Abstract
Objective
To determine matrix metalloproteinase-2 and survivin expressions in endometrial cancers, their relation to clinical and histologic parameters and to investigate any difference in the expression of these markers between endometrioid and nonendometrioid cancers.
Methods
Ninety-five patients with endometrial cancer, were included. Matrix metalloproteinase-2 and survivin expressions were analyzed immunohistochemically from paraffin-embedded tissues by using specific monoclonal antibodies.
Results
Survivin nuclear expression was higher in endometrioid cancer as compared to nonendometrioid cancer (p=0.040), but there was no difference for cytoplasmic survivin and matrix metalloproteinase-2 expressions between type I and type II carcinomas. Survivin cytoplasmic staining was significantly lower in patients with deep myometrial invasion (p=0.038). Nuclear expression of survivin is decreased in histologic grade 3 tumors compared to grade 1 and 2 tumors (p=0.013), but there is no difference between grade 1 and 2. We did not find any statistically significant difference between survivin or matrix metalloproteinase-2 expressions and survival.
Conclusion
Survivin and matrix metalloproteinase-2 are present in endometrioid and nonendometrioid cancers. Grade 1 and 2 tumors and carcinomas having myometrial invasion less than 50% have higher survivin expression. These results supports that, survivin may play an important role in early stage tumors and early phases of tumor development. We did not find any association between matrix metalloproteinase-2 expression and classical prognostic factors in endometrial cancer and both proteins were not associated with survival.
Keywords: Endometrial cancer, Matrix metalloproteinases, Survivin, Prognosis, Survival
INTRODUCTION
Endometrial cancer is the most common malignancy of the female reproductive tract in developed countries [1]. It affects mainly postmenopausal women and only 20% of women will be diagnosed before menopause. The prognosis is usually good because approximately 72% of cases are diagnosed at stage I [2]. It has commonly been classified into two major clinicopathologic types, type I and type II [3]. It is currently known that these two types of endometrial carcinomas show different molecular alterations. The most frequent early molecular defects of endometrioid carcinomas are mutations of PTEN, K-Ras, CTNNB1 (beta-catenin) genes and microsatellite instability (MSI), but they rarely exhibit p53 mutations [1,4,5]. In contrast, nonendometrioid carcinomas often exhibit p53 tumor suppressor gene mutations and loss of heterozygosity located on several chromosomes, but occasionally have MSI [6,7] Unfortunately, none of these molecular markers are prognostic enough for endometrial cancer, and new prognostic markers are necessary.
Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases which degrade the extracellular matrix and play an important role in tumor invasion and progression [8]. MMPs are expressed at low levels in normal adult tissues, except in tissues that undergo remodeling such as the cyclic endometrium and the skin during wound healing [9-11]. Also, MMP-2 expression has been shown to be increased in several human neoplasias such as breast, ovary and colon cancers, and it seems to correlate with their invasion and metastatic behaviours [9]. Recently, MMP-2 has been demonstrated in endometrial carcinoma cells but there are only a few studies comparing MMP-2 expression between type I and type II endometrial carcinoma [12,13].
Survivin is a novel and structurally unique member of the inhibitor apoptosis (IAP) family of antiapoptotic proteins. Its gene is located on chromosome 17q25 [14]. Unlike other IAP proteins, survivin was found during fetal development, is not detectable in normal adult tissues, and became reexpressed in all of the most common human cancers [15,16]. Recently, in several studies, overexpression of survivin has been shown to be associated with endometrial carcinoma, but there are conflicting results for the impact on prognosis of endometrial carcinoma [17-19]. The nuclear or cytoplasmic expression of survivin is controversial. Immunofluorescence studies showed that survivin was expressed mainly in the nucleus of endometrial carcinoma cells, whereas previous studies reported that it is restricted to the cytoplasm [18].
This study aimed to investigate the expression of MMP-2 and survivin in surgically treated endometrial cancer patients. MMP-2 and survivin expressions were compared in type I and type II tumors, and the correlation of these markers between well-established prognostic factors and the relationship with survival were analyzed.
MATERIALS AND METHODS
1. Patients and specimens
This study was approved by the institutional review board at Dokuz Eylul University Faculty of Medicine. For immunohistochemical analysis, ninety-five patients with primary endometrial carcinoma, including 74 endometrioid and 21 nonendometrioid carcinomas, were selected from the pathology archive. All patients were surgically treated, histologically diagnosed and given follow-up care at the Division of Gynecologic Oncology, Department of Obstetrics and Gynecology at Dokuz Eylul University Faculty of Medicine. Certain clinical and pathologic parameters were recorded, including details on age, histopathologic type, tumor stage, tumor grade, and follow-up. The tumors were classified according to the histologic typing of the female genital tract by the World Health Organization, staged and graded according to the International Federation of Gynecology and Obstetrics (FIGO) systems [20,21]. Standard hematoxylin and eosin (H&E)-stained sections of formalin-fixed, paraffin-embedded tumor tissues were reviewed to confirm the histopathologic diagnosis; 73 cases were classified as FIGO stage 1, 6 cases as stage 2 and 16 cases as stage 3. All patients were followed up until death or a median of 39 months (range, 9 to 151 months).
2. Immunohistochemistry
Five µm sections were taken onto poly-L lysin-coated slides from each representative archival paraffin-embedded tumor tissue for immunohistochemical staining. The standard streptavidin biotin immunoperoxidase method was performed using the primary antibodies against survivin (1/100, Diagnostic Biosystems, Pleasanton, CA, USA) and MMP-2 (1/50, Diagnostic Biosystems). Briefly, sections were deparaffinized in xylene, rehydrated in alcohol series, and immersed in distillated water. Endogenous peroxidase activity was blocked using a 0.3% solution of hydrogen peroxide in phosphate-buffered saline (0.01 mmol/L, pH 7.5) at room temperature for 15 minutes and rinsed with TRIS buffer. After antigen retrieval by heating in 10 mmol/L citrate buffer (pH 6.0) for 30 minutes, primary antibodies were applied for 60 minutes at room temperature and washed in TRIS buffer. Biotinylated secondary antibodies and streptavidin-peroxidase complex were added consecutively for 10 minutes at room temperature and washed in TRIS buffer. Peroxidase activity was visualized with 0.03% 3-3' diaminobenzidine tetrahydrochloride applied for 7 minutes. After rinsing in deionized water and counterstaining in Mayer's hematoxylin, the slides were dehydrated and mounted. Appropriate tissue sections as positive controls for each primary antibody were also stained simultaneously. As a negative control, sections were processed in the absence of primary antibody.
3. Immunohistochemical evaluation
Immunohistochemical staining in tumor cells was evaluated in a semiquantitative fashion. For the survivin antibody, both cytoplasmic and nuclear staining were observed and evaluated. For MMP-2, only cytoplasmic staining was observed and evaluated. The degree of staining for survivin and MMP-2 was evaluated by scoring on a scale from 0 to 4 for intensity (I) and for distribution (D). Tissues with IxD equal to 0 were considered negative, those with less than or equal to 4 were weakly positive, and those with greater than 4 were strongly positive [22]. Results were also further simplified as positive and negative.
4. Statistical analysis
Data analyses were performed using SPSS 15.0 (SPSS Inc., Chicago, IL, USA). The relationship between immunohistochemical scores, tumor stage and histologic and nuclear grade was investigated by using the chi-squared test. Survival analyses were calculated by using the Kaplan-Meier method. The long rank test was used for univariate, and Cox proportional hazards regression model for the multivariate evaluation. The probability level 0.05 or less was chosen to represent statistical significance.
RESULTS
A total of ninety-five patients with endometrial carcinoma were included in the present study. There were 74 (77.9%) endometrioid (type I) and 21 (22.1%) non-endometrioid (type II) carcinomas. The median age of the patients was 61 years (range, 34 to 87 years). Clinicopathologic characteristics of the cases are shown in Table 1.
Table 1.
Distribution of clinicopathologic characteristics in 95 patients with endometrial carcinoma
Values are presented as number (%) or mean±SD (range).
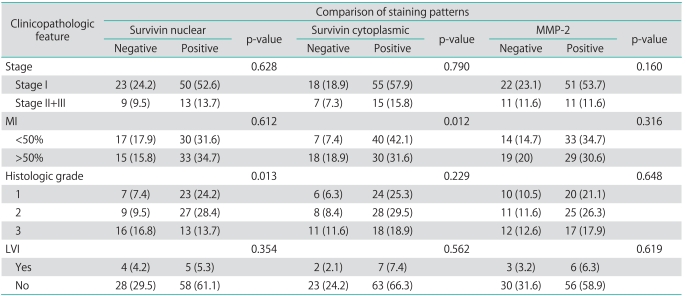
Distribution of MMP-2 and survivin staining in 95 endometrial tumors are shown in Table 2. For survivin, positive cytoplasmic and nuclear immunohistochemical staining was seen in 73.7% and 66.3% respectively, and also MMP-2 on 66.3% of all tumors (Figs. 1 and 2). Survivin nuclear expression was higher in endometrioid endometrial carcinoma (EEC) compared to non-EEC (NEEC), and this was statistically significant (p=0.040), but there was no statistically significant relationship between cytoplasmic survivin and MMP-2 expressions between type I and type II carcinomas. Table 3 summarizes the clinicopathologic characteristics of 95 patients with endometrial carcinoma and their association with the expression of survivin and MMP-2. There was no relationship between survivin cytoplasmic, survivin nuclear and MMP-2 expressions and lymphovascular space involvement and extrauterine disease. However survivin cytoplasmic staining was significantly lower in patients with deep myometrial invasion (p=0.038). The percentage of survivin nuclear staining cells in histologic grade 1, 2 and 3 tumors were 24.2%, 28.4% and 13.7%, respectively. Nuclear expression of survivin was decreased in histologic grade 3 tumors compared to grade 1 and 2 tumors, but there was no difference between grade 1 and 2. Adversely, the survivin cytoplasmic and MMP-2 expressions were decreased in grade 3 tumors but not statistically significant.
Table 2.
Distribution of MMP-2 and survivin staining in 95 patients in endometrial cancer and comparison with endometrioid and nonendometrioid type
Values are presented as number (%).
MMP, matrix metalloproteinases.
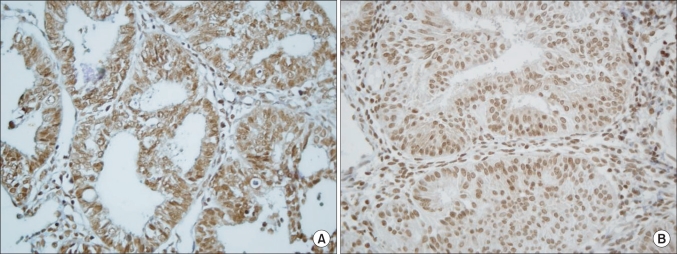
Fig. 1.
Survivin cytoplasmic (A) and nuclear (B) immunohistochemical staining in pT3a endometrioid endometrial adenocarcinoma (×400).
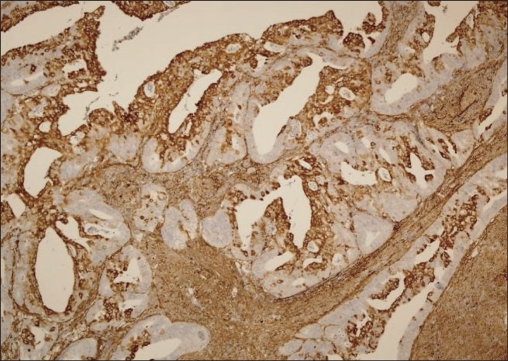
Fig. 2.
Matrix metalloproteinase-2 positivity in pT1b endometrioid endometrial adenocarcinoma (×100).
Table 3.
Clinicopathologic characteristics of 95 cases and association with survivin and MMP-2 expressions
Values are presented as number (%).
MMP, matrix metalloproteinases; MI, myometrial invasion; LVI, lymphovascular invasion.
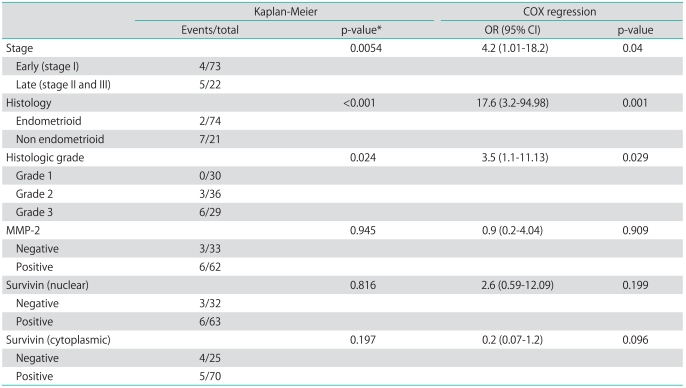
Follow-up data were available for all patients. The median duration of follow-up was 39 months (range, 9 to 151 months). During the follow-up, 3 local recurrences, 2 solid organ metastasis, and 3 lymph node metastasis were documented. Overall mean cumulative survival of the patients was 134.30±5.46 months, and the cumulative overall and disease-free survival rates were 0.822 and 0.821, respectively. The mean survival related to survivin nuclear, cytoplasmic and MMP-2 expression is shown in Table 4. While the mean cumulative survival was 140.96 months in stage I cases, it dropped to 110.22 months in the advanced stage group (p=0.0054). Also, the cumulative survival rates of patients with type I tumors were significantly higher than those of patients with type II tumors (p<0.001). Cox regression analysis showed that stage, grade and histological type have an independent impact on survival. We did not find any statistically significant difference between survivin or MMP-2 expressions and survival (p>0.05) (Table 4).
Table 4.
Results of survival analysis
OR, odds ratios; CI, confidence interval; MMP, matrix metalloproteinases.
*Log-rank test.
DISCUSSION
MMP-2 and survivin expressions and their relationships with the clinical behaviors and histologic types of endometrial cancers were investigated in this study. In our present study, MMP-2 expression did not show any difference between EEC and NEEC carcinomas. These results confirm those of Graesslin et al. [13]. On the other hand, Shaco-Levy et al. [23] found increased MMP-2 expression in NEEC as compared to EEC. In another study, Monaghan et al. [12] showed that significantly greater MMP-2 expression was present in EEC compared to NEEC. The difference between these two studies could be attributed to the fact that the two study groups were not homogenous in terms of tumor stage and grade. There have been only a few studies investigating the relationship between matrix metalloproteinase expression and survival in endometrial cancer patients. Previous studies have shown that, MMP -1, MMP-2 or MMP-9 and membrane-type 1 (MT1)-MMP proteins or mRNAs exist in endometrial cancer tissues [24,25]. Di Nezza et al. [26] revealed that MMP-2 and MMP-9 expressions in tumor cells are increased in the transition from histologic grade 1 to grades 2 and 3. These results are supported by other studies [13,25]. On the other hand, Lopata et al. [27], with use of gelatin zymography, revealed that elevated levels of latent and active forms of MMP-2 are detected in uterine lavage samples from patients with endometrial cancer, and they found no significant association between the MMP score and the histologic grade of tumor. Likewise, Honkavuori et al. [28] did not determine any correlation between MMP-2 immunostaining and histological grade. These results are consistent with our study. Furthermore, we did not find any correlation between the MMP-2 score and the FIGO stage, vascular/lymphatic invasion, or depth of myometrial invasion. These results agree with those of other authors [25,26,28,29]. Graesslin et al. [13] found that high MMP-2 expression is correlated with only lymph node metastasis, but that there is no association with other clinical features of endometrial cancer. Their results are in keeping with the study of Iurlaro et al. [30] Survival analyses have been rarely performed in previous studies. Moser et al. [24], Aglund et al. [25], and, recently, Honkavuori et al. [28] found no statistically significant correlation between MMP-2 expression and overall survival in endometrial cancer. Similarly, in the present study, we did not find any association between survival and the MMP-2 staining score.
Survivin, a member of the IAP family, has been detected to be over expressed in 60 human cancer types studied, including endometrial carcinoma [31,32]. Although some immunohistochemical studies suggested that survivin expression occurs primarily in the nucleus and partially in the cytoplasm of endometrial cancer cells, Pallares et al. [33] found that most of the survivin proteins were cytoplasmic. The present study did not determine statistically significant difference between cytoplasmic and nuclear expressions. In a small number of previous reports investigating the role of survivin in the endometrial cancer, controversial results have been obtained [17,18,33-35]. Saitoh et al. [34] demonstrated that levels of survivin mRNA in endometrial cancer samples were significantly higher than the levels that were seen in the normal endometrium, and did not show any correlation with histologic grade of the tumors. Similarly, in their study, Ai et al. [32] showed that survivin protein expression levels were significantly higher in endometrial cancer cells than atypical or normal endometrium, and were not different between type 1 and type 2 endometrial cancers. Also, they did not observe any association with patient age, tumor grade and stage, and these results were supported by Pallares et al. [33]. In our present study, unlike the results of Ai et al. [32], survivin nuclear expression was higher in EEC as compared to NEEC. This difference may be due to the nonhomogenous stage distribution in type 1 and type 2 patient groups in the above studies including ours. Lehner et al. evaluated survivin mRNA levels in both normal endometrium and endometrial cancer specimens, and suggested that survivin mRNA is not a specific marker of endometrial cancer. In contrast to the studies of Ai et al. [32] and Pallares et al. [33], Lehner et al. [35] suggested that survivin mRNA expression only correlates with the histologic grade of the tumors. The study of Takai et al. [18], supporting the results of Lehner et al. [35], showed that survivin protein expressions within endometrial carcinoma cells were significantly associated with significant clinicopathologic prognostic parameters and survival rates. Recently, at another immunohistochemical study of Erkanli et al. [17], they showed survivin expression to be increased in a spectrum from normal to hyperplastic endometrium and to endometrial cancer. Also, different from Takai et al. [18], they have reported no correlation between survivin and classical prognostic factors or survival rates in endometrial carcinomas. Eventually these authors concluded that these findings suggest that survivin overexpression is one of the most important factors in the developmental pathway of endometrioid carcinomas [17]. Likewise, in our study, we found that nuclear expression of survivin is decreased in histologic grade 3 tumors compared to both grade 1 and grade 2 tumors, and survivin cytoplasmic expression was significantly lower in patients with deep myometrial invasion (p=0.038). These results differ from other studies, supporting the suggestion in the study of Erkanli et al., and may be explained as survivin expression playing a role in the earlier stages or development of tumor, but not in tumor aggressiveness of it. Also, in our study, we did not find any relation between survivin expression and clinical prognostic factors including lymphovascular space involvement and extrauterine disease or survival. However, these results are not consistent with the study of Lambropoulou et al. [19]. They found a significant correlation survivin expression with myometrial invasion, histological grade, stage and survival rates. This difference may be due to different staining criteria.
In conclusion, differing from those of previous studies, we have shown that in histologic grade 1 and 2 tumors and cases with myometrial invasion less than 50%, survivin expression was found to be higher. These results support the hypothesis that survivin may play an important role in early stage tumors and early phases of tumor development. However, we did not find any association between MMP-2 expression and classical prognostic factors in endometrial carcinoma, additionally both proteins were not associated with survival. As far as we know our study includes the largest number of cases of both survivin and MMP-2 expression in the literature. Further studies with longer follow-up and larger number of cases are needed.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Prat J. Prognostic parameters of endometrial carcinoma. Hum Pathol. 2004;35:649–662. doi: 10.1016/j.humpath.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Sorosky JI. Endometrial cancer. Obstet Gynecol. 2008;111:436–447. doi: 10.1097/AOG.0b013e318162f690. [DOI] [PubMed] [Google Scholar]
- 3.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 4.Matias-Guiu X, Catasus L, Bussaglia E, Lagarda H, Garcia A, Pons C, et al. Molecular pathology of endometrial hyperplasia and carcinoma. Hum Pathol. 2001;32:569–577. doi: 10.1053/hupa.2001.25929. [DOI] [PubMed] [Google Scholar]
- 5.Sun H, Enomoto T, Fujita M, Wada H, Yoshino K, Ozaki K, et al. Mutational analysis of the PTEN gene in endometrial carcinoma and hyperplasia. Am J Clin Pathol. 2001;115:32–38. doi: 10.1309/7JX6-B9U9-3P0R-EQNY. [DOI] [PubMed] [Google Scholar]
- 6.Tashiro H, Isacson C, Levine R, Kurman RJ, Cho KR, Hedrick L. p53 gene mutations are common in uterine serous carcinoma and occur early in their pathogenesis. Am J Pathol. 1997;150:177–185. [PMC free article] [PubMed] [Google Scholar]
- 7.Tritz D, Pieretti M, Turner S, Powell D. Loss of heterozygosity in usual and special variant carcinomas of the endometrium. Hum Pathol. 1997;28:607–612. doi: 10.1016/s0046-8177(97)90084-8. [DOI] [PubMed] [Google Scholar]
- 8.Aznavoorian S, Murphy AN, Stetler-Stevenson WG, Liotta LA. Molecular aspects of tumor cell invasion and metastasis. Cancer. 1993;71:1368–1383. doi: 10.1002/1097-0142(19930215)71:4<1368::aid-cncr2820710432>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 9.Chang C, Werb Z. The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol. 2001;11:S37–S43. doi: 10.1016/s0962-8924(01)02122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parks WC. Matrix metalloproteinases in repair. Wound Repair Regen. 1999;7:423–432. doi: 10.1046/j.1524-475x.1999.00423.x. [DOI] [PubMed] [Google Scholar]
- 11.Rodgers WH, Osteen KG, Matrisian LM, Navre M, Giudice LC, Gorstein F. Expression and localization of matrilysin, a matrix metalloproteinase, in human endometrium during the reproductive cycle. Am J Obstet Gynecol. 1993;168:253–260. doi: 10.1016/s0002-9378(12)90922-9. [DOI] [PubMed] [Google Scholar]
- 12.Monaghan H, MacWhinnie N, Williams AR. The role of matrix metalloproteinases-2, -7 and -9 and beta-catenin in high grade endometrial carcinoma. Histopathology. 2007;50:348–357. doi: 10.1111/j.1365-2559.2007.02612.x. [DOI] [PubMed] [Google Scholar]
- 13.Graesslin O, Cortez A, Uzan C, Birembaut P, Quereux C, Darai E. Endometrial tumor invasiveness is related to metalloproteinase 2 and tissue inhibitor of metalloproteinase 2 expressions. Int J Gynecol Cancer. 2006;16:1911–1917. doi: 10.1111/j.1525-1438.2006.00717.x. [DOI] [PubMed] [Google Scholar]
- 14.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3:917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 15.Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581–8589. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- 16.Altieri DC, Marchisio PC. Survivin apoptosis: an interloper between cell death and cell proliferation in cancer. Lab Invest. 1999;79:1327–1333. [PubMed] [Google Scholar]
- 17.Erkanli S, Kayaselcuk F, Kuscu E, Bagis T, Bolat F, Haberal A, et al. Expression of survivin, PTEN and p27 in normal, hyperplastic, and carcinomatous endometrium. Int J Gynecol Cancer. 2006;16:1412–1418. doi: 10.1111/j.1525-1438.2006.00541.x. [DOI] [PubMed] [Google Scholar]
- 18.Takai N, Miyazaki T, Nishida M, Nasu K, Miyakawa I. Survivin expression correlates with clinical stage, histological grade, invasive behavior and survival rate in endometrial carcinoma. Cancer Lett. 2002;184:105–116. doi: 10.1016/s0304-3835(02)00190-8. [DOI] [PubMed] [Google Scholar]
- 19.Lambropoulou M, Papadopoulos N, Tripsianis G, Alexiadis G, Pagonopoulou O, Kiziridou A, et al. Co-expression of survivin, c-erbB2, and cyclooxygenase-2 (COX-2): prognostic value and survival of endometrial cancer patients. J Cancer Res Clin Oncol. 2010;136:427–435. doi: 10.1007/s00432-009-0673-6. [DOI] [PubMed] [Google Scholar]
- 20.Lee KR, Tavassoli FA, Prat J. Surface epithelial-stromal tumours. In: Tavassoli FA, Devilee P, editors. World Health Organization classification of tumours. Pathology and genetics of the breast and female genital organs. Lyon: IARC Press; 2003. pp. 117–145. [Google Scholar]
- 21.Benedet JL, Bender H, Jones H, 3rd, Ngan HY, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers: FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2000;70:209–262. [PubMed] [Google Scholar]
- 22.Gilcrease MZ, Truong L, Brown RW. Correlation of very late activation integrin and CD44 expression with extrarenal invasion and metastasis of renal cell carcinomas. Hum Pathol. 1996;27:1355–1360. doi: 10.1016/s0046-8177(96)90350-0. [DOI] [PubMed] [Google Scholar]
- 23.Shaco-Levy R, Sharabi S, Piura B, Sion-Vardy N. MMP-2, TIMP-1, E-cadherin, and beta-catenin expression in endometrial serous carcinoma compared with low-grade endometrial endometrioid carcinoma and proliferative endometrium. Acta Obstet Gynecol Scand. 2008;87:868–874. doi: 10.1080/00016340802253775. [DOI] [PubMed] [Google Scholar]
- 24.Moser PL, Hefler L, Tempfer C, Neunteufel W, Kieback DG, Gitsch G. Immunohistochemical detection of matrix metalloproteinases (MMP) 1 and 2, and tissue inhibitor of metalloproteinase 2 (TIMP 2) in stage I and II endometrial cancer. Anticancer Res. 1999;19(3B):2365–2367. [PubMed] [Google Scholar]
- 25.Aglund K, Rauvala M, Puistola U, Angstrom T, Turpeenniemi-Hujanen T, Zackrisson B, et al. Gelatinases A and B (MMP-2 and MMP-9) in endometrial cancer-MMP-9 correlates to the grade and the stage. Gynecol Oncol. 2004;94:699–704. doi: 10.1016/j.ygyno.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 26.Di Nezza LA, Misajon A, Zhang J, Jobling T, Quinn MA, Ostor AG, et al. Presence of active gelatinases in endometrial carcinoma and correlation of matrix metalloproteinase expression with increasing tumor grade and invasion. Cancer. 2002;94:1466–1475. doi: 10.1002/cncr.10355. [DOI] [PubMed] [Google Scholar]
- 27.Lopata A, Agresta F, Quinn MA, Smith C, Ostor AG, Salamonsen LA. Detection of endometrial cancer by determination of matrix metalloproteinases in the uterine cavity. Gynecol Oncol. 2003;90:318–324. doi: 10.1016/s0090-8258(03)00328-7. [DOI] [PubMed] [Google Scholar]
- 28.Honkavuori M, Talvensaari-Mattila A, Soini Y, Turpeenniemi-Hujanen T, Santala M. MMP-2 expression associates with CA 125 and clinical course in endometrial carcinoma. Gynecol Oncol. 2007;104:217–221. doi: 10.1016/j.ygyno.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 29.Shaco-Levy R, Sharabi S, Benharroch D, Piura B, Sion-Vardy N. Matrix metalloproteinases 2 and 9, E-cadherin, and beta-catenin expression in endometriosis, low-grade endometrial carcinoma and non-neoplastic eutopic endometrium. Eur J Obstet Gynecol Reprod Biol. 2008;139:226–232. doi: 10.1016/j.ejogrb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Iurlaro M, Loverro G, Vacca A, Cormio G, Ribatti D, Minischetti M, et al. Angiogenesis extent and expression of matrix metalloproteinase-2 and -9 correlate with upgrading and myometrial invasion in endometrial carcinoma. Eur J Clin Invest. 1999;29:793–801. doi: 10.1046/j.1365-2362.1999.00532.x. [DOI] [PubMed] [Google Scholar]
- 31.Tamm I, Wang Y, Sausville E, Scudiero DA, Vigna N, Oltersdorf T, et al. IAP-family protein survivin inhibits caspase activity and apoptosis induced by Fas (CD95), Bax, caspases, and anticancer drugs. Cancer Res. 1998;58:5315–5320. [PubMed] [Google Scholar]
- 32.Ai Z, Yin L, Zhou X, Zhu Y, Zhu D, Yu Y, et al. Inhibition of survivin reduces cell proliferation and induces apoptosis in human endometrial cancer. Cancer. 2006;107:746–756. doi: 10.1002/cncr.22044. [DOI] [PubMed] [Google Scholar]
- 33.Pallares J, Martinez-Guitarte JL, Dolcet X, Llobet D, Rue M, Palacios J, et al. Survivin expression in endometrial carcinoma: a tissue microarray study with correlation with PTEN and STAT-3. Int J Gynecol Pathol. 2005;24:247–253. doi: 10.1097/01.pgp.0000163849.37129.d4. [DOI] [PubMed] [Google Scholar]
- 34.Saitoh Y, Yaginuma Y, Ishikawa M. Analysis of Bcl-2, Bax and Survivin genes in uterine cancer. Int J Oncol. 1999;15:137–141. doi: 10.3892/ijo.15.1.137. [DOI] [PubMed] [Google Scholar]
- 35.Lehner R, Enomoto T, McGregor JA, Shroyer L, Haugen BR, Pugazhenthi U, et al. Correlation of survivin mRNA detection with histologic diagnosis in normal endometrium and endometrial carcinoma. Acta Obstet Gynecol Scand. 2002;81:162–167. doi: 10.1034/j.1600-0412.2002.810213.x. [DOI] [PubMed] [Google Scholar]