Abstract
Background
NovoTwist® (Novo Nordisk A/S, Bagsværd, Denmark) is an insulin pen needle that features a novel attachment and detachment system. The aim of this test was to assess overall preference and handling of NovoTwist compared with conventional screw-thread needles in people with type 1 or type 2 diabetes.
Methods
One hundred twenty adults with type 1 or type 2 diabetes and manual dexterity dysfunction who were currently self-injecting with an insulin pen were included in this open-label, randomized, crossover test. Participants were stratified according to the impact that manual dexterity problems had on their ability to inject insulin (1 = no effect at all; 4 = a lot), and those rated as 1 were excluded from subanalyses because of low numbers. Following instruction, participants attached the needle to Next Generation FlexPen® (Novo Nordisk A/S), made an injection into a foam cushion, and detached the needle; this process was repeated three times with NovoTwist and the participant's current screw-thread needle (or NovoFine® [Novo Nordisk A/S]) in a random order. Responses to questions on user experience with each needle were subsequently recorded on a 6-point rating scale (1 = very difficult; 6 = very easy).
Results
Significantly more respondents had a preference for NovoTwist (79%) compared with the conventional screw-thread needles (21%, P < 0.001). Significantly more respondents preferred NovoTwist for both ease of attachment (80%, P < 0.001) and ease of detachment (74%, P < 0.001). Most respondents found NovoTwist the most appropriate needle for performing everyday injections (71%, P < 0.001).
Conclusions
Such preference by patients has a positive impact on the treatment of diabetes as NovoTwist may alleviate the burden of performing everyday injections through its ease of use.
Introduction
Insulin injections for the management of diabetes can be traumatic and inconvenient, and many patients fear injections and lack confidence in their own ability to self-inject.1–5 Insulin pens reduce the fear of injection, improve the confidence of dose delivery, and remove the inconvenience associated with vial and syringes. Consequently, injection pens are increasingly preferred over conventional syringes and have become the predominant devices for insulin delivery, especially in Europe.6–11 Importantly, insulin pens provide more accurate and precise delivery of insulin doses compared with syringes, and this may assist in improving glycemic control.12–14 Collectively, these features of insulin pens have enabled diabetes patients to lead more flexible and less-restrictive lives, leading to improved quality of life.15–17
However, continued improvements in injection-system technology seek to improve both the ease of use and patient satisfaction with the injection process. Previous studies have indicated that the diameter and design of the needle play an important role in reducing injection pain and needle anxiety in subcutaneous insulin delivery and in improving patient satisfaction.18–20 In one study, significantly more patients preferred the shorter 31-gauge × 6-mm needles compared with a longer 29-gauge × 12.7-mm needles.21 Another study reported that 58% of patients had a preference for 32-gauge × 6-mm needles compared with 30-gauge × 8-mm needles, whereas 26% patients preferred the longer needle.20 Shorter needles increase patient acceptance and satisfaction with routine injections19,21,22 and are associated with fewer intramuscular injections than their longer counterparts.23 A positive correlation also exists between the diameter of a needle and the frequency of painful injection.24 For example, the insertion of a 27-gauge or 28-gauge needle was associated with pain in 50% of recipients, whereas the insertion of a narrower 31-gauge needle was associated with injection pain in 39% of recipients.22 In another study, the use of a 32-gauge needle significantly reduced the frequency of injection pain by more than 50% compared with the use of a 23-gauge needle.24 Thin-wall technology and microtapering of needles have also been shown to increase the flow of insulin through the needles and to reduce discomfort and injection pain.25,26
NovoTwist® (Novo Nordisk A/S, Bagsværd, Denmark) 5-mm and 8-mm needles fulfill many of the requirements for a less painful needle that administers subcutaneous insulin in both children and adults with a minimum risk of intramuscular injection. NovoTwist is attached via a bayonet fitting, where the needle is pressed down and turned a quarter of a revolution, compared with the conventional screw-thread needle, which requires several revolutions to attach and detach the needle correctly. NovoTwist is compatible with the prefilled pen Next Generation FlexPen® (Novo Nordisk A/S), which is simple to use and preferred over vial and syringe and other insulin pens.27–31 Next Generation FlexPen has a novel needle–pen interface making it compatible with conventional screw-thread needles and NovoTwist.32–35 Two surveys have shown that people with diabetes preferred NovoTwist needles to conventional screw-thread needles because of the ease of the attachment and detachment processes.35,36 Here we report the results of a test to evaluate the overall preference for NovoTwist 5-mm needles versus conventional screw-thread needles, when used with FlexPen, in adults with diabetes and impaired manual dexterity. Patients with reduced manual dexterity may have a particular need for an easy to use needle such as NovoTwist.
Subjects and Methods
Test design and participants
This was an open-label, randomized, crossover usability test in adults with impaired manual dexterity and with diabetes who were already self-injecting with an insulin pen. Participants were stratified according to the impact that manual dexterity problems had on their ability to inject insulin using a 4-point scale (from 1 = no effect at all to 4 = severe impact). The test was carried out at seven centers in Italy and nine centers in the United Kingdom.
Inclusion criteria
Male or female adults ≤18 years of age with type 1 or type 2 diabetes being treated with insulin and who were self-injecting using a pen device were eligible. Patients were included if they had difficulties due to impaired manual dexterity (neuropathy, arthritis, familial tremor, Parkinson's disease, stroke-induced partial paralysis, generalized lupus). Approximately 10% of the recruited patients were to be left-handed. Written informed consent and a confidentiality agreement regarding the participation in the test and test products were obtained before any test-related activities.
Exclusion criteria
Those with mental or physical incapacity, unwillingness, or language barriers that precluded an adequate understanding or co-operation in the test were excluded. Additional exclusion criteria included the following: participants with any disease or condition that may have interfered with completion of the test; blindness or visual impairment requiring assistance when injecting; Ypsomed (Burgdorf, Switzerland) Penfine® users; those with any personal or family ties to a pharmaceutical company or marketing research agency; and those who had participated in market research on diabetes within the last 3 months.
Materials and procedures
FlexPen with test medium was used in conjunction with either NovoTwist 32-gauge tip 5-mm needles or the patient's own screw-thread needles (if the patient did not bring his or her own needles, NovoFine® [Novo Nordisk A/S] 32-gauge 6-mm needles were used for the comparison). Following instruction, participants attached the needle to FlexPen, made an injection into a foam cushion, and detached the needle. This procedure was repeated three times, and a new needle was attached before each injection. This process was conducted with NovoTwist and the participant's current screw-thread needle (or NovoFine needle) in a random order.
Questionnaire and statistical analysis
The questionnaire used in the test contained two types of questions: (1) rating questions, in which each needle were evaluated immediately following handling; and (2) preference questions, which were asked following handling of both needles. Responses to questions on user rating were subsequently recorded on a 6-point rating scale. Some questions, such as “How easy/difficult was it to attach the needle?” had a rating scale scored from 1 = very difficult to 6 = very easy, with points 2 to 5 being assessed as in between these two parameters. The primary endpoint of the test was to evaluate the overall preference for NovoTwist versus screw-thread needles for adults with dexterity disabilities, as evaluated by the patients after the handling process. All preference question results were tested against a value of 50% from the null hypothesis, with a two-tailed, one-sample binomial test with a 95% confidence interval. Secondary endpoints included the perception of overall ease of handling and of attachment/detachment of the needle. In addition to the primary and secondary endpoints, further evaluation and safety objectives were assessed in the questionnaire, including rating of ease of handling, improvement on everyday injection, appropriateness for everyday use, and needle preference for safety.
Results
One hundred twenty adults with impaired manual dexterity were recruited into the usability test: 60 from Italy and 60 from the United Kingdom. The patient and disease characteristics are presented in Table 1. The majority of participants were right-handed and had been injecting insulin for a mean of 12.1 years (range, 0.2–53.9 years). Arthritis was the most common manual dexterity difficulty, while diabetic neuropathy and tremor were also common. Impaired manual dexterity affected the ability to inject insulin in 98% of participants (two participants indicated a manual dexterity rating of 1 and were not included in the subanalyses because of the small number), and 19 of the 120 (16%) participants indicated that impaired manual dexterity severely affected their ability to inject insulin.
Table 1.
Baseline Patient and Disease Characteristics
| Characteristic | Patients (%) |
|---|---|
| Mean age (years) | 60.7 |
| Type 1 diabetes | 33 |
| Type 2 diabetes | 67 |
| Left-handed | 14 |
| Duration of insulin use | |
| <1 year | 5 |
| >1–4 years | 14 |
| >4–7 years | 17 |
| >7–10 years | 20 |
| >10–20 years | 26 |
| >20 years | 18 |
| Manual dexterity affecting handsa | |
| Diabetic neuropathy | 32 |
| Arthritis | 48 |
| Tremor | 28 |
| Parkinson's disease | 5 |
| Partial paralysis after stroke | 6 |
| Generalized lupus | 3 |
| Other | 9 |
| Effect of impaired manual dexterity on ability to inject insulin | |
| 1 (not at all) | 2 |
| 2 | 51 |
| 3 | 32 |
| 4 (a lot) | 16 |
| Injection device used (number)b | |
| Autopen® | 6 (7) |
| FlexPen® | 31 (37) |
| HumaPen® | 12 (14) |
| Innolet® | 3 (4) |
| KwikPen® | 6 (6) |
| NovoPen® | 26 (31) |
| OptiClik® | 1 (1) |
| OptiPen® | 5 (6) |
| OptiSet® | 3 (3) |
| SoloStar® | 18 (21) |
| Other | 12 (14) |
| Needle used (number)c | |
| MicroFine® | 48 (57) |
| NovoFine® | 38 (46) |
| NovoFine® Autocover | 1 (1) |
| Unifine Pentip® | 3 (4) |
| Other/don't know | 10 (12) |
Some patients had more than one manual dexterity problem.
Autopen® is a registered trademark of Owen Mumford, Oxford, UK; FlexPen®, Innolet®, and NovoPen® are registered trademarks of Novo Nordisk A/S, Bagsvaerd, Denmark; Humapen® and KwikPen® are registered trademarks of Eli Lilly & Company, Indianapolis, IN; and OptiClik®, OptiPen®, OptiSet®, and SoloStar® are registered trademarks of Sanofi-Aventis, Paris, France.
MicroFine® is a registered trademark of Becton Dickinson, Oxford; NovoFine® and NovoFine® Autocover are registered trademarks of Novo Nordisk A/S; and Unifine Pentip® is a registered trademark of Owen Mumford.
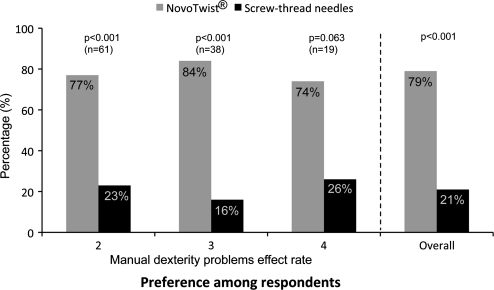
Significantly more respondents had an overall preference for NovoTwist (79%) compared with the conventional screw-thread needles (P < 0.001) (Fig. 1). Subgroup analysis showed that respondents regardless of severity of manual dexterity problems had a clear preference for NovoTwist compared with screw-thread needles (Fig. 1), although for respondents with severe manual dexterity problems (grade 4) the difference was not statistically significant.
FIG. 1.
Overall preference for use of NovoTwist compared with conventional screw-thread needles among people with diabetes and impaired manual dexterity (n = 118) and overall preference (n = 120).
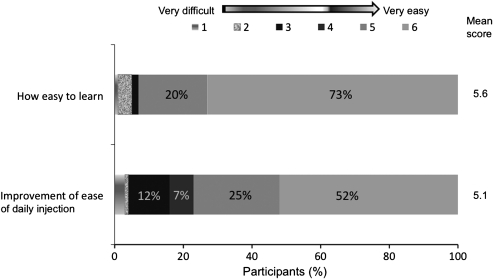
Most respondents (71%, P < 0.001) found that NovoTwist was easier to use than conventional screw-thread needles. A significant proportion of participants with manual dexterity problems rated at 2 (67%, n = 61, P = 0.01) and 3 (76%, n = 38, P = 0.002) found overall use of NovoTwist to be easier than conventional screw-thread needles. Most participants with severe dexterity problems (68%, n = 19) also found NovoTwist to be easier to use, but this was not statistically significant (P = 0.167). Patient perspectives of different aspects of ease of use are shown in Figure 2. Most respondents found NovoTwist to be easy to learn to use, with 73% of respondents giving the top score of 6, and the mean score was 5.6. Similarly, most respondents found NovoTwist to be an improvement for ease of daily injection compared with their usual needle with a mean score of 5.1.
FIG. 2.
Ease of use of NovoTwist among 120 people with diabetes and motor dysfunction. Evaluation was based on a 6-point rating scale, where 1 = very difficult and 6 = very easy.
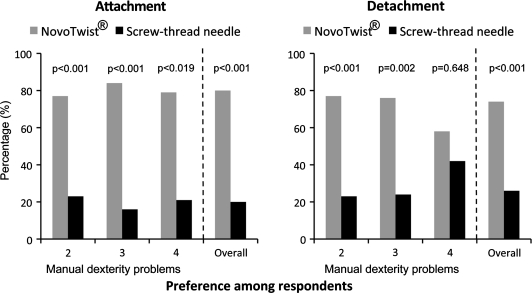
Most respondents preferred NovoTwist for both ease of attachment (80%, P < 0.001) and for ease of detachment (74%, P < 0.001) (Fig. 3). Subgroup analysis showed that participants with various degrees of manual dexterity problems preferred attachment/detachment of NovoTwist to conventional screw-thread needles (Fig. 3). Significantly, more respondents with severe manual dexterity effects rated as 4 found NovoTwist easier to attach (79%, P = 0.019) than conventional screw-thread needles. Most respondents with severe manual dexterity effects also found NovoTwist easier to detach than conventional screw-thread needles.
FIG. 3.
Preferences for (left panel) attachment and (right panel) detachment of NovoTwist compared with conventional screw-thread needles.
Among those with manual dexterity problems, NovoTwist was considered more appropriate for everyday injections by 69% (n = 61, P = 0.004), 76% (n = 38, P = 0.002), and 63% (n = 19, P = 0.359) of patients with manual dexterity effect ratings of 2, 3, and 4, respectively. In total, 71% of respondents found NovoTwist the most appropriate needle for performing everyday injections compared with 29% who found screw-thread needles more appropriate to handle (P < 0.001).
In everyday use, 59% of participants were very confident that they had correctly attached NovoTwist versus 47% of respondents for the conventional screw-thread needles. After removing NovoTwist from the pen, 70% of respondents found NovoTwist very easy to dispose of; 46% of respondent considered the disposal of screw-thread needles to be very easy. Fifty-nine percent of respondents also selected NovoTwist as the safest needle to handle compared with 41% who found screw-thread needles safer to handle (P = 0.055).
Discussion
This usability test assessed various aspects of NovoTwist versus conventional screw-thread needles, including ease of use, convenience, safety, and confidence of use. Adults with diabetes and manual dexterity preferred NovoTwist to conventional screw-thread needles. Despite no previous experience of this novel and innovative needle system, participants rated NovoTwist as easier to use, easier to attach/detach, and more appropriate for everyday use. Most respondents with manual dexterity problems also rated NovoTwist as a safer needle to handle compared with conventional screw-thread needles. The overall preference for NovoTwist was not significant in those with the highest manual dexterity disability, but there was a strong trend towards significance (P = 0.063). However, the lack of significance in those with the highest manual dexterity disability may simply reflect the small number of respondents in this group. Nevertheless, participant response to NovoTwist is encouraging as it is a novel needle with which the patients had no previous experience, compared with conventional screw-thread needles that patients may have used for several years.
The participants in this test represent users of several different pens and needles from a range of manufacturers. Therefore, it is reasonable to assume that respondents were not biased by previous experience of pens and needles from a single manufacturer. Indeed, a previous study showed that patients preferred attaching NovoTwist on Next Generation FlexPen compared with NovoFine needles.35 The present test expands on this, not only showing that this preference exists among patients with different levels of impaired manual dexterity, but also showing preference for NovoTwist versus a variety of other needles. Previous studies have shown that among users of insulin injection pens most preferred the use of Next Generation FlexPen,27–31,35,37 and the introduction of the NovoTwist needle is likely to increase patient preference for this system. However, no attempt was made in this test to determine how important needle preference may be in overall patient preference for pen–needle systems.
Manual dexterity disability is a frequent problem in patients with diabetes, with polyneuropathy eventually affecting approximately 40% of patients with diabetes.38–40 This may be an underestimate because locomotor disease, which may also affect manual dexterity in those with upper limb involvement, was found to be present in 75% of patients with diabetes.41 Furthermore, reduced manual dexterity was recently found to be a frequent comorbidity in both young and old age groups of patients with type 2 diabetes.42 In this test, there were several causes of manual dexterity disability, with peripheral neuropathy, arthritis, and tremor being the most common. However, despite the high incidence of manual dexterity disability in diabetes patients we were unable to find a standardized method of measuring manual dexterity. Therefore, a limitation of the test was the use of the subjective manual dexterity disability score, which was used in the absence of available standardized tools for measuring manual dexterity.
Several studies have reported reduced injection pain with insulin regimens using insulin pens compared with those using conventional syringes.43–49 Indeed, 5-mm × 32-gauge needles such as NovoTwist have previously been found to be much less painful than longer needles for the administration of insulin in children and adults with a minimum risk of intramuscular injection and without major backflow compared to 6-mm needles.18 Although pain perception was not assessed in this test, the reduced injection pain from 5-mm NovoTwist needles and reduced risk of intra-muscular injection may further enhance the perception of safety with NovoTwist in a real-life setting.
Collectively, the attributes of NovoTwist may alleviate the burden of performing everyday insulin injections in adults with manual dexterity through its ease of use with attachment and detachment and positive perception of safe handling. When used with FlexPen, the NovoTwist system may help to build confidence with self-injections and thereby increase adherence to therapy.
Acknowledgments
This test was supported by Novo Nordisk A/S, Bagsværd, Denmark. Editorial assistance was provided by John Clarke at ESP Bioscience (Sandhurst, UK) supported by Novo Nordisk A/S.
Author Disclosure Statement
S.K.L. and G.T.-B. are employees of Novo Nordisk A/S. B.H. has previously worked as a consultant for Novo Nordisk A/S.
References
- 1.Bashoff EC. Beaser RS. Insulin therapy, the reluctant patient. Overcoming obstacles to success. Postgrad Med. 1995;97:86–90. 93–96. [PubMed] [Google Scholar]
- 2.Fu AZ. Qiu Y. Radican L. Impact of fear of insulin or fear of injection on treatment outcomes of patients with diabetes. Curr Med Res Opin. 2009;25:1413–1420. doi: 10.1185/03007990902905724. [DOI] [PubMed] [Google Scholar]
- 3.Korytkowski M. Niskanen L. Asakura T. FlexPen: addressing issues of confidence and convenience in insulin delivery. Clin Ther. 2005;27(Suppl B):S89–S100. doi: 10.1016/j.clinthera.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Mollema ED. Snoek FJ. Ader HJ. Heine RJ. van der Ploeg HM. Insulin-treated diabetes patients with fear of self-injecting or fear of self-testing: psychological comorbidity and general well-being. J Psychosom Res. 2001;51:665–672. doi: 10.1016/s0022-3999(01)00229-x. [DOI] [PubMed] [Google Scholar]
- 5.Mollema ED. Snoek FJ. Heine RJ. van der Ploeg HM. Phobia of self-injecting and self-testing in insulin-treated diabetes patients: opportunities for screening. Diabet Med. 2001;18:671–674. doi: 10.1046/j.1464-5491.2001.00547.x. [DOI] [PubMed] [Google Scholar]
- 6.Albano S. Assessment of quality of treatment in insulin-treated patients with diabetes using a pre-filled insulin pen. The ORBITER Study Group. Acta Biomed. 2004;75:34–39. [PubMed] [Google Scholar]
- 7.Bohannon NJ. Insulin delivery using pen devices. Simple-to-use tools may help young and old alike. Postgrad Med. 1999;106:57–58. doi: 10.3810/pgm.1999.10.15.751. 61–64, 68. [DOI] [PubMed] [Google Scholar]
- 8.Chantelau E. Schiffers T. Schutze J. Hansen B. Effect of patient-selected intensive insulin therapy on quality of life. Patient Educ Couns. 1997;30:167–173. doi: 10.1016/s0738-3991(96)00964-0. [DOI] [PubMed] [Google Scholar]
- 9.Kadiri A. Chraibi A. Marouan F. Ababou MR. el Guermai N. Wadjinny A. Kerfati A. Douiri M. Bensouda JD. Belkhadir J. Arvanitis Y. Comparison of NovoPen 3 and syringes/vials in the acceptance of insulin therapy in NIDDM patients with secondary failure to oral hypoglycaemic agents. Diabetes Res Clin Pract. 1998;41:15–23. doi: 10.1016/s0168-8227(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 10.Lombardo F. Salzano G. Messina MF. De Luca F. Compliance and administration methods in management of type 1 diabetes. Acta Biomed. 2005;76(Suppl 3):66–69. [PubMed] [Google Scholar]
- 11.Summers KH. Szeinbach SL. Lenox SM. Preference for insulin delivery systems among current insulin users and nonusers. Clin Ther. 2004;26:1498–1505. doi: 10.1016/j.clinthera.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Coscelli C. Lostia S. Lunetta M. Nosari I. Coronel GA. Safety, efficacy, acceptability of a pre-filled insulin pen in diabetic patients over 60 years old. Diabetes Res Clin Pract. 1995;28:173–177. doi: 10.1016/0168-8227(95)01092-r. [DOI] [PubMed] [Google Scholar]
- 13.Keith K. Nicholson D. Rogers D. Accuracy and precision of low-dose insulin administration using syringes, pen injectors, and a pump. Clin Pediatr (Phila) 2004;43:69–74. doi: 10.1177/000992280404300109. [DOI] [PubMed] [Google Scholar]
- 14.Lteif AN. Schwenk WF. Accuracy of pen injectors versus insulin syringes in children with type 1 diabetes. Diabetes Care. 1999;22:137–140. doi: 10.2337/diacare.22.1.137. [DOI] [PubMed] [Google Scholar]
- 15.Hornquist JO. Wikby A. Andersson PO. Dufva AM. Insulin-pen treatment, quality of life and metabolic control: retrospective intra-group evaluations. Diabetes Res Clin Pract. 1990;10:221–230. doi: 10.1016/0168-8227(90)90065-2. [DOI] [PubMed] [Google Scholar]
- 16.Piscopo MA. Chiesa G. Bonfanti R. Viscardi M. Meschi F. Chiumello G. Quality of life and new devices in the management of type 1 diabetes in children and adolescents. Acta Biomed. 2003;74(Suppl 1):21–25. [PubMed] [Google Scholar]
- 17.Tallroth G. Karlson B. Nilsson A. Agardh CD. The influence of different insulin regimens on quality of life and metabolic control in insulin-dependent diabetics. Diabetes Res Clin Pract. 1989;6:37–43. doi: 10.1016/0168-8227(89)90055-7. [DOI] [PubMed] [Google Scholar]
- 18.Hofman PL. Behrensdorf Derraik JG. Pinto TE. Tregurtha S. Faherty A. Peart JM. Drury PL. Robinson E. Tehranchi R. Donsmark M. Cutfield WS. Defining the ideal injection techniques when using 5-mm needles in children and adults. Diabetes Care. 2010;33:1940–1944. doi: 10.2337/dc10-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwanaga M. Kamoi K. Patient perceptions of injection pain and anxiety: a comparison of NovoFine 32-gauge tip 6mm and Micro Fine Plus 31-gauge 5mm needles. Diabetes Technol Ther. 2009;11:81–86. doi: 10.1089/dia.2008.0027. [DOI] [PubMed] [Google Scholar]
- 20.McKay M. Compion G. Lytzen L. A comparison of insulin injection needles on patients' perceptions of pain, handling, and acceptability: a randomized, open-label, crossover study in subjects with diabetes. Diabetes Technol Ther. 2009;11:195–201. doi: 10.1089/dia.2008.0054. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz S. Hassman D. Shelmet J. Sievers R. Weinstein R. Liang J. Lyness W. A multicenter, open-label, randomized, two-period crossover trial comparing glycemic control, satisfaction, and preference achieved with a 31 gauge × 6 mm needle versus a 29 gauge × 12.7 mm needle in obese patients with diabetes mellitus. Clin Ther. 2004;26:1663–1678. doi: 10.1016/j.clinthera.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Gill HS. Prausnitz MR. Does needle size matter? J Diabetes Sci Technol. 2007;1:725–729. doi: 10.1177/193229680700100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solvig J. Needle length affects insulin deposition in normal and obese diabetes patients [abstract] Diabetes. 2001;50(Suppl 2):A132. [Google Scholar]
- 24.Arendt-Nielsen L. Egekvist H. Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosens Mot Res. 2006;23:37–43. doi: 10.1080/08990220600700925. [DOI] [PubMed] [Google Scholar]
- 25.Asakura T. Seino H. Handling and safety of two insulin injection pens (FlexPen® and OptiClik®) in insulin-naive type 2 diabetes patients [abstract] Diabetes. 2006;55(Suppl 1):A457. [Google Scholar]
- 26.Siegmund T. Blankenfeld H. Schumm-Draeger PM. Comparison of usability and patient preference for insulin pen needles produced with different production techniques: “thin-wall” needles compared to “regular-wall” needles: an open-label study. Diabetes Technol Ther. 2009;11:523–528. doi: 10.1089/dia.2009.0048. [DOI] [PubMed] [Google Scholar]
- 27.Asakura T. Seino H. A comparison of the usability of two types of disposable pen (FlexPen versus Humalog Kit) containing rapid-acting insulin analogues [abstract] Diabetes Metab. 2003;29:S236. [Google Scholar]
- 28.Dreyer M. Comparison of metabolic control, safety, handling and patient acceptance of FlexPen with NovoLet in type 1 and type 2 diabetes patients. J Clin Res. 2005;8:1–7. [Google Scholar]
- 29.Korytkowski M. Bell D. Jacobsen C. Suwannasari R. A multicenter, randomized, open-label, comparative, two-period crossover trial of preference, efficacy, and safety profiles of a prefilled, disposable pen and conventional vial/syringe for insulin injection in patients with type 1 or 2 diabetes mellitus. Clin Ther. 2003;25:2836–2848. doi: 10.1016/s0149-2918(03)80337-5. [DOI] [PubMed] [Google Scholar]
- 30.Niskanen L. Jensen LE. Rastam J. Nygaard-Pedersen L. Erichsen K. Vora JP. Randomized, multinational, open-label, 2-period, crossover comparison of biphasic insulin aspart 30 and biphasic insulin lispro 25 and pen devices in adult patients with type 2 diabetes mellitus. Clin Ther. 2004;26:531–540. doi: 10.1016/s0149-2918(04)90055-0. [DOI] [PubMed] [Google Scholar]
- 31.Rubin RR. Peyrot M. Quality of life, treatment satisfaction, and treatment preference associated with use of a pen device delivering a premixed 70/30 insulin aspart suspension (aspart protamine suspension/soluble aspart) versus alternative treatment strategies. Diabetes Care. 2004;27:2495–2497. doi: 10.2337/diacare.27.10.2495. [DOI] [PubMed] [Google Scholar]
- 32.Asakura T. Seino H. Kageyama M. Yohkoh N. Evaluation of injection force of three insulin delivery pens. Expert Opin Pharmacother. 2009;10:1389–1393. doi: 10.1517/14656560903018929. [DOI] [PubMed] [Google Scholar]
- 33.Pfützner A. Reimer T. Frokjaer LPF. Jorgensen C. Prefilled insulin device with reduced injection force: patient perception and accuracy. Curr Med Res Opin. 2008;24:2545–2549. doi: 10.1185/03007990802329264. [DOI] [PubMed] [Google Scholar]
- 34.Rissler J. Jorgensen C. Rye Hansen M. Hansen N-A. Evaluation of injection force dynamics of a modified prefilled insulin pen. Expert Opin Pharmacother. 2008;9:2217–2222. doi: 10.1517/14656566.9.13.2217. [DOI] [PubMed] [Google Scholar]
- 35.Sommavilla B. Jorgensen C. Jensen KH. Safety, simplicity and convenience of a modified prefilled insulin pen. Expert Opin Pharmacother. 2008;9:2223–2232. doi: 10.1517/14656566.9.13.2223. [DOI] [PubMed] [Google Scholar]
- 36.Lytzen L. Ostfeldt L. Comparative assessment of NovoTwist™, a novel insulin pen needle system [abstract] Diabetes. 2009;58(Suppl 1):A509. [Google Scholar]
- 37.Asakura T. Seino H. Nakano R. Muto T. Toraishi K. Sako Y. Kageyama M. Yohkoh N. A comparison of the handling and accuracy of syringe and vial versus prefilled insulin pen (FlexPen) Diabetes Technol Ther. 2009;11:657–661. doi: 10.1089/dia.2009.0006. [DOI] [PubMed] [Google Scholar]
- 38.Loseth S. Mellgren SI. Jorde R. Lindal S. Stalberg E. Polyneuropathy in type 1 and type 2 diabetes: comparison of nerve conduction studies, thermal perception thresholds and intraepidermal nerve fibre densities. Diabetes Metab Res Rev. 2010;26:100–106. doi: 10.1002/dmrr.1049. [DOI] [PubMed] [Google Scholar]
- 39.Miralles-Garcia JM. de Pablos-Velasco P. Cabrerizo L. Perez M. Lopez-Gomez V. Prevalence of distal diabetic polyneuropathy using quantitative sensory methods in a population with diabetes of more than 10 years' disease duration. Endocrinol Nutr. 2010;57:414–420. doi: 10.1016/j.endonu.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Vinik AI. Maser RE. Mitchell BD. Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26:1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 41.Ramchurn N. Mashamba C. Leitch E. Arutchelvam V. Narayanan K. Weaver J. Hamilton J. Heycock C. Saravanan V. Kelly C. Upper limb musculoskeletal abnormalities and poor metabolic control in diabetes. Eur J Intern Med. 2009;20:718–721. doi: 10.1016/j.ejim.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 42.Pfutzner J. Hellhammer J. Musholt PB. Pfutzner AH. Bohnke J. Hero T. Amann-Zalan I. Ganz M. Stephan P. Forst T. Pfutzner A. Evaluation of dexterity and cognitive capacity of patients with insulin treated diabetes mellitus. Diabetes. 2009;58(Suppl 1):A478–A479. doi: 10.1177/193229681100500122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Houtzagers CM. van der Velde EA. Multiple daily insulin injections: a multicentre study on acceptability and efficacy. Neth J Med. 1988;33:16–25. [PubMed] [Google Scholar]
- 44.Houtzagers CM. Visser AP. Berntzen PA. van der Stap H. van Maarschalkerweerd WW. Heine RJ. van der Veen EA. Multiple daily insulin injections improve self-confidence. Diabet Med. 1989;6:512–519. doi: 10.1111/j.1464-5491.1989.tb01219.x. [DOI] [PubMed] [Google Scholar]
- 45.Jefferson IG. Marteau TM. Smith MA. Baum JD. A multiple injection regimen using an insulin injection pen and pre-filled cartridged soluble human insulin in adolescents with diabetes. Diabet Med. 1985;2:493–495. doi: 10.1111/j.1464-5491.1985.tb00690.x. [DOI] [PubMed] [Google Scholar]
- 46.Murray DP. Keenan P. Gayer E. Salmon P. Tomkin GH. Drury MI. O'Sullivan DJ. A randomized trial of the efficacy and acceptability of a pen injector. Diabet Med. 1988;5:750–754. doi: 10.1111/j.1464-5491.1988.tb01102.x. [DOI] [PubMed] [Google Scholar]
- 47.Rex J. Jensen KH. Lawton SA. A review of 20 years' experience with the NovoPen family of insulin injection devices. Clin Drug Investig. 2006;26:367–401. doi: 10.2165/00044011-200626070-00001. [DOI] [PubMed] [Google Scholar]
- 48.Saurbrey N. Arnold-Larsen S. Moller-Jensen B. Kuhl C. Comparison of continuous subcutaneous insulin infusion with multiple insulin injections using the NovoPen. Diabet Med. 1988;5:150–153. doi: 10.1111/j.1464-5491.1988.tb00962.x. [DOI] [PubMed] [Google Scholar]
- 49.Su CC. Chen HS. Lin HD. Glycemic control with different premixed insulin in Taiwanese people with type two diabetes mellitus. J Chin Med Assoc. 2003;66:155–159. [PubMed] [Google Scholar]