ABSTRACT
Objective: The study compared two brands of tuberculin skin tests (TST): PPD RT 23, (SSI, Denmark) and PPD IC 65, (Cantacuzino Institute, Romania), 2 TU/ 0.1 ml each, with an interferon gamma release assay [IGRA], Quantiferon–TB Gold (QFT).
Material and methods: QFT was performed on whole blood samples, before TSTs, on 60 children with tuberculosis (TB), BCG vaccinated, admitted in a paediatric pneumophtisiology hospital. The proportion of boys (51.6 %) and girls (48.3 %) was nearly equal, the mean age of subjects was 9.44 years (SD= 5.37 years; variance= 28.83).
Results: With TST induration ≥ 10 mm considered as positive response, only 47.46 % of children classified positive with RT23 and 48.27 % with IC-65, were IFN-γ positive.We obtained a very good agreement between the two tuberculins (59/60 for RT 23 and 58/60 for IC 65), while for QFT, which confirmed as positives only 27/60, i.e. 45 % (18/60 were indetermined, 15/60 were negatives).
Conclusions: The tests did not agree on positive results, showing a low redundancy between in vitro and in vivo measurements, suggesting that independent aspects of anti-mycobacterial immunity are being measured by these tests.The specificities of the assays could not been calculated since all the children had TB, confirmed by bacteriological and/ or clinical and radiological data. Further comparison of TST and QFT, may determine whether such discordance reflect a higher specificity of QFT. Meantime, we are trying to obtain a recombinant PPD, using a cocktail of specific M. tuberculosis (M.tb) antigens, in order to eliminate any interference with BCG in skin test reactions.
Keywords: Tuberculin, quantiferon, sensitivity, diagnosis, paediatric tuberculosis
INTRODUCTION
Worldwide, tuberculin skin testing is the most frequently used screening assay for the diagnosis of tuberculosis (TB) or for detection of latent TB infection (LTBI) (1).
Due to the fact that Romania has a high TB incidence (2), for both clinical and epidemiological use, there is a need of periodic comparison of well-standardized tuberculin test reagents used in TST programmes. In a recent published study (3) we had evaluated the efficacy of two tuberculins, RT23, the tuberculin most widely used globally for skin testing (4), and IC-65 given the importance of having potent, well-standardized tuberculin test reagents available for clinical and epidemiological use.
Since 2000, advances in molecular biology have led to the development of new in vitro commercial assays that measures interferon (INF)- γ release (IGRAs) by sensitized T cells after stimulation with M. tuberculosis antigens (5), such as QuantiFERON--TB® Gold In -Tube Assay (QFT).
Some published data showed that even if the agreement between both tests was very good on negative results, the tests did not agree on positive results (6), while other data claim that IGRAs have greater specificity and similar sensitivity in comparison to TST (7).
Because few studies have been done in high incidence countries, and because paediatric data are limited (8,9) our aim was to compare the sensitivities of Tuberculin PPD RT 23 and PPD IC 65, 2 TU/0.1 ml, when simultaneously administered to hospitalized children with TB, with that of QFT.
The main question is: could QFT be a good supplement or even a total replacement for the TST in TB screening ? ❑
METHODS AND MATERIALS
Subjects: Hospitalized children (1-18 years old) / TB patients known to be infected with M. tb, bacteriologically and/or clinically & radiological confirmed. Since in Romania the vaccination with the bacille Calmette-Guerain (BCG) at birth is mandatory, we have considered that all the children were vaccinated. The study received the approval of local ethical committee (IRB #0002508) (Table 1).
Table 1.
Patient distribution according to age and sex
| Age | N | % |
|---|---|---|
| 1-4 years | 13 | 21.7 |
| 5-9 years | 15 | 25 |
| 10-14 years | 15 | 25 |
| 14-18 years | 17 | 28.3 |
| Gender | ||
| Girls | 29 | 48.3 |
| Boys | 31 | 51.7 |
At the enrolment, participants (or their family, when the children were underage) provided informed consent to participate in the study.
The proportion of boys (51.6 %) and girls (48.3 %) was nearly equal, the mean age of subjects was 9.44 years (SD= 5.37 years; variance= 28.83).
Mantoux method: TST was administered by trained nurses using the Mantoux method, following standard procedures. Briefly, 0.1 mL (2 TU) of purified protein derivate (PPD, RT23; Statens Serum Institute, Copenhagen, Denmark and IC-65, Cantacuzino Institute, Bucharest, Romania, were injected in the volar side of right and respectively, left forearm of the children and read 72 hours afterwards. The transverse diameter of the induration was measured by experienced personnel, by palpation using a millimetre ruler. Interpretation was based on current recommendations (1). TST+ indicates an induration with a transverse diameter of ≥ 10 mm 48-72 hours after the inoculation, otherwise TST-.
The test measures induration of the skin (mm) 72 hrs following intradermal inoculation of M. tuberculosis purified protein derivative (PPD) and represents a delayed-type hypersensitivity (DTH) response to PPD.
QFT assay. The QuantiFERON-TB Gold Assay (Cellestis Limited, Carnegie, Australia) was used. Venous blood was directly collected, prior to TST administration (8).
The test was performed (10) and interpreted according to the manufacturer`s criteria. The assay involved two stages: the first stage involved incubation of whole blood with antigens, and the second stage involved measurement of IFN-γ production in harvested plasma by ELISA. QFT + indicates that the IFN-γ concentrations of the control tubes met the criteria for a valid test and tuberculosis antigen response minus nil response ≥ 0.35 IU/ml; it also indicates a positive result on the test. QFT- indicates IFN-γ concentrations of the control tubes met the criteria for a valid test and tuberculosis antigen response minus nil response < 0.35 IU/ml. An indeterminate result, i.e. QFT results cannot be interpreted, was defined as <0.35 IU/ml and also, when QFT results cannot be interpreted as a responsible background response, when < 50 above nil.
Observers were blinded to the results of the TST results. ❑
STATISTICAL ANALYSIS
For ordered risks, the proportions of positive test results were compared using the Chi -square test of trend. The agreement between TST and QFT independent from the agreement by chance alone was assessed by calculating Kappa values for TST >10 mm. P <0.05 was considered statistically significant.
Because there is no gold standard for LTBI, concordance was evaluated between TST and QFT assay using two indices: proportion agreement, and kappa (k) coefficients. In addition, we calculated the sensitivity of the IFN-γ assay and compared that against the sensitivity of TST. Sensitivity was defined as the proportion of individuals that had a positive result in the cohort deemed as having laboratory confirmed active TB (laboratory confirmation involved either bacteriological or pathological verification) (8).
Concordance was assessed by the calculation of a kappa statistic and discordance by McNemar's test (11). Agreement between the tests was classified into categories; Kappa coefficient, poor (if κ <0.20), fair (κ 0.21–0.40), moderate (κ 0.41– 0.60), good (κ 0.61– 0.80), and very good (κ 0.81–1.00) (12). ❑
RESULTS
60 hospitalized children, with tuberculosis, confirmed bacteriologically and / or clinically and radiologically, were tested simultaneously with RT23 and IC-65 tuberculins, 2 TU/0.1 ml.
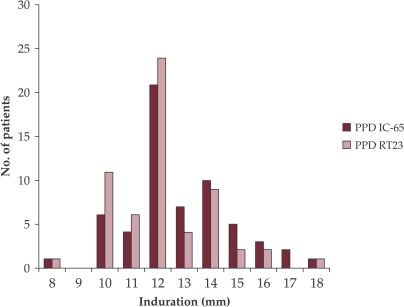
The distribution of the TST reaction size after simultaneous injection of both tuberculins is presented in Fig.1.
Figure 1. Reaction sizes observed after testing 60 TB patients with PPD IC-65 and RT23.
RT23 skin test reactions ranged from 8 to 18 mm (mean, 12.2 mm; SD = 1.8 mm; variance = 3.2; median, 12 mm) and IC-65 reactions ranged also from 8 to 18 mm (mean, 12.8 mm; SD =2 mm; variance = 3.9; median, 12 mm).
There were no significant differences in the mean reaction sizes by age and gender.
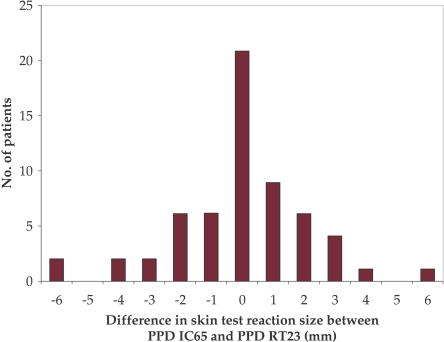
The paired differences in IC-65 minus RT23 reaction sizes for each patient are presented in Fig. 2. The mean difference in paired reaction sizes for the two reagents was 0.02 mm and was not statistically different from zero (P value, 0.06).
Figure 2. Difference in skin test reaction size between PPD IC-65 and PPD RT23.
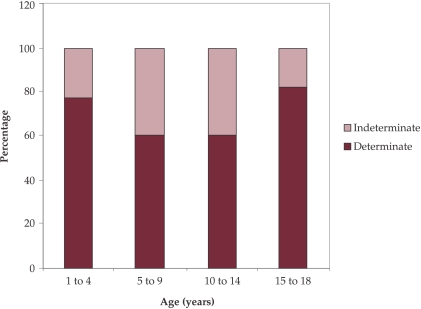
The QFT assay results on the same 60 children with TB, divided on age groups, are presented in Fig. 3. It could be seen that more indeterminate QFT results are in children groups aged between 5-14 years.
Figure 3. Proportion of undetermined QFT assay results on age groups of children with tuberculosis.
Table 2 presents the comparison between TST (PPD IC-65 and RT23) results and QFT-G assay among hospitalized children with tuberculosis.
Table 2.
Comparison between TST (PPD IC-65 and RT23) results and QFT-G assay among hospitalized children with tuberculosis
| PPD IC-65 | PPD RT23 | QFT | ||||
|---|---|---|---|---|---|---|
| n ( N=60) | % | n (N=60) | % | n (N=60) | % | |
| Positive | 58 | 96.7 | 59 | 98.3 | 27 | 45 |
| False negative | 2 | 3.3 | 1 | 1.7 | 15 | 25 |
| Indetermined | - | - | 18 | 30 | ||
| Both TSTs and QFT-G positives | 27 | 27 | 27 | |||
Current data underline the importance of diagnosis in children aged less than 5 years, since interferon-γ release assay evidence for this group is scarce (13). QFT sensitivity is poorly defined, and TST is preferred because the evidence for determining the reliability of negative results among this age group is still insufficient, and the consequences of missing a diagnosis of infection potentially are more severe than they would be for older children.
Our results confirm the fact that the sensitivity of TST is significantly higher than QFT in this age group (Table 3).
Table 3.
Comparison between QFT and TST results on 1-4 years old group of children with tuberculosis (n= 23)
| Positives | Negatives | Undetermined | |
|---|---|---|---|
| QFT | 2/13 | 9/13 | 2/13 |
| 15.4 % | 69.2 % | 15.4 % | |
| TST | 12/13 | 1/13 | - |
| 92.3 % | 7.7 % | - |
We calculated the concordance and discordance between each TST and QFT assay. There was good agreement between PPDs (κ = 0.66) and poor agreement for QFT and each PPD (κ = 0.03 – 0.05). There was discordance between each PPD and QFT (χ2 = 29-30, P value < 0.0001) and no statistical significant differences between both PPDs (χ2 = 0.000 with one degree of freedom, P value = 1.0000).
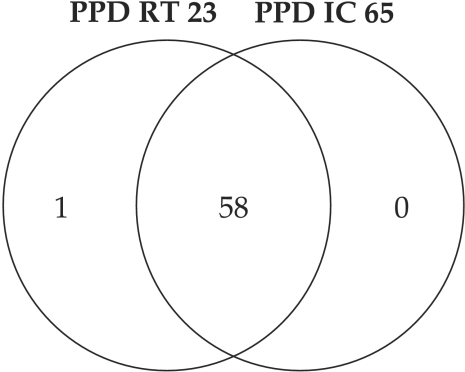
Fig. 4 presents the Ven diagram of PPD RT23 and IC-65 results comparison, showing a very good agreement between the two tuberculins (59/60 for RT 23 (i.e. 98.3%) and 58/60 for IC 65 (i.e. 96.7 %)).
Figure 4. Comparison of the results obtained with PPD IC 65 and PPD RT23.
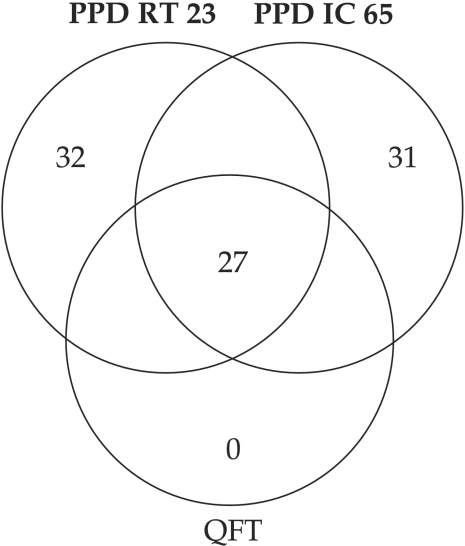
As for QFT and PPDs comparison, the Ven diagram (Fig 5) showed a significant discordance. QFT confirmed as positives only 27/60, i.e. 45 %, while 18/60 were indetermined and 15/60 were negatives. ❑
Figure 5. Comparison of the results obtained with PPD IC 65, PPD RT23 and QFT.
CONCLUSIONS
We found indistinguishable reaction-size distribution and median TST results for the two PPDs. Both reagents showed a unimodal distribution of reaction sizes, centering around 12 mm. No statistically significant difference was established for the efficacy of these two commercially available PPD TST reagents, both tuberculins appearing to have equivalent potency
The availability of high-quality reference standards is basic to the continued production of effective commercial preparations of PPD for public use.
We obtained a very good agreement between the two tuberculins (59/60 for RT 23 and 58/60 for IC 65), while for QFT, which confirmed as positives only 27/60, i.e. 45 % (18/60 were undetermined, 15/60 were negatives), the results showed a significant discordance.
The specificities of the assays could not been calculated since all the children had TB, confirmed by bacteriological and/ or clinical, radiological data.
Probably, in low risk populations, where the pre-test probability of a negative result is high, further comparison of TST and QFT may determine whether such discordance probably reflect a higher specificity of QFT. In the case of a population with high TB incidence, as in Romania, the sensitivity of TST proved to be higher than QFT, even with BCG vaccinated people. Many authors underlined that because QFT-GIT assay requires fewer visits, it may offer an advantage over the TST (14,15). They don't take into consideration the fact that for the countries with high TB incidence and low national income, the TST price is less than 0.1 % of QFT.
Our findings strongly supports Gallant et al (16) who suggested that in vitro and in vivo assays of antimycobacterial immunity are complementary rather than competing measures of antimycobacterial immunity.
It could be possible that the TST+/IGRA– discordance is due to a lower sensitivity of QFT than of TST, especially considering that the ESAT-6 and CFP-10 antigens used in IGRAs do not represent the whole spectrum of M.tb antigenicity. The IGRAs might reflect more recent, rather than remote, TB infections because once the antigen is cleared; the activated memory T lymphocytes that produce IFN-γ persist for a limited time in the peripheral circulation.
Exploring the immunology underlying this discordance may help in the development of more accurate and reliable immunodiagnostic tests for the diagnosis of TB in children. In addition, long-term follow-up studies are needed to determine the true predictive value of IGRA for the development of active TB disease. Connell et al. suggested that until such data are available, IGRA should not be used as replacement tests for the tuberculin skin testing in children (17).
Considering the limitations that TST and IGRA present, the best solution could be the use of both, using the IGRA higher specificity for confirming a positive TST, taking advantage of the best characteristics of each test.
Meantime, we are trying to obtain a recombinant PPD, using a cocktail of specific M.tb antigens, in order to eliminate any interference with BCG in skin test reactions. ❑
References
- 1.Dorman SE. New Diagnostic Tests for Tuberculosis: Bench, Bedside, and Beyond. Clinical Infectious Diseases. 2010;50(S3):S173–S177. doi: 10.1086/651488. [DOI] [PubMed] [Google Scholar]
- 2.Ibraim E, Stoicescu P, Popa Cr. Tuberculosis in Romania. Problems and solutions. Pneumologia. 2010;59(1):6–12. [PubMed] [Google Scholar]
- 3.Ulea I, Murgoci Gh, Stavri H, et al. Comparative study of RT-23 and IC-65 tuberculins tested on children with tuberculosis. Rom.Arch.Microbiol.Immunol. 2010;69(2):75–78. [PubMed] [Google Scholar]
- 4.Haslov K, Ponce-de-Leon Rosales S, Rangel-Frausto S, et al. Tuberculin PPD RT23: still going strong. Int J Tuberc Lung Dis. 1998;2:793–795. [PubMed] [Google Scholar]
- 5.Costa JT, Silva R, Sa R, et al. Comparison of interferon-γ release assay and tuberculin test for screening in healthcare workers. Rev Port Pneumol. 2010;XVI (2):211–221. [PubMed] [Google Scholar]
- 6.Cummings KJ, Smith TS, Shogren ES, et al. Prospective Comparison of Tuberculin Skin Test and QuantiFERON-TB Gold In-Tube Assay for the Detection of Latent Tuberculosis Infection among Healthcare Workers in a Low-Incidence Setting. Infect Control Hosp Epidemiol. 2009;30:1123–1126. doi: 10.1086/644754. [DOI] [PubMed] [Google Scholar]
- 7.Pai M, Zwerling A, Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177–184. doi: 10.7326/0003-4819-149-3-200808050-00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dogra S, Narang P, Mendiratta DK, et al. Comparison of a whole blood interferon-g assay with tuberculin skin testing for the detection of tuberculosis infection in hospitalized children in rural India. Journal of Infection. 2007;54:267–276. doi: 10.1016/j.jinf.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Stavri H, Ene L, Popa GL, et al. Comparison of tuberculin skin test with a whole-blood interferon gamma assay and ELISA, in HIV positive children and adolescents with TB. Roum.Arch.of Microbiol and Immunol. 2009;68(1):14–19. [PubMed] [Google Scholar]
- 10.Mori T, Sakatani M, Yamagishi F, et al. Specific detection of tuberculosis infection: an interferon-gamma-based assay using new antigens. Am. J. Respir. Crit. Care Med. 2004;170(1):59–64. doi: 10.1164/rccm.200402-179OC. [DOI] [PubMed] [Google Scholar]
- 11.Adetifa IM, Ota MO, Jeffries DJ, et al. Commercial Interferon Gamma Release Assays Compared to the Tuberculin Skin Test for Diagnosis of Latent Mycobacterium tuberculosis Infection in Childhood Contacts in the Gambia. MDPediatr Infect Dis J. 2010;29:1–5. doi: 10.1097/INF.0b013e3181cb45da. [DOI] [PubMed] [Google Scholar]
- 12.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 13.Lewinsohna DA, Lobatob MN, Jerebb JA. Interferon-g release assays: new diagnostic tests for Mycobacterium tuberculosis infection, and their use in children. Current Opinion in Pediatrics. 2010;22(1):1–6. doi: 10.1097/MOP.0b013e3283350301. [DOI] [PubMed] [Google Scholar]
- 14.Diel R, Schaberg T, Loddenkemper R, et al. Enhanced cost-benefit analysis of strategies for LTBI screening and INH chemoprevention in Germany. Respiratory Medicine. 2009;103(12):1838–1853. doi: 10.1016/j.rmed.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Detjen AK, Keil S, Roll R, et al. Interferon-gamma release assays improve the diagnosis of tuberculosis and nontuberculous mycobacterial disease in children in a country with a low incidence of tuberculosis. Clin Infect Dis. 2007;45(3):322–328. doi: 10.1086/519266. [DOI] [PubMed] [Google Scholar]
- 16.Gallant CJ, Cobat A, Simkin L, et al. Tuberculin skin test and in vitro assays provide complementary measures of anti-mycobacterial immunity in children and adolescents. Chest. 2010;137(5):1071–1077. doi: 10.1378/chest.09-1852. [DOI] [PubMed] [Google Scholar]
- 17.Connell TG, Tebruegge M, Ritz N, et al. Indeterminate Interferon-γ Release Assay Results in Children. The Pediatric Infectious Disease Journal. 2010;29(3):285–286. doi: 10.1097/INF.0b013e3181c4822f. [DOI] [PubMed] [Google Scholar]