Google Scholar]
- 35.Evers AS, Crowder CM, Balser JR. General anesthetics. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics. Eleventh. New York: McGraw-Hill; 2006. pp. 341–368. [Google Scholar]
- 36.Barros RC, Branco LG, Carnio EC. Respiratory and body temperature modulation by adenosine A1 receptors in the anteroventral preoptic region during normoxia and hypoxia. Respir Physiol Neurobiol. 2006;153:115–125. doi: 10.1016/j.resp.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Bischoff P, Schneider G, Kochs E. Anesthetics drug pharmacodynamics. In: Schuttler J, Schwilden H, editors. Handbook of Experimental Pharmacology. Berlin: Springer; 2008. pp. 379–408. [DOI] [PubMed] [Google Scholar]
- 38.Joseph V, Pequignot JM, Van Reeth O. Neurochemical perspectives on the control of breathing during sleep. Respir Physiol Neurobiol. 2002;130:253–263. doi: 10.1016/s0034-5687(02)00012-9. [DOI] [PubMed] [Google Scholar]
- 39.Chun HH, Spiegel ET, Solomon IC. Burst-to-burst variability in respiratory timing, inspiratory-phase spectral activity, and inspiratory neural network complexity in urethane-anesthetized C57BL/6 mice in vivo. Adv Exp Med & Biol. 2008;605:408–412. doi: 10.1007/978-0-387-73693-8_71. [DOI] [PubMed] [Google Scholar]
- 40.Strohl KP. Periodic breathing and genetics. Respir Physiol Neurobiol. 2003;135:179–185. doi: 10.1016/s1569-9048(03)00036-3. [DOI] [PubMed] [Google Scholar]
- 41.Gonsenhauser I, Wilson CG, Han F, Strohl KP, Dick T. Strain differences in murine ventilatory behavior persists after urethane anesthesia. J Appl Physiol. 2004;97:888–894. doi: 10.1152/japplphysiol.01346.2003. [DOI] [PubMed] [Google Scholar]
- 42.Morillo DS, Rojas JL, Crespo LF, Leon A, Gross N. Poincaré analysis of an overnight arterial oxygen saturation signal applied to the diagnosis of sleep apnea hypopnea syndrome. Physiol Meas. 2009;30:405–420. doi: 10.1088/0967-3334/30/4/005. [DOI] [PubMed] [Google Scholar]
- 43.Mitsis GD, Governo RJM, Rogers R, Pattinson KTS. The effect of remifentanil on respiratory variability, evaluated with dynamic modeling. J Appl Physiol. 2009;106:1038–1049. doi: 10.1152/japplphysiol.90769.2008. [DOI] [PubMed] [Google Scholar]
- 44.Fink BR. Influence of cerebral activity in wakefulness on regulation of breathing. J Appl Physiol. 1961;16:15–20. doi: 10.1152/jappl.1961.16.1.15. [DOI] [PubMed] [Google Scholar]
- 45.Chen X, Yamakage M, Namiki A. Inhibitory effects of volatile anesthetics on K+ and Cl− channel currents in porcine tracheal and bronchial smooth muscle. Anesthesiology. 2002;96:458–466. doi: 10.1097/00000542-200202000-00035. [DOI] [PubMed] [Google Scholar]
- 46.Ikeda SR, Jeong SW. Use of RGS-insensitive Gα subunits to study endogenous RGS protein action on G-protein modulation of N-type calcium channels in sympathetic neurons. Methods Enzymol. 2004;389:170–189. doi: 10.1016/S0076-6879(04)89011-6. [DOI] [PubMed] [Google Scholar]
- 47.Akhtar S, Brull SJ. Effect of isoflurane on endothelin-1 mediated airway smooth muscle contraction. Pulm Pharmacol Ther. 1998;11:227–230. doi: 10.1006/pupt.1998.0143. [DOI] [PubMed] [Google Scholar]
- 48.Fauroux B, Cordingley J, Hart N, Clement A, Moxham J, Lofaso F, M I P. Depression of diaphragm contractility by nitrous oxide in humans. Anesth Analg. 2002;94:340–345. doi: 10.1097/00000539-200202000-00020. [DOI] [PubMed] [Google Scholar]
- 49.Robert C. Knockout mice: advantages and limitations for biological modeling. Definitions, principles and examples. Ann Dermatog Venereol. 1998;125:946–947. [PubMed] [Google Scholar]
- 50.Crawley J. Behavioral Phenotyping of Transgenic and Knockout Mice. New York: Wiley-Liss; 2000. What's Wrong with my Mouse? [Google Scholar]
- 51.Han SB, Moratz C, Huang NN, Kelsall B, Cho H, Shi CS, Schwartz O, Kehrl JH. Rgs1 and Gnai2 regulate the entrance of B lymphocytes into lymph nodes and B cell motility within lymph node follicles. Immunity. 2005;22:343–354. doi: 10.1016/j.immuni.2005.01.017. [DOI] [PubMed] [Google Scholar]