Abstract
Green florescent protein (GFP) variants that are sensitive to changes in pH are invaluable reagents for the analysis of protein dynamics associated with both endo- and exocytotic vesicular trafficking. Ratiometric pHluorin is a GFP variant that displays a bimodal excitation spectrum with peaks at 395 and 475 nm and an emission maximum at 509 nm. Upon acidification, pHluorin excitation at 395 nm decreases with a corresponding increase in the excitation at 475 nm. GFP2, a GFP variant that contains mammalianized codons and the folding enhancing mutation F64L, displays ~8-fold higher florescence compared to pHluorin upon excitation at 395 nm. Using GFP2 as a template, an enhanced ratiometric pHluorin (pHluorin2) construct was developed to contain fully mammalianized codons, the F64L mutation and ten of the thirteen pHluorin-specific mutations. As a result, pHluorin2 displays markedly higher florescence when compared to pHluorin while maintaining the ratiometric pH-sensitivity. Unlike native pHluorin, pHluorin2 expressed in the ligand-binding domain of the parathyroid hormone 1 receptor is readily detectable by confocal microscopy and displays a marked increase in florescence upon ligand-induced endocytosis to intracellular vesicles. Thus, pHluorin2’s enhanced florescence while sustaining ratiometric pH-sensitivity represents a significant improvement for this methodological approach.
Introduction
The green florescent protein (GFP) from Aequorea victoria and its many engineered variants have revolutionized the analysis of protein dynamics (1). Proteins of interest fused to GFP, typically from either the amino- or carboxy terminal ends, facilitates cellular localization from either fixed samples or for real-time analysis using spinning disk confocal microscopy. GFPs have also enabled the analysis of real-time, intracellular protein-protein interactions using resonance energy transfer techniques, such as BRET (bioluminescence resonance energy transfer) and FRET (Forster resonance energy transfer) (2). Notably, GFPs fluoresce autonomously and thus do not require either exogenous or endogenous co-factors, which is an important attribute when analyzing protein localization in various cellular compartments. Several reports demonstrate that the excitation/emission profiles of specific GFP variants are sensitive to pH changes (3–6).
Using a directed mutagenesis approach in E. coli, Rothman and coworkers (3) developed two, pH-sensitive GFP variants, termed ecliptic- and ratiometric pHluorin, each containing clusters of mutations that direct pH sensitivity. Native GFP possesses a bimodal excitation profile with a major peak at 395 nm and a minor peak at 475 nm measured with an emission maximum at 509 nm. Under acidic conditions (pH < 6), ecliptic pHluorin is non-florescent, while shifting to a neutral environment enhances florescence at both peaks of excitation. Targeted expression of ecliptic pHluorin to acidic secretory vesicles allows for the real-time analysis of the exocytotic fusion of these vesicles to the plasma membrane where the pH becomes neutral, resulting in a corresponding increase in florescence (3). A fluorescently enhanced variant of GFP, termed EGFP, was developed through the inclusion of the F64L and S65T mutations, changes that promote protein folding at 37°C and a shift in the major excitation peak to 475 nm, respectively (7). Sankarararayanan et al (8) developed a superecliptic variant through incorporation of these same F64L and S65T mutations, changes that markedly enhance florescent output at the 475 nm excitation peak while maintaining pH sensitivity. The superecliptic pHluorin is ideal for analyzing exocytosis because florescence increases through progression of this process, i.e. acidic to neutral. However, using the same variant for the analysis of endocytosis would result in a loss of florescence over time, due to transit from a neutral environment (plasma membrane) to an acidic vesicle. The reduction of florescence could have two possible interpretations; the transit of tagged proteins to acidic vesicles or the proteolytic breakdown of these tagged proteins in lysosomes, leading to confounding analyses. Therefore, a pH-sensor that displays an inverse relationship between pH and florescence output would be ideal for the analysis of endocytotic processes.
The second, ratiometric variant developed by Miesenbock et al displays the desirable characteristics for analysis of endocytosis. With increasing acidity, the ratiometric pHluorin displays a dose-dependent decrease in the excitation at 395 nm with a concomitant increase in the excitation at 475 nm (3). These characteristics make ratiometric pHluorin an ideal reagent for monitoring cellular pathways that connect compartments that progress from neutral pH towards acidic ones, such as processes associated with endocytotic vesicular trafficking. Analysis of ligand-induced internalization of G protein coupled receptors (GPCRs) could benefit from this type of sensor. Analogous to the development of the superecliptic pHluorin (8), here we show that inclusion of mammalianized codons and the F64L mutation in the ratiometric pHluorin backbone markedly enhances florescence while maintaining the desired pH-sensitivity, yielding a super-ratiometric variant called pHluorin2.
Material and Methods
DNA constructs and cloning
The ratiometric pHluorin construct was a generous gift from Dr. Rothman and the GFP2 construct was from Perkin-Elmer (Boston, MA). Using PCR directed by pfu polymerase (Agilent Technologies, Santa Clara, CA), both pHluorin and GFP2 were cloned into pcDNA3.1 (Invitrogen, Carlsbad, CA) as single, soluble proteins. Compared to native GFP, the GFP2 construct contains the F64L mutation and mammalianized codons (i.e. codon usage that is fully optimized for translation on mammalian ribosomes). Using site-directed, PCR-mediated mutagenesis, pHluorin-specific mutations were incorporated into the GFP2 construct, yielding the pHluorin2 construct, which contains the F64L mutation and mammalianized codons. Relative to GFP2, the pHluorin2-specific mutations include S147E, N149L, I161T, V163A, N164I, K166Q, I167V, R168H, S202H and L231H. Native pHluorin mutations not included in the pHluorin2 construct are Q80R, E132D and S175G. The pHluorin2 cDNA was then cloned in-frame within exon 2 between amino acids 95 and 96 of the human parathyroid hormone 1 receptor, yielding the hPTH1R-pHluorin2 construct. Inclusion of EGFP within this domain does not interfere with ligand binding, internalization or signaling (9).
Florescence measurements
Excitation scans were generated using a Deltascan dual-wavelength fluorimeter from Photon Technology International (PTI; Birmingham, NJ) and the associated Felix software. Standard HEK293 cells were plated on 10 cm dishes and transiently transfected with 5 µg each of the soluble forms of GFP2, pHluorin and pHluorin2 using Fugene HD (Roche, Indianapolis, IN). Forty-eight hours post transfection, cells were washed with Hank’s Balanced Salt solution lacking calcium and magnesium ions and containing 2 mM EDTA. Cells were lifted from the plates using the same solution after a 10 minute incubation at 37°C and placed in a quartz cuvette with a stir bar. Excitation scans were performed between wavelengths 370 nm to 490 nm with the emission set at 510 nm. For the pH clamp experiments, cells were resuspended in a buffer containing 140 mM potassium chloride, 10 mM sodium phosphate with molar ratios of mono- and dibasic forms appropriate for the given pH and 30 mM nigericin (EMD Biosciences, Gibbstown, NJ). For the florescence plate reader studies, black, 96-well plates (Perkin-Elmer, Boston, MA) were loaded with 25,000 cells per well from the transfections described above and analyzed with dual excitation at 405 nm/8 nm and 485 nm/25 nm with a 535 nm/25 nm emission filter in an EnVision plate reader (Perkin-Elmer, Boston, MA). The narrow bandwidth of the 405 nm filter of 8 nm reduces the relative florescence compared to the larger bandwidth of the 485 nm filter. Despite this effect, the 405/485 nm ratios are still responsive to changes in intracellular pH.
For the hPTH1R endocytosis studies, HEK293 cells were sparsely plated into culture slides and transiently transfected with 100 ng of the hPTH1R-pHluorin2 construct using Fugene HD. Forty-eight hours post-transfection, cells were treated with either vehicle (acetic acid) or 100 nM parathyroid hormone containing amino acids 1 to 34 for 20 minutes. Cells were fixed with 3.7% paraformaldehyde and analyzed by confocal microscopy using a Radiance 2100 confocal microscope and the associated LaserSharp 2000 software (Bio-Rad Laboratories, Inc., Hercules, CA).
Results and Discussion
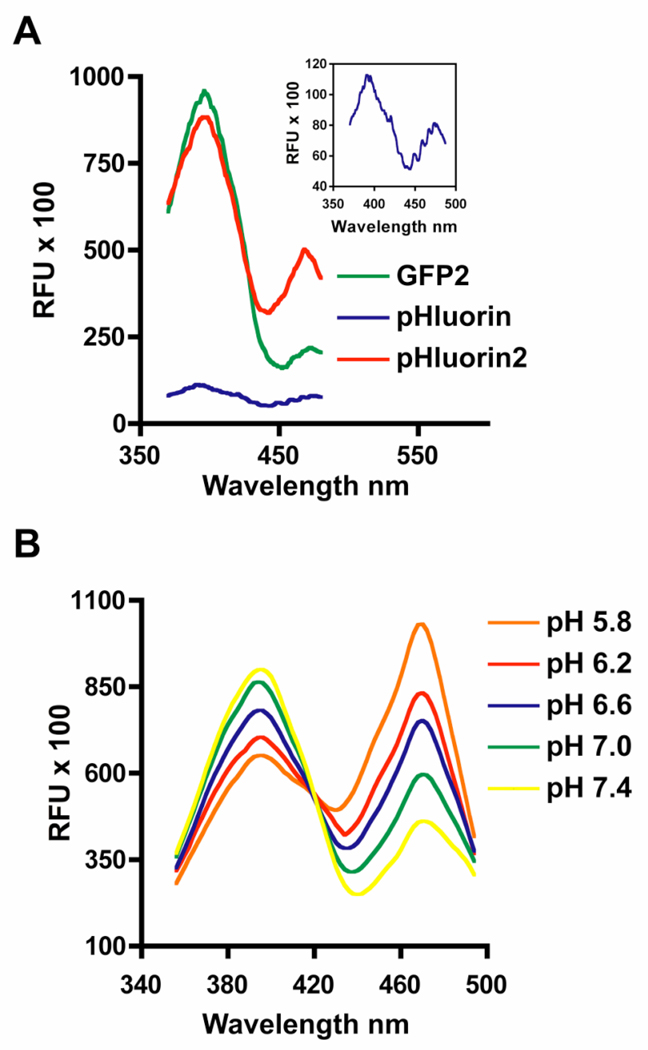
The excitation spectrum for native GFP is bimodal with a major peak at 395 nm and a minor peak at 475 nm with an emission maximum at 509 nm. GFP2, a variant developed for the use in BRET applications, contains fully mammalianized codons and the florescence enhancing mutation F64L. GFP2 and pHluorin display the same native GFP-specific excitation profile. However, the relative florescence of the 395 nm excitation peak of GFP2 is approximately 8-fold higher than pHluorin (Figure 1A). Several possible reasons regarding this marked difference in florescence exist. First, the several pHluorin-specific mutations, which impart the pH-sensitivity, may hamper the florescent output. However, this effect seems unlikely because pHluorin was selected based on its florescence characteristics when expressed in bacteria (3). This mutagenesis process, however, raises the possibility that the selected codons favor bacterial expression over those codons preferred by mammalian ribosomes. This possible effect is further compounded by the fact that pHluorin was developed from gene sequences consistent with the original jellyfish GFP cDNA. In contrast, the synthetic GFP2 construct contains fully mammalianized codons. Lastly, unlike pHluorin, all of the enhanced variants of GFP contain a leucine for phenylalanine substitution at amino acid 64 (F64L), including GFP2, enhanced cyan florescent protein (ECFP), enhanced green florescent protein (EGFP) and enhanced yellow florescent protein (EYFP) (7). It is thought that the F64L mutation markedly enhances florescence by promoting proper protein folding at 37°C (10).
Figure 1.
Compared to pHluorin, pHluorin2 displays a marked increase in florescence while maintaining ratiometric, pH-sensing. (A) HEK293 cells were transiently transfected with the cytoplasmic forms of GFP2, pHluorin and pHluorin2. Relative florescence units (RFU) of excitation scans between 370 nm and 490 nm with an emission set at 510 nm for GFP2 (green line), pHluorin (blue line and inset graph) and pHluorin2 (red line) are shown. (B) Excitation scans of cytoplasmic pHluorin2 expressed in HEK293 cells clamped at the indicated pH using K+ ions and nigericin are shown. Data are representative of three independent experiments.
Using PCR-mediated mutagenesis and the GFP2 cDNA as a template, an enhanced version of ratiometric pHluorin (pHluorin2) was developed. PHluorin2 contains the F64L mutation and ten out of the thirteen pHluorin-specific mutations (Figure 2). It is not known if the remaining mutations (Q80R, E132D and S175G) will have additional effects, but at this stage they have been determined to be unnecessary. Furthermore, the pHluorin2 cDNA contains fully mammalianized codons. As a result of these changes, the florescence output of pHluorin2 is 8-fold higher than native pHluorin when excited at 395 nm to levels that are comparable to GFP2 (Figure 1A). Importantly, pH clamp experiments using nigericin clearly demonstrate that the elements included to enhance pHluorin2 florescence do not affect the ratiometric, pH-sensitive characteristics attributed to the original pHluorin-specific mutations (Figure 1B). Compared to the pHluorin expressed in bacteria (3), the pH-dependent difference in the florescence maxima at 475 nm also appears greater for pHluorin2 when expressed in mammalian cells (Figure 1B). The markedly enhanced florescent output while maintaining pH-sensitivity makes pHluorin2 a valuable reagent for the analysis of changes in intracellular pH.
Figure 2.
Amino acid comparisons between GFP2, pHluorin and pHluorin2. Amino acid alignment of GFP2, pHluorin and pHluorin2 is shown. Amino acids in red are specific to GFP2 and pHluorin2. Amino acids in green are pHluorin-specific mutations incorporated into the pHluorin2 construct. The protein folding enhancing mutation, F64L, is boxed.
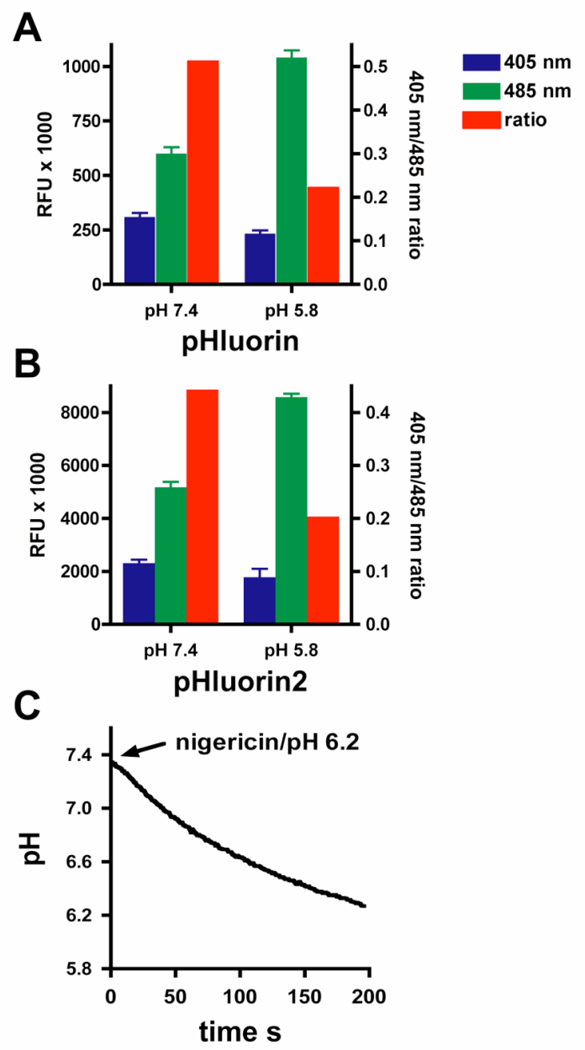
Similar to 2',7'-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF), pHluorin2 can monitor changes in intracellular pH using a standard plate florimeter. Consistent with the data described above, the relative florescence of the pHluorin2 is ~8-fold higher than pHluorin when analyzed with a plate florimeter (Figure 3A and B). Dual excitation at 405 nm and 485 nm yields 405 nm/485 nm ratios that decrease in response to lower intracellular pH for both pHluorin and pHluroin2. Notably, these ratios are similar for both proteins, illustrating the benefit of ratiometric analysis, which normalizes expression levels of the florescent protein. Using the nigericin pH clamp methodology, a pH versus the 405 nm/485 nm ratio standard curve was generated (data not shown). Applying this standard curve, cytoplasmic pHluorin2 is capable of monitoring nigercin-induced cellular acidification over time using a plate florimeter (Figure 3C).
Figure 3.
Monitoring intracellular pH using pHluorin2 and a plate florimeter. Cytoplasmic pHluorin (A) or pHluorin2 (B), expressed in HEK293 cells clamped at either pH 7.4 or 5.8 with nigericin, as indicated, was analyzed in a plate florimeter by dual excitation at 405 nm (blue bars) and 485 nm (green bars) with an emission filter of 535 nm. Ratios of 405 nm/485 nm (red bars) are shown on the right Y-axis. (C) Nigericin-induced cellular acidification of HEK293 cells expressing pHluorin2 in extracellular buffer set at pH 6.2 over time in seconds is shown. Data are representative of three independent experiments.
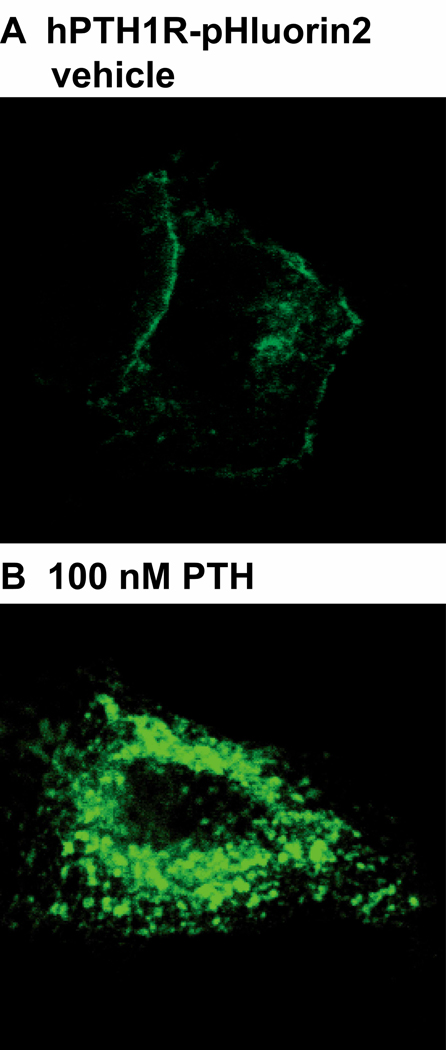
The parathyroid hormone 1 receptor (PTH1R), a primary regulator of mineral ion homeostasis, is a class b G protein coupled receptor (GPCR) that binds both parathyroid hormone (PTH) and parathyroid hormone relate protein (PTHrP). Recent reports demonstrate that PTH1R endocytosis plays an important role in the differential cellular responses mediated by these two prominent hormones (11). Thus, development of a high-throughput method capable of analyzing PTH1R endocytosis, or any GPCR for that matter, would greatly benefit this field of research. When EGFP is cloned in-frame within the second exon of the PTH1R, which is located in the extracellular ligand-binding domain, the receptor functions normally (9). However, EGFP florescence expressed as a chimera with the PTH1R is lower when compared to EGFP expressed alone (unpublished observations). Consistent with this phenomenon, pHluorin expressed in this same domain of the PTH1R yields florescence that is marginally detectable above background when analyzed by confocal microscopy (data not shown). These initial observations prompted the development of pHluorin2. As a result of the enhanced florescence, pHluorin2 cloned within this same domain of the PTH1R generates a receptor that is readily detected by confocal microscopy (Figure 4). Notably, ligand-induced endocytosis results in the formation of PTH1R-containing vesicles that are markedly brighter than when the receptor is on the cell surface, suggesting movement into an acidic vesicular environment (Figure 4B).
Figure 4.
Enhanced florescence in the FITC-channel upon ligand-induced endocytosis of hPTH1R-pHluorin2. HEK293 cells expressing hPTH1R-pHluorin2 were treated with either vehicle (A) or 100 nM PTH(1–34) (B). Representative confocal images using the FITC-channel with identical laser power and gain are shown.
Since the original discovery and cloning of the wild-type GFP, several variants have been engineered with the goal to improve the utility of this protein for the analysis of protein dynamics. Development of the pHluorins by Rothman and co-workers (3) represents seminal work in the achievement of these goals, especially as it relates to processes associated with compartmental changes in pH, such as exo- and endocytosis. Two straightforward modifications of the original ratiometric pHluorin were included in the development of pHluorin2. First, inclusion of the F64L mutation was desirable because this modification enhances florescence without changing the spectral characteristics. This is in contrast to the S65T mutation specific to EGFP, which markedly enhances florescence but does so through shifting the major excitation peak from 395 nm to 475 nm (7). Second, use of fully mammalianized codons assures that codon biases are not impeding full expression, especially when the original pHluorin construct was developed in bacteria.
With respect to ligand-induced receptor endocytosis, such as that demonstrated for GPCRs, high throughput methods monitoring transit from the neutral extracellular plasma membrane to acidic vesicles would certainly benefit from the use of pH-sensitive GFPs. For example, the enhanced yellow florescent protein (EYFP) displays a marked decrease in florescence upon acidification (4). Similar to ecliptic pHluorin, loss of florescence could signify receptor localization to acidic vesicles; however, as discussed earlier, a potential drawback is that one cannot rule receptor degradation as a possible explanation for decreased florescence. Therefore, use of the ratiometric forms of pHluorin is more desirable because the ligand-induced receptor transit from the cell surface to the endocytic vesicles is marked by an increase in florescence using the common FITC-type filter sets. Furthermore, the enhanced florescence displayed by pHluorin2 increases sensitivity and thus reduces the need to grossly over-express the receptor chimera of interest. Combined, the enhanced sensitivity and expression of pHluorin2 will increase the applicability and effectiveness of this construct.
Acknowledgements
I thank Dr. James Rothman for the ratiometric pHluorin construct. This work is supported by the NIDDK Program Project Grant 5P01DK011794-42 of Dr. John Potts. This paper is subject to the NIH Public Access Policy.
References
- 1.Shaner NC, Patterson GH, Davidson MW. Advances in fluorescent protein technology. Journal of Cell Science. 2007;120:4247–4260. doi: 10.1242/jcs.005801. [DOI] [PubMed] [Google Scholar]
- 2.Ciruela F. Fluorescence-based methods in the study of protein-protein interactions in living cells. Current Opinion in Biotechnology. 2008;19:338–343. doi: 10.1016/j.copbio.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Miesenbock G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 4.Llopis J, McCaffery JM, Miyawaki A, et al. Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proceedings of the National Academy of Sciences U S A. 1998;95:6803–6808. doi: 10.1073/pnas.95.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Awaji T, Hirasawa A, Shirakawa H, et al. Novel green fluorescent protein-based ratiometric indicators for monitoring pH in defined intracellular microdomains. Biochemical Biophysical Research Communications. 2001;289:457–462. doi: 10.1006/bbrc.2001.6004. [DOI] [PubMed] [Google Scholar]
- 6.Bizzarri R, Arcangeli C, Arosio D, et al. Development of a novel GFP-based ratiometric excitation and emission pH indicator for intracellular studies. Biophysics Journal. 2006;90:3300–3314. doi: 10.1529/biophysj.105.074708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heim R, Cubitt AB, Tsien RY. Improved green fluorescence. Nature. 1995;373:663–664. doi: 10.1038/373663b0. [DOI] [PubMed] [Google Scholar]
- 8.Sankaranarayanan S, De Angelis D, Rothman JE, et al. The use of pHluorins for optical measurements of presynaptic activity. Biophys Journal. 2000;79:2199–2208. doi: 10.1016/S0006-3495(00)76468-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tawfeek HA, Qian F, Abou-Samra AB. Phosphorylation of the receptor for PTH and PTHrP is required for internalization and regulates receptor signaling. Molecular Endocrinology. 2002;16:1–13. doi: 10.1210/mend.16.1.0760. [DOI] [PubMed] [Google Scholar]
- 10.Thastrup OTS, Kongsbak Poulsen L, Bjørn S. Fluorescent Proteins. US patent. 1995
- 11.Ferrandon S, Feinstein TN, Castro M, et al. Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nature Chemical Biology. 2009;5:734–742. doi: 10.1038/nchembio.206. [DOI] [PMC free article] [PubMed] [Google Scholar]