Abstract
Objective
To compare profiles of a prescreening and screening cohort of women with cervical cancer regarding histopathology and clinical variables in order to identify those remaining at risk despite successful screening programs. By analyzing these profiles we hope to improve future screening methods.
Methods
The prescreening and screening cohorts consisted of 5,046 and 1,174 women, respectively, treated for cervical cancer at the Department of Gynecological Oncology at Radiumhemmet, Karolinska University Hospital, during the periods 1944-1957 and 1990-2004.
Results
Mean age increased from 48.9 years to 55.3 years in the cohorts treated 1944-1957 and 1990-2004, respectively. The percentage of patients older than 69 years was 5.4% and 27.3% in the prescreening and screening period, respectively. A shift towards earlier stages at diagnosis, a reduction of squamous cervical cancer and an increase of adenocarcinoma were observed in the screening cohort. The percentage of adenocarcinoma was about 6 times higher among younger patients. Cases of stump cancer and cervical cancer associated with pregnancy have declined. Eighty-seven women in the screening cohort had a history of treatment for in situ carcinoma by conization; 28% of these cases developed cervical cancer within one year after conization.
Conclusion
The profile changed in the screening era indicating a need to refine screening for improved detection of in older women. This study, one of the largest clinical series of cervical cancer, provides an important baseline with which later studies can be compared to evaluate the effects of human papillomavirus vaccine and other important changes in this field.
Keywords: Cervical cancer, Screening, Age, Stage, Histology
INTRODUCTION
Despite promising reports from British Columbia [1] and Louisville, Kentucky [2] about the benefits of early pioneering screening programs for detection and treatment of precancerous stages of cervical cancer (CC), most critics did not accept the results and asked for randomized trials. However, for various reasons, including ethical, such trials were not feasible in large populations. Faced with this dilemma, most Nordic countries decided to introduce population-based screening programs. Early trials in a few counties in Sweden gave promising results [3-5]. Organized screening programs for detection of precancerous stages of CC using the Papanicolaou (Pap) smear were already underway in some Swedish counties by the late 1950s and early 1960s. In 1967 the Swedish Board of Health and Welfare recommended that all counties implement mass Pap screening of women aged 30-49 years and to repeat the examination every 4 years. The program rapidly gained acceptance and by 1973 the entire female population in the recommended age groups (except women in the city of Gothenburg) were offered testing. Many counties also offered screening to other age groups. Prior to 1974 the participation rate varied considerably in different geographic areas of Sweden from approximately 50% up to 90%, with an average of about 70%. Subsequently the program expanded to cover women aged 23 to 49 every 3 years and women aged 50-59 every 5 years. In addition, numerous opportunistic tests were often performed on the same age groups [4,6,7]. The incidence and mortality rates of CC in countries with well organized screening programs have decreased significantly since the introduction of screening. In Sweden the incidence of CC has declined from 20 per 100,000 women (world standard rate) in 1965 and seems to be leveling out at a rate of approximately 7 per 100,000 since the late 1990s [8]. The goal with well organized screening programs including treatment of detected precancerous lesions is that invasive cancer should be preventable. Although we still see significant numbers of CC, age, stage and histopathology profiles have changed. Consequently, and as a basis for refining screening, we need to carefully consider the new profile of these residual cases. The current study aims to compare the profile of histopathology and clinical variables of a prescreening cohort of CC treated at Radiumhemmet between 1944 and 1957 with a cohort treated at the same clinic during the screening era (1990-2004).
MATERIALS AND METHODS
Early twentieth century treatment of CC in Sweden was centralized to Radiumhemmet in Stockholm. Initially, patients were referred from all over Sweden, but gradually local treatment centers opened in other parts of Sweden. In more recent times, patients referred to Radiumhemmet come from hospitals in the Stockholm region, with its population of 1.8 million. The Stockholm method of treating CC became one of the world's leading methods. In order to obtain reliable data of treatment results, a group of pioneering doctors, including Prof. Heyman (1917-1947) and Prof. Kottmeier (1948-1971), established an early database of patients with CC that included information on tumor stage, histopathology, treatment and outcome. All cases were staged according to the International Federation of Gynecology and Obstetrics [FIGO] rules for clinical staging of CC [9] and confirmed histologically by biopsy. Follow-up continued until the end of life and the database is complete, with the exception of a small inevitable loss to follow-up due to emigration. Most patients were followed-up through office visits with annual examinations at Radiumhemmet. In some cases follow-up was maintained through correspondence with patients and their local doctors. Death certificates were obtained from local parishes and more recently, from the Swedish "cause of death" register. In all, for the 90-year period from 1914 to 2004 the register comprised 18,400 records. The prescreening cohort includes all 5,046 women treated for CC at the Department of Gynecological Oncology at Radiumhemmet, Karolinska University Hospital, Solna from 1944 to 1957. The 1,174 women treated from 1990 to 2004 at the same clinic comprise the screening period cohort. The two cohorts were chosen to cover a similar length of time. The time period of the screening cohort was selected 30 years after the introduction of screening activities, when the decline of incidence of CC had levelled out at a quite stable level, in order to detect any changes in the profile of the remaining CC cases. The time period of the pre-screening cohort was chosen to cover the years before the start of the screening programme. The variables under study are age, stage and histopathology, as well as occurrence of stump cancer and cancer associated with pregnancy (defined as CC diagnosed during pregnancy or within 6 months of delivery). In the screening cohort, the number of cases previously treated with conization for in situ carcinoma will also be recorded. In Sweden the Pap smear technique has been used for cytological sampling in the screening programme, liquid-based cytology and human papillomavirus (HPV) test have not been applied during the time period of this study.
1. Statistical analysis
The standard error (SE) of difference between percentages was calculated as: SE p1-p2=√p1 (100-p1)/n1+p2(100-p2)/n2
To test the difference between two percentages, the chi square test with one degree of freedom was used. Yates correction was applied. The SE of the difference between means was calculated by t-test.
RESULTS
About 400 women were treated annually during the 1944-1957 period (5,046 cases). Mean age was 48.9 years with a range of 16-91 years (SE, 0.17) and 271 women (5.4%) were older than 69 years.
The annual number of cases was less than 100 in the cohort treated during the 1990-2004 period (1,174 cases). Mean age was 55.3 years with a range of 23-95 years (SE, 0.50) and 320 women (27.3%) were older than 69 years. The difference between the mean age for women treated in the two periods was 6.4 years, which is statistically highly significant (p<0.01).
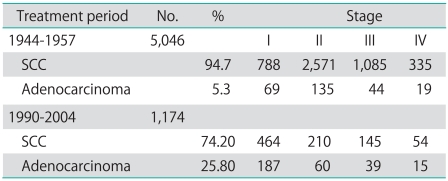
Women from the screening period are diagnosed at an earlier stage: 55% were diagnosed as stage I, compared with 17% during the prescreening period. Similarly, the proportion of advanced cases, stages III and IV, dropped from 29.3% to 21.2%. The same trend is observed for both squamous cervical carcinoma (SCC) (SE of diff 1.77 p<0.001) and adenocarcinoma (AC) (SE of diff 3.60 p<0.001) in the screening period (Table 1).
Table 1.
Stage distribution by histopathology and treatment period
SCC, squamous cervical carcinoma.
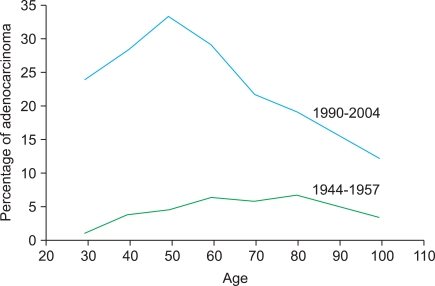
Table 1 shows the decrease in the proportion of SCC and the corresponding increase in the proportion of AC in the screening period compared with the prescreening period cohort. During the prescreening period AC accounted for 5.3% of cases on average. Fig. 1 shows that the percentage of AC of the uterine cervix varied between 2% and 7% across the prescreening cohort. During the screening period the AC cases increased significantly and accounted for 25.7% of cases on average (p<0.001), with a marked difference observed between younger and older women. In the screening cohort, the percentage of AC among younger women is about 6 times higher and in women ≥70 years about 3 times higher than in the prescreening cohort. Stump cancer cases were associated with a history of hysterectomy for benign diagnosis. The percentage dropped from 2.1% to 1.1% between study periods.
Fig. 1.
Percentage of adenocarcinoma of the uterine cervix by age and by treatment period.
Of the 1,174 women treated between 1990 and 2004, 87 had a history of prior treatment for in situ carcinoma by conization. In twenty-four cases (28%) conization had been performed in the same year that the invasive carcinoma was diagnosed and served as a basis for diagnosis. Of the 63 remaining cases, mean age 53.7 years (range, 30 to 86 years), the number of years between conization and diagnosis of CC were as follows: 1-2 years for 10 women, 3-4 years for 8 women, 5-10 years for 19 women, 11-20 years for 11 women and 21-36 years for 15 women.
Relative incidence of cervical cancer associated with pregnancy decreased between study cohorts from 1.4% (1944-1957) to 0.9% (1990-2004). The same trend is evident in the subgroup of women of childbearing age (<50 years).
DISCUSSION
The present study is based on one of the largest single institutional material of consecutive CC cases in the literature. Our results clearly show that cases treated during the prescreening period and during the screening period have different profiles regarding incidence and the distribution by age, stage and histopathology.
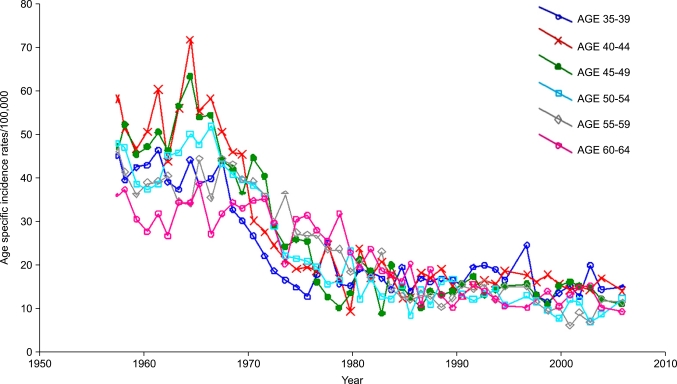
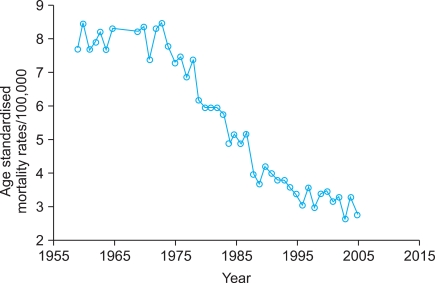
After years of doubts and criticism, it was finally shown that the Swedish screening program works. Today very few people in the field, if any, will deny its success and the results is comparable with that reported from other institutions worldwide involved in well organized screening [3,10]. Nevertheless, a considerable number of new cases of CC still occur. If we adopt the hypothetical assumption that every case should be preventable, it follows that each new case constitutes a failure. The annual number of cases treated at Radiumhemmet decreased from 400 in the 1950s to less than 100 for cases included in the screening period. The National Swedish Cancer Registry shows a drop in the age-specific rate for the 35-39 year age group from 46.0 per 100,000 women in 1958 to 15.5 in 2004 ??a 66% reduction (Fig. 2). In the 70-74 year age group the rate dropped from 27.0 to 11.4 ??a 42% reduction. The Swedish cause of death register shows an age standardized mortality rate of 7.7 per 100,000 women for CC in 1959 and 2.8 in 2004 (Fig. 3).
Fig. 2.
Carcinoma of the uterine cervix. Age specific incidence rates/100,000 in Sweden in age groups 35-39 to 60-64. Data from the National Swedish Cancer Registry.
Fig. 3.
Age standardized mortality rates for carcinoma of the uterine cervix in Sweden per 100,000 women. The Swedish Cause of Death Register.
Our study demonstrates a shift towards earlier stages of CC diagnosed during the screening period compared with the prescreening period. The same trend is observed for both SCC and AC in the screening period, contradicting a recent report from the Netherlands [11].
The change in incidence and stage distribution in the two cohorts is also connected with change in social conditions, availability to health care and in exposure to risk factors for CC. Increased knowledge in the etiology to CC (that CC mainly is a sexually transmitted disease caused by high risk HPV) have led to increased information how to prevent spread of gynaecological infections and thereby contributed to changed sexual behaviors and a decreased incidence of CC. In addition reduced tobacco use from the 1990s [12] has probably affected the incidence as it is known as a cofactor for cervical carcinogenesis.
We observed a change in the distribution of histopathology, the proportion of AC found in the screening cohort increased on average about fivefold, especially in the younger age groups, which is in accordance with data in other reports [10]. The reasons to the increase in AC have been discussed in the literature. Studies have shown that SCC and AC of the cervix seem to share most risk factors, with the exception of smoking (for which risk is elevated for SCC but not AC) [13-15]. Like SCC HPV appears to be the key risk factor for AC. However, it may be concluded that our understanding of the epidemiology and natural history of CC is still incomplete. The overwhelming majority of the literature addressing this subject to date has dealt with SCC. A study of age groups and birth cohorts, based on the Swedish National Cancer Registry for 1958-1980, of women born between 1879 and 1959 (including 17,100 cases of CC) showed an increase in incidence of AC in the 25-29 year age group from 0.7 per 100,000 for women born from 1935 to 1939, to 1.2 for women born from 1950 to 1954. Similarly, for the 30-34 year age group an increase was seen from 1.0 for women born between 1930 and 1934 to 1.9 for women born between 1945 and 1949 [7,16]. In a subsequent study, based on the same material but extended to 1992, a similar increase in incidence of AC was seen during the last decade as well [17]. This increase in AC in the later birth cohorts is probably related to greater exposure to HPV after the sexual revolution in the 1960s [18].
In the present study we also noted a change in age distribution between the two cohorts. In the prescreening cohort, 271 women (5.4%) were aged ≥70, which corresponds well to the percentage of the Swedish population at risk at that time (7.8%). In the screening cohort, both mean age (p< 0.001) and percentage of patients in older age groups are higher compared with the Swedish population at risk at that time. In all, 320 women (27.3%) were aged ≥70, which far exceeds the expected number (18.2%), considering the increase in population at risk within that age group in Sweden. With the implementation of screening and patient compliance with the proposed screening intervals, it was presumed that women would be protected for their entire lives. Our finding of an increased proportion of older women (≥70) among the women treated today may indicate that there is a need to refine the screening program to include older women. Furthermore, we find the proportion of AC among older women treated today to be lower than among younger women, while the proportion of AC was more evenly distributed among different age groups during the prescreening period. Under the tentative hypothesis that the higher percentage of ACs after introduction of screening may serve as an indicator of screening efficacy, it may be surmised that current screening programs do not provide the protection we had assumed for the older age groups. In other words, ACs in older age groups have been diluted by undetected cases of SCC. We must also take into consideration that postmenopausal women have undergone hormonally-related physiological changes including retraction of the transformation zone, which results in fewer desquamated cells on the Pap slide and makes it more difficult to obtain an adequate sample. Therefore we must ask if today's sampling methods are suitable for the postmenopausal population. In addition the cytological screening with Pap smear seem to be less effective in detecting precursor lesions of AC [18-20], than of SCC.
The Pap smear technique was described already in 1928 by both Babes [21] about the potential for using desquamated cellular material from the uterine cervix to serve as a diagnostic tool for detection of CC. Accuracy of the Pap smear technique for early detection of CC and precursor lesions has been evaluated through various means with differing results. False negative rates ranging from 5% to 40% have been reported [22]. Since all precursor lesions of ACs and nearly all invasive ACs seem to be positive for high risk HPV, the incorporation of high risk HPV testing in cervical cancer screening programmes is likely to decrease markedly the incidence of cervical AC [23]. Furthermore, a combination of HPV testing and cytological evaluation following conization has shown to significantly improve reliability of follow-up surveillance [24].
In 2006, over 60% of patients with cervical squamous carcinoma in Sweden occurred in postmenopausal women, aged 50 years or older [8]. Efficacy of cytological screening is known to be lower in higher age groups, and is only effective in 20% of women aged 50 years or older, compared with women aged 30??5 years old [25]. It was shown that the high risk HPV test was three times as sensitive as the Pap test in detecting grade 2 and 3 CIN lesions in women aged 50??5 years [26]. However in very old women the diagnostic difficulties may remain since the occurrence of HPV is not well-known and may be less common [10].
We must also realize that, even with good screening tools, some women will refuse to participate in screening and will not be covered by the screening programs, which has been suggested as the major reason for cervical cancer morbidity [27]. According to a recent report the coverage of the CC screening programs in the European Union member states was below 80% (range, 10 to 79%) [28]. In Sweden the coverage was reported to be 73% [28]. The category of women who avoid participation, despite being called to screening, often includes women of lower socioeconomic status, as well as women of higher socioeconomic status who do not consider themselves to be at risk [29-31]. Studies have shown that lack of knowledge and information about the benefits and role of screening are important factors for non-compliance [32,33]. Furthermore, we often see immigrant groups for whom screening was not available in their country of origin. More than 15% of newly diagnosed CC cases, treated at Radiumhemmet in 1987-88, were immigrant women, especially from non-Nordic countries [16]. Women in the "false negative" screening results category are sometimes incorrectly classified as having tumors that arise and grow rapidly. More thorough investigation may reveal that women with these rapidly growing tumors have a history of inadequately treated precancerous stages. Such treatment may have involved cold-knife, laser, or diathermy conization. In this study we noted cases of invasive cancer appearing 1-36 years after conization for previous in situ carcinoma. Consequently, these women, who were treated for precancerous stages, still comprise a high-risk group and should be carefully monitored. In a record linkage study using data from the National Swedish Cancer Registry, we found that among 56,116 women treated for CC in situ and followed-up by record-linkage with the invasive cancer register, an increasing number fell victim to invasive carcinoma over time. The relative risk for this group of women was found to be twice that of women in the general population after 20 years. In women ≥50 years when treated for in situ lesions, relative risk was 6 times higher compared with women at large [34]. Obviously, once women have been diagnosed with carcinoma in situ, they belong to a high-risk category for developing CC and should be carefully monitored.
We also found in the present study that stump cancer was being diagnosed long after subtotal hysterectomies for benign conditions. This subject was covered in an earlier study from our institution [35]. These women should be covered in the routine screening programs, and should be reminded to continue screening after the surgery for subtotal hysterectomy. Furthermore, we saw cases in conjunction with pregnancy and our results have recently been published [36]. Pregnant women should also be reminded to do routine cytological test according to the screening program.
In conclusion, the screening activities have markedly affected the incidence and we found an increase in ACs and CC in older women. Efficient use of screening resources necessitates continuous monitoring of incidence and mortality trends within different population strata. Quite obviously, conventional Pap smear technique, has been effective in significantly reducing incidence and mortality rates for CC. Nevertheless, the challenge remains to increase coverage to screening, provide protection for older women and to find a working technique that can be used for large-scale population screening, capable of identifying the remaining cases that currently still progress to invasive carcinomas. The next tier in the preventive work is to develop safe methods for the treatment of detected precancerous lesions. It is important to realize that to avoid losing what we have hitherto gained through screening, we must continue the battle against CC. This retrospective study, to our knowledge one of the largest in the literature, provides an important baseline for future improvements of the screening methods.
ACKNOWLEDGMENTS
This study was supported by the Swedish Cancer Foundation, KI, Cancer Strategic Grants, Swedish Research Council, Cancer Society, Radiumhemmet in Stockholm.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Boyes DA, Knowelden J, Phillips AJ. The evaluation of cancer control measures. Br J Cancer. 1973;28:105–107. doi: 10.1038/bjc.1973.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christopherson WM, Parker JE, Mendez WM, Lundin FE., Jr Cervix cancer death rates and mass cytologic screening. Cancer. 1970;26:808–811. doi: 10.1002/1097-0142(197010)26:4<808::aid-cncr2820260411>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 3.Dillner J. Cervical cancer screening in Sweden. Eur J Cancer. 2000;36:2255–2259. doi: 10.1016/s0959-8049(00)00320-8. [DOI] [PubMed] [Google Scholar]
- 4.Pettersson F, Bjorkholm E, Naslund I. Evaluation of screening for cervical cancer in Sweden: trends in incidence and mortality 1958-1980. Int J Epidemiol. 1985;14:521–527. doi: 10.1093/ije/14.4.521. [DOI] [PubMed] [Google Scholar]
- 5.Pettersson F, Naslund I, Malker B. Evaluation of the effect of Papanicolaou screening in Sweden: record linkage between a central screening registry and the National Cancer Registry. IARC Sci Publ. 1986;(76):91–105. [PubMed] [Google Scholar]
- 6.The National Board of Health and Welfare. Statistics Sweden. Stockholm: The National Board of Health and Welfare; 1976. [Google Scholar]
- 7.Pettersson F. Secondary prevention: screening for carcinoma of the uterine cervix. In: Kavanagh JJ, Singletary SE, Einhorn N, DePetrillo AD, editors. Cancer in women. Cambridge, MA: Blackwell Science; 1998. pp. 240–250. [Google Scholar]
- 8.The National Board of Health and Welfare. Cancer Incidence in Sweden 2008. Stockholm: The National Board of Health and Welfare; 2009. [Google Scholar]
- 9.Pettersson F. Annual report on the results of treatment in gynecologic cancer FIGO 1994. Stockholm: Radiumhemmet; 1995. [Google Scholar]
- 10.World Health Organization. Use of screening for cervical cancer: cervical cancer screening. Lyon: IARC Press; 2005. [Google Scholar]
- 11.Bulk S, Visser O, Rozendaal L, Verheijen RH, Meijer CJ. Cervical cancer in the Netherlands 1989-1998: decrease of squamous cell carcinoma in older women, increase of adenocarcinoma in younger women. Int J Cancer. 2005;113:1005–1009. doi: 10.1002/ijc.20678. [DOI] [PubMed] [Google Scholar]
- 12.The National Board of Health and Welfare. Stockholm: Socialstyrelsen; [cited 2011 May 20]. Socialstyrelsen: Statistics of Sweden [Internet] Available from: http://www.sos.se. [Google Scholar]
- 13.International Collaboration of Epidemiological Studies of Cervical Cancer. Appleby P, Beral V, Berrington de Gonzalez A, Colin D, Franceschi S, et al. Carcinoma of the cervix and tobacco smoking: collaborative reanalysis of individual data on 13,541 women with carcinoma of the cervix and 23,017 women without carcinoma of the cervix from 23 epidemiological studies. Int J Cancer. 2006;118:1481–1495. doi: 10.1002/ijc.21493. [DOI] [PubMed] [Google Scholar]
- 14.Berrington de Gonzalez A, Sweetland S, Green J. Comparison of risk factors for squamous cell and adenocarcinomas of the cervix: a meta-analysis. Br J Cancer. 2004;90:1787–1791. doi: 10.1038/sj.bjc.6601764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plummer M, Herrero R, Franceschi S, Meijer CJ, Snijders P, Bosch FX, et al. Smoking and cervical cancer: pooled analysis of the IARC multi-centric case-control study. Cancer Causes Control. 2003;14:805–814. doi: 10.1023/b:caco.0000003811.98261.3e. [DOI] [PubMed] [Google Scholar]
- 16.Pettersson BF. Adenocarcinoma of the uterine cervix. Changes in incidence. A cancer registry study [abstract]; Paper presented at: 38th Annual Meeting of the Society of Pelvic Surgeons; October 5-8, 1988; Toronto, ON. [Google Scholar]
- 17.Bergstrom R, Sparen P, Adami HO. Trends in cancer of the cervix uteri in Sweden following cytological screening. Br J Cancer. 1999;81:159–166. doi: 10.1038/sj.bjc.6690666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sasieni P, Adams J. Changing rates of adenocarcinoma and adenosquamous carcinoma of the cervix in England. Lancet. 2001;357:1490–1493. doi: 10.1016/S0140-6736(00)04646-8. [DOI] [PubMed] [Google Scholar]
- 19.Sasieni P, Castanon A, Cuzick J. Screening and adenocarcinoma of the cervix. Int J Cancer. 2009;125:525–529. doi: 10.1002/ijc.24410. [DOI] [PubMed] [Google Scholar]
- 20.Bray F, Carstensen B, Moller H, Zappa M, Zakelj MP, Lawrence G, et al. Incidence trends of adenocarcinoma of the cervix in 13 European countries. Cancer Epidemiol Biomarkers Prev. 2005;14:2191–2199. doi: 10.1158/1055-9965.EPI-05-0231. [DOI] [PubMed] [Google Scholar]
- 21.Babes A. Diagnostic du cancer du col uterin par Les-Frottis. Presse Med. 1928;36:451–454. [Google Scholar]
- 22.Naslund I, Auer G, Pettersson F, Sjovall K. Evaluation of the pulse wash sampling technique for screening of uterine cervical carcinoma. Acta Radiol Oncol. 1986;25:131–136. doi: 10.3109/02841868609136391. [DOI] [PubMed] [Google Scholar]
- 23.Zielinski GD, Snijders PJ, Rozendaal L, Daalmeijer NF, Risse EK, Voorhorst FJ, et al. The presence of high-risk HPV combined with specific p53 and p16INK4a expression patterns points to high-risk HPV as the main causative agent for adenocarcinoma in situ and adenocarcinoma of the cervix. J Pathol. 2003;201:535–543. doi: 10.1002/path.1480. [DOI] [PubMed] [Google Scholar]
- 24.Brismar S, Johansson B, Borjesson M, Arbyn M, Andersson S. Follow-up after treatment of cervical intraepithelial neoplasia by human papillomavirus genotyping. Am J Obstet Gynecol. 2009;201:17.e1–17.e8. doi: 10.1016/j.ajog.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Gustafsson L, Sparen P, Gustafsson M, Pettersson B, Wilander E, Bergstrom R, et al. Low efficiency of cytologic screening for cancer in situ of the cervix in older women. Int J Cancer. 1995;63:804–809. doi: 10.1002/ijc.2910630610. [DOI] [PubMed] [Google Scholar]
- 26.Gyllensten U, Lindell M, Gustafsson I, Wilander E. HPV test shows low sensitivity of Pap screen in older women. Lancet Oncol. 2010;11:509–510. doi: 10.1016/S1470-2045(10)70064-4. [DOI] [PubMed] [Google Scholar]
- 27.Andrae B, Kemetli L, Sparen P, Silfverdal L, Strander B, Ryd W, et al. Screening-preventable cervical cancer risks: evidence from a nationwide audit in Sweden. J Natl Cancer Inst. 2008;100:622–629. doi: 10.1093/jnci/djn099. [DOI] [PubMed] [Google Scholar]
- 28.Anttila A, Ronco G Working Group on the Registration and Monitoring of Cervical Cancer Screening Programmes in the European Union; within the European Network for Information on Cancer (EUNICE) Description of the national situation of cervical cancer screening in the member states of the European Union. Eur J Cancer. 2009;45:2685–2708. doi: 10.1016/j.ejca.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 29.Clegg LX, Reichman ME, Miller BA, Hankey BF, Singh GK, Lin YD, et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control. 2009;20:417–435. doi: 10.1007/s10552-008-9256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen KE, Hannibal CG, Nielsen A, Jensen A, Nohr B, Munk C, et al. Social inequality and incidence of and survival from cancer of the female genital organs in a population-based study in Denmark, 1994-2003. Eur J Cancer. 2008;44:2003–2017. doi: 10.1016/j.ejca.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 31.van der Aa MA, Siesling S, Louwman MW, Visser O, Pukkala E, Coebergh JW. Geographical relationships between sociodemographic factors and incidence of cervical cancer in the Netherlands 1989-2003. Eur J Cancer Prev. 2008;17:453–459. doi: 10.1097/CEJ.0b013e3282f75ed0. [DOI] [PubMed] [Google Scholar]
- 32.Eaker S, Adami HO, Sparen P. Reasons women do not attend screening for cervical cancer: a population-based study in Sweden. Prev Med. 2001;32:482–491. doi: 10.1006/pmed.2001.0844. [DOI] [PubMed] [Google Scholar]
- 33.Idestrom M, Milsom I, Andersson-Ellstrom A. Knowledge and attitudes about the Pap-smear screening program: a population-based study of women aged 20-59 years. Acta Obstet Gynecol Scand. 2002;81:962–967. doi: 10.1080/j.1600-0412.2002.811011.x. [DOI] [PubMed] [Google Scholar]
- 34.Pettersson F, Malker B. Invasive carcinoma of the uterine cervix following diagnosis and treatment of in situ carcinoma: record linkage study within a National Cancer Registry. Radiother Oncol. 1989;16:115–120. doi: 10.1016/0167-8140(89)90028-5. [DOI] [PubMed] [Google Scholar]
- 35.Hellstrom AC, Sigurjonson T, Pettersson F. Carcinoma of the cervical stump. The radiumhemmet series 1959-1987: treatment and prognosis. Acta Obstet Gynecol Scand. 2001;80:152–157. [PubMed] [Google Scholar]
- 36.Pettersson BF, Andersson S, Hellman K, Hellstrom AC. Invasive carcinoma of the uterine cervix associated with pregnancy: 90 years of experience. Cancer. 2010;116:2343–2349. doi: 10.1002/cncr.24971. [DOI] [PubMed] [Google Scholar]