ABSTRACT
Atherosclerosis is a chronic inflammatory disease started by endothelial injury and defined by arterial wall load with free and esterified cholesterol, followed by subintimal focal recruitment of circulating monocytes and T-lymphocytes that heals by fibrosis and calcification. Inflammation plays a crucial role in atherogenesis either by local cellular mechanisms or humoral consequences easily measurable in plasma. In most cases inflammation and endothelial dysfunction are triggered by cardiovascular risk factors: hypercholesterolemia, hypertension, smoking or diabetes. In other cases inflammation precedes atherosclerotic changes that occur in autoimmune diseases, as systemic lupus erythematosus and rheumatoid arthritis. In these diseases atherogenesis is mostly independent from conventional risk factors. Irrespective of its cause systemic inflammation is correlated with cardiovascular events, but currently there are controversial results regarding inflammatory markers and early atherosclerotic process. We designed a study to identify if the amplitude of inflammation expressed by multiple serum markers is correlated with the severity of the atherosclerotic process measured by coronary atheroma volume and carotid intima-media thickness. The selected inflammatory markers are associated with different pathogenic steps in atherogenesis: acute phase reactants (C-reactive protein); pro-inflammatory cytokines (TNF-alpha, interleukin-6 and -18); endothelium activation markers (soluble VCAM-1, ICAM-1); and specific factors (anticardiolipinic antibodies). We aim to enrol the two different patient subsets with early atherosclerosis: one with conventional risk factors and one with autoimmune diseases without traditional risk factors, in whom inflammation is part of the systemic disease progression.
Keywords: systemic inflammation, early atheroma formation
INTRODUCTION
THE RELATIONSHIP BETWEEN INFLAMMATION AND ATHERO-GENESIS
Atherosclerosis is a chronic inflammatory disease that has a silent course for a few decades before reaching clinical significance (1); this is generally associated with athero-thrombotic complications, such as acute coronary syndromes or stroke. Atherosclerotic plaque development and progression is associated with inflammatory changes from early stages, both locally in the arterial wall or systemically. The latter could be identified by an early increase of serum inflammatory markers. Considering the demonstrated relationship between inflammation and atherosclerosis it is of uttermost importance to identify these early activity markers in order to prevent disease progression.
Inflammatory markers correlated with the atherosclerotic process can be divided in few categories: endothelial activation factors (soluble VCAM-1, ICAM-1 and CD40L); non-specific pro-inflammatory factors that induce hepatic synthesis of acute phase reactants (TNF-alpha, interleukin-1, -6 and -18); acute phase reactants synthesized by the liver (C reactive protein, serum amyloid A and fibrinogen); thrombotic endothelial dysfunction factors (PAI-1); and specific factors associated with autoimmune diseases, such as the anticardiolipinic antibodies. Some inflammatory factors are synthesized locally by mononuclear cells that invade vessel wall or by dysfunctional endothelial cells. Others are produced by hepatocytes stimulated by chemokines such as Il-6 systemically released by activated macrophages (2). The relationship between inflammation and athero genesis was studied up to date mainly looking at prognostic influence of inflammatory markers with respect to conventional risk factors, and much less to the possible pathogenic effect on progression of atherosclerotic plaque.
THE RELATIONSHIP BETWEEN INFLAMMATION AND CARDIOVASCULAR PROGNOSIS
C-reactive protein (CRP) is the most widely studied inflammation marker related to cardio-vascular prognosis (table 1); it is currently the only widely available biomarker used to quantify risk (3). Epidemiologic studies conclusively showed that the risk of major CV events increases with every milligram above 0.5-1 mg/l up to 20 mg/l; the upper limit of normal is 3 mg/l (4). A meta-analysis showed that cardiovascular risk of patients that have CRP in the upper tertile is two times higher than that of patients with a CRP value in the lower tertile. The most impressive relationship between inflammatory markers and atherosclerosis is observed in acute coronary syndromes: serum CRP, Il-6 or serum amyloid A are 10 to 20 times higher than baseline values (5).
Table 1.
The main factors that can influence serum CRP concentration
| Increase concentration | Lower concentration |
|---|---|
| Acute and chronic infection – inflammation (acute phase reactant) | Chronic moderate alcohol ingestion |
| Systemic hypertension | Physical training (daily, strenuous) |
| Overweight / obesity (mainly abdominal) | Starvation or chronic weight loss |
| Diabetes mellitus | Drugs: low dose aspirin; statins; fibrates; RAS-blockers |
| Low HDL and high triglycerides levels | |
| Oral contraceptives | |
| Chronic inflammation in auto-immune disease (RA, SLE) |
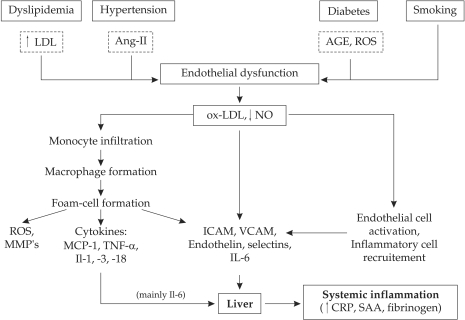
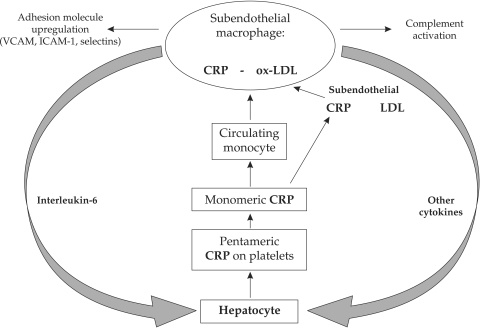
CRP may have direct pathogenic influence on atherogenesis by its synthesis in the macrophages from the lipid core and is associated with fibrous cap thinning (6). CRP is also a key mediator of complement activation by the ox-LDL in early atherosclerotic lesions and induces vessel wall damage and increases local inflammation. In early stages of atherogenesis CRP has multiple pathogenic effects: it promotes increased expression of adhesion molecules (VCAM-1, ICAM-1, E-selection) on endothelial surface, increases monocyte adhesion and migration, determines synthesis of chemotactic factors (MCP-1) and induces endothelial secretion of other pro-inflammatory factors (NF-KB, Il-6, Il-8) (figure 1 and 2). Other pathogenic effects are related to increased LDL oxidation and macrophage uptake of LDL, reactive oxygen species formation, AT1 receptor activation and smooth muscle cell proliferation and migration in the subendothelial space (7). Despite the clear relationship between CRP-levels and atherothrombotic complications, genetically determined higher levels of CRP do not correlate well with the risk of ischemic events, arguing with the proposed direct pathogenic effect of this biomarker. Hs-CRP was measured in 50,816 subjects with four CRP genetic variants that explained a difference in plasma CRP levels of 64% (8). Despite the expected association between CRP levels and cardiovascular disease was confirmed, there was no genetic CRP variant associated with the risk of ischemic events. Although polymorphisms of the CRP gene may induce a marked increase of CRP levels, they are not associated with an increased risk of atherothrombotic events (8). These genetic and epidemiological data are supported by experimental studies in which continuously infused human CRP in apo-E deficient mice did not have pro-atherosclerotic or pro-inflammatory effects (9).
Figure 1. The connections between risk factors, endothelial dysfunction and atherogenesis that promote systemic inflammation.
Ang-II: angiotensin-II; AGE: advanced glycation end-products; ROS: reactive oxygen species; NO: nitric oxyde; MMP: matrix metalloproteinases; MCP-1: monocyte chemotactic protein-1; TNF: tumor necrosis factor; Il: interleukins; SAA: serum amyloid A
Figure 2. The pathogenic role of CRP in the mechanisms of atherogenesis. CRP is fixed on platelets as a pentameric integrin and it is transformed in a monomer that atracts monocytes and stimulates their adhesion and transmigration in the subendothelial space. Here CRP co-localizes with LDL and it is specifically uptaken in the macrophages by specific receptors. Macrophages subsequently upregulate adhesion molecules, sinthesize tissue factor and activate complement. Interleukin-6 is the main citokyne responsible for increased liver synthesis of CRP.
Except CRP that has been extensively investigated because of widely available, well standardized lab kits, the prognostic influence of other inflammatory markers is less correlated with cardiovascular risk. The presence of these inflammatory markers is well correlated with CV events, as it resulted from a study in which sICAM-1, Il-6 and serum amyloid A were related with cardiac death, myocardial infarction, nonfatal stroke and coronary revascularization at a mean follow-up of 3 years in a group of 28,263 healthy women, without history of prior cardiovascular disease (10). However in routine clinical practice measurement of serum cytokine levels or acute phase reactants is not recommended to stratify cardiovascular risk with the exception of CRP, based on a Scientific Statement of American Heart Association issued in 2004 (class IIa recommendation) (3-11). Serum cytokines (Il-1, Il-6, Il-10, Il-18, TNF-alpha, and MCP-1) can be measured by ELISA techniques and should be determined with research purposes . These molecules have low stability after the blood sample was drawn and it is mandatory to quickly separate serum and conserve it at -70 degrees C until the test can be done. A similar situation is observed with the adhesion molecules E- and P-selectins, sVCAM-1 and sICAM-1.
SYSTEMIC INFLAMMATION AND ATHEROSCLEROSIS: ARE THEY RELATED?
Although epidemiological studies proved a clear correlation between systemic inflammation and adverse CV events, there is no definite relationship between serum levels of inflammatory markers and the volume of atherosclerotic lesions. In some experimental studies it a correlation between CRP and early atherosclerotic plaques in the aorta of genetically Apo-E deficient mice was found (12). In clinical studies serum CRP was not related with the severity of carotid atherosclerosis assessed by ultrasound or with the calcium score determined by multislice CT (13). Some studies demonstrated a positive relationship (14,15), while others found a link between CRP and intima – media thickness only with advanced atherosclerotic lesions (16). The ICARAS trial enrolled 1268 patients in whom serum CRP and amyloid A were correlated with rapid development of carotid atherosclerosis at a mean follow-up of only 7.5 months (17).
Some studies found a correlation between sICAM-1 and sVCAM-1 and carotid intima – media thickness (18), but this was denied by other clinical trials (19). In other studies there was a relationship between circulating platelet P-selectin concentration and intima – media thickness in 517 subjects with multiple cardiovascular risk factors (20) or between serum MCP-1 and coronary calcification in 3499 patients with classical risk factors for atherosclerosis (21).
These rather confusing results regarding the relationship between inflammatory markers and the development of atherosclerosis are an argument to design a study in which systemic inflammation should be investigated with respect to volumetric atherosclerotic plaque assessment.
INFLAMMATION AND AUTOIMMUNE DISEASES
The pathogenic mechanisms of autoimmune disorders include an important localized or systemic inflammatory response. This may trigger as an "innocent bystander" reaction a peculiar type of endothelial injury that predisposes to atherogenesis. Many of these diseases are associated with early, accelerated atherosclerosis. This can also be due to concomitant presence of conventional risk factors, but is determined mainly by specific autoimmune and pro-inflammatory mechanisms or by specific medication (i.e. long term systemic corticosteroid use) (22). In these cases atherosclerosis occurs in population subgroups traditionally protected from the atherosclerotic process, as young women that develop systemic lupus erythematosus. Atherothrombosis became the main cause of mortality in autoimmune disorders (23).
The mechanisms implicated in the pathogenesis of accelerated atherosclerosis in autoimmune diseases are excessive expression of adhesion molecules (selections, VCAM and ICAM) on endothelial surface; the excess of cytokine-secreting cells in the atherosclerotic plaque; the presence of a particular subset of T cells (CD4+28-) that has marked pro-inflammatory and tissue destructive effects. The latter are stimulated by endothelial antigens and can be found both in unstable angina and in a subset of patients with rheumatoid arthritis. Endothelial dysfunction found in early stages of athero genesis in autoimmune diseases is independent from traditional risk factors, depends only on the severity of systemic inflammation and can be easily assessed by measuring carotid intimae – media thickness (24).
THE ACCELERATED ATHEROSCLEROSIS IN RHEUMATOID ARTHRITIS (RA)
Coronary artery disease is the main cause of mortality in patients with RA (23). The prevalence of asymptomatic carotid atherosclerosis is 3 times higher in these patients (44% vs. 15%) in the presence of similar cardiovascular risk factors in non-RA subjects. The amplitude of atherosclerotic process depends on the duration of disease, the presence of extra-articular complications and is inversely correlated with the administration of monoclonal anti-TNF-alpha antibodies (25). Coronary calcification is related to disease duration (26), but RA patients exhibit early endothelial activation and rapid progression of intima media thickness (27). The number of circulating endothelial progenitor cells responsible for vessel wall regeneration is significantly lower in patients with active disease (28). These data suggest that the severity and duration of inflammatory process induces early atherosclerotic changes in RA. The prevalence of carotid atherosclerosis in patients with RA was found to be the same with that of diabetics (25). Thus patients with RA should also have aggressive treatment targets with respect to blood pressure and serum cholesterol similar to those observed in secondary prophylaxis.
Important evidence that chronic inflammation is responsible for accelerated atherosclerosis in RA is the fact that methotrexate treatment lowers CV risk in these patients. Also patients with RA treated with anti-TNF-alpha antibodies have low incidence of mortality and CV events, suggesting that aggressive anti-inflammatory treatment can reduce clinical consequences of CV disease (29).
Despite all these evidence today we have no clear recommendations to seek for early atherosclerotic changes without clinical signs in patients with RA, mainly because today these patients are treated aggressively with biologic agents or methotrexate, both associated with CV risk reduction.
ATHERO GENESIS IN SYSTEMIC LUPUS ERYTHEMATOSUS (SLE)
In SLE the prevalence of coronary atherosclerosis is between 6 and 10%, and the risk to develop it is 4 to 8 times higher than in control patients (30). Post mortem studies demonstrated extensive atherosclerosis in 50% of patients with SLE independent of the cause of death. Although the incidence of conventional risk factors (hypertension, smoking, diabetes, dyslipidemia) is higher in lupus patients than in general population, the accelerated atherosclerotic process does not depend on these to occur. The factors related to the atherosclerotic process in SLE are advanced age, disease duration, duration of corticosteroid treatment and serum oxLDL and homocysteine levels (31).
As with RA, asymptomatic carotid atherosclerosis and coronary calcifications occur very early in SLE and can be directly attributed to the auto-immune disease. Atherosclerosis progression rate is double in lupus patients and is directly correlated with disease duration and serum levels of homocysteine (32).
SLE is also associated with the presence of lupus anticoagulant factor well known to have a prominent role in initiation and progression of atherosclerosis (33). Anti-cardiolipinic antibodies in SLE cross-react with multiple plasma and tissue antigens (anti-HDL, anti-Apo-A1) that can play a role in accelerating athero-genesis. In the anti-phospholipidic syndrome the main tissue antigen is beta-2-glicoprotein-I that forms tissue complexes with oxLDL. Auto-antibodies anti beta-2-GPI/oxLDL are important players in athero-genesis (34). The pathogenic significance of anti-phospholipidic antibodies in SLE is emphasized by experimental data showing that administration of IgG monoclonal antibodies anti-phospholipidic factors has a protective effect against atherosclerosis (35).
PROPOSED TRIAL TO INVESTIGATE INFLAMMATION – ATHEROSCLEROSIS RELATIONSHIP
Considering the incompletely clarified relationship between early atherosclerosis and inflammation we designed a clinical study that is enrolling two different patient subgroups. The first group consists of patients with traditional risk factors for athero-genesis in whom inflammation presumably accompanies the pathological arterial wall process. The second patient group consists of patients with autoimmune diseases (SLE and RA) without traditional risk factors and balanced for age and sex with the previous subgroup. In this second subset of patients the initial cause of athero-genesis is known to be related to chronic systemic inflammation.
The purpose of the study is to investigate if inflammation assessed by a wide panel of systemic markers is associated with arterial atheroma volume measured by ultrasound. We assume that athero-genesis is different in these two patient subsets: due to the presence of conventional risk factors with secondary inflammation in the former subgroup and induced by pure systemic inflammation and secondary endothelial dysfunction in the autoimmune subgroup.
We will include patients less than 60 years-old with symptomatic coronary artery disease (angina and ischemic ECG changes) and reversible myocardial ischemia demonstrated by a positive stress test, if clinically indicated. Patients should have an indication for invasive angiographic study according to current guidelines and should provide an informed consent for participation in the trial. Two subgroups will be considered: one with conventional risk factors (hypertension, smoking, hypercholesterolemia, family history for complicated atherosclerosis), but without diabetes mellitus. The second subgroup will have an autoimmune disease (SLE or RA) without conventional risk factors; their disease should be in a remission stage, under standard therapy. Only patients without systolic heart failure should be included. Exclusion criteria from the study are: acute coronary syndromes because constant systemic inflammation associated with complicated atherosclerotic plaques is present in these cases; previous myocardial infarction and coronary revascularization procedures by angioplasty or coronary artery by-pass grafting generally associated with advanced atherosclerotic process; statin treatment in the previous 6 months because of the possible effect this might have on systemic inflammation and atheroma volume, and systolic heart failure with an ejection fraction < 40% because of activation of inflammatory systems (i.e. increase in serum concentration of TNF-alpha). Patients with systemic disease that might influence endothelial dysfunction and inflammatory markers should also be excluded: diabetes mellitus because of early atherosclerotic process; renal failure with a creatinin clearance < 60 ml/min (MDRD formula) due to accelerated athero-genesis; evolving or previous neoplastic disease; and recent or evolving infectious disease < 3 months prior to inclusion. ❑
STUDY PROTOCOL
The study protocol consists of three steps. In step 1 the assessment of serum inflammation markers will be performed. Serum will be separated by centrifugation after venous blood is obtained. The following markers will be assessed by currently available ELISA kits: acute phase reactants (C-reactive protein); pro-inflammatory factors (TNF-alpha, interleukin-6, interleukin-18); endothelial activation factors (soluble VCAM-1, ICAM-1); endothelial dysfunction markers (PAI-1); and specific factors (anti-cardiolipinic antibodies).
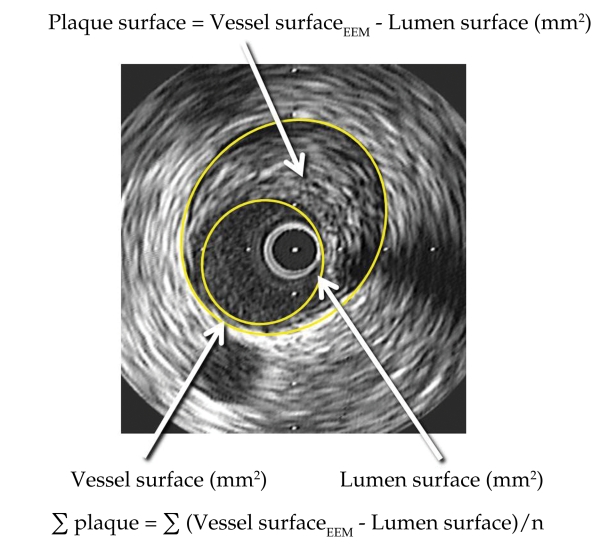
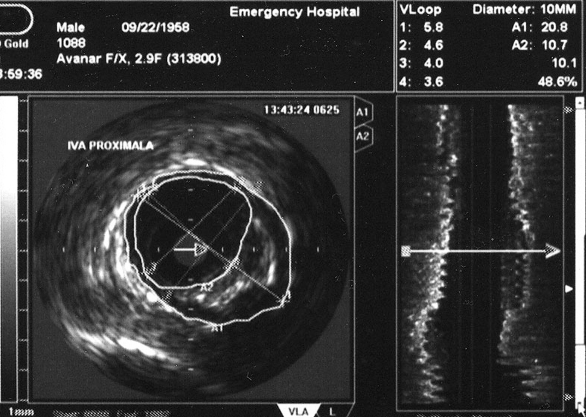
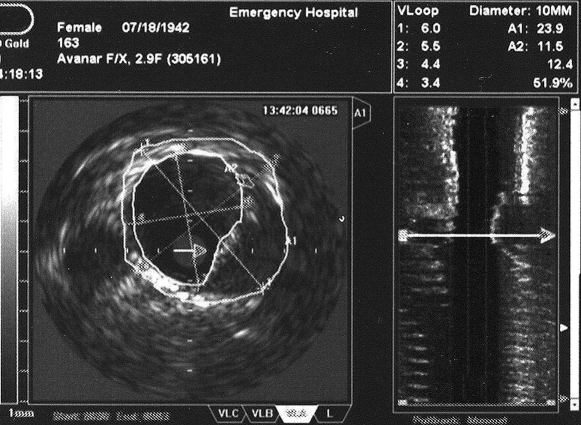
In step 2, coronary angiography with IVUS examination to assess the volume of atherosclerotic plaques will be performed. After coronary angiography an IVUS examination will be done in all three major coronary arteries advancing the probe to the most distal segments of the vessel possibly accessible. After intracoronary nitro-glycerin and IV heparin a single motorized pull-back maneuver with 0.5 mm/sec will be recorded. By off-line analysis of the recorded loop the volume of the atherosclerotic plaque will be assessed by measuring plaque surface (plaque plus media to the level of the external elastic membrane from which luminal surface will be subtracted) on every frame multiplying it with total distance of the examined vessel segment (figure 3). In figure 4 we present two cases of patients enrolled in our trial.
Figure 3. Practical way to assess the surface of coronary atheroma by IVUS planimetry of the blood-vessel interface (lumen surface) and vessel surface at the level of external elastic membrane (EEM).
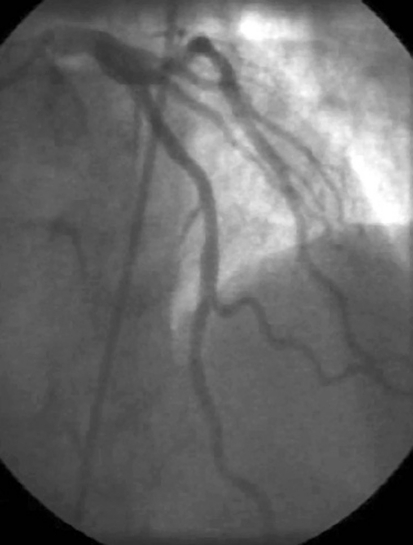
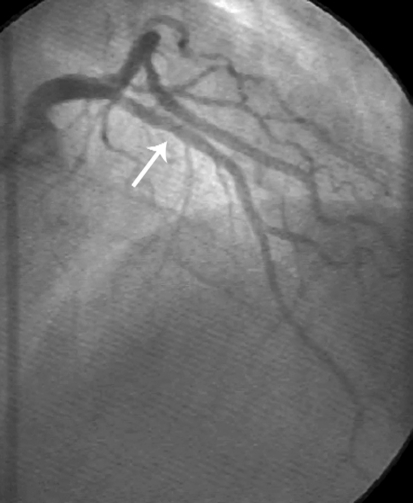
Figure 4. Angiographic aspect and intracoronary ultrasound of two cases of patients enrolled in our on-going trial. In figure 4A coronary angiography shows only mild lumen irregularities, while IVUS (figure 4B) demonstrates a large mixed atherosclerotic plaque in the proximal LAD in a 52 year-old hypertensive patient with stable angina and hypercholesterolemia. In figure 4C the same apparent lack of significant atherosclerotic involvement of the LAD is shown by the angiogram in a 68 year-old rheumatoid artrhitis female patient; IVUS shows a large excentric soft plaque in the proximal LAD that does not significantly reduce coronary lumen.
A.
B.
B.
B.
In step 3, carotid ultrasound will be performed to assess early non-coronary atherosclerosis. A mode B carotid ultrasound will be performed to measure IMT by recording a longitudinal image at the level of both common and internal carotid arteries. IMT will be assessed as the maximal value of intimae – media diameter from the level of posterior wall 1 cm under common carotid bifurcation.
The patients will be followed up for a mean interval of 18 months to determine prognostic significance of recorded parameters. The clinical endpoints that will be recorded are cardio-vascular mortality, unstable angina, non-fatal myocardial infarction or stroke and any myocardial revascularization procedure. The study is ongoing.
INTRACORONARY ULTRASOUND TO ASSESS ATHEROSCLEROTIC PLAQUES
In the last decade intravascular ultrasound (IVUS) is widely used in the cathlab to assess the severity of coronary atherosclerosis and to investigate the structure of the atherosclerotic plaque. Current 30 MHz probe resolution allows sufficient quality to evaluate the atherosclerotic plaque; special software algorithms were validated to precisely define structural composition of the plaque (virtual histology) (36).
Up to date IVUS has already been used to study progression of the atherosclerotic process in patients aggressively treated with statins (37). In REVERSAL, a randomized double-blind multicenter study, administration of Atorvastatin 80 mg/day was associated with lack of progression of coronary atheroma versus Pravastatin 40 mg/day at 18 months (38). Another IVUS trial showed that administration of Rosuvastatin 40 mg/day for 2 years determined a reduction by 14.7% of total coronary atheroma volume (39).
In another IVUS study it was demonstrated that statins have a negative remodeling effect on the atherosclerotic plaque in a process that is independently determined by the reduction of serum CRP. The lack of effect on the plaque volume while the vessel surface was reduced at the level of the external elastic membrane was considered to be due to anti-inflammatory effects of the statin (40). IVUS parameters also show a good correlation between coronary atheroma volume and the presence of CV risk factors (hypertension, diabetes and male sex) (41).
Considering the relationship between systemic inflammation and atheroma volume measured by IVUS, this has not been thoroughly investigated. In the REVERSAL study CRP, the only reported inflammatory marker, decreased by 36.4% with Atorvastatin 80 mg and only by 5.2% by Pravastatin 40 mg (38). Supplemental studies as the one we are submitting may be necessary to fully investigate the relationship between inflammatory markers and coronary atherosclerosis. In the ASTEROID trial there is no mention about serum inflammatory markers related to the volume of coronary atherosclerotic plaque (39).
Today IVUS is the most reliable modality to assess the atherosclerotic process in vivo and can supply precise volumetric data that can be easily correlated with systemic inflammation, the main purpose of the present study. ❑
INTIMAE – MEDIA THICKNESS
Intimae-media thickness (IMT) is measured by B mode vascular ultrasound as the dimension of intimae – media complex in mm up to the external elastic membrane. When IMT is larger than 0.9 mm it is associated with early atherosclerotic process and the risk of stroke or myocardial infarction. When it is larger than 1.3 - 1.5 mm it already has the meaning of a fully blown atherosclerotic plaque. IMT is depending on sex (it is higher in men) and it increases with age. IMT can be expressed as mean value of multiple measurements at the level of common and internal carotid arteries or as a maximal value, independent from location (42).
IMT is strongly correlated with 5-year cardiovascular events. The risk of myocardial infarction increases by 10-15% and that of stroke increases by 13-18% for every 0.1 mm IMT increase (43). The risk of CV complications associated with IMT increase has no organ specificity, because early atherosclerotic changes do not predict the location of future major vascular events.
Just as with coronary atherosclerosis it was demonstrated in some studies that aggressive statin treatment can reduce IMT in patients with familial hypercholesterolemia, without making any correlation with serum inflammatory markers (44).
Controversial data is available regarding the relationship between IMT and inflammation. In a trial that enrolled 5888 patients older than 65 years old and who did not have proven CV disease on trial entry a positive relationship between serum CRP and carotid IMT was noticed at 12 years follow up (16). Some other studies did not confirm any relationship between IMT and inflammatory parameters expressed by serum CRP (45,46). In another recent study based on the results obtained from 2885 patients from the Framingham cohort there was a modest correlation between internal carotid IMT and multiple inflammatory markers (CRP, sICAM, Il-6, MCP-1, P-selectin and CD40L) (47).
Thus we believe that a trial looking at the relationship between IMT and inflammatory markers could provide supplemental data that would shed some light in this issue. ❑
CONCLUSION
Although a well known correlation between inflammations and atherosclerosis exists, there is no definite data regarding the amplitude of inflammation and early atherosclerotic changes. This happens despite it is clearly proven that inflammation accompanies atherogenesis from its early phases ("fatty streak") to its fully developed lesion ("fibrous plaque") and progression towards complications ("thin-cap fibroatheroma" and ulcerated plaque). We are currently investigating the relationship between some serum markers of inflammation and the early atherosclerotic changes at the level of coronary and carotid arteries in patients with different pathogenesis of the disease: the common pathway determined by traditional risk factors and the autoimmune pathway induced by systemic inflammation when classic risk factors are lacking. ❑
ACKNOLEDGENENTS
This work is supported by a CNCSIS – UEFISCSU Grant, project number PNII – IDEI, code 257/2008.
References
- 1.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Kinlay S, Egido J. Inflammatory biomarkers in stable atherosclerosis. Am J Cardiol. 2006;98(Suppl):2P–8P. doi: 10.1016/j.amjcard.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Smith S, Anderson J, Cannon R, et al. DC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease Application to Clinical and Public Health Practice Report From the Clinical Practice Discussion Group. Circulation. 2004;110:e550–e553. doi: 10.1161/01.CIR.0000148981.71644.C7. [DOI] [PubMed] [Google Scholar]
- 4.Ridker P, Cook N. Clinical usefulness of very high and very low levels of C-reactive protein across the full range of Framingham risk scores. Circulation. 2004;109:1955–1959. doi: 10.1161/01.CIR.0000125690.80303.A8. [DOI] [PubMed] [Google Scholar]
- 5.Ray K, Cannon C, Ganz P. Beyond lipid lowering: what have we learned about the benefits of statins from the acute coronary syndromes trials? Am J Cardiol. 2006;98(Suppl):18P–25P. doi: 10.1016/j.amjcard.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Burke A, Tracy R, Kolodgie F, et al. Elevated C-reactive protein values and atherosclerosis in sudden coronary death: association with different pathologies. Circulation. 2002;105:2019–2033. doi: 10.1161/01.cir.0000015507.29953.38. [DOI] [PubMed] [Google Scholar]
- 7.Eisenhardt S, Habersberger J, Peter K A, Eisenhardt S, Habersberger J, Peter K R, Eisenhardt S, Habersberger J, Peter K F, et al. Monomeric C-reactive protein generation on activated platelets: the missing link between inflammation and atherothrombotic risk. 2009;19:232–237. doi: 10.1016/j.tcm.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Zacho J, Tybjaerg-Hansen A, Jensen J, et al. Genetically elevated C-reactive protein and ischemic vascular disease. N Engl J Med. 2008;359:1897–1908. doi: 10.1056/NEJMoa0707402. [DOI] [PubMed] [Google Scholar]
- 9.Ortiz M, Campana G, Woods J, et al. Continuously-infused human C-reactive protein is neither proatherosclerotic nor proinflammatory in apolipoprotein E-deficient mice. Exp Biol Med. 2009;234:624–631. doi: 10.3181/0812-RM-347. [DOI] [PubMed] [Google Scholar]
- 10.Ridker P, Hennekens C, Buring J, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 11.Myers G, Rifai N, Tracy R, et al. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: Report From the Laboratory Science Discussion Group. Circulation. 2004;110:e545–e549. doi: 10.1161/01.CIR.0000148980.87579.5E. [DOI] [PubMed] [Google Scholar]
- 12.Paul A, Ko K, Li L, et al. C-reactive protein accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2004;109:647–655. doi: 10.1161/01.CIR.0000114526.50618.24. [DOI] [PubMed] [Google Scholar]
- 13.Hunt M, O'Malley P, Vernalis M, et al. C-reactive protein is not associated with the presence or extent of calcified subclinical atherosclerosis. Am Heart J. 2001;141:206–210. doi: 10.1067/mhj.2001.112488. [DOI] [PubMed] [Google Scholar]
- 14.Gronholdt M, Sillesen H, Wiebe B, et al. Increased acute phase reactants are associated with levels of lipoproteins and increased carotid plaque volume. Eur J Vasc Endovasc Surg. 2001;21:227–234. doi: 10.1053/ejvs.2001.1321. [DOI] [PubMed] [Google Scholar]
- 15.Zouridakis E, Avanzas P, Arroyo-Espliguero R, et al. Markers of inflammation and rapid coronary artery disease progression in patients with stable angina pectoris. Circulation. 2004;110:1747–1753. doi: 10.1161/01.CIR.0000142664.18739.92. [DOI] [PubMed] [Google Scholar]
- 16.Cao J, Arnold A, Manolio T, et al. Association of carotid artery intimae-media thickness, plaques, and C-reactive protein with future cardiovascular disease and all-cause mortality. The Cardiovascular Health Study. Circulation. 2007;116:32–38. doi: 10.1161/CIRCULATIONAHA.106.645606. [DOI] [PubMed] [Google Scholar]
- 17.Schillinger M, Exner M, Mlekusch W, et al. Inflammation and Carotid Artery-Risk for Atherosclerosis Study (ICARAS) Circulation. 2005;111:2203–2209. doi: 10.1161/01.CIR.0000163569.97918.C0. [DOI] [PubMed] [Google Scholar]
- 18.Kondo K, Kitagawa K, Nagai Y, et al. Associations of soluble intercellular adhesion molecule-1 with carotid atherosclerosis progression. Atherosclerosis. 2005;179:155–160. doi: 10.1016/j.atherosclerosis.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Luc G, Arveiler D, Evans A, et al. For the Prospective Epidemiological Study of Myocardial Infarction (PRIME) Study Group. Circulating soluble adhesion molecules ICAM-1 and VCAM-1 and incident coronary heart disease: the PRIME Study. Atherosclerosis. 2003;170:169–176. doi: 10.1016/s0021-9150(03)00280-6. [DOI] [PubMed] [Google Scholar]
- 20.Koyama H, Maeno T, Fukumoto S, et al. Platelet P-selectin expression is associated with atherosclerotic wall thickness in carotid artery in humans. Circulation. 2003;108:524–529. doi: 10.1161/01.CIR.0000081765.88440.51. [DOI] [PubMed] [Google Scholar]
- 21.Deo R, Khera A, McGuire D, et al. Association among plasma levels of monocyte chemoattractant protein-1, traditional cardiovascular risk factors, and subclinical atherosclerosis. J Am Coll Cardiol. 2004;44:1812–1818. doi: 10.1016/j.jacc.2004.07.047. [DOI] [PubMed] [Google Scholar]
- 22.Shoenfeld Y, Gerli R, Doria A, et al. Accelerated atherosclerosis in autoimmune rheumatic diseases. Circulation. 2005;112:3337–3347. doi: 10.1161/CIRCULATIONAHA.104.507996. [DOI] [PubMed] [Google Scholar]
- 23.Solomon D, Karlson E, Rimm E, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–1307. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 24.del Rincon I, Williams K, Stern M, et al. Association between carotid atherosclerosis and markers of inflammation in rheumatoid arthritis patients and healthy subjects. Arthritis Rheum. 2003;48:1833–1840. doi: 10.1002/art.11078. [DOI] [PubMed] [Google Scholar]
- 25.Roman M, Moeller E, Davis A, et al. Preclinical carotid atherosclerosis in patients with rheumatoid arthritis: prevalence and associated factors. Ann Intern Med. 2006;144:249–256. doi: 10.7326/0003-4819-144-4-200602210-00006. [DOI] [PubMed] [Google Scholar]
- 26.Chung C, Oeser A, Raggi P, et al. Increased coronary-artery atherosclerosis in rheumatoid arthritis: relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005;52:3045–3053. doi: 10.1002/art.21288. [DOI] [PubMed] [Google Scholar]
- 27.Södergren A, Karp K, Boman K, et al. Atherosclerosis in early rheumatoid arthritis: very early endothelial activation and rapid progression of intima media thickness. Arthritis Research & Therapy. 2010;12:R158–R158. doi: 10.1186/ar3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grisar J, Aletaha D, Steiner C, et al. Depletion of endothelial progenitor cells in the peripheral blood of patients with rheumatoid arthritis. Circulation. 2005;111:204–211. doi: 10.1161/01.CIR.0000151875.21836.AE. [DOI] [PubMed] [Google Scholar]
- 29.Jacobsson L, Turesson C, lfe G, et al. Treatment with tumor necrosis factor blockers is associated with a lower incidence of first cardiovascular events in patients with rheumatoid arthritis. J Rheumatol. 2005;32:1213–1218. [PubMed] [Google Scholar]
- 30.Manzi S, Meilahn E, Rairie J, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 31.de Leeuw K, Freire B, Smit A, et al. Traditional and non-traditional risk factors contribute to the development of accelerated atherosclerosis in patients with systemic lupus erythematosus. Lupus. 2006;15:675–682. doi: 10.1177/0961203306069972. [DOI] [PubMed] [Google Scholar]
- 32.Roman M, Crow M, Lockshin M, et al. Rate and determinants of progression of atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2007;56:3412–3419. doi: 10.1002/art.22924. [DOI] [PubMed] [Google Scholar]
- 33.Farzaneh-Far A, Roman M, Lockshin M, et al. Relationship of antiphospholipid antibodies to cardiovascular manifestations of systemic lupus erythematosus. Arthritis Rheum. 2006;54:3918–3925. doi: 10.1002/art.22265. [DOI] [PubMed] [Google Scholar]
- 34.Nojima J, Masuda Y, Iwatani Y, et al. Arteriosclerosis obliterans associated with anti-cardiolipin antibody/beta2-glycoprotein I antibodies as a strong risk factor for ischaemic heart disease in patients with systemic lupus erythematosus. Rheumatology. 2008;47:684–689. doi: 10.1093/rheumatology/ken124. [DOI] [PubMed] [Google Scholar]
- 35.Nicolo D, Goldman B, Monestier M. Reduction of atherosclerosis in low-density lipoprotein receptor-deficient mice by passive administration of antiphospholipid antibody. Arthritis Rheum. 2003;48:2974–2978. doi: 10.1002/art.11255. [DOI] [PubMed] [Google Scholar]
- 36.Nasu K, Tsuchikane E, Katoh O, et al. Accuracy of in vivo coronary plaque morphology assessment. A validation study of in vivo virtual histology compared with in vitro histopathology. J Am Coll Cardiol. 2006;47:2405–2412. doi: 10.1016/j.jacc.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 37.Nicholls S, Tuzcu E, Wolski K, et al. Coronary artery calcification and changes in atheroma burden in response to established medical therapies. J Am Coll Cardiol. 2007;49:263–270. doi: 10.1016/j.jacc.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 38.Nissen S, Tuzcu E, Schoenhagen P, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis. A randomized controlled trial. JAMA. 2004;291:1071–1080. doi: 10.1001/jama.291.9.1071. [DOI] [PubMed] [Google Scholar]
- 39.Nissen S, Nicholls S, Sipahi I, et al. For the ASTEROID Investigators. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis. The ASTEROID Trial. JAMA. 2006;295:1556–1565. doi: 10.1001/jama.295.13.jpc60002. [DOI] [PubMed] [Google Scholar]
- 40.Schoenhagen P, Tuzcu E, Apperson-Hansen C, et al. Determinants of arterial wall remodeling during lipid-lowering therapy serial intravascular ultrasound observations from the Reversal of Atherosclerosis With Aggressive Lipid Lowering Therapy (REVERSAL) Trial. Circulation. 2006;113:2826–2834. doi: 10.1161/CIRCULATIONAHA.105.585703. [DOI] [PubMed] [Google Scholar]
- 41.Nicholls S, Tuzcu E, Crowe T, et al. Relationship between cardiovascular risk factors and atherosclerotic disease burden measured by intravascular ultrasound. J Am Coll Cardiol. 2006;47:1967–1975. doi: 10.1016/j.jacc.2005.12.058. [DOI] [PubMed] [Google Scholar]
- 42.Hollander M, Hak A, Koudstaal P, et al. Comparison between measures of atherosclerosis and risk of stroke: the Rotterdam Study. Stroke. 2003;34:2367–2372. doi: 10.1161/01.STR.0000091393.32060.0E. [DOI] [PubMed] [Google Scholar]
- 43.Lorenz M, Markus H, Bots M, et al. Prediction of clinical cardiovascular events with carotid intima-media thickness. A systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 44.Smilde T, Wissen S, Wollersheim H, et al. Effect of aggressive versus conventional lipid lowering on atherosclerosis progression in familial hypercholesterolaemia (ASAP): a prospective, randomised, double-blind trial. Lancet. 2001;357:577–581. doi: 10.1016/s0140-6736(00)04053-8. [DOI] [PubMed] [Google Scholar]
- 45.Lorenz M, Karbstein P, Markus H, et al. High-sensitivity C-reactive protein is not associated with carotid intima-media progression. The Carotid Atherosclerosis Progression Study. Stroke. 2007;38:1774–1779. doi: 10.1161/STROKEAHA.106.476135. [DOI] [PubMed] [Google Scholar]
- 46.Martinez L, Miname M, Bortolotto L, et al. No correlation and low agreement of imaging and inflammatory atherosclerosis' markers in familial hypercholesterolemia. Atherosclerosis. 2008;200:83–88. doi: 10.1016/j.atherosclerosis.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 47.Thakore A, Guo C, Larson M, et al. Association of multiple inflammatory markers with carotid intimal medial thickness and stenosis (from the Framingham Heart Study) Am J Cardiol. 2007;99:1598–1602. doi: 10.1016/j.amjcard.2007.01.036. [DOI] [PubMed] [Google Scholar]