Abstract
Background
Fluctuations in 24-hour cardiovascular hemodynamics, specifically heart rate (HR) and blood pressure (BP), are thought to reflect autonomic nervous system (ANS) activity. Persons with spinal cord injury (SCI) represent a model of ANS dysfunction, which may affect 24-hour hemodynamics and predispose these individuals to increased cardiovascular disease risk.
Objective
To determine 24-hour cardiovascular and ANS function among individuals with tetraplegia (n = 20; TETRA: C4–C8), high paraplegia (n = 10; HP: T2–T5), low paraplegia (n = 9; LP: T7–T12), and non-SCI controls (n = 10). Twenty-four-hour ANS function was assessed by time domain parameters of heart rate variability (HRV); the standard deviation of the 5-minute average R–R intervals (SDANN; milliseconds/ms), and the root-mean square of the standard deviation of the R–R intervals (rMSSD; ms). Subjects wore 24-hour ambulatory monitors to record HR, HRV, and BP. Mixed analysis of variance (ANOVA) revealed significantly lower 24-hour BP in the tetraplegic group; however, BP did not differ between the HP, LP, and control groups. Mixed ANOVA suggested significantly elevated 24-hour HR in the HP and LP groups compared to the TETRA and control groups (P < 0.05); daytime HR was higher in both paraplegic groups compared to the TETRA and control groups (P < 0.01) and nighttime HR was significantly elevated in the LP group compared to the TETRA and control groups (P < 0.01). Twenty-four-hour SDANN was significantly increased in the HP group compared to the LP and TETRA groups (P < 0.05) and rMSSD was significantly lower in the LP compared to the other three groups (P < 0.05). Elevated 24-hour HR in persons with paraplegia, in concert with altered HRV dynamics, may impart significant adverse cardiovascular consequences, which are currently unappreciated.
Keywords: Paraplegia, Tetraplegia, Autonomic nervous system, Heart rate, Blood pressure, Heart rate variability, Diurnal variation, Spinal cord injuries, Cardiovascular risk
Introduction
The autonomic nervous system (ANS) is believed to play an integral role in the maintenance of 24-hour cardiovascular hemodynamics, and changes in sympathetic nerve activity (SNA) relate to daytime versus nighttime heart rate (HR), and blood pressure (BP).1
Reductions in nocturnal SNA, HR, and BP and relative increases in vagal tone2 during non-rapid eye movement (REM) sleep are thought to be cardioprotective. Absence or diminution of nocturnal reductions in HR, BP, and SNA are associated with increased incidence of stroke,3 congestive heart failure,4 and cardiovascular morbidity.5 Therefore, 24-hour, daytime and nighttime variation in cardiovascular hemodynamics and autonomic function may provide insight into long-term cardiovascular health and/or disease risk.
Cardiovascular disease (CVD) has emerged as a leading cause of mortality in persons with chronic spinal cord injury (SCI), exceeding renal and pulmonary complications.6–8 Increased morbidity due to CVD, especially coronary heart disease (CHD), is reported in individuals with SCI7 and the incidence of CVD may occur earlier in life compared to the non-SCI population.6 Current literature on 24-hour cardiovascular and autonomic function in the SCI population has focused on individuals with tetraplegia9–14 and one paper considered the model of paraplegia as the control.15 Nevertheless, there is evidence suggesting that individuals with paraplegia are at increased risk for CVD,16 silent ischemia,17 and CHD,18 and the relative risk of CHD has been described as 70% greater in individuals with chronic paraplegia compared to individuals with chronic tetraplegia.7 Furthermore, a recent series of publications found that a third of the Swedish paraplegic population is at greater risk for CVD disease than age-matched non-SCI controls.19–21
The evidence of increased CVD risk in persons with paraplegia and the paucity of literature focused on 24-hour cardiovascular hemodynamic and autonomic function in these individuals have prompted this study. The objectives were to describe 24-hour HR, systolic BP (SBP), and time-domain parameters of HR variability (HRV) in individuals with high paraplegia and low paraplegia compared to individuals with tetraplegia and age-matched non-SCI controls. We hypothesized that 24-hour SBP would be significantly lower in the group with tetraplegia, but would not differ among the paraplegic and control groups. Although empirical data are lacking, we hypothesized that 24-hour HR would be elevated in subjects with HP and LP compared to the tetraplegic and control groups. Finally, we hypothesized that 24-hour HRV would be reduced in the tetraplegic and paraplegic groups compared to the non-SCI controls, thus implicating autonomic dysregulation as a potential contributor to the cardiovascular dysfunction.
Methods
Subjects
Forty-nine individuals volunteered to participate in the investigation: 20 with tetraplegia (TETRA: C4–C8), 10 with high paraplegia (HP: T2–T5), and 9 with low paraplegia (LP: T7–T12) and 10 non-SCI (controls). Subjects within each study group were matched for gender, age, height, and weight and were recruited based on a convenience sample of the population at the two testing sites. Study subjects did not have a history of cardiovascular disease or diabetes mellitus; they were not taking prescription medications for any cardiovascular or autonomic condition; and all were current non-smokers. Subjects with SCI were neurologically stable, at least 1-year post-injury and used a wheelchair as their primary means of mobility. The level and completeness of SCI were determined by a complete neurological examination conducted as per the International Standards at the time of testing in 70% of the study sample; in the other 30% the American Spinal Injury Association (ASIA) Impairment Scale (AIS) classification score was based on a clinical examination within the preceding 12 months. Because level of physical fitness may contribute to 24-hour cardiovascular and autonomic function, we assessed individual exercise participation in all subjects with a self-report survey. Study participants were recruited from the Center of Excellence for the Medical Consequences of SCI at the James J Peters Veterans Affairs Medical Center and Kessler Institute for Rehabilitation; study procedures were approved by the Institutional Review Boards at both institutions. Written informed consent was obtained prior to performing any study procedure, which followed institutional guidelines.
Protocol procedures
Subjects visited the laboratory between 8:00 am and 10:00 am after a light breakfast and having avoided caffeine, alcohol, and heavy exertion (i.e. exercise) for 24 hours prior to arrival. The study involved two consecutive visits to the laboratory: Day 1, subjects were instrumented with two ambulatory monitors for recording BP and HR over a 24-hour period, Day 2, subjects returned the ambulatory monitors and discussed the events of the prior 24-hour period. During the 24-hour observation, subjects were instructed to maintain their routine activities while avoiding exercise and excessive physical exertion. Subjects with SCI who perform regimented bowel care routines were asked to avoid scheduling testing on these days, if possible, and control subjects were asked to avoid standing and remained in a wheelchair during the daytime to avoid significant orthostatic influences on cardiovascular hemodynamics or autonomic function.
Subjects maintained a log to indicate the times that they went to bed (recumbancy), fell asleep, and woke-up in the morning. Although the subjects were instructed to have a ‘normal’ night of sleep and to stay awake during the day, cases of daytime sleep and nighttime insomnia were noted. As it has been reported that daytime sleep can underestimate nocturnal dipping status,22 naps were excluded from the daytime averages and periods of insomnia were excluded from nighttime averages. As such, wakeful and sleep periods were individualized per subject, regardless of the time when an individual went to bed.
Twenty-four hour blood pressure
BP was measured with an Ambulatory Blood Pressure Monitor (90207 ABPM; SpaceLabs Corp., Troy, MI, USA), and in most subjects the brachial artery of the non-dominant arm was used. The monitor was programmed to obtain BP readings every 20 minutes during the day and at 20–30-minute intervals during the night. The BP values were averaged hourly and a summary of systolic (SBP), diastolic (DBP), and mean arterial pressure (MAP) was obtained for the 24 hour, daytime and nighttime periods. As mentioned above, manual review and adjustment was made to the average daytime and nighttime periods based on individual sleep and wake logs. The nocturnal dip in SBP (SBPdip) was calculated as follows: (average SBP nighttime − average SBP daytime)/average SBP daytime) × 100.23
Twenty-four-hour HR
HR was assessed using a Holter monitor (Brentwood IQMark EZ Holter, Midmark Diagnostics Group, Torrance, CA, USA); a three-lead continuous electrocardiogram (ECG) provided the average hourly HR, and daytime and nighttime HR was calculated based on the sleep and wake log for each subject. The nocturnal dip in HR (HRdip) was calculated as: (average HR nighttime − HR average daytime)/average HR daytime) × 100.23
Twenty-four-hour time-domain heart rate variability
The Holter monitor also provided 24-hour time-domain parameters of heart rate variability (HRV), which reflect autonomic cardiac tone.24–26 The 24-hour ECG recordings were reviewed and edited for ectopic and missing beats and artifact. The parameters reported include: the standard deviation of the 5-minute average R–R intervals (SDANN; millisecond/ms) and the root-mean square of the standard deviation of adjacent R–R intervals (rMSSD; ms). SDANN reflects the standard deviation of the R–R intervals across the data set while the rMSSD is the standard deviation of the difference in adjacent R–R intervals throughout the data segment. It has been suggested that the SDANN measures cycle length variability (CLV), which is influenced by diurnal trends in HR, reflecting short-term sympathetic influences and vagal tone. On the other hand, the rMSSD measures variability in adjacent cardiac cycles and has been demonstrated to strongly reflect vagal tone in healthy controls and post-myocardial infarction.26–28
Data analysis
Data were analyzed using a statistical analysis program (StatView, SAS Institute, Berkley, CA). Factorial ANOVA was used to test for significant group differences in demographic parameters and mean 24-hour cardiovascular and autonomic data. Mixed ANOVA models were created to test for significant main effects for group (TETRA, HP, LP, controls) and time (hour), and significant interaction effects for HR, SBP, and HRV over the entire 24-hour period, as well as for the individualized daytime and nighttime periods. Multiple regression analyses were used to determine the influence of 24 hour HR on group differences in SDANN and rMSSD, the Bryant–Paulson generalization of the Tukey HSD procedure was used to determine significant pair-wise comparisons on the adjusted group means. Statistical significance was set at an alpha level of P < 0.05.
Results
Mean demographic data are presented for the study groups (Table 1). There were no statistically significant differences for age, height, or body mass among the groups, and 84% of the study population was male. The number of female subjects was comparable, and did not exceed 25% in any one study group. Findings on 24-hour cardiovascular and autonomic function did not differ between genders; however, this may be attributable to the small representation of females. Individual demographic data for the subjects with SCI are presented (Table 2). All subjects with SCI were chronically injured (2–37 years) and 31 of 39 subjects were classified as having complete loss of motor and sensory function (AIS A). Since the data for 24-hour HR, SBP, and HRV did not differ by completeness of injury (data not shown) we have included both categorizations of injury in the analyses. Individual exercise training regimens are presented for the 10 moderately active subjects (Table 3). Participation in a regular exercise training regimen did not significantly improve 24 hour cardiovascular hemodynamics or autonomic function in the active compared to the less active subjects.
Table 1.
Subject demographic characteristics
| Controls (n = 10) | Low paraplegia (n = 9) | High paraplegia (n = 10) | Tetraplegia (n = 20) | |
|---|---|---|---|---|
| Age (years) | 40 ± 10 | 43 ± 10 | 43 ± 13 | 42 ± 8 |
| Height (cm) | 173 ± 10 | 180 ± 6 | 179 ± 6 | 176 ± 9 |
| Weight (kg) | 79 ± 15 | 84 ± 17 | 91 ± 21 | 80 ± 21 |
| Male number (%) | 8 (80) | 7 (78) | 9 (90) | 17 (85) |
| BMI (kg/m2) | 26.6 ± 5.1 | 26.0 ± 4.7 | 28.2 ± 6.0 | 25.8 ± 5.9 |
| Level of injury | – | T7–T12 | T2–T5 | C4–C8 |
| DOI (years) | – | 12 ± 11 | 14 ± 11 | 19 ± 11 |
Data are means ± SD; BMI, body mass index; DOI, duration of injury.
Table 2.
Characteristics of the spinal cord injury
| Age | Level | Duration | Comp/inc | AIS |
|---|---|---|---|---|
| Tetraplegia | ||||
| 45 | C6 | 29 | Incomplete | B |
| 42 | C5 | 25 | Complete | A |
| 46 | C5 | 20 | Complete | A |
| 42 | C5 | 10 | Complete | A |
| 54 | C6 | 37 | Incomplete | B |
| 41 | C4 | 2 | Complete | A |
| 30 | C7 | 2 | Complete | A |
| 35 | C5 | 17 | Incomplete | C |
| 48 | C5 | 19 | Complete | A |
| 38 | C4 | 13 | Complete | A |
| 47 | C5 | 22 | Incomplete | D |
| 51 | C5 | 37 | Complete | A |
| 36 | C5 | 17 | Complete | A |
| 43 | C8 | 13 | Incomplete | B |
| 34 | C5 | 17 | Complete | A |
| 34 | C5 | 9 | Complete | A |
| 43 | C4 | 37 | Incomplete | C |
| 60 | C8 | 28 | Complete | A |
| 38 | C5 | 20 | Complete | A |
| 26 | C5 | 8 | Complete | A |
| High paraplegia | ||||
| 50 | T3 | 5 | Complete | A |
| 25 | T3 | 2 | Complete | A |
| 49 | T2 | 17 | Complete | A |
| 46 | T5 | 7 | Complete | A |
| 33 | T2 | 7 | Complete | A |
| 59 | T3 | 26 | Complete | A |
| 59 | T3 | 31 | Incomplete | B |
| 27 | T2 | 7 | Complete | A |
| 30 | T3 | 5 | Incomplete | B |
| 54 | T4 | 28 | Complete | A |
| Low paraplegia | ||||
| 46 | T10 | 2 | Complete | A |
| 50 | T7 | 5 | Complete | A |
| 58 | T12 | 28 | Complete | A |
| 33 | T10 | 5 | Complete | A |
| 42 | T8 | 21 | Complete | A |
| 27 | T11 | 11 | Complete | A |
| 35 | T10 | 4 | Complete | A |
| 46 | T7 | 28 | Complete | A |
| 48 | T9 | 6 | Complete | A |
Table 3.
Description of physical activity
| Activity | Duration (minutes) | Frequency |
|---|---|---|
| Tetraplegia (n = 3) | ||
| Pulleys, hand bike, passive cycling, and bench press | 90 | 2× week |
| Hand cycle/wheelchair racing | 30–40 | 3–4× week |
| FES | 30–60 | |
| Weight training | 30–60 | 3–4× week |
| Weight training | 90 | 4–5× week |
| Wheelchair pushing | 60 | 1× week |
| High paraplegia (n = 2) | ||
| Wheel-cycle pushing/racing | 60 | 5–7× week |
| Weight training | 30–40 | 3× week |
| Weight training | 60 | 5–7× week |
| Low paraplegia (n = 3) | ||
| Hand cycle | 60 | 1× week |
| Thera-cycle | 30 | 1× week |
| Weight training | 30 | 3× week |
| Basketball | 60–120 | 2× week |
| Hand cycling/wheelchair racing | 30–60 | 2–3× week |
| Ambulate | 15–30 | 3× week |
| Weight training | 30–60 | |
| Controls (n = 2) | ||
| Bicycling | 60 | 3× week |
| Bicycling | 60 | 2–3× week |
Systolic blood pressure
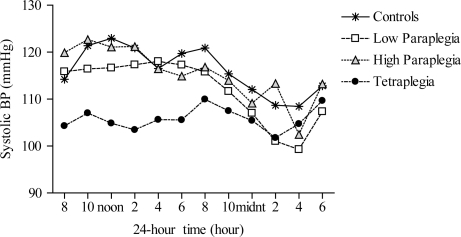
To test our first hypothesis we ran a 1 × 4 ANOVA on the average 24-hour, daytime and nighttime SBP data (Table 4). On average, 24-hour SBP was not different among the groups; however, mixed measures ANOVA indicated significant main effects for group (P < 0.05) and time (P < 0.0001). Post-hoc analysis demonstrated that 24-hour SBP was significantly lower in the TETRA group compared to the control (P < 0.05) and HP (P < 0.05) groups. There were no significant differences for 24-hour SBP among the HP, LP, and control groups.
Table 4.
Mean 24-hour data
| Controls | Low paraplegia | High paraplegia | Tetraplegia | Significance | |
|---|---|---|---|---|---|
| 24-hour HR (bpm) | 70 ± 9 | 83 ± 12 | 80 ± 6 | 69 ± 10 | 1,2,5,6 |
| Daytime HR | 75 ± 10 | 87 ± 13 | 87 ± 9 | 70 ± 10 | 1,2,5,6 |
| Nighttime HR | 62 ± 10 | 75 ± 13 | 70 ± 6 | 64 ± 9 | 1,5 |
| Nocturnal HRdip | −17 ± 8 | −14 ± 8 | −19 ± 7 | −8 ± 7 | 3,6 |
| SDANN (ms) | 112 ± 31 | 96 ± 34 | 140 ± 45 | 103 ± 31 | 4,6 |
| rMSSD (ms) | 61 ± 20 | 30 ± 13 | 46 ± 10 | 51 ± 20 | 1,4,5 |
| 24-hour SBP (mmHg) | 115 ± 8 | 113 ± 13 | 116 ± 8 | 107 ± 11 | NS |
| Daytime SBP | 119 ± 8 | 114 ± 16 | 115 ± 7 | 103 ± 12 | 3,5,6 |
| Nighttime SBP | 107 ± 8 | 102 ± 11 | 108 ± 11 | 106 ± 10 | NS |
| Nocturnal SBPdip | −10 ± 5 | −10 ± 11 | −6 ± 10 | 3 ± 10 | 3,5,6 |
Data are means ± SD; HR, heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; SDANN, standard deviation of the average normal R–R interval; RMSSD, root mean square of the standard deviation; AI, autonomic index.
1, control versus low paraplegia; 2, control versus high paraplegia; 3, control versus tetraplegia; 4, low paraplegia versus high paraplegia; 5, low paraplegia versus tetraplegia; 6, high paraplegia versus tetraplegia.
Additional mixed ANOVAs were run on daytime and nighttime SBP. The results indicate a significant group main effect for daytime SBP (P < 0.01), such that SBP was lower in the TETRA group compared to the control (P < 0.01), HP (P < 0.01), and LP (P < 0.05) groups. Although the group main effect for nighttime SBP was not significant, the main effect for time was significant, regardless of group (P < 0.0001), suggesting a nocturnal dip in SBP. Further analysis confirmed previous findings10–14 of a diminished nocturnal SBPdip in the TETRA group, which differed significantly from the other groups (P < 0.05). (Fig. 1)
Figure 1.
Twenty-four-hour SBP among the groups; controls (asterisks), low paraplegia (open squares), high paraplegia (closed triangles), and tetraplegia (closed circles). Main effects for group (P < 0.05) and time (P < 0.0001) were significant; such that 24-hour SBP was significantly lower in the tetraplegic group compared with the control and HP groups (P < 0.05).
Heart rate
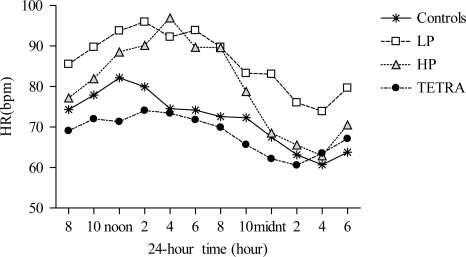
To test our second hypothesis, mean HR data from the 24-hour Holter monitor were compared across the groups (Table 4). On average 24-hour HR was significantly increased in the LP and HP groups compared to the non-SCI and TETRA groups. Twenty-four-hour HR data are presented by group (Fig. 2), and results of the mixed measures ANOVA revealed significant main effects for group (P < 0.01), time (P < 0.0001), and a significant interaction effect (P < 0.0001). Post-hoc analysis indicated that 24-hour HR was significantly elevated in the LP group compared to the control (P < 0.01) and TETRA (P < 0.001) groups and was elevated in the HP group compared to the control (P < 0.05) and TETRA (P < 0.01) groups.
Figure 2.
Twenty-four-hour HR among the groups; controls (asterisks), low paraplegia (open squares), high paraplegia (closed triangles), and tetraplegia (closed circles). Effects for group (P < 0.01), time (P < 0.0001), and the interaction (P < 0.0001) were significant; such that 24-hour HR was significantly elevated in the HP group compared to the control (P < 0.05) and TETRA (P < 0.01) groups, and in the LP group compared to the control (P < 0.01) and TETRA groups (P < 0.001).
Average daytime and nighttime HR data are presented (Table 4). Results of the mixed measures ANOVA for daytime HR revealed significant effects for group (P < 0.001), time (P < 0.0001) and the interaction (P < 0.01). Post-hoc analysis indicated that daytime HR was significantly elevated in both the HP and LP groups compared to the control (P < 0.01) and TETRA (P < 0.001) groups. Similar significant effects for group (P = 0.01), time (P < 0.0001), and the interaction (P < 0.0001) were found for nighttime HR, such that the LP group displayed persistently elevated HR during the nighttime compared to the control (P < 0.01) and TETRA (P < 0.01) groups. Differences for nighttime HR did not attain statistical significance comparing the HP group with the control (P = 0.13) or TETRA (P = 0.13) groups.
Although the nocturnal HRdip did not differ between the paraplegic and control groups, individual peak HRs were significantly elevated in the HP (111 ± 14 (range: 91–134) bpm; P < 0.0001) and LP groups (103 ± 13 (range: 77–121) bpm; P < 0.01) compared to the control (84 ± 13 (range: 70–114) bpm) and TETRA groups (83 ± 13 (range: 56–107) bpm). The individual nadir HRs were also statistically higher in the LP group (69 ± 13 (range: 53–95) bpm) compared to the control (58 ± 9 (range: 49–75) bpm; P < 0.05), HP groups (59 ± 6 (range: 51–70) bpm; P < 0.05), and TETRA (58 ± 10 (range: 32–72) bpm; P < 0.01) groups.
Time-domain HRV parameters
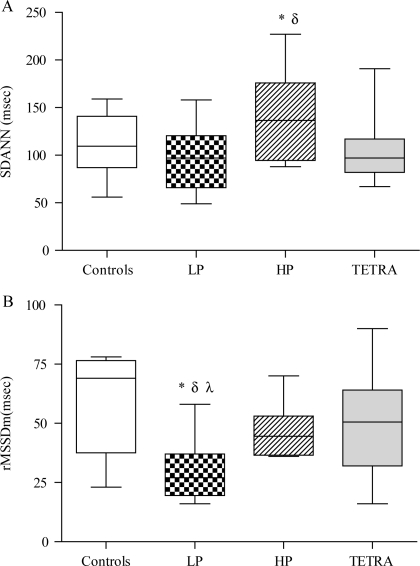
Elevated HR in the paraplegic groups may be suggestive of abnormal autonomic tone,26,29 and 24-hour time-domain parameters of HRV are presented (Table 4). We hypothesized that 24-hour HRV data would be reduced in the TETRA, HP, and LP groups compared to the non-SCI controls; however, the HRV index reflective of CLV (i.e. SDANN) was significantly elevated in the HP group compared to the LP and TETRA groups (Fig. 3A). In addition, 24-hour SDANN was marginally increased in the HP compared to the control group (P = 0.0734). On the other hand, 24-hour rMSSD (i.e. an indirect measure of vagal tone) was significantly reduced in the LP compared to the control (P < 0.001), HP (P = 0.05), and TETRA (P < 0.01) groups, and was marginally lower in the HP group compared to the controls (P = 0.0692) (Fig. 3B).
Figure 3.
(A) SDANN (ms) in the control (open box), LP (dotted box), HP (hatched box), and TETRA (gray box). *: P < 0.01 versus LP and TETRA; δ: P < 0.08 versus controls. (B) rMSSD (ms) in the control (open box), LP (dotted box), HP (hatched box), and TETRA (gray box). *: P < 0.01 versus TETRA; δ: P < 0.001 versus controls; λ: P = 0.05 versus HP.
Because it has been suggested that basal HR may influence these HRV parameters, adjustment for 24-hour HR with multiple regression analyses was used to determine group differences in SDANN and rMSSD. The results suggest that, after controlling for differences in 24-hour HR, the group main effect for SDANN remained significant (P < 0.01) and was marginally significant for rMSSD (P = 0.057). After statistically controlling for 24-hour HR, post-hoc analysis with the Bryant–Paulson procedure indicated that SDANN was significantly increased in the HP group compared to the TETRA and control groups and rMSSD was significantly reduced in the LP compared to the control group.
Discussion
To our knowledge, this is the first report on 24-hour cardiovascular and autonomic function in individuals with high paraplegia and low paraplegia compared with non-SCI controls and persons with tetraplegia. To date, the majority of available literature on 24-hour cardiovascular autonomic function following SCI focuses on persons with tetraplegia, and one paper considered the model of paraplegia as the control.15 However, the data reported here suggest that, although 24-hour SBP did not differ in individuals with high (T2–T5) and low (T7–T12) paraplegia compared to controls and individuals with tetraplegia, 24-hour HR was significantly elevated. Furthermore, 24-hour HRV data suggest alterations in autonomic tone that may predispose these individuals to persistently elevated HR and cardiovascular disease risk.
As hypothesized and previously reported,10–14 24-hour SBP was significantly reduced in the tetraplegic group compared to the control and HP groups. Literature in the general population suggests an association between chronic hypotension and cognitive deficits,30–34 poorer reporting of general health,35,36 increased reporting of fatigue,35–37 impaired social well-being,38 and a higher incidence of depression.35 In addition, because daytime SBP was significantly reduced in the TETRA group, but nighttime SBP did not differ from the other groups, the nocturnal SBPdip was diminished. The absence of a nocturnal dip in BP was independently and significantly associated with cognitive impairments39 and evidence of ischemic brain injury40 in hypertensive individuals. Therefore, we speculate that persistent hypotension and the lack of an appropriate nocturnal SBP dip may predispose individuals with tetraplegia to ischemic brain injury and cognitive deficits, associations that should be studied in greater detail.
As hypothesized, we report significantly elevated 24-hour HR in individuals with HP and LP compared to controls. Elevated HR was evident during the daytime in both paraplegic groups and persisted into the nighttime in the LP group. Although there is empirical evidence of elevated HR in persons with paraplegia,41–43 little attention has been given to these reports. That said, there is emerging evidence that elevated HR may be an independent cardiovascular risk factor,44–47 and an inverse relationship between HR and survival time was reported in 30 000 individuals over a period of 5–36 years.48 However, in the general population, a direct link between elevated HR and cardiovascular risk has been difficult to determine because of the complex interactions among the various risk factors.44,45,49 Associations between chronically elevated HR and increased systolic pulsatility, arterial stiffness and endothelial dysfunction are reported in the general population,46,49–52 and although there is evidence of increased arterial stiffness in persons with SCI,53,54 increased HR was not reported. The long-term effects of persistently elevated HR in the paraplegic population is unknown, but evidence in the non-SCI literature of a possible link between increased HR and cardiovascular disease risk is compelling and warrants further investigation in this population.
Theoretically, elevated HR may reflect ANS dysfunction, with a shift toward sympathetic dominance, thus promoting the development of atherosclerotic lesions and cardiovascular events.49,55 However, whether accelerated HR is a marker of autonomic dysfunction or an independent cardiovascular risk factor is not easily discerned. Individuals with SCI represent a model of autonomic dysfunction, which may be characterized by diminished sympathetic activity; however, individuals with LP (defined as T5 and below) were observed to have elevated resting catecholamine concentrations compared to controls.43,56 Therefore, persistently elevated 24-hour HR in the paraplegic groups may relate to increased sympathetic activity or may reflect vagal dysfunction, or a combination of both sympathetic and vagal pathology.
The general health of the heart can be ascertained from the analysis of HRV, and it has been suggested that calculation of HRV from 24-hour monitoring of HR may be more sensitive in detecting abnormalities in autonomic tone, because short-term data collection techniques do not detect changes in diurnal influences.25,26 The various time-domain parameters of HRV reflect different autonomic cardiac influences and parameters, which measure CLV, such as SDANN, reflect diurnal, and secular trends in HR,26 whereas indices that examine variance around adjacent cardiac cycles (i.e. rMSSD) are thought to strongly reflect vagal tone.28 Counter to our hypothesis, we found significantly increased SDANN in the HP group compared to the LP and T groups and marginally increased SDANN compared to the controls. This finding presumably reflects greater fluctuation in diurnal variability because daytime HR was significantly elevated but there was an appropriate nocturnal fall in HR in the HP group. However, increased SDANN does not necessarily confer reduced risk in the HP group because 24-hour rMSSD was marginally lower compared to age-matched controls. Thus, elevated HR and increased diurnal variability in the HP group most likely reflects increased daytime sympathetic activity without significant influence from preserved or increased vagal tone.
However, perhaps more alarming was the persistently elevated HR and diminished rMSSD in the LP group. Previous reports have suggested that individuals with paraplegia have comparable 24-hour HR and HRV compared to age-matched controls.9,10 However, these studies either did not distinguish between HP and LP or failed to control for the activities of daily living in the control group (orthostatic influences), which would confound both the HR and HRV data. Our control group was confined to a wheelchair for the duration of the study thus limiting the effects of gravitational stressors on cardiovascular regulation. Although we cannot account for the stress of upper-body ambulation in the control group, the daytime HR and BP data do not suggest increased cardiovascular work. Therefore, the significantly elevated HR in the LP group compared to controls, with evidence of diminished vagal tone, may impart significant adverse cardiovascular consequences that are currently unappreciated.
Perspectives
Anatomically, the sympathetic nervous system (SNS) would be expected to be affected by a spinal cord lesion and the degree of cardiovascular dysfunction loosely reflects the level of SNS injury.57,58 However, since cranial nerve integrity should be unaffected by SCI, parasympathetic cardiac control mechanisms should be anatomically intact, and several reports document normal resting vagal tone and comparable vagal responses to provocation following SCI.9,59–61 Yet others suggest vagal pathology in persons with SCI, and attribute these findings to compensatory parasympathetic suppression or cardiovascular deconditioning.62–64 Moreover, increased resting plasma catecholamine concentrations have been observed in individuals with T5–T11 paraplegia, reflecting increased post-ganglionic sympathetic activity.43,56 The present evidence of elevated HR may therefore be a harbinger of autonomic dysfunction that precedes clinically measurable cardiovascular disease processes. It is our strong opinion that cardiovascular autonomic scrutiny and clinical surveillance should be an integral part of the annual physical examination in individuals with paraplegia.
Limitations
Because clinical medicine and research lack a tool for the accurate measurement and diagnosis of SNS dysfunction, we are unable to determine the extent of autonomic cardiovascular impairment in our subjects with SCI. In addition, we did not include any observation of peripheral sympathetic activity and can only speculate regarding the possible association between accelerated HR and increased plasma catecholamine concentrations. Although evidence in the general population on the relationship between persistently elevated HR and arterial stiffness is convincing, and may apply to the SCI population, we did not measure any vascular morphology or physiological function and can only speculate on this possible association. Finally, because the study population was comprised of mostly men and the spinal cord injuries were largely complete, applicability of the findings to females or a cohort of individuals with incomplete SCI cannot be ascertained.
Conclusion
The present study demonstrated that although 24-hour SBP is not altered following paraplegia, 24-hour HR is persistently elevated, which may predispose these individuals to significant cardiovascular risk. In addition, the 24-hour assessment of autonomic function suggests diminished vagal tone in individuals with low paraplegia, which, in addition to reported elevations in plasma catecholamines, suggests significant adverse autonomic changes that may contribute to long-term cardiovascular demise. Furthermore, our results suggest that autonomic cardiovascular dysfunction differs in persons with tetraplegia and paraplegia and clinical practice guidelines should account for these differences with respect to cardiovascular disease progression. However, before clinical guidelines can be established, longitudinal studies should be performed to establish the cardiovascular consequences of chronically elevated HR in these individuals. In addition, studies should be designed to investigate the short-term and long-term effects of various interventions (e.g. exercise training, pharmacological interventions) on normalization of 24-hour cardiovascular and autonomic function in individuals with paraplegia.
Acknowledgements
We thank Dr Joseph Weir for his support with the statistical analyses. This research was supported by the Veterans Affairs Rehabilitation Research and Development Service (grant no. A6161W, B3203R and B4162C).
References
- 1.Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 1993;328(5):303–7 [DOI] [PubMed] [Google Scholar]
- 2.Elsenbruch S, Harnish MJ, Orr WC. Heart rate variability during waking and sleep in healthy males and females. Sleep 1999;22(8):1067–71 [DOI] [PubMed] [Google Scholar]
- 3.O'Brien E, Sheridan J, O'Malley K. Dippers and non-dippers. Lancet 1988;2(8607):397. [DOI] [PubMed] [Google Scholar]
- 4.Ingelsson E, Bjorklund-Bodegard K, Lind L, Arnlov J, Sundstrom J. Diurnal blood pressure pattern and risk of congestive heart failure. J Am Med Assoc 2006;295(24):2859–66 [DOI] [PubMed] [Google Scholar]
- 5.Verdecchia P, Schillaci G, Gatteschi C, Zampi I, Battistelli M, Bartoccini C, et al. Blunted nocturnal fall in blood pressure in hypertensive women with future cardiovascular morbid events. Circulation 1993;88(3):986–92 [DOI] [PubMed] [Google Scholar]
- 6.Myers J, Lee M, Kiratli J. Cardiovascular disease in spinal cord injury: an overview of prevalence, risk, evaluation, and management. Am J Phys Med Rehabil 2007;86(2):142–52 [DOI] [PubMed] [Google Scholar]
- 7.Groah SL, Weitzenkamp D, Sett P, Soni B, Savic G. The relationship between neurological level of injury and symptomatic cardiovascular disease risk in the aging spinal injured. Spinal Cord 2001;39(6):310–7 [DOI] [PubMed] [Google Scholar]
- 8.Samsa GP, Patrick CH, Feussner JR. Long-term survival of veterans with traumatic spinal cord injury. Arch Neurol 1993;50(9):909–14 [DOI] [PubMed] [Google Scholar]
- 9.Bunten DC, Warner AL, Brunnemann SR, Segal JL. Heart rate variability is altered following spinal cord injury. Clin Auton Res 1998;8(6):329–34 [DOI] [PubMed] [Google Scholar]
- 10.Munakata M, Kameyama J, Kanazawa M, Nunokawa T, Moriai N, Yoshinaga K. Circadian blood pressure rhythm in patients with higher and lower spinal cord injury: simultaneous evaluation of autonomic nervous activity and physical activity. J Hypertens 1997;15(12 Pt 2):1745–9 [DOI] [PubMed] [Google Scholar]
- 11.Christ JE. An analysis of circadian rhythmicity of heart rate in tetraplegic human subjects. Paraplegia 1979;17(2):251–8 [DOI] [PubMed] [Google Scholar]
- 12.Goswami R, Krishan K, Suryaprakash B, Vaidyanathan S, Rao K, Rao MS, et al. Circadian desynchronization in pulse rate, systolic and diastolic blood pressure, rectal temperature and urine output in traumatic tetraplegics. Indian J Physiol Pharmacol 1985;29(4):199–206 [PubMed] [Google Scholar]
- 13.Krum H, Louis WJ, Brown DJ, Jackman GP, Howes LG. Diurnal blood pressure variation in quadriplegic chronic spinal cord injury patients. Clin Sci (Lond) 1991;80(3):271–6 [DOI] [PubMed] [Google Scholar]
- 14.Nitsche B, Perschak H, Curt A, Dietz V. Loss of circadian blood pressure variability in complete tetraplegia. J Hum Hypertens 1996;10(5):311–7 [PubMed] [Google Scholar]
- 15.Wang YH, Huang TS, Lin JL, Hwang JJ, Chan HL, Lai JS, et al. Decreased autonomic nervous system activity as assessed by heart rate variability in patients with chronic tetraplegia. Arch Phys Med Rehabil 2000;81(9):1181–4 [DOI] [PubMed] [Google Scholar]
- 16.Nash MS, Mendez AJ. A guideline-driven assessment of need for cardiovascular disease risk intervention in persons with chronic paraplegia. Arch Phys Med Rehabil 2007;88(6):751–7 [DOI] [PubMed] [Google Scholar]
- 17.Bauman WA, Raza M, Spungen AM, Machac J. Cardiac stress testing with thallium-201 imaging reveals silent ischemia in individuals with paraplegia. Arch Phys Med Rehabil 1994;75(9):946–50 [PubMed] [Google Scholar]
- 18.Bauman WA, Spungen AM, Raza M, Rothstein J, Zhang RL, Zhong YG, et al. Coronary artery disease: metabolic risk factors and latent disease in individuals with paraplegia. Mt Sinai J Med 1992;59(2):163–8 [PubMed] [Google Scholar]
- 19.Wahman K, Nash MS, Lewis JE, Seiger A, Levi R. Cardiovascular disease risk and the need for prevention after paraplegia determined by conventional multifactorial risk models: the Stockholm spinal cord injury study. J Rehabil Med 2011;43(3):237–42 [DOI] [PubMed] [Google Scholar]
- 20.Wahman K, Nash MS, Lewis JE, Seiger A, Levi R. Increased cardiovascular disease risk in Swedish persons with paraplegia: the Stockholm spinal cord injury study. J Rehabil Med 2010;42(5):489–92 [DOI] [PubMed] [Google Scholar]
- 21.Wahman K, Nash MS, Westgren N, Lewis JE, Seiger A, Levi R. Cardiovascular disease risk factors in persons with paraplegia: the Stockholm spinal cord injury study. J Rehabil Med 2010;42(3):272–8 [DOI] [PubMed] [Google Scholar]
- 22.Ben-Dov IZ, Bursztyn M. Daytime sleeping and night-time urinating obscure normal dipping. Nephrol Dial Transplant 2006;21(1):226–7 [DOI] [PubMed] [Google Scholar]
- 23.Seo WS, Oh HS. The circadian rhythms of blood pressure and heart rate in the hypertensive subjects: dippers and non-dippers. Yonsei Med J 2002;43(3):320–8 [DOI] [PubMed] [Google Scholar]
- 24.Kleiger RE, Bigger JT, Bosner MS, Chung MK, Cook JR, Rolnitzky LM, et al. Stability over time of variables measuring heart rate variability in normal subjects. Am J Cardiol 1991;68(6):626–30 [DOI] [PubMed] [Google Scholar]
- 25.Kleiger RE, Stein PK, Bigger JT., Jr Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol 2005;10(1):88–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleiger RE, Stein PK, Bosner MS, Rottman JN. Time domain measurements of heart rate variability. Cardiol Clin 1992;10(3):487–98 [PubMed] [Google Scholar]
- 27.Bigger JT, Jr, Kleiger RE, Fleiss JL, Rolnitzky LM, Steinman RC, Miller JP. Components of heart rate variability measured during healing of acute myocardial infarction. Am J Cardiol 1988;61(4):208–15 [DOI] [PubMed] [Google Scholar]
- 28.Cook JR, Bigger JT, Jr, Kleiger RE, Fleiss JL, Steinman RC, Rolnitzky LM. Effect of atenolol and diltiazem on heart period variability in normal persons. J Am Coll Cardiol 1991;17(2):480–4 [DOI] [PubMed] [Google Scholar]
- 29.Ferri C, Emdin M, Giuggioli D, Carpeggiani C, Maielli M, Varga A, et al. Autonomic dysfunction in systemic sclerosis: time and frequency domain 24 hour heart rate variability analysis. Br J Rheumatol 1997;36(6):669–76 [DOI] [PubMed] [Google Scholar]
- 30.Weisz N, Schandry R, Jacobs AM, Mialet JP, Duschek S. Early contingent negative variation of the EEG and attentional flexibility are reduced in hypotension. Int J Psychophysiol 2002;45(3):253–60 [DOI] [PubMed] [Google Scholar]
- 31.Costa M, Stegagno L, Schandry R, Bitti PE. Contingent negative variation and cognitive performance in hypotension. Psychophysiology 1998;35(6):737–44 [PubMed] [Google Scholar]
- 32.Duschek S, Matthias E, Schandry R. Essential hypotension is accompanied by deficits in attention and working memory. Behav Med 2005;30(4):149–58 [DOI] [PubMed] [Google Scholar]
- 33.Duschek S, Weisz N, Schandry R. Reduced cognitive performance and prolonged reaction time accompany moderate hypotension. Clin Auton Res 2003;13(6):427–32 [DOI] [PubMed] [Google Scholar]
- 34.Duschek S, Schandry R. Reduced brain perfusion and cognitive performance due to constitutional hypotension. Clin Auton Res 2007;17(2):69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrett-Connor E, Palinkas LA. Low blood pressure and depression in older men: a population based study. Br Med J 1994;308(6926):446–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pilgrim JA, Stansfeld S, Marmot M. Low blood pressure, low mood? Br Med J 1992;304(6819):75–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wessely S, Nickson J, Cox B. Symptoms of low blood pressure: a population study. Br Med J 1990;301(6748):362–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosengren A, Tibblin G, Wilhelmsen L. Low systolic blood pressure and self perceived wellbeing in middle aged men. Br Med J 1993;306(6872):243–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto Y, Akiguchi I, Oiwa K, Hayashi M, Ohara T, Ozasa K. The relationship between 24-hour blood pressure readings, subcortical ischemic lesions and vascular dementia. Cerebrovasc Dis 2005;19(5):302–8 [DOI] [PubMed] [Google Scholar]
- 40.Schwartz GL, Bailey KR, Mosley T, Knopman DS, Jack CR, Jr, Canzanello VJ, et al. Association of ambulatory blood pressure with ischemic brain injury. Hypertension 2007;49(6):1228–34 [DOI] [PubMed] [Google Scholar]
- 41.Houtman S, Oeseburg B, Hughson RL, Hopman MT. Sympathetic nervous system activity and cardiovascular homeostatis during head-up tilt in patients with spinal cord injuries. Clin Auton Res 2000;10(4):207–12 [DOI] [PubMed] [Google Scholar]
- 42.Jacobs PL, Mahoney ET, Robbins A, Nash M. Hypokinetic circulation in persons with paraplegia. Med Sci Sports Exerc 2002;34(9):1401–7 [DOI] [PubMed] [Google Scholar]
- 43.Schmid A, Huonker M, Barturen JM, Stahl F, Schmidt-Trucksass A, Konig D, et al. Catecholamines, heart rate, and oxygen uptake during exercise in persons with spinal cord injury. J Appl Physiol 1998;85(2):635–41 [DOI] [PubMed] [Google Scholar]
- 44.Palatini P. Elevated heart rate in cardiovascular diseases: a target for treatment? Prog Cardiovasc Dis 2009;52(1):46–60 [DOI] [PubMed] [Google Scholar]
- 45.Palatini P. Elevated heart rate: a ‘new’ cardiovascular risk factor? Prog Cardiovasc Dis 2009;52(1):1–5 [DOI] [PubMed] [Google Scholar]
- 46.Park BJ, Lee HR, Shim JY, Lee JH, Jung DH, Lee YJ. Association between resting heart rate and arterial stiffness in Korean adults. Arch Cardiovasc Dis 2010;103(4):246–52 [DOI] [PubMed] [Google Scholar]
- 47.Fox K, Borer JS, Camm AJ, Danchin N, Ferrari R, Lopez Sendon JL, et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol 2007;50(9):823–30 [DOI] [PubMed] [Google Scholar]
- 48.Reil JC, Bohm M. The role of heart rate in the development of cardiovascular disease. Clin Res Cardiol 2007;96(9):585–92 [DOI] [PubMed] [Google Scholar]
- 49.Palatini P, Parati G. Persistently elevated heart rate accelerates the progression of arterial stiffness. J Hypertens 2010;28(4):653–6 [DOI] [PubMed] [Google Scholar]
- 50.Sa Cunha R, Pannier B, Benetos A, Siche JP, London GM, Mallion JM, et al. Association between high heart rate and high arterial rigidity in normotensive and hypertensive subjects. J Hypertens 1997;15(12 Pt 1):1423–30 [DOI] [PubMed] [Google Scholar]
- 51.Albaladejo P, Asmar R, Safar M, Benetos A. Association between 24-hour ambulatory heart rate and arterial stiffness. J Hum Hypertens 2000;14(2):137–41 [DOI] [PubMed] [Google Scholar]
- 52.Chen W, Srinivasan SR, Berenson GS. Differential impact of heart rate on arterial wall stiffness and thickness in young adults: the Bogalusa Heart Study. J Am Soc Hypertens 2008;2(3):152–7 [DOI] [PubMed] [Google Scholar]
- 53.Miyatani M, Masani K, Oh PI, Miyachi M, Popovic MR, Craven BC. Pulse wave velocity for assessment of arterial stiffness among people with spinal cord injury: a pilot study. J Spinal Cord Med 2009;32(1):72–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wecht JM, Weir JP, DeMeersman RE, Spungen AM, Bauman WA. Arterial stiffness in persons with paraplegia. J Spinal Cord Med 2004;27(3):255–9 [DOI] [PubMed] [Google Scholar]
- 55.Palatini P. Heart rate as an independent risk factor for cardiovascular disease: current evidence and basic mechanisms. Drugs 2007;67Suppl 2:3–13 [DOI] [PubMed] [Google Scholar]
- 56.Schmid A, Huonker M, Stahl F, Barturen JM, Konig D, Heim M, et al. Free plasma catecholamines in spinal cord injured persons with different injury levels at rest and during exercise. J Auton Nerv Syst 1998;68(1–2):96–100 [DOI] [PubMed] [Google Scholar]
- 57.Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil 2000;81(4):506–16 [DOI] [PubMed] [Google Scholar]
- 58.Claydon VE, Krassioukov AV. Orthostatic hypotension and autonomic pathways after spinal cord injury. J Neurotrauma 2006;23(12):1713–25 [DOI] [PubMed] [Google Scholar]
- 59.Koh J, Brown TE, Beightol LA, Ha CY, Eckberg DL. Human autonomic rhythms: vagal cardiac mechanisms in tetraplegic subjects. J Physiol 1994;474(3):483–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takahashi M, Matsukawa K, Nakamoto T, Tsuchimochi H, Sakaguchi A, Kawaguchi K, et al. Control of heart rate variability by cardiac parasympathetic nerve activity during voluntary static exercise in humans with tetraplegia. J Appl Physiol 2007;103(5):1669–77 [DOI] [PubMed] [Google Scholar]
- 61.Takahashi M, Sakaguchi A, Matsukawa K, Komine H, Kawaguchi K, Onari K. Cardiovascular control during voluntary static exercise in humans with tetraplegia. J Appl Physiol 2004;97(6):2077–82 [DOI] [PubMed] [Google Scholar]
- 62.Wecht JM, Weir JP, DeMeersman RE, Schilero GJ, Handrakis JP, LaFountaine MF, et al. Cold face test in persons with spinal cord injury: age versus inactivity. Clin Auton Res 2009;19(4):221–9 [DOI] [PubMed] [Google Scholar]
- 63.Grimm DR, De Meersman RE, Almenoff PL, Spungen AM, Bauman WA. Sympathovagal balance of the heart in subjects with spinal cord injury. Am J Physiol 1997;272(2 Pt 2):H835–42 [DOI] [PubMed] [Google Scholar]
- 64.Inoue K, Ogata H, Hayano J, Miyake S, Kamada T, Kuno M, et al. Assessment of autonomic function in traumatic quadriplegic and paraplegic patients by spectral analysis of heart rate variability. J Auton Nerv Syst 1995;54(3):225–34 [DOI] [PubMed] [Google Scholar]