ABSTRACT
Introduction: Hepatocellular carcinoma (HCC) represents the fifth most common cancer worldwide, while being the third leading cause of death by cancer. The primary risk factor for HCC seems to be liver cirrhosis. A large majority of these patients have a history of viral hepatitis.
Materials and methods: We selected a study lot consisting of 244 patients diagnosed with HCC, admitted between 2006 and October 2009 in the Emergency County Hospital of Craiova, Romania along with an age and gender matched control group, consisting of patients with no history of HCC or other malignancies. We interviewed all subjects regarding their alcohol consumption and background environment. All subjects underwent hepatitis B surface antigen (Hbs Ag) and anti-HCV antibodies (Anti-HCV Atb) serological determinations.
Results: The study group consisted of 148 males and 96 females. Liver cirrhosis (LC) was present in 84% of the study lot, 10% associated viral B hepatitis (HBV) and 6% viral C hepatitis (HCV), with no signs of LC. We found LC to be an important risk factor for HCC (RR 6.53, CI 95% 3.18–13.38). The RR and 95% CI of HCC were 4.51 (2.48–8.21) for HbsAg positivity. We noticed a strong correlation (Chi-square test, p<0.001) between the rural environment and the association with LC. HVB was also more present in patients coming from rural areas (p< 0.01). Alcohol intake was present in 89% of the whole lot, being more correlated with the presence of LC as well as with HbsAg positivity (RR 9.165, CI 95% 4.43–18.92).
Conclusion: Cirrhosis proved to be the primary risk factors for HCC. We underline the fact that HCC was found to be directly associated with viral hepatitis, without evident LC. Further studies are needed in order to establish if intensified HCC screening, especially in rural areas, is required in patients with newly diagnosed viral hepatitis. The increased prevalence of HBV infections might encourage HBV vaccinations as an efficient tool to prevent HCC.
Keywords: Hepatocellular carcinoma, liver cirrhosis, viral hepatitis, HbsAg positivity, Anti-HCV Atb positivity, alcohol abuse, background environment
INTRODUCTION
Hepatocellular carcinoma (HCC) represents a major health burden worldwide. Currently, it represents the fifth most common cancer, while being the third leading cause of death by cancer (1-6). Approximately half a million deaths by HCC occur annually on the globe (3,4). Several regions in Asia and Africa display a particular increase in the number of cases, being currently regarded as endemic areas for HCC. These areas display 40-fold more cases based on the age-adjusted incidence rate, when compared with different continents (1,3,4). Recently, the incidence of HCC has been rising worldwide, particularly in highly industrialized countries such as the United States or Japan (2-5).
A gender-dependency seems to exists, as current statistics indicate men are more affected than women, the ratio being roughly 2:1 cases (1,3-5,7). Worldwide, HCC accounts for 7.5% of cancer in men and approximately 3.5% in women. No differences have been found in the afore-mentioned endemic areas, hepatitis playing a major role in all these cases (2,3,6). Data is yet scarce on the mechanisms behind this correlation, several mechanisms being proposed. The first hypothesis includes a hormonal interaction as main mechanism; however it was not proven by attempted hormonal manipulation (8). Recent studies suggest it is more likely this is a result of gender-related inflammatory response (8-10).
The primary risk factor for HCC seems to be represented by liver cirrhosis, as it is present in about 80 to 90% of all HCC patients (11-15). A large majority of these cirrhosis patients have a history of viral hepatitis (15-17). Roughly two-thirds of the total cirrhotic patients with a viral background is attributed to hepatitis B viral (HBV) infections, while the remaining third has a hepatitis C viral (HCV) infection as cause (11,12,15-17). In the United States, Western countries or Japan, however, HCV infection plays a major role, among high prevalence of non-alcoholic fatty liver disease (NAFLD) cases (18-21).
Our aim was to assess the importance liver cirrhosis and hepatitis, HbsAg and anti-HCV Atb positivity as well as social and economic risk factors, such as alcohol and living environment, have on the development of HCC in a heterogeneous population group. ❑
PATIENTS AND METHODS
We included in the study lot all patients newly diagnosed with HCC between 2006 and October 2009 admitted in the First Medical Clinic of the Emergency County Hospital of Craiova, Romania. All 244 patients who fitted the inclusion criteria above gave written informed consent for all investigations and inquiries used in the study. The hospital ethic committee also approved the study. We used the Barcelona 2000 EASL set of criteria for diagnosing HCC. Ultrasound and contrast CT was performed for all patients, while histology or cytology was available for 191 (78.24%) patients. We assessed liver cirrhosis (LC) using clinical and ultrasound criteria accepted worldwide. Upper endoscopy was performed in all patients.
We selected a control group consisting of 244 patients with no documented history of hepatocellular carcinoma or any neoplasm, who attended the hospital in the same time-frame. Controls were matched in gender and age with the study lot.
We interviewed all subjects regarding their alcohol consumption, using an in-house questionnaire adapted from the interview version of the Alcohol Use Disorders Identification Test. We regarded a sustained alcohol intake of over 70 grams of ethanol per day in the last five years as "heavy". Patients were also questioned about their background environment (rural/non-rural residence).
All subjects underwent hepatitis B surface antigen (HBsAg) and anti-HCV antibodies serological determinations, performed using standard enzyme-linked immunosorbent assay (ELISA) kits.
We used student's t-test in order to compare means of continuous variables, along with the chi-square and Fisher's exact test for proportions. We calculated the relative risk (RR) within a 95% confidence interval (CI) for determining the risk for the development of HCC in HBV and HCV infected patients, as well as the importance of alcohol as a risk factor. Statistical significance was achieved for a p value <0.05. The statistical packaged for social sciences (SPSS) version 17 (SPSS, Chicago, IL, USA) was used for data analysis. ❑
RESULTS
DESCRIPTIVE STATISTICS
The study group consisted of 148 (60.65%) males and 96 (39.35%) females, 182 (74.59%) patients coming from rural areas. Mean age was 54.43 years, with a standard deviation (SD) of ±12.43 years (minimum age: 32, maximum: 89). The control lot was perfectly matched in gender and age.
The incidence of risk factors among men and women in the study group is illustrated in table 1.
Table 1.
Distribution of risk factors in the study and control groups
| Study lot | Control lot | ||||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| Alcohol abuse | 155 | 62 | 8 | 3 | |
| Social background | Urban | 43 | 19 | 49 | 23 |
| Rural | 143 | 39 | 122 | 50 | |
| Cirrhosis | Hbs Ag + | 134 | 27 | 4 | 3 |
| Anti - HCV Atb + | 24 | 7 | 2 | 1 | |
| Other | 9 | 3 | 0 | 0 | |
| Hepatitis | Hbs Ag + | 11 | 13 | 5 | 3 |
| Anti - HCV Atb + | 8 | 8 | 2 | 2 | |
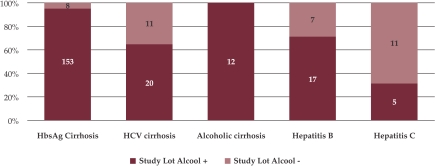
We found a history of heavy alcohol consumption in 217 (89%) patients of the whole study lot, 195 (95.12%) of them also having LC (153 patients with HbsAg positivity and 20 with anti-HCV antibodies). From the 22 heavy drinking patients with no signs of LC, 17 patients presented HbsAg positivity and 5 anti-HCV Atb positivity (Figure 1).
Figure 1. Alcohol abuse among the study group.
Of the 244 HCC patients in the study group, 204 (83.6%) patients presented with liver cirrhosis, 24 (9.83%) associated HBV and 16 (6.17%) presented HCV infections with no sign of cirrhosis. The mean age for the LC subgroup was 69±7 years, for HBV 61±6 years and for HCV 64±9 years. From the 204 patients presenting LC, 161 (78.92%) had HbsAg positivity, 31 (15.12%) anti-HCV Atb positivity, and 12 (5.86%) were diagnosed with alcohol-induced cirrhosis.
PREVALENCE OF VIRAL MARKERS AND HEAVY ALCOHOL USE IN CONTROLS
From the control lot, 10 patients displayed signs of liver cirrhosis, all having viral etiology: HbsAg positivity was found in 7 patients while 3 presented anti-HCV antibodies. We did not register any co-infections. Eight patients were discovered with HBV and four with HCV. Heavy alcohol usage was registered in 11 of the control group subjects. Out of these, five had HbsAg positivity and none had HCV markers present. Nine of them presented signs of LC.
INCIDENCE AND PREVALENCE
The RR and 95% CI of HCC were 6.53 (3.18–13.38) for cirrhosis, thus proving that cirrhosis represents a major risk factor for the development of primary liver tumors.
The RR and 95% CI of HCC were 4.51 (2.48–8.21) for HbsAg positivity. We found no significant risk increase in subjects who were anti-HCV Atb positive (RR of 1.91 and 95% CI 0.84–4.33), because the CI contained 1.0.
For alcohol, we registered a RR and 95% CI of HCC of 9.165 (4.43–18.92).
We found alcohol to be connected to HbsAg positivity in both cirrhosis and hepatitis (Chi-square test, p<0.001). The rural environment proved to be related with a higher incidence of HbsAg positivity, as well as with a higher alcohol consumption (Chi-square test, p<0.001).
TUMOR MORPHOLOGY AND ALPHA-FETOPROTEIN LEVELS IN HCC PATIENTS WITH AND WITHOUT CIRRHOSIS
Multi-nodular HCC was found in 203 (83.19%) patients of the study lot. Out of these, 182 (74.59%) patients presented with LC (Table 2). We found multi-nodular HCC to be more frequently associated with cirrhosis (p<0.001). In the case of a solitary nodule, we could not determine any difference between tumor diameters when comparing various risk factors.
Table 2.
Incidence of solitary and multi-nodular HCC in patients with and without LC
| Patients with LC | Patients without LC | |
|---|---|---|
| Multinodular HCC | 182 | 21 |
| Solitary HCC | 22 | 19 |
Alpha-fetoprotein (AFP) was present in all patient subgroups. No particularities were found in this regard, proving that the presence of this serological marker is not dependent of any particular etiology. ❑
DISCUSSION
HCC is the most common type of primary liver cancer, representing the third leading cause of cancer death. It is necessary to detect early stage HCC, as it has been shown that curative treatments (resection, percutaneous therapy, liver transplantation) improve the 5-year survival rates above 50% to 70% (22-25). But nevertheless the most important aspect remains to detect risk factors and try to eliminate them.
The major factors increasing the risk of HCC are chronic hepatitis B and C as well as cirrhosis, irrespective of its etiology (11-15). In North America, Europe, and other areas of low prevalence, most patients have underlying cirrhosis unrelated to HBV or HCV infection. Obesity and diabetes are also increasing the risk among those with chronic viral hepatitis, especially in high industrialized countries such as the United States and Japan (18-21). The GLOBCAN 2002 database (26) reports similar HCC incidence and mortality rates for Central and Eastern European countries (crude rates for males – 6.8%, mortality 7.5%; crude rate for women – 4.3%, mortality 5.0%). Romania has the highest crude incidence rate among these countries: 14.2% for males and 6.8% for women. Mortality rates are similarly high when compared to other Eastern European countries, with a 10% crude rate for men and a 7.0% crude rate for women. HCC is usually diagnosed in late stages. The most prevalent risk factor in Romania is represented by liver cirrhosis, predominantly of HBV etiology. A smaller percentage of HCC cases are not associated with cirrhosis, viral hepatitis being the primary affect. NAFLD and metabolic disease have a far less important role in HCC development. Alcohol consumption represents a major concern among these patients. A study recently published by Kumar et al, (15) presenting their findings in a lot of Indian patients, proved that the main risk factors for HCC are represented primary by HBV, followed by HCV infection. In our patient group, liver cirrhosis represented the primary risk factor associated with HCC; however an important percentage presented HCC with hepatitis alone. The presence of HbsAg positivity proved to be the most relevant factor for HCC development. In a study published in 2004 by Ayoola et al, (27) HBV infection proved to be the most important risk factor for HCC, in a group of Saudi Arabian patients.
Alcohol acts synergistically with chronic viral hepatitis in increasing the risk of HCC (15-17,28). In our study, heavy alcohol usage positively correlated with the presence of HCC in all age groups. Other studies further support this finding, proving alcohol to be not only a potentiating agent, but a risk factor in itself, as heavy alcohol consumption leads to a precarious lifestyle which directly favors HBS especially, as well as HCV infection.
The particularities of the socio-economic situation in our country showed an increase incidence of cirrhosis and alcohol abuse, as well as HbsAg positivity, in rural areas. Patients coming from this environment showed an increase correlation and synergistic action of these risk factors.
Men seem to be more affected by HCC than women (it is estimated that there were 564000 new cases of HCC in the year 2000, accounting for 7.5% of cancer in men and 3.5% of cancer in women worldwide). Our findings support this observation, the men to woman ratio being 1.54.
Most recently, chronic hepatitis C virus (HCV) infection has been recognized as an important factor in the genesis of hepatocellular carcinoma. The mechanism of carcinogenesis is unknown because HCV is an RNA virus and (unlike HBV) is not incorporated into the host genome (29). The tumor may evolve from fibro genesis rather than from HCV infection itself because cirrhosis is already established in almost all cases.
In areas where HBV is endemic, most HCC are eventually associated with markedly elevated alpha-fetoprotein levels, although levels are often normal in early-stage disease; in low prevalence areas, high levels are less frequent (30-32). We found no correlation between the presence of alpha-fetoprotein and the presence of a particular risk factor, thus proving that the presence of alpha-fetoprotein cannot be used as a discriminating test between etiologic agents.
Cirrhosis proved to be the primary risk factors for HCC. We underline the fact that HCC was found to be directly associated with viral hepatitis, without evident LC. Further studies are needed in order to establish if intensified HCC screening, especially in rural areas, is required in patients with newly diagnosed viral hepatitis. The risk of HCC is increased with male gender, older age, cirrhosis, alcohol intake. Thus, the best strategy is eliminating modifiable risk factors (alcohol, tobacco, obesity, diabetes) and to treat with antiviral agents. Use of vaccines against HBV should eventually be of benefit, especially in endemic areas (33). Another important issue is whether antiviral therapy can prevent HCC. Early treatment intervention is necessary to prevent damaging liver cells and decrease viral genome integration. ❑
Footnotes
Conflicts of interest None declared.
References
- 1.Bosch FX, Ribes J, Borras J. Epidemiology of primary liver cancer. Semin Liv Dis. 1999;19:271–285. doi: 10.1055/s-2007-1007117. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. – Cancer Facts and Figures. Atlanta: American Cancer Society; 2007
- 3.Parkin DM, Bray F, Ferlay J. Global cancer statistics, 2002. CA Cancer J Clin. 2005;25:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.McGlynn KA, London WT. Epidemiology and natural history of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2005;19:3–23. doi: 10.1016/j.bpg.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Sherman M. Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin Liv Dis. 2005;25:143–154. doi: 10.1055/s-2005-871194. [DOI] [PubMed] [Google Scholar]
- 6.Bosch FX, Ribes J, Díaz M, et al. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Gish RG. Risk factors and treatment for hepatocellular carcinoma. Gastroenterol Hepatol. 2006;2:477–478. [PMC free article] [PubMed] [Google Scholar]
- 8.De Maria N, Manno M, Villa E. Sex hormones and liver cancer. Mol Cell Endocrinol. 2002;193:59–63. doi: 10.1016/s0303-7207(02)00096-5. [DOI] [PubMed] [Google Scholar]
- 9.Rogers AB, Theve EJ, Feng Y, et al. Hepatocellular carcinoma associated with liver-gender disruption in male mice. Cancer Res. 2007;67:11536–11546. doi: 10.1158/0008-5472.CAN-07-1479. [DOI] [PubMed] [Google Scholar]
- 10.Yu MW, Chang HC, Chang SC, et al. Role of reproductive factors in hepatocellular carcinoma: Impact on hepatitis B- and C-related risk. Hepatology. 2003;38:1393–1400. doi: 10.1016/j.hep.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 11.Kemp W, Pianko S, Nguyen S, et al. Survival in hepatocellular carcinoma: impact of screening and etiology of liver disease. J Gastroenterol Hepatol. 2005;20:873–881. doi: 10.1111/j.1440-1746.2005.03844.x. [DOI] [PubMed] [Google Scholar]
- 12.Schlaeger C, Longerich T, Schiller C, et al. Etiology-dependent molecular mechanisms in human hepatocarcinogenesis. Hepatology. 2008;47:511–520. doi: 10.1002/hep.22033. [DOI] [PubMed] [Google Scholar]
- 13.Yu MW, Yeh SH, Chen PJ, et al. Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst. 2005;97:265–272. doi: 10.1093/jnci/dji043. [DOI] [PubMed] [Google Scholar]
- 14.Velazquez RF, Rodriguez M, Navascues CA, et al. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology. 2003;37:520–527. doi: 10.1053/jhep.2003.50093. [DOI] [PubMed] [Google Scholar]
- 15.Kumar M, Kumar R, Hissar SS, et al. Risk factors analysis for hepatocellular carcinoma in patients with and without cirrhosis: A case–control study of 213 hepatocellular carcinoma patients from India. J Gastroenterol Hepatol. 2007;22:1104–1111. doi: 10.1111/j.1440-1746.2007.04908.x. [DOI] [PubMed] [Google Scholar]
- 16.Fassio E, Míguez C, Soria S, et al. Etiology of hepatocellular carcinoma in Argentina: results of a multicenter retrospective study. Acta Gastroenterológica Latinoamericana. 2009;39(1):47–52. [PubMed] [Google Scholar]
- 17.Lopez PM, Villanueva A, Llovet JM. Systematic review: evidencebased management of hepatocellular carcinoma—an updated analysis of randomized controlled trials. Aliment Pharmacol Ther. 2006;23:1535–1547. doi: 10.1111/j.1365-2036.2006.02932.x. [DOI] [PubMed] [Google Scholar]
- 18.Bugianesi E, Leone N, Vanni E, et al. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123:134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 19.Marrero JA, Fontana RJ, Su GL, et al. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349–1354. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 20.Poonawala A, Nair SP, Thuluvath PJ. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a casecontrol study. Hepatology. 2000;32:689–692. doi: 10.1053/jhep.2000.17894. [DOI] [PubMed] [Google Scholar]
- 21.Caldwell SH, Crespo DM, Kang HS, et al. Obesity and hepatocellular carcinoma. Gastroenterology. 2004;127:S97–S103. doi: 10.1053/j.gastro.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 22.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–524. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 23.Bruix J, Sherman M. Management of hepatocellular carcinoma. Practice guidelines committee, American Association for the Study of Liver Disease. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 24.Yeo W, Mo KF, Chan SL, et al. Hepatitis B viral load predicts survival of HCC patients undergoing systemic chemotherapy. Hepatology. 2007;45:1382–1389. doi: 10.1002/hep.21572. [DOI] [PubMed] [Google Scholar]
- 25.Lo CM, Liu CL, Chan SC, et al. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma. Ann Surg. 2007;245:831–842. doi: 10.1097/01.sla.0000245829.00977.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.GLOBCAN 2002 Cancer statistics - http://www-dep.iarc.fr/ - accessed December 15th 2009.
- 27.Ayoola EA, Gadour MO. Hepatocellular carcinoma in Saudi Arabia: Role of hepatitis B and C Infection. J of Gastroenterol and Hepatol. 2004;19:665–669. doi: 10.1111/j.1440-1746.2003.03334.x. [DOI] [PubMed] [Google Scholar]
- 28.Hassan MM, Hwang L-Y, Hatten CJ, et al. Risk factors for hepatocellular carcinoma: synergism of alcohol with viral hepatitis and diabetes mellitus. Hepatology. 2002;36:1206–1213. doi: 10.1053/jhep.2002.36780. [DOI] [PubMed] [Google Scholar]
- 29.Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- 30.Izumi R, Shimizu K, Kiriyama M, et al. Alpha-fetoprotein production by hepatocellular carcinoma is prognostic of poor patient survival. J Surg Oncol. 1992;49:151–155. doi: 10.1002/jso.2930490305. [DOI] [PubMed] [Google Scholar]
- 31.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519–524. doi: 10.1053/jhep.2002.32089. [DOI] [PubMed] [Google Scholar]
- 32.Fen-Yu Ren, Xi-Xu Piao, Ai-Lian Jin J, Fen-Yu Ren, Xi-Xu Piao, Ai-Lian Jin JM, Fen-Yu Ren, Xi-Xu Piao, Ai-Lian Jin JM. Efficacy of ultrasonography and alpha-fetoprotein on early detection of hepatocellular carcinoma. World J Gastroenterol. 2006 Aug;12(29)(7):4656–4659. doi: 10.3748/wjg.v12.i29.4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang M-H, Shau W-Y, Chen C-J, et al. Hepatitis B vaccination and hepatocellular carcinoma rates in boys and girls. JAMA. 2000;284:3040–3042. doi: 10.1001/jama.284.23.3040. [DOI] [PubMed] [Google Scholar]