Abstract
Our studies involve computational simulations, a reconstructed plasma/platelet proteome, whole blood in vitro and blood exuding from microvascular wounds. All studies indicate that in normal hemostasis, the binding of tissue factor (Tf) with plasma factor (f) VIIa (extrinsic fXase complex) results in the INITIATION PHASE of the procoagulant response. This phase is negatively regulated by tissue factor pathway inhibitor (TFPI) in combination with antithrombin (AT) and the protein C (PC) pathway. The synergy between these inhibitors provides a threshold-limited reaction in which a stimulus of sufficient magnitude must be provided for continuation of the reaction. With sufficient stimulus, the fXa produced activates some prothrombin. This initial thrombin activates the procofactors and platelets required for presentation of the intrinsic fXase (fVIIIa-fIXa) and prothrombinase (fVa-fXa) complexes which drive the subsequent PROPAGATION PHASE; continuous downregulation of which is provided by AT and the thrombin-thrombomodulin-PC complex. FXa generation during the PROPAGATION PHASE is largely (>90%) provided by the intrinsic fXase complex. Tf is required for the INITIATION PHASE of the reaction but becomes non-essential once the PROPAGATION PHASE has been achieved. The PROPAGATION PHASE catalysts (fVIIIa-fIXa and fVa-fXa) continue to drive the reaction as blood is resupplied to the wound site by flow. Ultimately, the control of the reaction is governed by the pro- and anticoagulant dynamics and the supply of blood reactants to the site of a perforating injury. Our systems have been utilized to examine the qualities of hypothetical and novel antihemorrhagic and anticoagulation agents in epidemiologic studies of venous and arterial thrombosis and the hemorrhagic pathology.
Keywords: Thrombin, blood coagulation, hemostasis, numerical models, phenotype variability
Introduction
The maintenance of blood flow is essential to life. The loss of blood flow may occur because the heart that pumps the blood fails, the blood vessels become obstructed or blood leakage occurs. The coagulation system has evolved to deal with the latter two events under normal circumstances by providing qualities which maintain blood in a fluid state within the vasculature, while at the same time addressing leaks.
The blood itself is the primary supplier of pro- and anti- coagulant proteins in plasma and the source of platelets which are the principle blood elements contributing to the hemostatic process. When the endothelial lining is disrupted by a perforating injury, the extravascular compartment and blood interact to rapidly produce a vigorous local coagulation response which attenuates blood loss and initiates the vascular repair process.
The Hemostatic Process
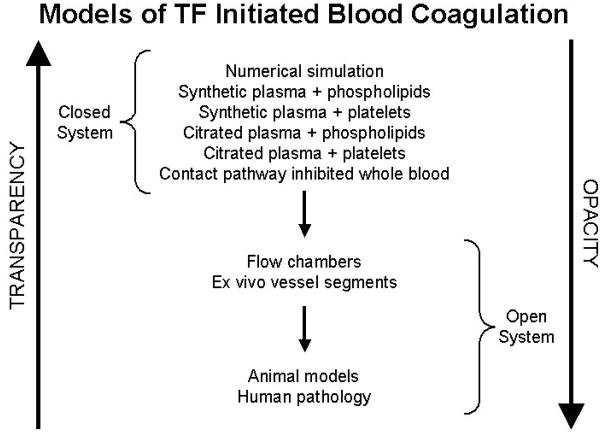
The dynamics of the coagulation system and its significance in defining hemorrhagic and thrombotic phenotypes and potential interventions are explored in our laboratory utilizing various techniques: a synthetic plasma proteome, a computational model and whole human blood in vitro and in vivo(1). Each system has specific advantages and limitations. Numerically based computer models are completely transparent, can be rapidly solved for a given set of conditions but have the lowest level of biological authenticity (Figure 1). In contrast, studies of blood in vitro and blood exuding from a peripheral wound in vivo are authentic biological models which are technically difficult, time consuming, expensive and, while clearly biologically applicable, maintain a high level of opacity with respect to complete knowledge of what is going on. An intermediate phase is represented by the synthetic plasma proteome which attempts to mimic biological reality in vitro.
Figure 1. Schematic of models of blood coagulation.
The relationship between biochemical transparency and biological fidelity proceeding from numerical models to human biology. (With permission from Mann et al. Blood Cells, Mol and Dis; 2006:36:108).
The synthetic coagulation proteome mimics coagulation by utilizing mixtures of the vitamin K dependent zymogens, their (pro)cofactors, and the stoichiometric inhibitors with a membrane source of either platelets or synthetic lipid membranes.(2–4) The reaction is started by the addition of relipidated tissue factor (Tf) or activated monocytes.
Computational simulations of the coagulation system based upon individual reaction mechanisms and the concentrations of pro- and anti-coagulants rapidly determine the fate of all protein products.(1;5) These simulations are the least expensive and the most rapid method for analyses. These models provide insights into reaction processes when quantitative and qualitative variables are altered and by the inclusion of pharmacologic or pathophysiologic interventions and are useful in the design of empirical experiments.
Contact pathway inhibited but otherwise unaltered whole blood induced to clotting by Tf is studied using a system of immunoassays which follow pro- and anti-coagulant products generated during the time course of the reaction.(6;7) This technique has been used to study hemorrhagic and thrombotic pathologies and the influence of pharmacologic management in both pathologies.(8–10) The vascular component is absent and this is a closed system with no flow.
The most biologically relevant model is an in vivo bleeding time model of the hemostatic system, in which whole blood exuding from a microvascular wound is sequentially sampled for relevant product formation.(11–14) The vascular and extravascular components are present, and an injury, induced by the Simplate device, is the source of coagulation initiation by the perforation of a blood vessel.
The intricate blood coagulation mechanisms that govern normal hemostasis can be categorized into three overlapping phases: the INITIATION of coagulation, its PROPAGATION and the TERMINATION of the procoagulant response. These phases are analyzed utilizing the models described above.
Consistency between the results obtained from each of these models implies that the paradigms used to predict and evaluate the dynamics of the coagulation system are reasonably correct. Discontinuities, when observed, provide insights into new biology/biochemistry associated with this exceedingly complicated system.
Procoagulant Enzymatic Complexes
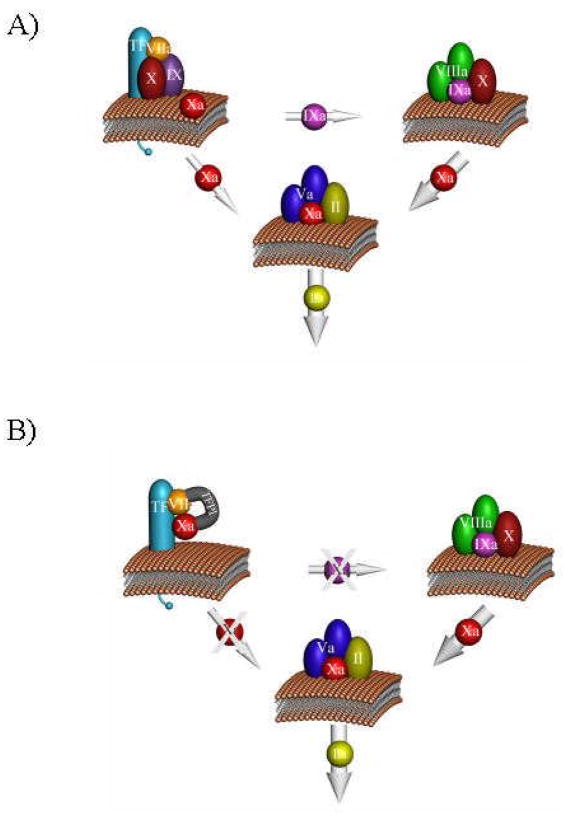
The dynamics of the blood coagulation system are dominated by three membrane-bound proteolytic procoagulant complexes which assemble at the site of vascular injury on platelets, the damaged endothelium and subvascular tissue(15) (Figure 2). The presentation and regulation of these complexes and their opposing anticoagulant systems dictate the response of the coagulation system.
Figure 2. Complex catalysts.
Three complexes are shown: the extrinsic fXase (Tf-fVIIa); the intrinsic fXase (fVIIIa-fIXa); and prothrombinase (fVa-fXa). The fXa generated by the fVIIa-Tf complex activates complex a small amount of thrombin which activates fV and fVIII leading to the presentation of the intrinsic fXase and prothrombinase complexes (panel A). The thick arrow representing fXa generation by the intrinsic fXase complex illustrates the ~50-fold more efficient fXa generation by this catalyst. The tissue factor pathway inhibitor, TFPI, interacts with the fVIIa-Tf-fXa product complex to block the Tf-initiated activation of both fIX and fX leaving the fIX-fVIIIa complex as the only viable catalyst for fX activation (panel B). Used with permission from the “Dynamics of Hemostasis” Haematologic Technologies, K.G. Mann, 2002.
Following vascular injury and the exposure of subvascular Tf, the Tf-fVIIa (Tf-fVIIa) extrinsic fXase complex is formed which activates fX and fIX to their respective enzymatic products (16;17) (Figure 2a). This reaction is tightly regulated by the tissue factor pathway inhibitor (TFPI) which binds the fVIIa-Tf-fXa product complex(18) (Figure 2b). The small amount of fXa initially formed activates prothrombin which activates additional platelets, fVIII and fV to their respective cofactor forms.(19) Subsequently, the combination of activated platelets and the formation of the intrinsic fXase (fVIIIa-fIXa) and prothrombinase (fVa-fXa) complexes dominate fXa and thrombin generation respectively (Figure 2a). Cofactor-protease assembly on membrane surfaces yields enhancements in the rates of substrate processing ranging from 105–109 fold relative to rates observed when the same reactions are limited to solution phase bimolecular interactions between the individual proteases (fVIIa, fIXa and fXa) and their corresponding substrates.(20–22)
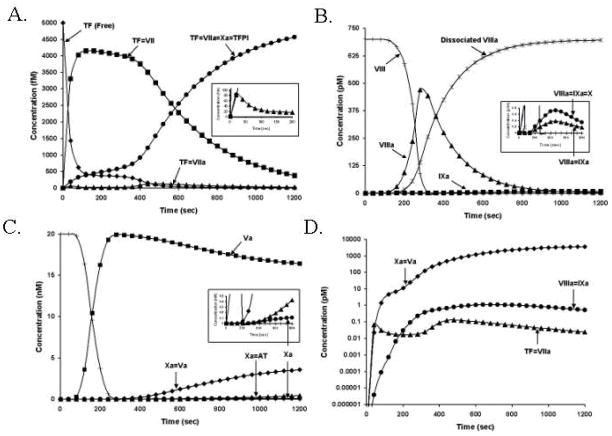
The dynamics of procoagulant complex formation are illustrated in the four panels of Figure 3 which show the formation and the life spans of the complexes during the coagulation process after the presentation of Tf to blood. The insets on each figure illustrate the concentrations of active complexes which drive thrombin generation.(19) Dominant features include the observation that in every case the limiting reactant in complex formation is the protease component of the complex. The concentration of each complex is extremely low, relative to its potential, ranging from less than 100 femtomolar (10−15M, ~2% of potential total) for the concentration of Tf-fVIIa (extrinsic fXase complex) to 0.7 pM (10−12M, ~1% of potential total) for the fVIIIa-fIXa (intrinsic fXase complex) and approximately 3 nM (10−9M, ~15% of potential total) for fVa-fXa (prothrombinase complex). The time-scales of formation and concentrations of the procoagulant complexes are illustrated in Figure 3 panel D. Note that the vertical axis in panel D is on an exponential scale. Functional levels of the extrinsic fXase complex are limited by both the competitive binding of fVII to Tf which initially occupies ~90% of the Tf pool and by TFPI which efficiently inhibits the extrinsic fXase complex via fXa dependent reaction.(23) Levels of the intrinsic fXase complex appear controlled by the instability of fVIIIa and the offset between the timing of peak fVIIIa levels and the production of fIXa.
Figure 3. The process of procoagulant complex formation.
The reaction in electronic plasma is started by addition of 5 pM Tf. Data are expressed as metabolite concentration (molar) vs time (s). Panel A. The extrinsic fXase complex. Inert complexes: Tf not bound to other proteins (◆); Tf-fVII (■); Tf-fVIIa-fXa-TFPI (●); also shown Tf-factor VIIa (▲). The inset shows the first 200s of the process of Tf-fVIIa complex formation (▲) and the generation of the TFPI inhibited complex. (●). Note that the extrinsic fXase peak level is ~80 fM or ~2% of the available Tf cofactor. Panel B. The intrinsic fXase complex. FVIII consumption (+), fVIIIa formation (▲), fVIIIa inactivation via dissociation (
 ) and fIXa formation (■) are illustrated over a 1200s time frame. The inset shows the time courses for the fVIIIa=fIXa complex (▲) and the fVIIIa-fIXa-fX complex (●). The formation of the intrinsic fXase complex over 1200s results in sub picomolar levels. Panel C. The prothrombinase complex. FV consumption (+), fVa formation (■), fXa formation (●), fXa-fVa complex (◆), and Xa-AT complex (▲) are shown. The inset highlights the formation of free fXa and the inhibited complex. Panel D. Time courses of active procoagulant complex formation. Note: The concentrations of fXa-fVa (◆), fVIIIa-fIXa (●), and Tf-fVIIa (▲) are shown on a logarithmic scale.
) and fIXa formation (■) are illustrated over a 1200s time frame. The inset shows the time courses for the fVIIIa=fIXa complex (▲) and the fVIIIa-fIXa-fX complex (●). The formation of the intrinsic fXase complex over 1200s results in sub picomolar levels. Panel C. The prothrombinase complex. FV consumption (+), fVa formation (■), fXa formation (●), fXa-fVa complex (◆), and Xa-AT complex (▲) are shown. The inset highlights the formation of free fXa and the inhibited complex. Panel D. Time courses of active procoagulant complex formation. Note: The concentrations of fXa-fVa (◆), fVIIIa-fIXa (●), and Tf-fVIIa (▲) are shown on a logarithmic scale.
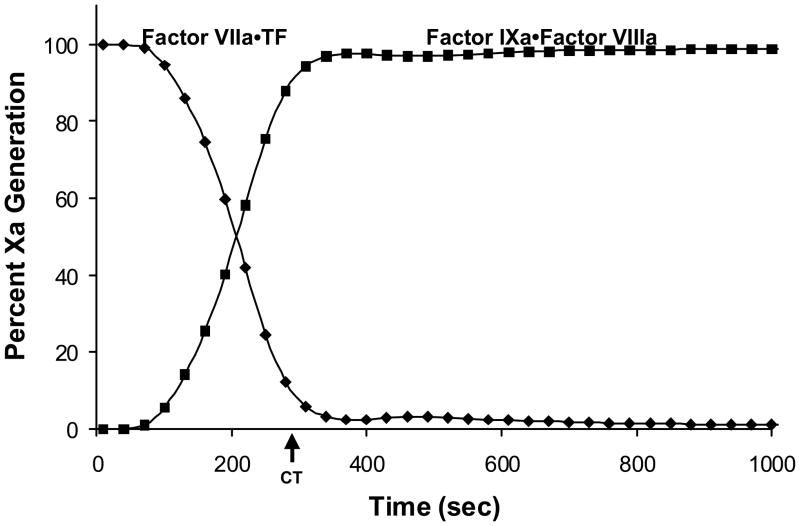
A representative comparison of the proportion of fXa generated by the two fXa activating complexes is illustrated in Figure 4, which shows that at the point of clot formation over 90% of the fXa is produced by the intrinsic fXase complex.(1)
Figure 4. Two pathways to fXa production.
A computational estimation of the temporal dependence of the relative fXa production by the extrinsic fXase (◆) and the intrinsic fXase (■) complexes. The clot time (CT, arrow) represents the time at which free thrombin levels are predicted to reach 2nM. (With permission from Hockin et al. 2002, JBC 277:18322).
Resupply
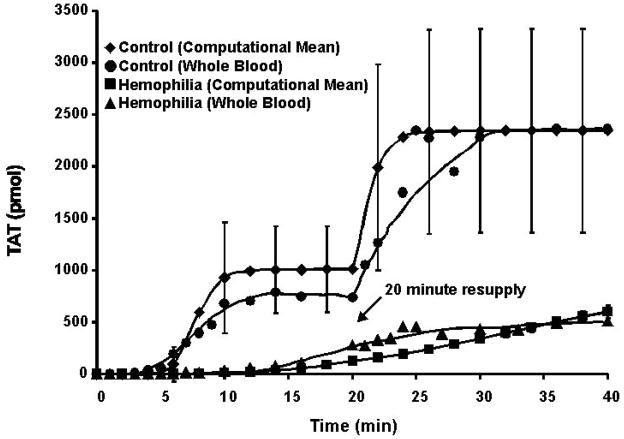
The illustrations and descriptions in the preceding section correspond to the generation of thrombin in closed systems. In contrast, the biological processes of coagulation occur in an open system in which the reaction site is constrained only by protein and membrane binding associated with the region of the vascular wound. We have provided partial insight into the continuity of the process (with respect to thrombin generation) which might occur in an open system by utilizing the three in vitro models and resupplying the quiescent Tf-initiated reaction systems with fresh aliquots of electronic, real or synthetic blood. An experimental result of this sort is illustrated in Figure 5 which shows a closed system computational simulation of thrombin-antithrombin (TAT) product accumulation in response to Tf-initiation and subsequent resupply.(24) The simulated time course of thrombin formation, using the mean values for all coagulation pro- and anti-coagulant factors is shown along with the standard deviation reflecting what happens when computational simulations are conducted using clinically accepted low and high values (Table 1) for each protein. Data for an actual whole blood experiment conducted utilizing blood from a single healthy individual are superimposed. Also presented is a whole blood experiment and the mean value (minus fVIII) computational simulations for a closed system with hemophilia blood. Consistent with the computational analysis of the temporal dependence of the contributions of the extrinsic and intrinsic fXase complexes (Figure 4), hemophilia blood (Figure 5), which lacks the intrinsic fXase complex, does not display a propagation phase of thrombin formation, as this deficiency does not permit the necessary expanded generation of fXa by fVIIIa-fIXa.
Figure 5. Resupply of a Tf-initiated blood coagulation reaction after the cessation of prothrombin consumption: empirical and simulated results.
Time courses of TAT formation are shown after addition of 5 pM Tf to both electronic plasma and contact pathway inhibited whole blood. The resupply response was evaluated in both electronic plasma and whole blood. Either a fresh aliquot of contact pathway phlebotomy blood from the same individual or an electronic volume of fresh zymogens, cofactors and inhibitors, without additional Tf, was added 20 minutes after Tf initiation. TAT levels are presented as total moles of product present versus time in order to conform to the methodology of the empirical experiments. Computational simulations were performed using the mean values of zymogens, cofactors and inhibitors or the clinically accepted low and high ranges for each of the zymogens, cofactors and inhibitors. The simulated data using mean values are shown for a control (◆) and a hemophilic (−fVIII, ■) with the standard deviation of the accepted high and low values for all constituents shown for the control numerical simulation. Empirical TAT formation data were obtained from contact pathway suppressed whole blood collected from one healthy control (●) and one fVIII deficient individual (▲). (With permission from Orfeo et al. 2008, JBC 283:9776).
Table 1.
Patient laboratory report Fletcher Allen Health Care (University of Vermont hospital). Coagulation factor levels for a healthy volunteer and the normal range values for each factor.
| Analyte | Patient Value | Normal Range |
|---|---|---|
| Factor X | 125% | 60–140% |
| Factor II | 112% | 60–140% |
| Factor V | 98% | 60–140% |
| Factor VII | 107% | 60–140% |
| Factor VIII | 107% | 64–232% |
| Factor IX | 86% | 69–151% |
| Antithrombin | 112% | 86–128% |
| Fibrinogen | 292 mg/dL | 180–433 mg/dL |
| Protime | 12.8s | 11.7–13.1 s |
| INR | 1.1 ratio | 0.8–1.2 ratio |
| PTT | 27s | 23–33s |
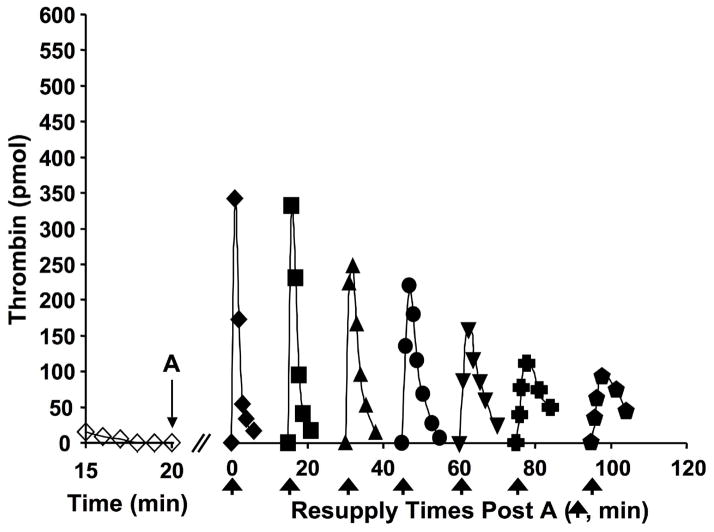
The resupply protocol involves the addition of a fresh aliquot of normal or hemophilia blood or its electronic equivalent, after the Tf-initiated reaction becomes completely quiescent with respect to further thrombin production (20 minutes). In the normal resupply case, there is rapid accumulation of thrombin-antithrombin with the rate and extent of accumulation being larger than that initially observed over the first 20 minutes. The source of this additional thrombin generating activity is due to the prothrombinase complex which remains active over the initial 20 minute time course. This is illustrated by a series of resupply reactions using the synthetic plasma proteome model(24) (Figure 6) in which sequential additions of only prothrombin, antithrombin and phospholipid are provided to the reaction system. A new burst of thrombin is observed with each new addition with the intensity of thrombin production reduced with each sequential aliquot, reflecting the slow decay of prothrombinase activity in the presence of antithrombin. Were a complete proteome mixture used for resupply instead of only prothrombin and antithrombin added, the intensity of the response would not show this decrease because of embedded and expanding intrinsic prothrombinase produced by regenerating the intrinsic fXase complex in the reaction system.
Figure 6. Resupply of the synthetic coagulation proteome.
A 5 pM Tf-initiated reaction mixture was subdivided after 20 min (A), and the seven separate aliquots resupplied either immediately (20 min → t=0, (◆)) or after an additional 15 (■), 30 (▲), 45 (●), 60 (▼), 75 (
 ) or 95 (
) or 95 (
 ) min of incubation. Resupply was conducted with an equal volume of a solution comprised of 1.4 μM fII/3.4 μM AT/2 μM PCPS. Thrombin levels for the final 5 min of the Tf-initiated episode are also shown (◇). Thrombin levels are expressed as total picomoles of active thrombin to normalize for the volume change. (With permission from Orfeo et al. 2008, JBC 283:9776).
) min of incubation. Resupply was conducted with an equal volume of a solution comprised of 1.4 μM fII/3.4 μM AT/2 μM PCPS. Thrombin levels for the final 5 min of the Tf-initiated episode are also shown (◇). Thrombin levels are expressed as total picomoles of active thrombin to normalize for the volume change. (With permission from Orfeo et al. 2008, JBC 283:9776).
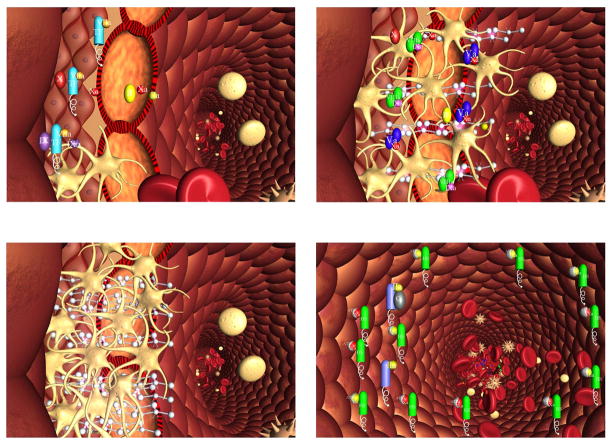
The overall interpretation of these resupply experimental systems is illustrated in Figure 7 in which the flow regulation component of the coagulation system is hypothesized. Upon disruption of the vasculature, platelets adhere to extravascular matrix proteins and the extrinsic fXase complex is formed after the presentation of plasma fVIIa to extravascular Tf (Figure 7a). Platelets and the procoagulant complexes and fibrin are accumulated in the breach (Figure 7b) with the reaction continuing as long as flow is maintained to the extravascular space. Once a plug has been completely formed (Figure 7c), blood and reactant supply is denied to the accumulated complexes on the extravascular side of the barrier plugging the wound causing the reaction to stop. The abundant anticoagulants present in the vasculature and in plasma will consume any residual procoagulants on the intravascular face of the plug during the normal hemostatic event, thus preventing occlusion of the vessel and loss of blood flow (Figure 7d).
Figure 7. Schema of a two compartment model of the regulation of TF-initiated blood coagulation.
A cross section of a blood vessel showing the luminal space, endothelial cell layer and extravascular region is presented at the site of a perforation. The blood coagulation process in response is depicted in four stages. Extrinsic fXase complex, TF•VIIa; prothrombinase complex, Xa•Va; intrinsic fXase, VIIIa•IXa; AT-endothelial cell heparan sulfate proteoglycan complex bound to thrombin or fXa, HS•AT•(IIa or Xa); protein C bound to thrombomodulin-thrombin, TM•IIa•PC.
Stage 1. Perforation results in delivery of blood, and with it circulating factor VIIa and platelets, to an extravascular space rich in membrane bound TF. Platelets adhere to collagen and von Willebrand factor associated with the extravascular tissue, and TF binds factor VIIa, initiating the process of factor IX and factor X activation. Factor Xa activates small amounts of prothrombin to thrombin that activates more platelets and converts factor V and factor VIII to factor Va and factor VIIIa.
Stage 2. The reaction is propagated by platelet-bound intrinsic factor Xase and prothrombinase with the former being the principle factor Xa generator. Initial clotting occurs and fibrin begins to fill in the void in cooperation with activated platelets.
Stage 3. A barrier composed of activated platelets ladened with procoagualant complexes and enmeshed in fibrin scaffolding is formed. The reaction in the now filled perforation is terminated by reagent consumption attenuating further thrombin generation but functional procoagulant enzyme complexes persist because they are protected from the dynamic inhibitory processes found on the intravascular face.
Stage 4. View downstream of the perforation. Enzymes escaping from the plugged perforation are captured by antithrombin-heparan complexes and the protein C system is activated by residual thrombin binding to endothelial cell thrombomodulin, initiating the dynamic anticoagulant system. These intravascular processes work against occlusion of the vessel despite the continuous resupply of reactants across the intravascular face of the thrombus. (With permission from Orfeo et al. 2005, JBC 280:42877).
Phenotypic Heterogeneity in the Blood Coagulation System
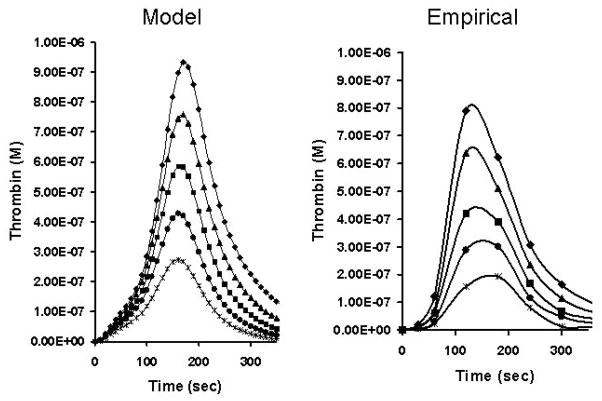
Laboratory tests for the defects in the coagulation system are largely confined to the contributions by various proteins to hemostasis while a limited ensemble of genetic defects is associated with thrombophillia.(25) However, in the majority of the healthy population the concentrations of pro- and anticoagulants vary significantly. This is illustrated in Table 1 which shows clinical coagulation laboratory values for the “normal” ranges of the healthy population. The basis for this heterogeneity is probably a consequence of genetic and environmental factors which influence the biosynthesis, utilization and pharmacokinetics of the coagulation proteins.(12;26;27) The molecules in blood which deal with coagulation are extremely complicated and require a significant variety of posttranslational and proteolytic processing steps in their overall lifetime. As a consequence of these multiple alterations, the dynamics of the coagulation proteome can be anticipated to be highly phenotypically variable. The consequences of this variety of “normal values” is illustrated in numerical and empirical model assessments of thrombin generation for the range of prothrombin concentrations from 50–150%(1;28) (Figure 8). Over this normal range of prothrombin concentrations one would observe a substantially different amount of thrombin produced for the same Tf initial stimulus. It should also be noted that the numerical model (left side of figure) is reasonably represented by the empirical proteome model on the right with far lower personnel and reagent costs in a much shorter time period.
Figure 8. Normal range of thrombin generation.
The concentration of active thrombin as a function of time in the presence of prothrombin at 150% (◆), 125% (▲), 100% (■), 75% (●) and 50% (
 ) of its mean physiological concentration. Represented are the empirical experiments of Butenas et al. (59) and a computational representation of the same experiment (With permission from Hockin et. al. 2002, JBC, 277: 18322).
) of its mean physiological concentration. Represented are the empirical experiments of Butenas et al. (59) and a computational representation of the same experiment (With permission from Hockin et. al. 2002, JBC, 277: 18322).
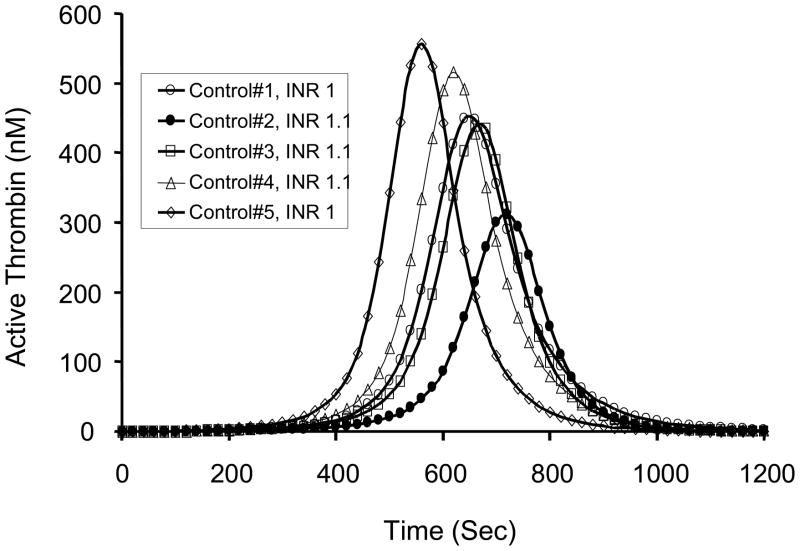
Heterogeneity is further illustrated in a numerical simulation of the compositional data for five healthy individuals (INR ~1) in terms of active thrombin anticipated to be generated following the challenge by the equivalent concentration of electronic Tf(29) (Figure 9). This hypothetical variety of thrombin generating responses in the healthy population has been verified by the examination of Tf-induced thrombin accumulation in blood samples from healthy male individuals over a six-month period. These data, illustrated in Figure 10, show that the phenotypic presentation of thrombin generation is fairly constant within any given individual, but highly variable between individuals, suggesting that the stimulus-coagulation coupling response in the healthy population may have utility in predicting risk following a prothrombotic challenge.
Figure 9. Thrombin generation and normal INR.
Numerical simulations representing active thrombin as a function of time based upon the composition of five normal individuals all with International Normalized Ratio (INR) of 1±0.1. (With permission from Mann et al. 2004, JTH 2:1727).
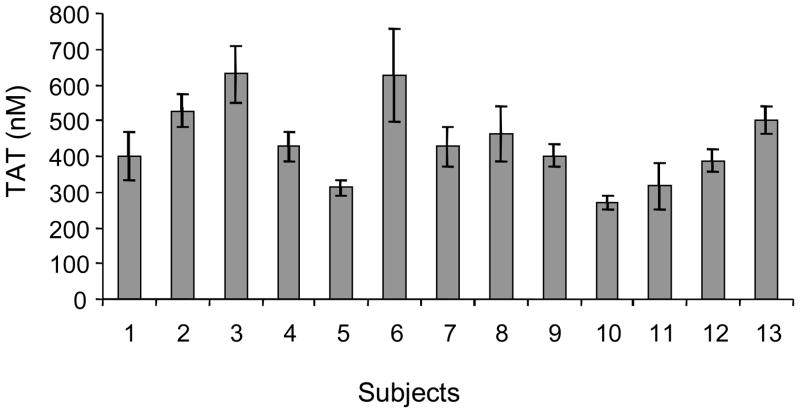
Figure 10. Thrombin generation in individuals.
Thrombin generation (measured as thrombin-antithrombin complex (TAT) formation) from contact pathway inhibited whole blood at a 20 minute time point (mean±SD, n=6) in thirteen individuals over a 6 month time frame. (With permission from Brummel-Ziedins et al. 2004, JTH 2:281).
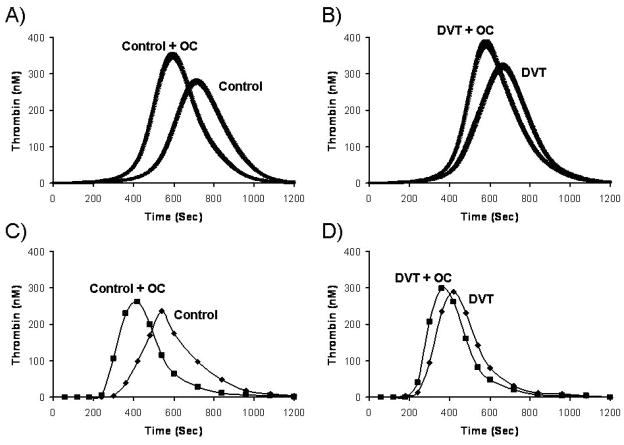
Potential predisposing effects of the compositions of the coagulation proteome and propensity for thrombotic disease has been evaluated. We performed a computational evaluation of the controls and cases from the Leiden Thrombophilia Study (LETS). For the control population (472 individuals), significant variations in hypothetical thrombin generation with a Tf insult were observed.(30) This variability was not explained by any one plasma factor. In a companion paper studying the thrombosis population (n=472) from LETS, the risk associations obtained gave odds ratios of 3.9 in men, 2.1 in women and 2.9 in women on oral contraceptives (OC).(31) As shown in Figure 11A and 11B, OC use created extreme shifts in thrombin generation in both the control OC populations and OC women with thrombosis suggesting an interaction of OC use in cases with underlying prothrombotic abnormality, consistent with the known risk of OC use. These observations also suggest that a multiplicity of events presage thrombosis of which blood chemistry is but one, as both cases and controls on OC showed similar enhanced thrombin generation in blood.
Figure 11. Oral contraceptive (OC) effect on theoretical and empirical thrombin generation.
Thrombin generation simulations are shown as the mean and the 95% confidence interval for (A) control women with (n=47) and without (n=90) OC use and (B) Women with deep venous thrombosis (DVT) with (n=30) and without (n=40) OC use. The mean factor levels of the selected populations shown in panel A and B were recapitulated in a synthetic plasma model (C and D) (With permission from Brummel-Ziedins et al. 2005, JTH 3:2497).
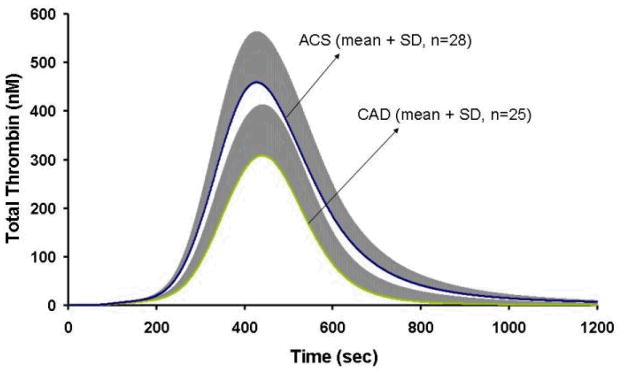
We have also shown that Tf-initiated computational blood coagulation was able to distinguish between acute and stable coronary artery disease by evaluating thrombin generation(32) (Figure 12).
Figure 12. Thrombin generation in acute coronary syndrome.
Computationally derived total thrombin generation is shown as the mean for the acute coronary syndrome (ACS, n=28) and coronary artery disease (CAD, n=25) population. The positive standard deviation is shown in grey.(With permission from Brummel-Ziedins et al. 2008, JTH, 6:104).
Anticoagulant Targeting
The search for new anticoagulants has been a frustrating and costly adventure for many pharmaceutical companies. An inspection of Figure 5 illustrates resupply in an already activated coagulation system. The data in this figure suggest that the choice and intensity of an anticoagulant might be different when one is using the drug in a prophylactic sense i.e. before the initiating thrombotic event compared to the treatment of an ongoing thrombotic complication with thrombin generation already occurring within the vasculature.(24) For the purposes of discussion, we have defined the latter case as a therapeutic intervention.
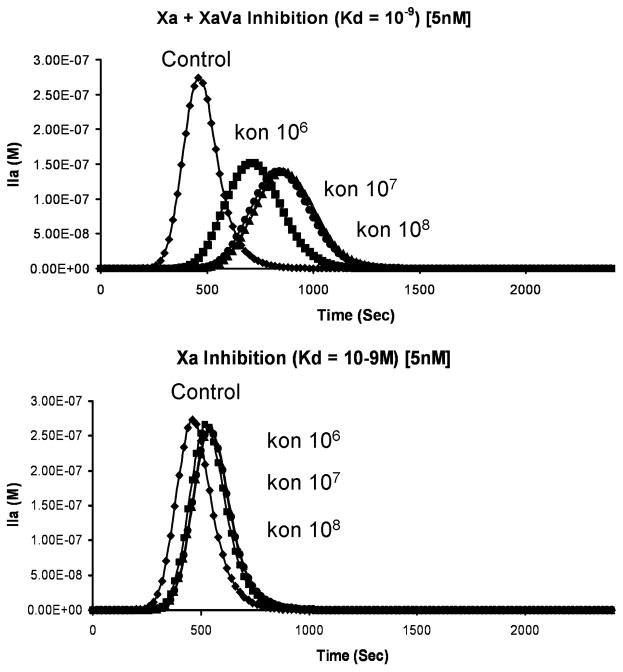
Figure 13 illustrates the circumstance(33) in which a family of inhibitors all with hypothetical Kds of 5 nM, but characterized by different on rates, targets either free fXa or free fXa in complex with fVa. Because of the large excess of fVa in the reaction system, virtually all fXa is in complex with fVa (see Figure 4). As a consequence, an inhibitor selective only for fXa would not be very effective regardless of the on rate.
Figure 13. The influence of factor Xa inhibitor on thrombin generation.
When both free fXa and fXa in prothrombinase complex is inhibited (upper panel) or only free fXa is inhibited (lower panel). (With permission from Mann et al. 2006, Blood Cells Mol. Dis. 36:108).
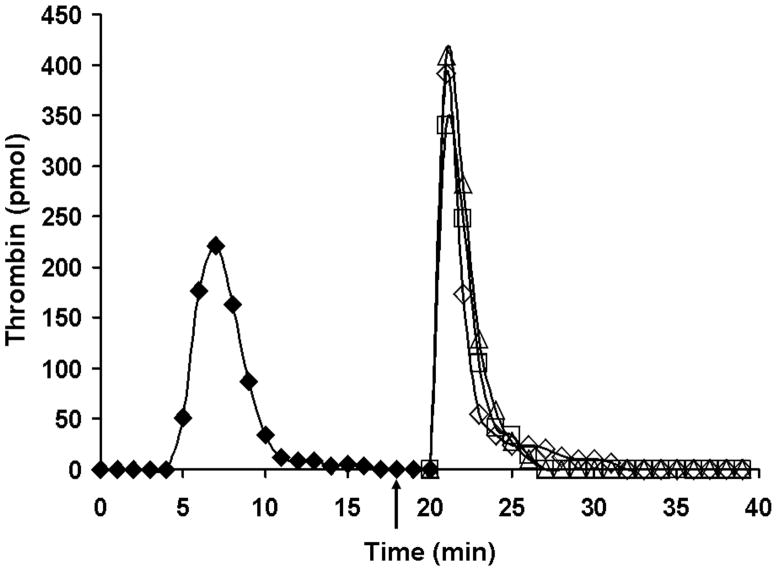
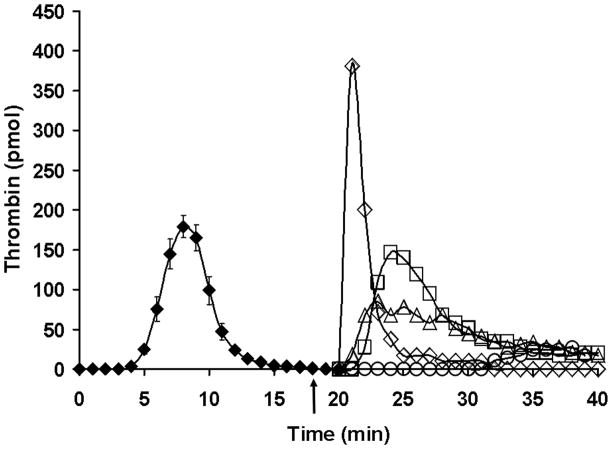
For a therapeutic intervention, the enzyme complex is already formed(24) and able to generate more product as more blood is supplied. Figure 14 illustrates targeting fIXa with an inhibitory antibody in a therapeutic intervention during resupply. These data are in contrast to antibody inhibition of fXa (Figure 15). Even better inhibition is obtained with a small molecule prothrombinase and fXa inhibitor.
Figure 14. Resupply of the synthetic coagulation proteome: the effect of an inhibitory α-fIX antibody.
Time courses of thrombin formation are presented for synthetic coagulation proteome mixtures which have been initiated with 5 pM Tf reagent and then resupplied after 20 min with an equal volume of synthetic coagulation proteome/2 μM PCPS without Tf. Shown are: normal resupply control (◇); resupply after 0.1 mg/mL (□) or 0.2 mg/mL (△) α-fIX-91 was added 2 min prior to resupply (see arrow). Thrombin levels at each time are expressed as total picomoles of active thrombin to normalize for the volume change. (With permission from Orfeo et al. 2008, JBC 283: 9776).
Figure 15. Resupply of the synthetic coagulation proteome: the effect of inhibitors targeting fXa and fXa in the prothrombinase complex.
Time courses of thrombin generation are presented for reactions initiated with 5 pM Tf reagent and then resupplied at 20 min with an equal volume of synthetic coagulation proteome/2 μM PCPS without Tf: control resupply (◇); 320 nM C921-78 included in the synthetic coagulation proteome/2 μM PCPS resupply mixture (○); 0.1mg/mL α-bfX-2 added 2 min prior to resupply with synthetic coagulation proteome/2 μM PCPS (□); and 0.1mg/mL α-bfX-2 added 2 min prior to resupply with 1.4 μM fII/3.4 μM AT/2 μM PCPS (△). The arrow indicates the time of α-bfX-2 addition. Thrombin generation over the initial 20 min is presented as the mean ± SD of 4 determinations. Thrombin levels at each time are expressed as total moles of active thrombin to normalize for the volume change. (With permission from Orfeo et al. 2008, JBC 283: 9776).
Summary
All in vitro blood tests of the coagulation system are severely limited because of the absence of contributions of the vasculature and surrounding tissue to hemostasis and thrombosis. In human in vivo tests incur inherent pathophysiologic risk although they employ all of the physiology impacting on the coagulation system and mechanistic interpretations are frequently only superficial. The partnership of carefully constructed numerical, in vitro and in vivo evaluations of the blood coagulation physiology and pathophysiology provides a useful marriage of technologies which assist in decision making in altering the blood coagulation system.
Acknowledgments
The study was supported by grants P01 HL46703 and R01 HL34575 from the National Institutes of Health.
Reference List
- 1.Hockin MF, Jones KC, Everse SJ, Mann KG. A model for the stoichiometric regulation of blood coagulation. J Biol Chem. 2002;277(21):18322–18333. doi: 10.1074/jbc.M201173200. [DOI] [PubMed] [Google Scholar]
- 2.Lawson JH, Kalafatis M, Stram S, Mann KG. A model for the tissue factor pathway to thrombin. I. An empirical study. J Biol Chem. 1994;269(37):23357–23366. [PubMed] [Google Scholar]
- 3.van ‘t Veer C, Mann KG. Regulation of tissue factor initiated thrombin generation by the stoichiometric inhibitors tissue factor pathway inhibitor, antithrombin- III, and heparin cofactor-II. J Biol Chem. 1997;272(7):4367–4377. doi: 10.1074/jbc.272.7.4367. [DOI] [PubMed] [Google Scholar]
- 4.Butenas S, van ‘t Veer C, Mann KG. Evaluation of the initiation phase of blood coagulation using ultrasensitive assays for serine proteases. J Biol Chem. 1997;272(34):21527–21533. doi: 10.1074/jbc.272.34.21527. [DOI] [PubMed] [Google Scholar]
- 5.Jones KC, Mann KG. A model for the tissue factor pathway to thrombin. II. A mathematical simulation. J Biol Chem. 1994;269(37):23367–23373. [PubMed] [Google Scholar]
- 6.Rand MD, Lock JB, van ‘t Veer C, Gaffney DP, Mann KG. Blood clotting in minimally altered whole blood. Blood. 1996;88(9):3432–3445. [PubMed] [Google Scholar]
- 7.Brummel KE, Paradis SG, Butenas S, Mann KG. Thrombin functions during tissue factor-induced blood coagulation. Blood. 2002;100(1):148–152. doi: 10.1182/blood.v100.1.148. [DOI] [PubMed] [Google Scholar]
- 8.Cawthern KM, van ‘t Veer C, Lock JB, DiLorenzo ME, Branda RF, Mann KG. Blood coagulation in hemophilia A and hemophilia C. Blood. 1998;91(12):4581–4592. [PubMed] [Google Scholar]
- 9.Butenas S, Cawthern KM, van’t Veer C, DiLorenzo ME, Lock JB, Mann KG. Antiplatelet agents in tissue factor-induced blood coagulation. Blood. 2001;97(8):2314–2322. doi: 10.1182/blood.v97.8.2314. [DOI] [PubMed] [Google Scholar]
- 10.Brummel-Ziedins K, Rivard GE, Pouliot RL, Butenas S, Gissel M, Parhami-Seren B, et al. Factor VIIa replacement therapy in factor VII deficiency. J Thromb Haemost. 2004;2(10):1735–1744. doi: 10.1111/j.1538-7836.2004.00922.x. [DOI] [PubMed] [Google Scholar]
- 11.Undas A, Brummel KE, Musial J, Mann KG, Szczeklik A. Simvastatin depresses blood clotting by inhibiting activation of prothrombin, factor V, and factor XIII and by enhancing factor Va inactivation. Circulation. 2001;103(18):2248–2253. doi: 10.1161/01.cir.103.18.2248. [DOI] [PubMed] [Google Scholar]
- 12.Undas A, Brummel K, Musial J, Mann KG, Szczeklik A. Blood coagulation at the site of microvascular injury: effects of low- dose aspirin. Blood. 2001;98(8):2423–2431. doi: 10.1182/blood.v98.8.2423. [DOI] [PubMed] [Google Scholar]
- 13.Undas A, Brzezinska-Kolarz B, Brummel-Ziedins K, Musial J, Szczeklik A, Mann KG. Factor XIII Val34Leu polymorphism and gamma-chain cross-linking at the site of microvascular injury in healthy and coumadin-treated subjects. J Thromb Haemost. 2005;3(9):2015–2021. doi: 10.1111/j.1538-7836.2005.01509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Undas A, Szuldrzynski K, Brummel-Ziedins KE, Tracz W, Zmudka K, Mann KG. Systemic blood coagulation activation in acute coronary syndromes. Blood. 2008 doi: 10.1182/blood-2008-07-167411. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mann KG, Nesheim ME, Church WR, Haley P, Krishnaswamy S. Surface-dependent reactions of the vitamin K-dependent enzyme complexes. Blood. 1990;76(1):1–16. [PubMed] [Google Scholar]
- 16.Jesty J, Silverberg SA. Kinetics of the tissue factor-dependent activation of coagulation Factors IX and X in a bovine plasma system. J Biol Chem. 1979;254(24):12337–12345. [PubMed] [Google Scholar]
- 17.Osterud B, Rapaport SI. Activation of factor IX by the reaction product of tissue factor and factor VII: additional pathway for initiating blood coagulation. Proc Natl Acad Sci U S A. 1977;74(12):5260–5264. doi: 10.1073/pnas.74.12.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu G, Broze GJ, Jr, Krishnaswamy S. Formation of factors IXa and Xa by the extrinsic pathway: differential regulation by tissue factor pathway inhibitor and antithrombin III. J Biol Chem. 2004;279(17):17241–17249. doi: 10.1074/jbc.M312827200. [DOI] [PubMed] [Google Scholar]
- 19.Orfeo T, Brufatto N, Nesheim ME, Xu H, Butenas S, Mann KG. The factor V activation paradox. J Biol Chem. 2004;279(19):19580–19591. doi: 10.1074/jbc.M400727200. [DOI] [PubMed] [Google Scholar]
- 20.Nesheim ME, Taswell JB, Mann KG. The contribution of bovine Factor V and Factor Va to the activity of prothrombinase. J Biol Chem. 1979;254(21):10952–10962. [PubMed] [Google Scholar]
- 21.van Dieijen G, Tans G, Rosing J, Hemker HC. The role of phospholipid and factor VIIIa in the activation of bovine factor X. J Biol Chem. 1981;256(7):3433–3442. [PubMed] [Google Scholar]
- 22.Komiyama Y, Pedersen AH, Kisiel W. Proteolytic activation of human factors IX and X by recombinant human factor VIIa: effects of calcium, phospholipids, and tissue factor. Biochemistry. 1990;29(40):9418–9425. doi: 10.1021/bi00492a016. [DOI] [PubMed] [Google Scholar]
- 23.van ‘t Veer C, Golden NJ, Mann KG. Inhibition of thrombin generation by the zymogen factor VII: implications for the treatment of hemophilia A by factor VIIa. Blood. 2000;95(4):1330–1335. [PubMed] [Google Scholar]
- 24.Orfeo T, Brummel-Ziedins KE, Gissel M, Butenas S, Mann KG. The nature of the stable blood clot procoagulant activities. J Biol Chem. 2008;283(15):9776–9786. doi: 10.1074/jbc.M707435200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brummel-Ziedins K, Orfeo T, Jenny NS, Everse SJ, Mann KG. Blood coagulation and fibrinolysis. In: Greer JP, Foerster J, Lukens J, Rodgers GM, Paraskevas F, Glader B, editors. Wintrobe’s Clinical Hematology. Philidelphia: Lippencott Williams & Wilkins; 2003. pp. 677–774. [Google Scholar]
- 26.Undas A, Sydor WJ, Brummel K, Musial J, Mann KG, Szczeklik A. Aspirin alters the cardioprotective effects of the factor XIII Val34Leu polymorphism. Circulation. 2003;107(1):17–20. doi: 10.1161/01.cir.0000047062.03282.a3. [DOI] [PubMed] [Google Scholar]
- 27.Undas A, Brummel K, Musial J, Mann KG, Szczeklik A. Pl(A2) polymorphism of beta(3) integrins is associated with enhanced thrombin generation and impaired antithrombotic action of aspirin at the site of microvascular injury. Circulation. 2001;104(22):2666–2672. doi: 10.1161/hc4701.099787. [DOI] [PubMed] [Google Scholar]
- 28.Butenas S, van’t Veer C, Mann KG. “Normal” thrombin generation. Blood. 1999;94(7):2169–2178. [PubMed] [Google Scholar]
- 29.Mann KG, Brummel-Ziedins K, Undas A, Butenas S. Does the genotype predict the phenotype? Evaluations of the hemostatic proteome. J Thromb Haemost. 2004;2(10):1727–1734. doi: 10.1111/j.1538-7836.2004.00958.x. [DOI] [PubMed] [Google Scholar]
- 30.Brummel-Ziedins K, Vossen CY, Rosendaal FR, Umezaki K, Mann KG. The plasma hemostatic proteome: thrombin generation in healthy individuals. J Thromb Haemost. 2005;3(7):1472–1481. doi: 10.1111/j.1538-7836.2005.01249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brummel-Ziedins KE, Vossen CY, Butenas S, Mann KG, Rosendaal FR. Thrombin generation profiles in deep venous thrombosis. Journal of Thrombosis and Haemostasis. 2005;3(11):2497–2505. doi: 10.1111/j.1538-7836.2005.01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brummel-Ziedins K, Undas A, Orfeo T, Gissel M, Butenas S, Zmudka K, et al. Thrombin generation in acute coronary syndrome and stable coronary artery disease: dependence on plasma factor composition. J Thromb Haemost. 2008;6(1):104–110. doi: 10.1111/j.1538-7836.2007.02799.x. [DOI] [PubMed] [Google Scholar]
- 33.Mann KG, Brummel-Ziedins K, Orfeo T, Butenas S. Models of blood coagulation. Blood Cells Mol Dis. 2006;36:108–117. doi: 10.1016/j.bcmd.2005.12.034. [DOI] [PubMed] [Google Scholar]