Abstract
Objective
To compare two forms of device-specific training – body-weight-supported (BWS) ambulation on a fixed track (TRK) and BWS ambulation on a treadmill (TM) – to comprehensive physical therapy (PT) for improving walking speed in persons with chronic, motor-incomplete spinal cord injury (SCI).
Methods
Thirty-five adult subjects with a history of chronic SCI (>1 year; AIS ‘C’ or ‘D’) participated in a 13-week (1 hour/day; 3 days per week) training program. Subjects were randomized into one of the three training groups. Subjects in the two BWS groups trained without the benefit of additional input from a physical therapist or gait expert. For each training session, performance values and heart rate were monitored. Pre- and post-training maximal 10-m walking speed, balance, muscle strength, fitness, and quality of life were assessed in each subject.
Results
All three training groups showed significant improvement in maximal walking speed, muscle strength, and psychological well-being. A significant improvement in balance was seen for PT and TRK groups but not for subjects in the TM group. In all groups, post-training measures of fitness, functional independence, and perceived health and vitality were unchanged.
Conclusions
Our results demonstrate that persons with chronic, motor-incomplete SCI can improve walking ability and psychological well-being following a concentrated period of ambulation therapy, regardless of training method. Improvement in walking speed was associated with improved balance and muscle strength. In spite of the fact that we withheld any formal input of a physical therapist or gait expert from subjects in the device-specific training groups, these subjects did just as well as subjects receiving comprehensive PT for improving walking speed and strength. It is likely that further modest benefits would accrue to those subjects receiving a combination of device-specific training with input from a physical therapist or gait expert to guide that training.
Keywords: Spinal cord injuries, Ambulation training, Body weight support, Rehabilitation, Walking speed
Introduction
The majority of persons sustaining traumatic spinal cord injury (SCI) ultimately recover some volitional lower-limb motor function (i.e. they are ‘motor-incomplete’).1,2 A subset of these persons may benefit from rehabilitation designed to improve their walking ability,3,4 considered herein to be reflected by an increase in maximum walking speed. However, the optimal ambulation therapy approach remains an open question. Wernig and colleagues described substantial functional improvement in patients with SCI receiving treadmill (TM)-based ambulation training while a portion of their body weight was supported in a harness,5,6 stating, ‘The present results show that the novel (TM) therapy can improve locomotive capabilities of the spinal cord injured persons far beyond the outcome of conventional rehabilitation’.6 The latter approach is known to enhance functional performance of SCI individuals by means of exercising and compensation.7,8
More recent studies utilizing body-weight support (BWS) and TM training have reported an improvement in overground walking ability at both sub-acute (i.e. weeks to months post-injury)4 and chronic (i.e. more than 1 year post-injury)3,9–12 stages following SCI. Body-weight-supported training on a TM has become more common in SCI rehabilitation, whereby the near-constant speed of the TM belt helps to emphasize the rhythmicity of voluntary movements.13–15 Unfortunately, most TMs preclude the use of assistive ambulatory devices (e.g. walker, cane, Lofstrand crutches), and they also minimize the ground reaction forces that would normally come into play during overground walking. Systematic comparison of BWS training on a TM versus overground has not been reported after SCI or other neurological disorders. Moreover, published studies utilizing BWS for gait-training in persons with chronic SCI are few in number. Although early initiation of physical activity after SCI could be crucial,16 chronically injured individuals may still benefit from a concentrated period of ambulation training.3,10,12,13,17,18 Based on animal studies, training-mediated functional recovery after SCI appears to be task specific.16 Consequently, for human therapy one might expect any intervention which influences and/or incorporates the act of walking to be effective in promoting locomotor recovery. In the recent Spinal Cord Injury Locomotor Trial (SCILT), comparison of conventional physical therapy (PT) and BWS TM ambulation training did not reveal significant differences in improving walking ability after acute SCI.4,19,20 However, the conclusion by Wernig and colleagues that use of a training device (i.e. TM + BWS) is far superior to conventional PT for improving walking in persons with chronic SCI remains uncorroborated by an independent laboratory.
In the present study, we compared three training methods for improving walking speed in individuals with chronic, motor-incomplete SCI. For two of these training methods, we limited the amount of expert advice made available to subjects, focusing on the role played by the training device for mediating any improvements seen. One method utilized comprehensive PT; this can be considered as a study comparison group, in that it is consistent with conventional rehabilitation methods. The other two methods each used BWS, either over a moving TM, or over fixed ground using an overhead monorail track and trolley apparatus (TRK). We included the BWS + TM method as an example of a ‘new’ rehabilitation approach for improving walking after SCI, and in an attempt to replicate the findings of Wernig and colleagues5,6 in a more stringent study design. Finally, we included the third training approach (BWS + overground walking) as a still more novel, but also more realistic, method of rehabilitation after SCI that – at the time this study was conceived – had not been previously described within a randomized clinical trial.
Methods
The study was carried out at The Miami Project to Cure Paralysis (‘TMP’) of the University of Miami School of Medicine, and at the Department of Neurosurgery of Upstate Medical University (‘Upstate’). All subjects gave written informed consent to participate. The identical study protocol was approved by each institution's institutional review board.
Inclusion/exclusion criteria
The study included subjects who: (1) were 16–70 years old; (2) had sustained injury to the spinal cord at or rostral to the Tl0 (vertebral) level; (3) were injured at least one year prior to enrollment; (4) had voluntary movement in at least one leg; (5) were able to rise to standing from a seated position with no more than moderate assistance, and independently advance at least one leg; (6) agreed to maintain their current routine of medications and activity levels while training; and (7) received medical clearance from the study physician (assessment of general health and bilateral hip/knee radiographs to rule out significant pathology, e.g. significant osteoarthritis; heterotopic ossification; joint subluxation).
Exclusion criteria were: (1) degenerative myelopathy, neoplasm, or congenital spinal cord anomalies; (2) prior gait training with BWS; (3) the need for bilateral knee-ankle-foot orthoses for standing; and (4) the ability to jog or run.
Recruitment and randomization
Subjects were recruited regionally, nationally, and internationally. If the subject resided regionally, he or she visited the lab to demonstrate functional mobility. If the subject was unable to visit, we asked them to have their mobility videotaped and sent in for review. In both cases, if appropriate subjects met the inclusion mobility criteria, candidates were eligible for training assignment. In groups of three, subjects were randomly assigned to the PT, TRK, or TM training group. Assignment was done by a staff member not associated with the study, who drew printed labels from a box.
Procedure
Subjects in the PT group received a structured program delivered by a licensed physical therapist. Therapy was individualized for each subject and typically involved gait, balance, and functional activity modalities as well as strengthening, stretching, and aerobic (e.g. arm-crank) exercises. The physical therapist used verbal and tactile cues to improve different aspects of the subject's mobility. The therapist maintained a detailed log of activity and duration for each training session, along with the average heart rate (see below).
The BWS training groups used 30% BWS provided by a parachute-type harness (Maine AntiGravity Systems, Portland, ME, USA; Biodex Medical Systems, Shirley, NY, USA), adjusted to be tight across the lower pelvis, but loose about the thighs (thereby allowing for unrestricted hip flexion and extension). It was common for the harness to slip during a training session, requiring adjustment. The amount of body weight being supported was determined using either load-cells attached to the lifting bar (all TM and some TRK subjects) or force plates along the walking path (remaining TRK subjects). Load-cells provided a continual measure of BWS. Force plate measures were updated once per lap of the monorail track. For track training, the harness was supported directly under a six-wheel trolley mounted on a 4″ I-beam monorail supported by a free-standing series of 4″ schedule-80 stainless-steel pipes and fittings. The track length was ∼26 m, consisting of two 10-m straight sections connected on either end with 3-m lengths curved to 180° (curve radius ∼0.8 m). For TM training, suspension was accomplished by a ceiling-mounted pulley system powered by an electric winch. The support rails on either side of the TM were removed to prevent subject unloading through the arms, but grab-handles at the front of the TM (directly under the TM control panel) were left in place; subjects were free to use these for stabilization as desired. If subjects desired to use the TM grab-handles but lacked adequate grip-force to hold them, their hands were fastened to the grab-handles with elastic wraps (i.e. ‘Ace’ bandages).
Subjects in the BWS groups were helped by an assistant who did not have any formal training in rehabilitation. This person provided encouragement to the subjects during training sessions, but was told not to offer any training-specific advice. Our intent through this restriction was to test the training modality (track or TM) by itself in either of these groups against the more broad-ranging approach that the physical therapist could adopt.
For all groups, training was delivered 3 days/week for 13 weeks (39 sessions). A period of 13 weeks was chosen based on the time to plateau of overground walking speed and distance for a study using functional electrical stimulation for ambulation in a population with thoracic-level SCI.21 Each session was restricted to a maximum of 60 minutes of therapy, in order to mimic a typical outpatient rehabilitation schedule. All subjects walked at a self-selected pace. Within each session, they were free to change their training pace, and to take as many rests as they desired. The duration of training was recorded for each session, as was the distance walked (for subjects in the two BWS groups). When subjects in the TRK or TM groups needed manual assistance to advance the leg during walking, this was provided and documented by the training assistant. In all cases, assistance was provided at the subject's foot/ankle; assistance at the knee or pelvis was never provided. Subjects in the PT and TRK groups were permitted to change the type of assistive ambulatory devices they used during training sessions. For subjects in the PT group, change in the use of assistive ambulatory devices was typically recommended by a therapist, whereas for the TRK-based subjects, an assistive ambulatory device was added or changed only at that person's request. When training, all subjects wore a heart-rate monitor (Polar Electro Oy, Finland) to record the heart rate (beats/minute). For each session, the total number of heart beats, as an indirect measure of total work performed,22 was calculated as the product of average heart-rate X training time. Additional information obtained at each training session included the subject's weight, levels of activity outside of the lab setting, changes in health status (e.g. pain; spasticity; illness) and current medications. Subjects in all three groups wore socks and runners. When an ankle-foot orthosis was used, it was the molded type and was worn inside the shoe.
Outcome measures
The maximum walking speed averaged over a 10-m distance served as the primary outcome measure for this study. Secondary outcome measures included: (1) balance; (2) fitness (peak oxygen uptake during arm-crank); (3) muscle strength; (4) functional independence; and (5) quality of life. All measures – including maximum walking speed – were conducted by persons blinded to each subject's training assignment. Tests were carried out over a 2-day period, immediately before training started, and following its conclusion.
For the walking test, subjects were asked to perform three 2-minute walks at self-selected paces: (1) much slower than normal; (2) normal; and (3) as quickly as possible. Each trial was separated by a 3–5-minute resting period during which the subject was seated, and the heart rate was monitored for recovery. The maximal walking speed in meters/second (m/second) was calculated based upon the time needed to traverse the first 10-m distance covered during the final 2-minute walk. All subjects performed the same duration of walking at submaximal levels (effectively serving as a warm-up) prior to walking at their maximal speed to generate this study's primary outcome measure (maximum walking speed). During this test, subjects wore a BWS harness and walked underneath the monorail track, but they did not receive any BWS (i.e. the harness was used solely as a safety device). Subjects were free to use whatever assistive device(s) they desired for their initial walking test (e.g. AFO; walker), with the caveat that they must use the same device(s) during the final evaluation.
On a different day, subjects performed an upper body maximal graded exercise test (GXT) in order to measure peak work capacity and oxygen uptake. The arm ergometry protocol was performed in the upright position with a starting resistance of 200 kg/m/minute (33 W) increasing 50 kg/m/minute (8 W) every 3 minutes in a thermoneutral laboratory. In Miami, testing was performed on a Monarch 881E (Varberg, Sweden) upper body ergometer (UBE) and metabolic data were collected and analyzed with a Beckman MMC Horizon system (Anaheim, CA, USA). In Syracuse, testing was done with a Scifit Pro 1000 UBE (Tulsa, OK, USA) and metabolic data were collected and analyzed using a Cosmed Quark b2 system (Rome, Italy).
Testing at both sites involved calibration of both UBEs to manufacturer specifications to ensure valid and reliable workloads during testing. Seat position and ergometer adjustments were made individually so that the fulcrum was horizontal with the subject's shoulder, and elbow and shoulder were not fully extended when the crank arm was in the away position. In subjects who could not maintain an upright posture on the UBE, stabilizing straps were placed around the trunk to secure the subject against the ergometer's seatback. In subjects who could not grasp the crank handles, Ace bandages were used to secure the hands to the ergometer's crank handles.
Both the MMC Horizon and Cosmed Quark b2 metabolic cart systems were calibrated per manufacturer's guidelines prior to each test session using gases with the same known concentrations (16% O2 and 5% CO2). The turbine calculation for both systems was made with a 3-l syringe to ensure accurate volume measurements of the turbine flowmeter. Peak VO2 values (defined as the highest VO2 (averaged over the last 10 s) attained during the test) obtained at both sites fell within a common range for both the pre- and post-testing, indicating consistency of instrumentation and methodology across both study sites.
Standard 12-lead electrocardiogram and cardiopulmonary responses were obtained during the 2-minute rest, 2-minute warm-up, exercise test, and 5-minute recovery. Heart rate was continually recorded during testing and recovery. The Borg 6–20 perceived exertion scale was recorded during the last minute of each stage and at peak exercise. In addition, blood pressure was recorded at rest (supine, sitting, and standing), peak exercise, and recovery.
After the ergometer adjustments were made, the subject was fitted with the facemask over the mouth and nose and told to sit quietly for 2 minutes to adjust to the breathing pattern of the facemask. After rest, the subject was instructed to crank the UBE at 60 rpm at 0 kg/m/minute (0 W) for 2 minutes to acclimate to the cranking rpm. After the 2-minute warm-up, the resistance was increased to 200 kg/m/minute (33 W). Resistance was increased 50 kg/m/minute (8 W) every 3 minutes until volitional fatigue or failure to maintain a cadence of 60 rpm for 5 continuous seconds.
Smaller power output increases of 50 kg/m/minute (8 W) per 3-minute stage were utilized so that the targeted test duration was from 8 to 12 minutes, which is more reflective of functional ability. In addition, the smaller power outputs were selected because of the known inability to stimulate the autonomic and cardiovascular systems to support aerobic metabolism that is present in persons with SCI at or rostral to the T6 neurologic segment.23 American College of Sports Medicine (ACSM) guidelines suggest smaller increments in power output from 5 to 20 W per stage depending on the functional level of SCI, muscular strength, and conditioning level of the subject.24
The Tinetti scale25 was used to assess balance. This test measures movements of the trunk in the forwards, backwards, and sideways directions with eyes open versus closed, and with arms relaxed versus being held in the air, in both standing and sitting positions. A value ranging from 0 to 2 was assigned to each movement, and the total score (maximum = 16) was calculated.
The ASIA International Standard (AIS) manual muscle test (MMT) was used to assess muscle contraction strength.26 The upper and lower extremity motor scores (UEMS and LEMS, range 0–50 each) and the total MMT score (sum of UEMS and LEMS; range 0–100) were determined.
The motor domain component of the FIM measure was used to quantify self-reported activities of daily living, including self-care, sphincter control and mobility. Of the 13 original motor items, 11 were included for analysis. Subjects were asked about how much assistance they needed for eating, grooming, bathing, dressing, toileting, transfers, and management of bowel and bladder.
The quality-of-life survey incorporated a subset of items from two existing questionnaires (Satisfaction with Abilities and Well-Being Scale (SAWS) and the SF-36 Short-Form Health Survey). Thirteen components from the SAWS scale27 were used to assess subjective well-being (Table 3-1). Subjects were also asked to respond to each of four questions taken from the SF-36 test,28 addressing general health at the present time and one year ago (Table 3-2), a mental component of health perception (Table 3-4), and vitality (energy and fatigue) over the past 4 weeks (Tables 3-3 and 3-5). In addition to conducting this survey immediately before and after training, this survey was also mailed to subjects for completion 1 month after finishing the training protocol.
Table 3.
Changes in QOL scores after ambulation training
| Quality-of-life components | PT | TRK | TM | All 3 groups |
|---|---|---|---|---|
| 1. Satisfaction with disabilities and well-being (SAWS) | ||||
| Pre-training | 35.6 ± 9.9 | 35.9 ± 6.9 | 39.3 ± 8.3 | 36.7 ± 8.3 |
| Post-training | 29.0 ± 7.9 | 32.4 ± 7.6 | 35.2 ± 8.7 | 32.0 ± 8.1 |
| Re-evaluation 1 month later | 31.4 ± 5.5 | 32.4 ± 6.4 | 31.2 ± 7.8 | 31.7 ± 6.1 |
| 2. General health perception | ||||
| Pre-training | 3.2 ± 0.8 | 2.6 ± 0.8 | 2.4 ± 1.0 | 2.8 ± 0.9 |
| Post-training | 2.8 ± 0.8 | 2.5 ± 0.7 | 2.6 ± 1.1 | 2.6 ± 0.8 |
| Re-evaluation 1 month later | 2.9 ± 0.7 | 2.6 ± 1.0 | 2.2 ± 1.3 | 2.6 ± 1.0 |
| 3. Energy | ||||
| Pre-training | 12.8 ± 4.1 | 12.4 ± 3.6 | 10.9 ± 3.9 | 12.2 ± 3.8 |
| Post-training | 11.8 ± 2.9 | 10.8 ± 3.0 | 10.9 ± 3.2 | 11.1 ± 3.0 |
| Re-evaluation 1 month later | 11.4 ± 2.7 | 14.7 ± 2.7 | 9.8 ± 4.5 | 12.1 ± 3.7 |
| 4. Mental health perception | ||||
| Pre-training | 7.6 ± 2.0 | 8.1 ± 1.8 | 8.9 ± 1.8 | 8.1 ± 1.8 |
| Post-training | 7.5 ± 1.6 | 8.0 ± 1.9 | 8.7 ± 1.7 | 8.0 ± 1.8 |
| Re-evaluation 1 month later | 7.3 ± 1.7 | 7.7 ± 2.0 | 7.0 ± 1.9 | 7.3 ± 1.7 |
| 5. Fatigue | ||||
| Pre-training | 24.0 ± 2.7 | 23.4 ± 3.7 | 25.1 ± 3.2 | 24.0 ± 3.2 |
| Post-training | 24.6 ± 2.8 | 24.6 ± 2.5 | 24.4 ± 3.2 | 24.6 ± 2.7 |
| Re-evaluation 1 month later | 23.6 ± 3.4 | 23.2 ± 3.9 | 25.0 ± 3.7 | 23.8 ± 3.5 |
For measures #1–3, a lower score is desirable; for measures #4–5, a higher score is desirable. Scores are mean ± SD. Measures #2–5 are derived from SF-36. Post-training scores that are significantly-better than pre-training values are in italics. There were no values that were significantly worse than pre-training values.
Balance, strength, functional independence, and quality of life were assessed by the same physical therapist, who was blinded to training assignment. In order to minimize investigator bias, this person was on-site only for testing sessions; she did not take part in subject training, nor was she aware of the training assignment for each subject. The same was true for testing of arm-ergometry and walking performance: investigators performing measurements did not know the training groups of individual subjects.
Analysis
This study generally utilizes a mixed-factorial experimental design, with one independent measures factor representing participant's group and one repeated-measures factor representing the multiple observations over time. We assessed each outcome separately; however, in all cases we submitted participant's repeated outcomes observations to a two-factor mixed-model analysis of variance (ANOVA), setting two-tailed alpha to 0.05, with Bonferroni adjustments for multiple comparisons. Prior to analysis, we examined the distributions of our data in order to assess whether data transformations would be necessary in order to meet the assumption of the ANOVA statistic that the data be normally distributed. Measures of speed were significantly positively skewed and required a square root transformation for normalization.29 Spearman's correlation was used to investigate paired relationships (e.g. maximum speed versus balance). Statistical analysis was done with SPSS.
Results
Subjects
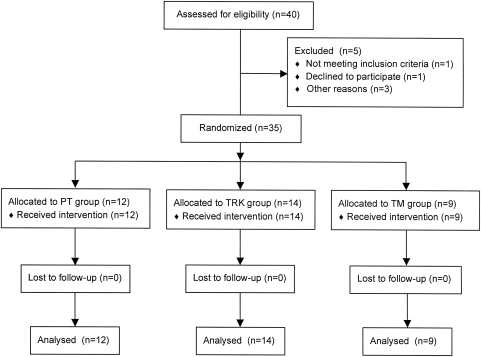
A total of 40 individuals with SCI consented to participate in the study and were randomized, as illustrated in Fig. 1. Thirty-five subjects (5 female, 30 male) completed the training program and follow-up assessments. Four of the five subjects who did not complete the study had been randomized to the TM group; the remaining subject was randomized to the PT group. Demographic characteristics of the study participants are listed in Table 1. There was no significant difference across training groups in subject age or gender, and each group was dominated by persons in the AIS ‘D’ category. Consistency across groups was lacking in the distribution by injury level, however, in that a high percentage of persons in the TM group had sustained cervical injury (89%), whereas the incidence of cervical injury was considerably lower within the TRK (57%) and PT (58%) training groups. Table 2 details the pre-training functional walking status of our subjects. All study participants prior to training initiation were able to ambulate at least 10 m overground, with the exception of subject #33. When visiting our laboratory for testing and training, eight subjects chose to ambulate with assistive devices as their primary mode of mobility, causing us to classify them as ‘wheelchair-independent’ in Table 2. Each of these subjects did use a wheelchair when circumstances called for it. The remaining subjects were dependent upon their wheelchair for their primary mobility at all times.
Figure 1.
CONSORT diagram summarizing numbers of subjects recruited and randomized to each of the three study arms.
Table 1.
Demography
| Subject | Group | Sex | Age | Years post-injury | SCI level | Etiology | AIS score | Spasticity/medication | Pain/medication |
|---|---|---|---|---|---|---|---|---|---|
| 1 | PT | F | 42 | 14 | C6 | Transverse myelitis | D | +/Baclofen | + |
| 2 | PT | M | 22 | 4 | T7 | Trauma | D | +/Baclofen | − |
| 3 | PT | M | 37 | 13 | C7 | Trauma | C | + | +/Carbamazepine |
| 4 | PT | M | 36 | 16 | C5 | Trauma | D | + | + |
| 5 | PT | M | 24 | 4 | C4 | Trauma | D | + | − |
| 6 | PT | M | 29 | 7 | T7 | Vascular | D | + | + |
| 7 | PT | M | 63 | 6 | T7 | Vascular | D | + | − |
| 8 | PT | M | 59 | 2 | C5 | Trauma | D | +/Baclofen | +/Gabapentin |
| 9 | PT | M | 39 | 3 | C5 | Trauma | D | − | + |
| 10 | PT | M | 29 | 1.3 | T4 | Trauma | C | +/Baclofen | + |
| 11 | PT | M | 26 | 1.2 | C5 | Trauma | D | +/Baclofen | − |
| 12 | PT | F | 42 | 25 | T10 | Trauma | D | +/Baclofen | + |
| 13 | TRK | M | 61 | 3 | C3 | Trauma | D | +/Clonidine | − |
| 14 | TRK | F | 24 | 1.2 | T10 | Trauma | C | + | + |
| 15 | TRK | M | 27 | 4 | C6 | Trauma | C | +/Baclofen, tizanidine | + |
| 16 | TRK | M | 30 | 5 | C5 | Trauma | D | +/Baclofen | +/Amitriptyline |
| 17 | TRK | M | 32 | 14 | C6 | Trauma | C | +/Baclofen | − |
| 18 | TRK | M | 23 | 1.4 | T8 | Trauma | C | + | − |
| 19 | TRK | M | 33 | 12 | T3 | Trauma | D | + | +/Acetaminophen |
| 20 | TRK | M | 53 | 37 | C2 | Trauma | D | +/Baclofen | − |
| 21 | TRK | M | 44 | 16 | C3 | Trauma | D | + | + |
| 22 | TRK | M | 38 | 5 | C7 | Trauma | D | + | + |
| 23 | TRK | F | 38 | 6 | T4 | Trauma | C | +/Baclofen | + |
| 24 | TRK | M | 21 | 1.0 | T8 | Trauma | D | + | − |
| 25 | TRK | M | 57 | 2 | C3 | Trauma | D | +/Baclofen | + |
| 26 | TRK | M | 29 | 1.1 | T4 | Trauma | D | +/Baclofen | − |
| 27 | TM | M | 60 | 6 | C4 | Trauma | D | +/Baclofen | +/Rofecoxib |
| 28 | TM | M | 63 | 2 | C4 | Trauma | D | + | +/Acetaminophen |
| 29 | TM | M | 56 | 1.5 | C5 | Trauma | D | +/Baclofen | − |
| 30 | TM | M | 40 | 1.0 | C4 | Trauma | D | +/Baclofen, tizanidine | − |
| 31 | TM | M | 31 | 8 | T8 | Trauma | D | + | + |
| 32 | TM | M | 56 | 5 | C5 | Trauma | D | +/Baclofen | +/Gabapentin |
| 33 | TM | F | 35 | 3 | C3 | Trauma | C | +/Baclofen | + |
| 34 | TM | M | 30 | 12 | C4 | Trauma | D | + | + |
| 35 | TM | M | 19 | 2 | C5 | Trauma | D | + | − |
Abbreviations: PT: physical therapy; TRK: body-weight supported ambulation on a fixed track; TM: body-weight supported ambulation on a treadmill; M: male; F: female; AIS: ASIA International Standards.
Table 2.
Initial functional status
| Subject | Group | Orthoses | Assistive device | WISCI II | Community ambulation |
|---|---|---|---|---|---|
| 1 | PT | AFO | Walker(S) | 9 | W/D |
| 2 | PT | AFO | Walker(S) | 9 | W/D |
| 3 | PT | AFO | Walker(S) | 9 | W/D |
| 4 | PT | AFO | Walker(S) | 9 | W/D |
| 5 | PT | Walker(S) | 13 | W/D | |
| 6 | PT | AFO | Cane | 15 | Ind |
| 7 | PT | 2 AFOs | Lofstrands | 12 | W/D |
| 8 | PT | Quad cane | 19 | Ind | |
| 9 | PT | Walker(S) | 13 | W/D | |
| 10 | PT | AFO | Walker(R) | 9 | W/D |
| 11 | PT | 2 AFOs | Lofstrands | 13 | W/D |
| 12 | PT | – | 20 | Ind | |
| 13 | TRK | Walker(R) | 13 | W/D | |
| 14 | TRK | AFO, KAFO | Walker(S) | 9 | W/D |
| 15 | TRK | AFO | Lofstrands | 12 | W/D |
| 16 | TRK | KAFO | Walker(S) | 9 | W/D |
| 17 | TRK | KAFO | Walker(S) | 9 | W/D |
| 18 | TRK | 2 AFOs | Walker(S) | 9 | W/D |
| 19 | TRK | Lofstrands | 16 | Ind | |
| 20 | TRK | Lofstrands | 16 | W/D | |
| 21 | TRK | AFO | Lofstrands | 12 | Ind |
| 22 | TRK | AFO | Cane | 15 | W/D |
| 23 | TRK | AFO | Walker(S) | 9 | W/D |
| 24 | TRK | AFO | Walker(R) | 9 | W/D |
| 25 | TRK | Cane | 19 | Ind | |
| 26 | TRK | KAFO | Walker(S) | 9 | W/D |
| 27 | TM | 2 AFOs | Walker(S) | 9 | W/D |
| 28 | TM | AFO | Cane | 15 | Ind |
| 29 | TM | KAFO | Walker(S) | 9 | W/D |
| 30 | TM | 2 AFOs | Walker(S) | 9 | W/D |
| 31 | TM | AFO | Lofstrands | 12 | Ind |
| 32 | TM | AFO | Quad cane | 15 | W/D |
| 33 | TM | KAFO | Walker(R) | 3 | W/D |
| 34 | TM | Walker(R) | 13 | W/D | |
| 35 | TM | AFO | Lofstrands | 12 | W/D |
Abbreviations: WISCI II: walking index for spinal cord injury; AFO: ankle-foot orthosis; KAFO: knee-ankle-foot orthosis; Walker(S): standard (4-point) walker, Walker(R): rolling walker; W/D: wheelchair dependent; Ind: wheelchair independent.
Training data
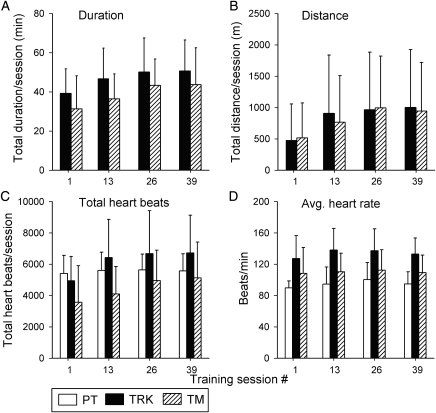
Fig. 2 summarizes several training parameters for different stages of the training protocol (averaged across subjects for sessions 1, 13, 26, and 39). On average, subjects in the TRK and TM groups tended to increase their duration of training (Fig. 2A) throughout the first 26 training sessions, and then plateau beyond this time. Actual durations for subjects in the PT group are not illustrated, because sessions almost always lasted a full 60 minutes.
Figure 2.
Summary of training parameters and heart-rate data for training sessions #1, 13, 26 and 39 for subjects in the TRK (filled bars), TM (diagonal bars), and PT (open bars) groups. Values are the group averages (±SD) of the total duration of ambulation (A; in minutes), total distance of ambulation (B; in meters), total number of heart beats while training (C), and average heart-rate (D) during the time spent in training. Note that duration and distance measures are provided for the TRK and TM groups only, since subjects in the PT group routinely trained for 1 hour each session, and walking distances within each PT training session were not tallied.
At training session #39, there was an extremely wide range of total distance walked (Fig. 2B) per session for subjects in both the TRK (range = 20–3828 m) and TM (range = 4.2–3218 m) groups, yet the mean distances (888 m; 843 m) were remarkably similar for the two groups. Fig. 2B shows that – like walking duration – mean walking distance also increased in a steady manner through training session #26, and then appeared to plateau. No walking distances are plotted for the PT group, since the walking route frequently changed, and stairs were sometimes incorporated into the walk-training portion of the PT sessions.
We measured heart rate to gain some sense of the metabolic demands imposed by the different training methods. Each group showed increases in total heart beats through training session #26 (Fig. 2C). Surprisingly, the total heart beat value was higher in the PT group than in the TM group. However, after correcting for training duration, subjects in the TRK group achieved the highest average heart rate during training, whereas those in the PT group had the lowest average heart rate (Fig. 2D).
For subjects in the PT group, the average durations (in minutes) for key modalities were as follows: walking: 15.5 ± 7.9; strengthening: 17.0 ± 7.1; stretching: 13.2 ± 4.0; cycling (arm-crank (mostly) and stationary bike): 15.5 ± 6.3; balance training: 12.6 ± 5.9; and functional activities (e.g. sit-to-stand; transfers): 5.1 ± 5.0 minutes. Note that the sum of these averaged totals exceeds 60 minutes, because not all modalities were included within each training session. In practice, individual PT training sessions were strictly limited to a maximum of 60-minute duration.
We documented each subject's requirement for assistance during gait, and the reliance on particular types of assistive devices by subjects in all three groups. At the beginning of training, two of the nine subjects in the TM group needed manual assistance to advance their weaker leg during stepping. By training session 24 (i.e. after ∼8 weeks), both of these subjects could train without need of any manual assistance. All subjects in the TM group used the forward-placed grab handles for stabilization during gait. However, the position of these handles precluded any useful unloading of the lower limbs during gait.
None of the subjects in the TRK group required assistance to advance their leg, even during the initial training sessions. At their own initiative, two of these subjects adopted a less-restrictive assistive device during training, both shifting from a walker to Lofstrand crutches.
Ten of the 11 subjects in the PT group adopted a less-restrictive assistive ambulatory device – or abandoned their original device altogether – during the ‘walking’ component of their PT session.
Speed
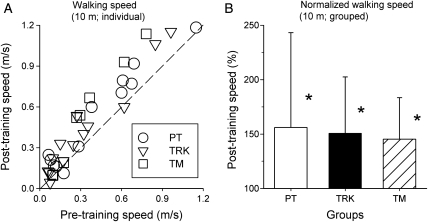
The range of maximum walking speeds at the outset of training varied from a low of 0 m/second (one subject (#33) was unable to take any overground steps initially) to a high of 1.2 m/second. There was no significant difference in pre-training walking speed among PT, TRK, and TM groups. Almost all subjects – regardless of training group – increased their maximum 10-m walking speed after completing the training program (Fig. 3A). Combining all subjects, independent of training group, the average pre- and post-training maximal walking speeds over a 10-m distance were 0.35 ± 0.29 and 0.47 ± 0.35 m/second, respectively. Broken down to group means, the pre-training speeds were: (1) PT: 0.41 ± 0.34 m/second; (2) TRK: 0.33 ± 0.29 m/second; and (3) TM: 0.30 ± 0.26 m/second. The mean post-training speeds were: (1) PT: 0.51 ± 0.36 m/second; (2) TRK: 0.44 ± 0.33 m/second; and (3) TM: 0.46 ± 0.40 m/second. Fig. 3B illustrates the percent change in mean walking speed for each of the three groups. Analysis revealed a significant increase in walking speed across groups (P < 0.001); however, the Group main effect and the Interaction of Group × Time were not significant. Bonferroni-adjusted contrasts revealed significant increases in speed Pre-to-Post in all three groups (P < 0.01 for each comparison). Thus, each of the three training groups showed a significant increase in maximum walking speed over a 10-m distance, but there were no significant differences between groups in the amount of improvement.
Figure 3.
Results of the 10-m maximal walking speed test in subjects of three training groups before and after completion of training. Individual results for each subject in the PT (○), TRK (∇), and TM (□) groups are plotted in ‘A’, where each data point is positioned at the intersection of each individual's post-training (ordinate) and pre-training (abscissa) values. The dashed line shows where data points would lie if there was no difference between pre-training and post-training values. In ‘B’, each subject's post-training measure was expressed as a percentage of his/her pre-training data, then averaged within each training group and plotted as group averages (±SD). All three groups showed a significant (*) post-training increase in walking speed over this 10 m distance.
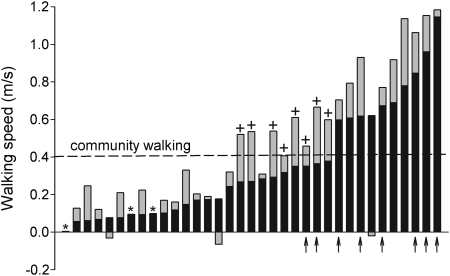
Fig. 4 summarizes additional features of walking speed not evident in Fig. 3. Most subjects (n = 30) walked at a faster speed after training. A walking speed of 0.4 m/second (indicated by dashed horizontal line) is considered to be a minimal speed for walking in the community.30 The majority of subjects (n = 25) were unable to walk at this speed at the beginning of training, but 8 of these 25 subjects (indicated by ‘+’ signs) did achieve a post-training maximum walking speed that equaled or exceeded 0.4 m/second by the end of the training sessions. Of these eight subjects, six were wheelchair-dependent prior to training onset. Four had trained on the track, three were from the TM group, and one subject had been in the PT group.
Figure 4.
Maximal 10-m walking speeds before and after training for all 35 subjects studied. Data are arranged from the slowest to the fastest pre-training walking speed (i.e. independent of training group). The pre-training walking speed is shown by black bars. Gray bars show the amount by which the final walking speed changed from the pre-training value. Thus most gray bars are stacked above the black bars, showing that final walking speeds were higher than initial values for most subjects. This was not the case for three subjects, who had slower walking speeds after training compared to pre-training values; these are shown where the gray-filled bar lies below the zero-value line. A few subjects had improvements in final walking speed that were too small (≤0.005 m/second) to be resolved in this figure; these cases are indicated with an ‘*’. The dashed horizontal line represents a widely cited ‘Most-limited community’ walking speed of 0.4 m/second, considered the minimum necessary to be functional in the community (30). Eight subjects in this study (indicated by ‘+’) began training with a walking speed below this 0.4 m/second value, but reached or exceeded this value by the end of training. Finally, the arrow (↑) symbols indicate those subjects who were wheelchair-independent ambulators at the beginning of training.
Two subjects who had low pre-training walking speeds of ∼0.1 m/second (cases #7 and #9 from the Y-axis of Fig. 4; indicated by asterisks) showed such a small improvement in post-training walking speed that this increase could not be resolved in Fig. 4. Another subject (case #1; the other asterisk in Fig. 4) was unable to complete any overground steps prior to training onset, resulting in a pre-training walking speed of zero. By the end of training (TM group), she walked ∼10 feet overground, without assistance or BWS. Finally, three subjects showed a decline in maximum walking speed (indicated by a down-going shaded bar in Fig. 4) following training. In the first two cases (first and second instances from the Y-axis origin), both subjects had developed walking-related injuries (see Results – Training complications). The third subject in this category (seventh bar from the right of Fig. 4) deliberately chose to walk at a slower pace in the final few weeks of training, concentrating on improving the symmetry of his gait by limiting hip circumduction. These adjustments led to a slight decline in total training distance in the last few weeks of his study participation. It is highly probable that had he walked as quickly as he was capable of, without regard to walking mechanics, the post-training walking speed of this subject (#22 from Table 1) would have increased compared to his pre-training value.
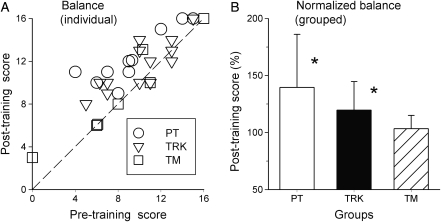
Balance
There was a wide range of pre-training balance scores (0–16) between subjects (average = 10.2 ± 3.9). Mean pre-training balance scores (PT = 10.1 ± 3.6; TRK = 10.5 ± 3.4; TM = 9.8 ± 5.4) were not significantly different between groups. Post-training balance scores all improved (PT = 12.9 ± 2.7; TRK = 11.9 ± 2.5; TM = 10.4 ± 5.0). Fig. 5A shows individual post-training balance scores plotted against pre-training values. As for walking speed, values lying above the dashed line show improvement, and the majority of data points lie in this region. Fig. 5B shows that group mean post-training balance scores (normalized) increased the most for the PT group, with TRK and TM groups showing progressively smaller average improvements.
Figure 5.
Results of the Tinetti balance test for the three training groups. Description as for Fig. 3. Note that the difference in balance scores was not significant for the TM group. Note also that one subject in the TM group (□) was unable to maintain standing balance prior to training onset (thereby scoring a ‘zero’), but scored a ‘3’ in this measure following training. Since normalization was not possible for this measure, it was not included in the average for the TM group.
With ANOVA testing, we found a significant Time × Group interaction (P < 0.05), suggesting that the groups differ in terms of their Pre/Post balance differences. Bonferroni-adjusted pairwise testing revealed significant improvements in balance Pre-to-Post in the PT group (P < 0.001) and in the Track group (P < 0.01), but no significant difference Pre-to-Post for the TM group (P = 0.23).
There was a good correlation between the pre- and post-training measures of maximal 10-m walking speed and balance (pre-training r = 0.79; post-training r = 0.83). The correlation coefficient was somewhat weaker for comparisons with Walking Index for Spinal Cord Injury (WISCI) II scores of maximum pre-training walking speed (r = 0.58) and balance (r = 0.67). Only the pre-training WISCI II scores were considered, since our post-training walking assessment constrained subjects to use the same assistive devices and/or orthose(s) as they used during the pre-training assessment.
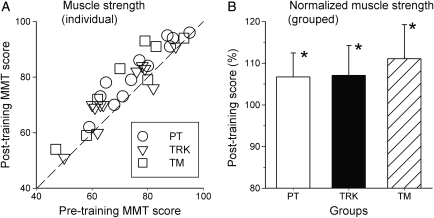
Strength
There was no significant difference in pre-training measures of muscle strength among PT, TRK, and TM group subjects, with the average MMT score being 76.3 ± 11.6 in the PT group, 69.5 ± 12.1 in the TRK group, and 71.5 ± 15.1 in the TM group. Most subjects showed an increase in strength after training (Fig. 6A). Post-training MMT score averaged 81.8 ± 11.0, 73.3 ± 11.5, and 78.1 ± 15.3 in PT, TRK, and TM groups, respectively. Fig. 6B shows that the mean change in muscle strength (normalized to pre-training values) increased by 6–9% across groups. There was a significant increase in strength across groups (P < 0.01), but no difference between groups. That is, the magnitude of strength increase within each group was roughly equal, regardless of training condition. Pairwise testing showed that strength improvements were significant for each of the three training groups (P < 0.05 for each).
Figure 6.
Results of aggregate muscle strength testing (upper-limbs + lower-limbs) in subjects of three training groups before and after completion of training. Description as for Fig. 3. All three groups showed a significant (*) post-training increase in muscle strength after training, and there were no differences between groups.
Most of the observed improvement in strength across subjects was confined to the lower limbs. Average pre- and post-training LEMS were 31.2 ± 10.4 and 35.1 ± 9.9, 25.3 ± 12.3 and 28.3 ± 10.9, and 33.5 ± 11.2 and 37.4 ± 10.7 for the PT, TRK, and TM groups, respectively. Not surprisingly, leg muscles were significantly stronger in subjects who were already community ambulators at the time of study enrollment (pre-training LEMS: 37.3 ± 8.4) compared with wheelchair-dependent subjects (LEMS: 27.8 ± 11.4), but both groups showed comparable gains in strength following training (42.8 ± 4.9 for wheelchair-independent and 30.9 ± 10.7 for wheelchair-dependent subjects).
Fitness
Values of pre-training VO2peak for the population as a whole ranged from ∼10 to 26 ml/kg/minute (Fig. 7A). Comparisons of normalized VO2peak values across training groups are illustrated in Fig. 7B. In all three groups there was a modest post-training increase in average normalized VO2peak (∼12% in each group), but these differences were not significant according to ANOVA.
Figure 7.
Results of the graded maximum arm-ergometry test showing averaged peak VO2 test for the three training groups. Description as for Fig. 3. None of the differences were significant within- or between-groups.
Quality-of-life and functional independence
Table 3 summarizes results of the questionnaires on quality-of-life issues that were completed before, immediately after, and 1 month after the training protocol. Immediately following the training program, 28 out of the 35 study participants (i.e. 80%) reported better satisfaction with abilities and well-being (SAWS; category 1 in Table 3). The degree of score improvement (where a lower value reflects higher satisfaction) was roughly constant across training groups, and the overall effect across groups was significant (P = 0.03). These improvements were retained, for the most part, at the 1-month follow-up evaluation.
None of the other measures in Table 3 showed significant change either between groups or within groups following training. Also unchanged by training were FIM motor scores, where the overall average before-training score (66.3 ± 17.2) showed only a slight increase after training (68.4 ± 13.5).
To test whether there was a relationship between SAWS measures and functional improvement, changes in walking speed (Fig. 8A) and balance (Fig. 8B) are plotted against change in SAWS score for all the subjects tested before and immediately after training, independent of training group. This figure shows that changes in performance were not always consistent with SAWS score. For example, Fig. 8A shows that in 3 out of 28 subjects, improvement in SAWS scores occurred in the absence of post-training increase in walking speed, whereas the 6 subjects who reported a worse SAWS score after training all showed an increase in maximum walking speed. For balance, the trend was that improved balance scores were associated with a better SAWS score, but again there were a few exceptions, as seen in Fig. 8B.
Figure 8.
Percent change in individual post-training score relative to pre-training score for walking speed (‘A’; ordinate) versus Satisfaction with Abilities and Well-Being Scale (SAWS; abscissa) and for balance score (‘B’) versus SAWS score. For most subjects, both speed and balance scores showed improvement, as did SAWS scores. However, there were subjects who showed improvement in walking speed (n = 6) and/or balance (n = 3) compared to their pre-training value (i.e. data points above the x-axis), yet they reported no change or a decrease in satisfaction with their abilities and/or well-being (i.e. data points to the left of the y-axis).
Training complications
Many subjects experienced a variety of aches and pains while in the study, but in most cases these did not hinder the training routine. Two subjects had to drop out of the training protocol shortly (1–2 weeks) after starting; each experienced an episode of acute cardiorespiratory distress on the weekend (i.e. this did not occur during a training session), leading to an emergency department visit. In both cases, subjects were treated with cardiac artery stents and underwent cardiac rehabilitation. Both subjects ultimately returned to successfully complete the protocol, re-starting no sooner than 6 months following cardiac rehabilitation.
There were two subjects whose post-training walking speeds were slower than their pre-training values, due to injury. Both had experienced an exacerbation of spasticity over the final ∼25% of their training sessions. The decrement in post-training performance was mirrored by a decline in the length of time spent actually training in each session, a reduction in average heart-rate (indicating less work being done), and an increase in self-reported symptoms of generalized leg weakness and increased spasticity.
Discussion
Our goal in this study was not to design the optimal intervention that would maximize functional recovery in subjects with chronic, motor-incomplete SCI. Rather it was to test the ability of two different devices – a TM and an overground track – coupled with BWS to facilitate functional improvements in walking, without the benefit of additional coaching, targeted strength training, or assistive-device fitting that are the hallmarks of PT. If either device on its own could match the improvements seen in the PT group, we would safely conclude that the effect was robust and could be generalized to the clinic setting, where control of study variables might not be as tightly maintained.
We found a robust effect of both devices, in spite of the study's design elements that prevented establishment of an ideal or optimal training paradigm. The main findings were: (1) overground maximum walking speed following training was improved by a significant amount in each of the three training groups, with no difference between groups in the amount of improvement; (2) balance showed a significant improvement in persons receiving PT and TRK training; (3) all three groups showed a significant increase in strength, with no difference between groups in the amount of improvement; (4) some persons were able to achieve functional walking speeds as a result of training; (5) satisfaction with abilities and well-being showed significant improvement in all three groups; and (6) there were no significant training effects on fitness. Since all subjects were at least 1 year post-injury and did not take part in any other SCI-related therapeutic studies, we attribute the observed improvements to training.
Subject population
A defining characteristic of the study population was the extremely wide range of pre-training walking speed (i.e. see Table 2; Figs 3 and 4), despite having rather specific inclusion criteria. Thirty-four of the 35 subjects who completed the training protocol were able to walk immediately prior to training onset. Even so, nine of these subjects could not even achieve a walking speed of 0.1 m/second, considered the minimum threshold for ‘Physiological’ walking.30 More than 50% of all subjects could not achieve a ‘Most-limited community’ walking speed of 0.4 m/second30 before training.
Training adaptations during the protocol
As illustrated in Fig. 2, the average values for three of the four training parameters we followed (walking distance and duration; total heart-beats) showed a plateau by training session #26 (roughly 9 weeks into the protocol). For comparison, a different study in persons with chronic SCI subjects utilized a BWS/TM training protocol that lasted an entire year,10 with subjects plateauing in speed and distance between 9 and 12 months. However, subjects in that study were almost all unable to stand or walk at the beginning of training,10 whereas only one of our subjects (#33) fits this description. In light of these substantial differences in initial walking capabilities between the two subject populations, a direct comparison of optimal training durations between the present study and that by Hicks and colleagues10 is not warranted. Another group used BWS-supported TM training in persons with incomplete SCI, showing a very wide range of training durations – from 10 to 28 weeks – during which improvements in overground locomotion continued to accrue.17,31 These studies17,31 did not employ a consistent protocol, however, so direct comparisons to the present study are not possible.
Training schedule
Animal studies of activity-dependent spinal cord plasticity routinely utilize daily exercise sessions,32,33 and even twice-daily sessions have been described.34 It is possible that we limited the potential training gains among our population by adopting an alternate-day training regimen. However, the impractical aspects of daily exercise for both subjects (e.g. transportation; bowel program) and the investigative team (e.g. staffing; data management), combined with the physical fatigue experienced by most subjects after each training session, caused us to avoid a daily training schedule. As a case in point, and in response to informal questioning, many subjects reported that they felt stronger and better-able to exercise on the Monday of a given training week. This was attributed to the extra day's rest afforded by the prior weekend. We chose the training frequency (3 times/week) and total duration (13 weeks; 39 sessions) for reasons related to neuromuscular adaptation to exercise (see Methods; Procedure), but more importantly to be consistent with a typical real-world outpatient rehabilitation setting, for which reimbursement on a continual basis could not be expected.
Walking speed and balance
Walking speed is considered a reproducible measure for assessing walking ability in patients with neurological movement disorders. The 10-m speed test used in our study evaluates short-duration ambulation ability and, along with other timed tests (e.g. Timed Up and Go; 6-minute walking), has been validated to assess walking function after SCI.35–38 In agreement with our findings, these tests have been shown to correlate with each other as well as with the WISCI II index, especially in those patients who are independent walkers.35,39
Without regard for training group, there was a significant and positive correlation in the present study between the subject's pre-training walking speed and balance. Following training, this relationship was even stronger. As a contributor to improvements in both maximum speed and balance, all three groups showed a significant increase in strength after training. Since walking requires a combination of stability and mobility, balance (both static and dynamic) improvement of subjects with movement disorders is a common goal of therapists.40 In our study, post-training balance showed greatest improvement in subjects who received either PT- or TRK-based training; improvements were significant for both groups. It comes as no surprise that balance improved the most in subjects in the PT group, because the therapist was free to concentrate on specific deficiencies in each subject's function, and this always included core balance and coordination exercises. Significant improvements in balance for subjects in the TRK-based group likely reflected their need to continually reposition both themselves and their assistive ambulatory devices in order to move forward. While the overhead suspension apparatus provided direct vertical support, the support cable was not restricted in lateral or anterior/posterior movements, such that the subject could stumble if he/she was not careful.
In contrast, and in contradiction to numerous other studies,3,6,13,18,41 the post-training balance scores of subjects in the TM group did not improve significantly from pre-training values. One possibility for this is that we allowed these subjects to use handles at the front of the TM. In addition, and as seen in Table 1, a high percentage of subjects randomized to the TM group had sustained cervical injury. We cannot rule out the possibility of a neurological explanation for the absence of balance improvement in the TM group. However, the similar initial walking capabilities of subjects in the TM group compared with the two other groups would argue against this being the primary cause of the difference in balance improvements seen.
Strength
A number of studies utilizing BWS for improving walking in persons with SCI have reported improvements in lower-limb strength for both chronic SCI3,13,17,42 and after sub-acute injury.4,43 Consistent with these reports, the present study further expands on the notion that PT alone after chronic and neurologically incomplete SCI can also lead to significant strength gains. Similar conclusions have been previously arrived at from studies on locomotor training after acute SCI.4,43
Leg strength is a good predictor of walking ability in persons with SCI, with aggregate scores of <20 (of a maximum 50) being associated with poor ambulation capability, whereas scores >30 were common in persons with functional (or ‘community’) ambulation.44,45 Subjects in the present study who were 100% wheelchair-dependent for mobility had average lower-limb motor scores of 27 (pre-training), while those who were wheelchair-independent had a mean aggregate lower-limb motor score of 37. There was no significant relationship between improvement in lower-limb motor scores and improvement in walking speed for either group, in agreement with other studies.44,45
Fitness
Subjects in the TRK group showed the highest mean heart-rate across all training sessions, suggesting they were working the hardest during their training sessions, whereas subjects in the PT group had the lowest average heart rate. Other than this measure, though, direct comparisons across all three training groups of the training-related change in work performance are difficult.
The significant training-related improvements in walking speed, balance, and strength described thus far did not carry over to peak oxygen uptake (VO2peak). Both pre- and post-training VO2peak values in our SCI subject population are comparable to measures reported by others,46,47 but are far below typical values of an untrained able-bodied cohort (e.g. a man aged 40–49 years at the 50th percentile would be expected to have a VO2peak of 40.4 ml/kg/minute).48 Despite the significant improvement in maximum walking speed for all three groups, and the doubling of training distance walked for two groups (TRK and TM) in which this metric was available, there was no increase in peak oxygen consumption within groups. The simplest explanation for this outcome is that training was insufficient in volume and/or intensity to induce positive adaptations in central cardiorespiratory functioning. Subjects with thoracic-level injury demonstrated higher VO2peak values than most persons with cervical injury (not shown), but there were no consistent improvements in these values after training. Another explanation reflects the necessity to test VO2peak using arm ergometry, whereas training was directed primarily to the legs. It is widely recognized that arm-based ergometry results in VO2peak values that are only 70–80% of those obtained during TM-based testing in able-bodied subjects.49 It is possible in the present study that the limiting factor in defining peak workload was arm strength and/or fatigue resistance, rather than a limitation in oxygen delivery. As mentioned previously, autonomic dysfunction in persons with SCI rostral to T6 could limit HR increase, also contributing to a limitation of peak work output.23 Thus the arm-based test for the population studied may have lacked sensitivity to detect any improvement in cardiorespiratory function that might have been caused by subject's participation in this protocol. This conclusion stands in contrast to numerous other studies of exercise after SCI, in which multiple cardiorespiratory parameters have been reported to show significant improvement following strengthening exercises,50–52 FES-assisted ambulation,47 and BWS ambulation.53,54
Quality-of-life measures
The quality-of-life measure that we used combined elements from the SAWS and SF-36 instruments, largely to avoid employment-related questions. Most of the subjects in our study traveled from out of town. The few subjects who were employed on a regular basis at the time of study participation had to take a leave-of-absence to participate. Therefore, we decided it was better to eliminate these work-related questions entirely.
The majority of our subjects reported enhanced satisfaction with their abilities and well-being (SAWS) after completing the training program, irrespective of the training method. Other training studies after SCI have reported similar findings.10,13,55–57
The positive effects on SAWS seen immediately after training conclusion were maintained at the 1-month follow-up evaluation, in agreement with other studies.10,50,57 Other quality-of-life measures at the 1-month follow-up were unchanged from pre-training or immediately post-training results.
Activities of daily living such as self-care, sphincter control, and mobility were assessed by the physical therapist via Functional Independence Measure (FIM).58 We excluded the FIM cognition domain, because of the known ceiling effect in persons with SCI.59 A ceiling effect for FIM motor scores (74.9 ± 1.7 of a possible 77 during pre-training testing) in our subject pool explains the absence of significant improvement following training in this study. Consistent with our findings, locomotion training failed to influence measures of the Instrumental Activities of Daily Living (IADL) in BWS-trained individuals with chronic SCI,10 and FIM scores were stable throughout a protocol involving gait training in persons with stroke.60 An alternative explanation is that FIM may not be sensitive to detect changes in walking ability following ambulation therapy in persons with motor-incomplete (i.e. AIS ‘C’ and ‘D’) SCI,38 especially in those who are able to ambulate more independently.3,4
Other BWS-based training studies
An early and influential study reported that BWS/TM training in 89 persons with motor-incomplete SCI (AIS ‘C’ and ‘D’ grades) improved ambulation to a much greater degree than conventional therapy.6 In that study, improvement was observed in subjects when training started at both sub-acute and chronic stages following injury. That study6 did not directly compare TM-based training to PT, was not randomized, and subjects participated for varying lengths of time. One study that did compare BWS/TM training to PT after acute SCI concluded that the former approach was more effective than PT alone, but in this case the TM training was combined with functional electrical stimulation to facilitate the stepping motion.43 One case report examining the impact of ∼7 months of TM training sessions with BWS on a subject with chronic AIS ‘B’ SCI showed evolution of muscle recruitment patterns more consistent with a walking pattern versus simple stretch-reflex activities.61
More recent – and arguably more carefully designed – studies of TM training with BWS have not been able to replicate the positive findings summarized above.6 The SCILT compared conventional PT for mobility versus the same duration and intensity of BWS/TM training in subjects with acute SCI graded AIS ‘B’, ‘C’, and ‘D’.4,19,20 This randomized multi-center study did not reveal significant group differences for its primary outcome measures, which were the level of independence for ambulation and, for those who were able to walk, the maximal walking speed over a 50-foot (∼15 m) distance.4 Similarly, in individuals with sub-acute stroke, the BWS/TM training method was no more effective than PT at improving gait.62,63
To our knowledge, there have been no randomized studies comparing equal durations of BWS-based gait training to PT in persons with chronic SCI, nor have the two forms of BWS training used in the present study been independently studied. We were encouraged to see that the great majority of subjects in all three groups improved in their walking ability, sometimes by considerable amounts. Based on our results and those from previous studies that have utilized different gait training protocols,3,10,12,13,17,18,64,65 there can no longer be any doubt that ambulatory individuals with chronic SCI are capable of improving their walking ability following intensive ambulation-oriented therapy, regardless of the training method used. In some cases – depending on their level of disability at the onset of training – this can lead to marked improvements in functional walking capabilities. In spite of the improvements seen, we are not suggesting that methods employed in the study described herein were optimal for improving walking function in our subjects, as expanded on in a following section.
Lastly, most subjects showed improvement in walking speed, even though the two BWS groups trained at a self-selected walking pace. The published data on the effect of training speed on locomotor recovery after neurological injury are inconsistent. After SCI or stroke, it has been reported that walking is improved to a greater degree when training is at speeds approaching normal.3,66,67 Conversely, the benefit of using slower training speeds for improving walking function after SCI and stroke has also been reported.13,17,18,43,68,69 While the initial maximal walking speed of our subjects (0.36 m/second) was only slightly slower than the minimum speed (0.4 m/second) associated with functional walking,30 this value was skewed upwards by much higher initial walking speeds in a small number of our subjects. Other subjects in our study were initially incapable of walk-training at even one-half of this group average. Therefore, given that a minimally normal walking speed was not achievable by the majority of our subjects prior to training onset, a self-selected training pace was adopted.
Summary, study limitations, and recommendations
The present study has demonstrated that many persons with neurologically incomplete SCI can improve their walking ability and psychological well-being following a concentrated period of ambulation therapy – even when initiated years after injury – regardless of the training method. Improvement in walking speed appeared to be due to a combination of increased balance and muscle strength. These individuals appear to have movement capabilities that are not fully exploited, and which can be improved upon with regular training involving relatively unsophisticated techniques. That is, the improvements seen with devices incorporating BWS-based gait training as tested herein – either overground or on a TM – match but do not surpass the benefits associated with traditional, comprehensive PT for improving walking speed in this population.
Given the significant gains seen in walking speed, balance and strength of subjects receiving PT training alone, it is possible that the benefits that accrued to subjects randomized to either of the devices utilizing BWS-based protocols would have been enhanced had the study protocol allowed some level of training advice or recommendations from a physical therapist or other gait expert. Although our decision to withhold such training recommendations was a deliberate part of the study design, we recognize that this decision probably provided some amount of advantage to subjects in the PT group. Another potential study weakness is that two different ergometer and metabolic systems were used to measure peak work capacity, but standard operating procedures were identical and calibration with the same known references gases was performed. Finally, several of the instruments we used for assessing our subject's level of satisfaction with the study and their outcomes have not been validated, because we selected only a portion of each instrument for use.
Based on our findings, we suggest that the ‘optimal’ training approach for improving walking ability in persons with neurologically incomplete SCI of long-standing origin is as follows: overground walk-training with 30% BWS, with training sessions lasting 1 hour, 3 times per week, for 10 weeks. The cost-savings of restricting the overall training duration to 10 rather than 13 weeks would, in our opinion, outweigh the limited further gains a minority of subjects might experience through participating in a longer training program. This shorter study duration would also be less likely to cause training-related injuries. In addition, a physical therapist would make weekly observations and recommendations regarding the walk-training (e.g. use of orthoses; assistive devices; gait strategies), and would spend 1 hour per week working hands-on with the subject to target specific factors limiting walking performance, providing the subject with instructions for working independently on non-training days on these and related tasks. Clearly, this strategy will not be appropriate for persons with more significant neurological disability, for whom advancing even one leg for one step would be problematic; for these persons, a treadmill-based training strategy would be called for.4,20
Acknowledgements
This work was supported by the NIH (U01 HD37460), by the State University of New York – Upstate Medical University, and by the Miami Project to Cure Paralysis – The University of Miami. The skill and dedication of Corrine Luria, Rosemary Grabowski, and Todd Charland – providers of the physical therapy treatment arm – were instrumental to this study, and their efforts are gratefully acknowledged.
References
- 1.Jackson AB, Dijkers M, DeVivo MJ, Poczatek RB. A demographic profile of new traumatic spinal cord injuries: change and stability over 30 years. Arch Phys Med Rehabil 2004;85(11):1740–8 [DOI] [PubMed] [Google Scholar]
- 2.Calancie B, Molano MR, Broton JG. Epidemiology and demography of acute spinal cord injury in a large urban setting. J Spinal Cord Med 2005;28(2):92–6 [DOI] [PubMed] [Google Scholar]
- 3.Behrman AL, Harkema SJ. Locomotor training after human spinal cord injury: a series of case studies. Phys Ther 2000;80(7):688–700 [PubMed] [Google Scholar]
- 4.Dobkin B, Apple D, Barbeau H, Basso M, Behrman A, Deforge D, et al. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology 2006;66(4):484–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wernig A, Müller S. Laufband locomotion with body weight support improved walking in persons with severe spinal cord injuries. Paraplegia 1992;30(4):229–38 [DOI] [PubMed] [Google Scholar]
- 6.Wernig A, Müller S, Nanassy A, Cagol E. Laufband therapy based on ‘rules of spinal locomotion’ is effective in spinal cord injured persons. Eur J Neurosci 1995;7(4):823–29 [DOI] [PubMed] [Google Scholar]
- 7.Somers MF. Spinal cord injury: functional rehabilitation. Norwalk, CN: Appleton & Lange; 1992 [Google Scholar]
- 8.Mehrholz J, Kugler J, Pohl M. Locomotor training for walking after spinal cord injury. Spine (Phila Pa 1976) 2008;33(21):E768–77 [DOI] [PubMed] [Google Scholar]
- 9.Wirz M, Colombo G, Dietz V. Long term effects of locomotor training in spinal humans. J Neurol Neurosurg Psychiatry 2001;71(1):93–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hicks AL, Adams MM, Martin GK, Giangregorio L, Latimer A, Phillips SM, et al. Long-term body-weight-supported treadmill training and subsequent follow-up in persons with chronic SCI: effects on functional walking ability and measures of subjective well-being. Spinal Cord 2005;43(5):291–8 [DOI] [PubMed] [Google Scholar]
- 11.Jayaraman A, Shah P, Gregory C, Bowden M, Stevens J, Bishop M, et al. Locomotor training and muscle function after incomplete spinal cord injury: case series. J Spinal Cord Med 2008;31(2):185–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nooijen CF, Ter Hoeve N, Field-Fote EC. Gait quality is improved by locomotor training in individuals with SCI regardless of training approach. J Neuroeng Rehabil 2009;6:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Field-Fote EC. Combined use of body weight support, functional electric stimulation, and treadmill training to improve walking ability in individuals with chronic incomplete spinal cord injury. Arch Phys Med Rehabil 2001;82(6):818–24 [DOI] [PubMed] [Google Scholar]
- 14.Harkema SJ. Plasticity of interneuronal networks of the functionally isolated human spinal cord. Brain Res Rev 2008;57(1):255–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beres-Jones JA, Harkema SJ. The human spinal cord interprets velocity-dependent afferent input during stepping. Brain 2004;127(Pt 10):2232–46 [DOI] [PubMed] [Google Scholar]
- 16.Edgerton VR, Tillakaratne NJ, Bigbee AJ, deLeon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci 2004;27:145–67 [DOI] [PubMed] [Google Scholar]
- 17.Thomas SL, Gorassini MA. Increases in corticospinal tract function by treadmill training after incomplete spinal cord injury. J Neurophysiol 2005;94(4):2844–55 [DOI] [PubMed] [Google Scholar]
- 18.Wirz M, Zemon DH, Rupp R, Scheel A, Colombo G, Dietz V, et al. Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: a multicenter trial. Arch Phys Med Rehabil 2005;86(4):672–80 [DOI] [PubMed] [Google Scholar]
- 19.Dobkin BH, Apple D, Barbeau H, Basso M, Behrman A, Deforge D, et al. Methods for a randomized trial of weight-supported treadmill training versus conventional training for walking during inpatient rehabilitation after incomplete traumatic spinal cord injury. Neurorehab Neural Repair 2003;17(3):153–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobkin B, Barbeau H, Deforge D, Ditunno J, Elashoff R, Apple D, et al. The evolution of walking-related outcomes over the first 12 weeks of rehabilitation for incomplete traumatic spinal cord injury: the multicenter randomized Spinal Cord Injury Locomotor Trial. Neurorehab Neural Repair 2007;21(1):25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klose KJ, Jacobs PL, Broton JG, Guest JG, Needham-Shropshire BM, Lebwohl N, et al. Evaluation of a training program for persons with SCI paraplegia using the Parastep 1 ambulation system. Part 1. Ambulation performance and anthropometric measures. Arch Phys Med Rehabil 1997;78(8):789–93 [DOI] [PubMed] [Google Scholar]
- 22.Fredrickson E, Ruff RL, Daly JJ. Physiological cost index as a proxy measure for the oxygen cost of gait in stroke patients. Neurorehab Neural Repair 2007;21(5):429–34 [DOI] [PubMed] [Google Scholar]
- 23.Krassioukov A, Eng JJ, Warburton DE, Teasell R, Spinal Cord Injury Rehabilitation Evidence Research Team A systematic review of the management of autonomic dysreflexia after spinal cord injury. Arch Phys Med Rehabil 2009;90(4):682–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Figoni SF, Kiratli BJ, Sasak R. ACSM's resources for clinical exercise physiology: musculoskeletal, neuromuscular, neoplastic, immunologic, and hematologic conditions. 2nd ed., Baltimore: Lippincott, Williams & Wilkins; 2010 [Google Scholar]
- 25.Tinetti ME. Performance oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc 1986;34(2):119–26 [DOI] [PubMed] [Google Scholar]
- 26.Waring WP, 3rd, Biering-Sorensen F, Burns S, Donovan W, Graves D, Jha A, et al. 2009 Review and revisions of the International Standards for the Neurological Classification of Spinal Cord Injury. J Spinal Cord Med. 2010;33(4):346–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katz PP, Alfieri WS. Satisfaction with abilities and well-being: development and validation of a questionnaire for use among persons with rheumatoid arthritis. Arthritis Care Res 1997;10(2):89–98 [DOI] [PubMed] [Google Scholar]
- 28.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30(6):473–83 [PubMed] [Google Scholar]
- 29.Tabachnick BG, Fidell LS. Using multivariate statistics. 2nd ed., New York: Harper & Collins; 1989 [Google Scholar]
- 30.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke 1995;26(6):982–9 [DOI] [PubMed] [Google Scholar]
- 31.Gorassini MA, Norton JA, Nevett-Duchcherer J, Roy FD, Yang JF. Changes in locomotor muscle activity after treadmill training in subjects with incomplete spinal cord injury. J Neurophysiol 2009;101(2):969–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reese NB, Skinner RD, Mitchell D, Yates C, Barnes CN, Kiser TS, et al. Restoration of frequency-dependent depression of the H-reflex by passive exercise in spinal rats. Spinal Cord 2006;44(1):28–34 [DOI] [PubMed] [Google Scholar]
- 33.Ying Z, Roy RR, Edgerton VR, Gomez-Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp Neurol 2005;193(2):411–19 [DOI] [PubMed] [Google Scholar]
- 34.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci 2004;20(10):2580–90 [DOI] [PubMed] [Google Scholar]
- 35.van Hedel HJ, Wirz M, Dietz V. Assessing walking ability in subjects with spinal cord injury: validity and reliability of 3 walking tests. Arch Phys Med Rehabil 2005;86(2):190–6 [DOI] [PubMed] [Google Scholar]
- 36.van Hedel HJ, Wirz M, Curt A. Improving walking assessment in subjects with an incomplete spinal cord injury: responsiveness. Spinal Cord 2006;44(6):352–6 [DOI] [PubMed] [Google Scholar]
- 37.Steeves JD, Lammertse D, Curt A, Fawcett JW, Tuszinski MH, Ditunno JF, et al. Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord 2007;45(3):206–21 [DOI] [PubMed] [Google Scholar]
- 38.Jackson AB, Carnel CT, Ditunno JF, Read MS, Boninger ML, Schmeler MR, et al. Outcome measures for gait and ambulation in the spinal cord injury population. J Spinal Cord Med 2008;31(5):487–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marino RJ, Scivoletto G, Patrick M, Tamburella F, Read MS, Burns AS, et al. Walking index for spinal cord injury version 2 (WISCI-II) with repeatability of the 10-m walk time: inter- and intrarater reliabilities. Am J Phys Med Rehabil 2010;89(1):7–15 [DOI] [PubMed] [Google Scholar]
- 40.Bohannon RW. Standing balance, lower extremity muscle strength, and walking performance of patients referred for physical therapy. Percept Mot Skills 1995;80(2):379–85 [DOI] [PubMed] [Google Scholar]
- 41.Visintin M, Barbeau H. The effects of body weight support on the locomotor pattern of spastic paretic patients. Can J Neurol Sci 1989;16(3):315–25 [DOI] [PubMed] [Google Scholar]
- 42.Dietz V, Wirz M, Colombo G, Curt A. Locomotor capacity and recovery of spinal cord function in paraplegic patients: a clinical and electrophysiological evaluation. Electroencephalogr Clin Neurophysiol 1998;109(2):140–53 [DOI] [PubMed] [Google Scholar]
- 43.Postans NJ, Hasler JP, Granat MH, Maxwell DJ. Functional electric stimulation to augment partial weight-bearing supported treadmill training for patients with acute incomplete spinal cord injury: a pilot study. Arch Phys Med Rehabil 2004;85(4):604–10 [DOI] [PubMed] [Google Scholar]
- 44.Waters RL, Adkins R, Yakura J, Vigil D. Prediction of ambulatory performance based on motor scores derived from standards of the American Spinal Injury Association. Arch Phys Med Rehabil 1994;75(7):756–60 [PubMed] [Google Scholar]
- 45.Kim CM, Eng JJ, Whittaker MW. Level walking and ambulatory capacity in persons with incomplete spinal cord injury: relationship with muscle strength. Spinal Cord 2004;42(3):156–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.El-Sayed MS, Younesian A. Lipid profiles are influenced by arm cranking exercise and training in individuals with spinal cord injury. Spinal Cord 2005;43(5):299–305 [DOI] [PubMed] [Google Scholar]
- 47.Jacobs PL, Nash MS, Klose KJ, Guest RS, Needham-Shropshire BM, Green BA. Evaluation of a training program for persons with SCI paraplegia using the Parastep 1 ambulation system: part 2. Effects on physiological responses to peak arm ergometry. Arch Phys Med Rehabil 1997;78(8):794–8 [DOI] [PubMed] [Google Scholar]
- 48.Thompson WR. editor. ACSM's guidelines for exercise testing & prescription. 8th ed., Philadelphia, PA: Wolters Kluwer; 2010 [Google Scholar]
- 49.Franklin BA. Exercise testing, training and arm ergometry. Sports Med 1985;2(2):100–19 [DOI] [PubMed] [Google Scholar]
- 50.Hicks AL, Martin KA, Ditor DS, Latimer AE, Craven C, Bugaresti J, et al. Long-term exercise training in persons with spinal cord injury: effects on strength, arm ergometry performance and psychological well-being. Spinal Cord 2003;41(1):34–43 [DOI] [PubMed] [Google Scholar]
- 51.Zoeller RF, Jr, Riechman SE, Dabayebeh IM, Goss FL, Robertson RJ, Jacobs PL. Relation between muscular strength and cardiorespiratory fitness in people with thoracic-level paraplegia. Arch Phys Med Rehabil 2005;86(7):1441–6 [DOI] [PubMed] [Google Scholar]
- 52.Jacobs PL. Effects of resistance and endurance training in persons with paraplegia. Med Sci Sports Exerc 2009;41(5):992–7 [DOI] [PubMed] [Google Scholar]
- 53.Stewart BG, Tarnopolsky MA, Hicks AL, McCartney N, Mahoney DJ, Staron RS, et al. Treadmill training-induced adaptations in muscle phenotype in persons with incomplete spinal cord injury. Muscle Nerve 2004;30(1):61–8 [DOI] [PubMed] [Google Scholar]
- 54.Ditor DS, Macdonald MJ, Kamath MV, Bugaresti J, Adams M, McCartney N, et al. The effects of body-weight supported treadmill training on cardiovascular regulation in individuals with motor-complete SCI. Spinal Cord 2005;43(11):664–73 [DOI] [PubMed] [Google Scholar]
- 55.Lannem AM, Sorensen M, Froslie KF, Hjeltnes N. Incomplete spinal cord injury, exercise and life satisfaction. Spinal Cord 2009;47(4):295–300 [DOI] [PubMed] [Google Scholar]
- 56.Effing TW, van Meeteren NL, van Asbeck FW, Prevo AJ. Body weight-supported treadmill training in chronic incomplete spinal cord injury: a pilot study evaluating functional health status and quality of life. Spinal Cord 2006;44(5):287–96 [DOI] [PubMed] [Google Scholar]
- 57.Ditor DS, Latimer AE, Ginis KA, Arbour KP, McCartney N, Hicks AL. Maintenance of exercise participation in individuals with spinal cord injury: effects on quality of life, stress and pain. Spinal Cord 2003;41(8):446–50 [DOI] [PubMed] [Google Scholar]
- 58.Ditunno JF., Jr Functional assessment measures in CNS trauma. J Neurotrauma 1992;9Suppl 1:s301–5 [PubMed] [Google Scholar]
- 59.Hall KM, Cohen ME, Wright J, Call M, Werner P. Characteristics of the functional independence measure in traumatic spinal cord injury. Arch Phys Med Rehabil 1999;80(11):1471–6 [DOI] [PubMed] [Google Scholar]
- 60.Peurala SH, Tarkka IM, Pitkanen K, Sivenius J. The effectiveness of body weight-supported gait training and floor walking in patients with chronic stroke. Arch Phys Med Rehabil 2005;86(8):1557–64 [DOI] [PubMed] [Google Scholar]
- 61.Forrest GF, Sisto SA, Barbeau H, Kirshblum SC, Wilen J, Bond Q, et al. Neuromotor and musculoskeletal responses to locomotor training for an individual with chronic motor complete AIS-B spinal cord injury. J Spinal Cord Med 2008;31(5):509–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hesse S, Werner C, von Frankenberg S, Bardeleben A. Treadmill training with partial body weight support after stroke. Phys Med Rehabil Clin N Am 2003;141 Suppl:S111–23 [DOI] [PubMed] [Google Scholar]
- 63.Dickstein R. Rehabilitation of gait speed after stroke: a critical review of intervention approaches. Neurorehabil Neural Repair 2008;22(6):649–60 [DOI] [PubMed] [Google Scholar]
- 64.Hornby TG, Zemon DH, Campbell D. Robotic-assisted, body-weight-supported treadmill training in individuals following motor incomplete spinal cord injury. Phys Ther 2005;85(1):52–66 [PubMed] [Google Scholar]
- 65.Adams MM, Ditor DS, Tarnopolsky MA, Phillips SW, McCartney N, Hicks AL. The effect of body weight-supported treadmill training on muscle morphology in an individual with chronic, motor-complete spinal cord injury: a case study. J Spinal Cord Med 2006;29(2):167–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Visintin M, Barbeau H. The effects of parallel bars, body weight support and speed on the modulation of the locomotor pattern of spastic paretic gait. A preliminary communication. Paraplegia 1994;32(8):540–53 [DOI] [PubMed] [Google Scholar]
- 67.Sullivan KJ, Knowlton BJ, Dobkin BH. Step training with body weight support: effect of treadmill speed and practice paradigms on poststroke locomotor recovery. Arch Phys Med Rehabil 2002;83(5):683–91 [DOI] [PubMed] [Google Scholar]
- 68.Hesse S, Bertelt C, Jahnke MT, Shaffrin A, Baake P, Malezic M, et al. Treadmill training with partial body weight support compared with physiotherapy in nonambulatory hemiparetic patients. Stroke 1995;26(6):976–81 [DOI] [PubMed] [Google Scholar]
- 69.Field-Fote EC, Lindley SD, Sherman AL. Locomotor training approaches for individuals with spinal cord injury: a preliminary report of walking-related outcomes. J Neurol Phys Ther 2005;29(3):127–37 [DOI] [PubMed] [Google Scholar]