ABSTRACT
Background: After acute myocardial infarction (AMI), left ventricular (LV) function is a well-established prognostic marker. Recent studies indicate that serum levels of brain natriuretic peptide (BNP) also represent an prognostic marker in this setting but so far without a precise cut-off value.
Objective: The aim of this study was to assess the predictive value of BNP serum levels for LV function assessed by echocardiography in STEMI patients undergoing revascularization.
Methods: We prospectively studied a cohort of 88 consecutive patients (mean age 51.6 years, 88.6% males) hospitalized in our clinic for STEMI in Killip class I (50% anterior infarction), who underwent reperfusion therapy. Serum BNP levels were measured on admission, at 24h and at 30 days after reperfusion. Detailed echocardiography was performed at baseline, at 24 hours after reperfusion, on discharge and at follow-up at 1 month. Left ventricular systolic and diastolic dysfunction were defined by LVEF < 45% and E/A ratio respectively.
Results: ROC curve analysis showed that BNP measurements on admission and at 24 hours after revascularization have no predictive value neighter for diastolic LV dysfunction in anteior or inferior AMI patients, nor for systolic LV dysfunction in inferior AMI patients. Only BNP levels at 24 hours after revascularization can predict systolic LV dysfunction in anterior AMI patients with a 90.3% sensitivity and a 60% false positive rate at a cutt off value of 90pg/ml.
Conclusions: Early measurement of BNP levels may allow early prediction of anterior STEMI patients at risk of developing systolic LV dysfunction after revascularization therapy.
Keywords: myocardial infarction, brain natriuretic peptide (BNP), revascularization, LV dysfunction
BACKGROUND
Early and successful myocardial reperfusion by primary percutaneous coronary intervention or by thrombolysis is the most effective strategy for reducing the infarct size and improving clinical outcome in STEMI patients (1).
Serum levels of brain natriuretic peptide (BNP), a cardiac neurohormone that is synthesized in ventricular myocardium and released in response to increased ventricular wall stress (3-6), are elevated both in patients with heart failure and acute coronary syndromes – acute myocardial infarction (AMI) and unstable angina, and represent an important marker of clinical outcome and cardiovascular mortality in patients with myocardial infarction, of recent research interest (7-12).
Previous studies had shown the prognostic value of BNP, measured in the subacute phase (13-15) on mortality of STEMI patients, while the value of early measurements of BNP has recently been studied (16,17).
There is no precise cut-off value in the literature for BNP levels from which it predicts clinical outcomes or cardiovascular mortality in STEMI patients, most of the studies using BNP as a dichotomous result at 80pg/ml (12,18-20). ❑
OBJECTIVES
The aim of this study was to assess the predictive value of BNP serum levels for left ventricular function assessed by echocardiography in STEMI patients undergoing revascularization. ❑
MATERIAL AND METHODS
Study Population and treatment
From January 2005 until July 2006 we prospectively studied a cohort of 88 consecutive patients, with mean age 51.6 years, 88.6% males (Table 1) hospitalized in our clinic for STEMI in Killip class I (50% anterior infarction), who underwent reperfusion therapy within a mean time from symptoms onset of 3.82 hours (range 1-12 hours).
Table 1.
Demographic and clinical features of studied patients
| Variable | p value * | |
|---|---|---|
| Sex | ||
| • Male | 88.6% | p = 0.0001 |
| • Female | 11.4% | |
| Age (years) | 51.6 (26-78) | |
| BMI (Kg/m2) | 27.58 (19.81 – 39.34) | |
| Smoker status | ||
| • Non-smoker | 20.5% | p = 0.0001 |
| • Smoker | 79.5% | |
| Diabetes | ||
| • Absent | 87.5% | p = 0.0001 |
| • Present | 12.5% | |
| Dyslipidemia | ||
| • Absent | 47.7% | p = 0.670 |
| • Present | 52.3% | |
| Hypertension | ||
| • Absent | 51.1% | p = 0.831 |
| • Present | 48.9% | |
| Late angina | ||
| • Absent | 52.3% | p = 0.0001 |
| • Present | 47.7% | |
| AMI location | ||
| • Anterior | 50% | p = 1 |
| • Inferior | 50% | |
Data are presented as a percentage for categorical variables and as mean value (range) for continuous variables. BMI = body mass index; * = chi square test
Thrombolysis was used in 87.5% of cases (30.3% streptokinase, 69.7% tissue plasminogen activator) and primary percutaneous coronary intervention (PTCA) in 11.5% of cases. Successful reperfusion (R) assessed non-invasively by classical criteria, mainly by ST-segment resolution, was obtained in 88.6% of patients, of whom 19.3% had reperfusion injury (RI) defined by acute heart failure and episodes of arrhythmias requiring intervention. In the remaining 11.4% there was lack of reperfusion (NR) (Table 2).
Table 2.
Reperfusion therapy
| Variable | p value * | |
|---|---|---|
| TTT (hours) | 3.82 (1 – 12) | |
| Revascularization | ||
| • Thrombolysis | 87.5% | p = 0.0001 |
| • PPTCA | 12.5% | |
| Thrombolitics | ||
| • AT | 25% | p = 0.713 |
| • RE | 21% | |
| • SK | 30.3% | |
| • TE | 23.7% | |
| RTR | ||
| • NR | 11.4% | p = 0.0001 |
| • R | 69.3% | |
| • RI | 19.3% | |
Data are presented as a percentage for categorical variables and as mean value (range) for continuous variables; TTT = time from symptom onset to therapy; RTR = reperfusion therapy results; NR = no reperfusion; R = reperfusion; RI = reperfusion injury; PPTCA = primary percutaneous coronary angioplasty; AT = alteplase; RE = reteplase; SK = streptokinase; TE = tenecteplase; * = chi square test
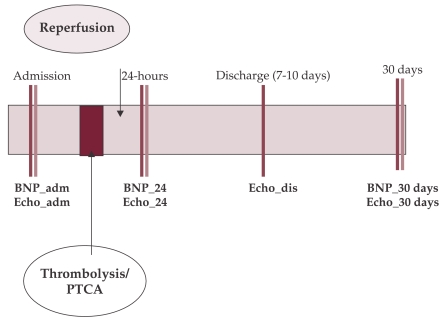
Serum BNP levels were measured on admission (BNP0), at 24h (BNP24) and at 30 days (BNP30) after reperfusion. Detailed echocardiography was performed at baseline, at 24 hours after reperfusion, on discharge (at 7 – 10 days) and at follow-up at 1 month. Left ventricular (LV) systolic and diastolic dysfunction were defined by LVEF < 45% and E/A ratio respectively (figure 1). ❑
Figure 1. Study design.
BNP = B-type Natriuretic peptide; 0 = on admission; 24 = at 24 hours after revascularization, 30 = at 30 days after revascularization; PTCA = percutaneous coronary angioplasty; Echo = echocardiography; dis = discharge
Statistics
Data analysis was performed using Statistical Package for Social Sciences (SPSS 15.0) software (SPSS Inc., Chicago, IL, USA), at a significance level of p ≤ 0.05.
Kolmogorov-Smirnov test was used to analyze continuous data distribution, according to which appropriate tests were further used in analysis: independent samples t-test or Mann-Whitney U test for differences between means of 2 independent groups, and paired-samples t-test or Wilcoxon test for differences between means of 2 related groups. Chi-square test was used to analyze differences between categorical data.
Person's or Spearman's correlation coefficients were calculated in order to test the association between variables.
The predictive value of BNP for detecting and systolic / diastolic left ventricular dysfunction was assessed using receiver-operating characteristics (ROC) analysis, identifing the cut point value that maximizes sensitivity and specificity. ❑
RESULTS
BNP levels and systolic/diastolic LV dysfunction
During hospitalization, 51.1% of all patients presented systolic LV dysfunction (SLVD-H: defined by LVEF < 45% on at least one of three measurements: on admission, at 24 hours after reperfusion therapy and on discharge) and 61.4% of all patients presented diastolic LV dysfunction (DLVD-H: defined by E/A < 1 or E/A > 2 on at least one of three measurements: on admission, at 24 hours after reperfusion therapy and on discharge).
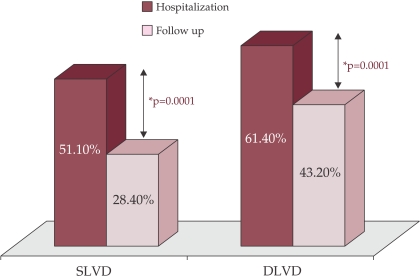
At follow-up (30 days after reperfusion therapy) there was a significant reduction in proportion of patients with both systolic and diastolic LV dysfunction [28.4% SLVD-30 vs. 51.1% SLVD-H; p = 0.0001; and respectively 43.2% DLVD-30 vs. 61.4% DLVD-H; p = 0.0001] (figure 2).
Figure 2. LV dysfunction during hospitalization and at follow up.
SLVD = systolic left ventricular dysfunction; DLVD-H = diastolic left ventricular dysfunction
Systolic left ventricular dysfunction during hospitalization was found significantly more frequent in patients with anterior AMI comparing to those with inferior location [76.2% vs. 33.3%; p = 0.0001] while at 30 after revascularization, the proportion of cases with systolic left ventricular dysfunction was statistically similar in both groups [39.5% vs. 21.1%; p = 0.072].
The proportion of cases with diastolic left ventricular dysfunction was statistically similar for both anterior and inferior AMI patients during hospitalization [88.9% vs. 78.6%; p = 0.259] while, at follow, up it was significantly higher in anterior AMI patients [72.2% vs. 44.4%; p = 0.026] (table 3).
Table 3.
Systolic and diastolic left ventricular dysfunction by AMI location
| ANT. AMI | INF.AMI | p value | |
|---|---|---|---|
| SLVD-H (%) | 76.2 | 33.3 | p = 0.0001* |
| DLVD-H (%) | 88.9 | 78.6 | p = 0.259* |
| SLVD-30 (%) | 39.5 | 21.1 | p = 0.072* |
| DLVD-30 (%) | 72.2 | 44.4 | p = 0.026* |
Data are presented as a percentage; SLVD-H = systolic left ventricular dysfunction during hospitalization; DLVD-H = diastolic left ventricular dysfunction during hospitalization; SLVD-30 = systolic left ventricular dysfunction at 30 days after revascularization; DLVD-30 = diastolic left ventricular dysfunction at 30 days after revascularization; ANT.AMI = anterior acute myocardial infarction; INF. AMI = inferior acute myocardial infarction; * = chi square test
Both on admission and at 24 hours after reperfusion, patients with anterior AMI had the highest BNP levels [BNP0: 84.33 pg/ml vs. 34.83 ng/ml; p = 0.033; BNP24: 259.32 pg/ml vs. 178.86 pg/ml; p = 0.044], while at 30 days after revascularization therapy BNP levels were similar in both groups [BNP30: 197.49 pg/ml vs. 188.3 pg/ml; p = 0.857] (table 4).
Table 4.
Differences in BNP levels in patients with anterior or inferior AMI
| BNP dynamics (pg/ml) | ANT AMI | INF AMI | p value |
|---|---|---|---|
| BNP0 | 84.33 (4-589) | 34.83 (4-157) | p = 0.033* |
| BNP24 | 259.32 (9.1-946) | 178.86 (7.9-690) | p = 0.044* |
| BNP30 | 197.49 (10.2-861) | 188.30 (12.9-1380) | p = 0.857* |
Data are presented as mean value (range); BNP = B-type Natriuretic peptide; 0 = on admission; 24 = at 24 hours after revascularization, 30 = at 30 days after revascularization; ANT.AMI = anterior acute myocardial infarction; INF. AMI = inferior acute myocardial infarction; * = independent samples t test
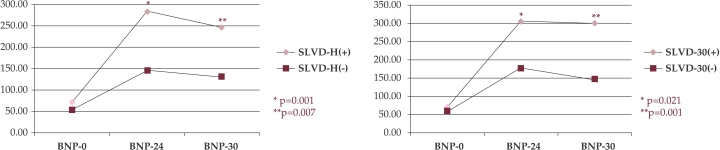
Starting from statistically similar BNP levels on admission, patients whom presented systolic LV dysfunction during hospitalization and at follow up, had significantly higher BNP24 and BNP30 levels compared to those without systolic LV dysfunction. [SLVD on admission absent vs. present: BNP0: 50.45 pg/ml vs. 70.77 pg/ml; p = 0.599; BNP24: 147.03 pg/ml vs. 282.46 pg/ml; p = 0.001; BNP30: 130.47 pg/ml vs. 246.47 pg/ml; p = 0.007; SLVD at follow up absent vs. present: BNP0: 56.04 pg/ml vs. 70.99 pg/ml; p = 0.906; BNP24: 175.90 pg/ml vs. 305.76 pg/ml; p = 0.021; BNP30: 147.56 pg/ml vs. 298.92 pg/ml; p = 0.001].(figure 3).
Figure 3. BNP levels and LV dysfunction.
BNP = B-type Natriuretic peptide; 0 = on admission; 24 = at 24 hours after revascularization, 30 = at 30 days after revascularization; SLVD-H = systolic left ventricular dysfunction during hospitalization; SLVD-30 = systolic left ventricular dysfunction at 30 days after revascularization; (+) = with; (-) = without
In anterior AMI patients there was a direct association of medium strength between BNP levels at 24 hours and at 30 days after the reperfusion therapy and systolic LV dysfunction during hospitalization [BNP0 – SLVD-H: rs= 0.396; rs2 = 0.156; p = 0.010; BNP30– SLVD: rs = 0.317; rs2 = 0.100 p = 0.044]. There were no statistic significant association between BNP levels and dyastolic LV dysfunction in this group of patients.
In inferior AMI patients there was a direct association of medium strength between BNP levels at 30 days after the reperfusion therapy and both systolic LV dysfunction during hospitalization and diastolic LV dysfunction at follow up [BNP30– SLVD-H: rs= 0.332; rs2 = 0.110 p = 0.048; BNP30– DLVD-30: rs= 0.383; rs2 = 0.146 p = 0.049]. There were no statistic significant association between BNP0 and BNP24 levels and systolic or diastolic LV dysfunction during hospitalization in this group of patients.
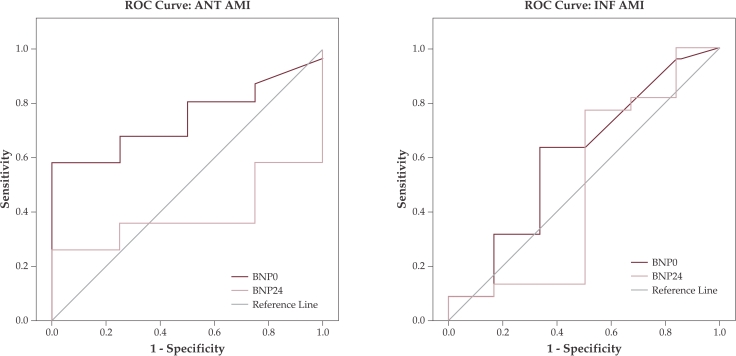
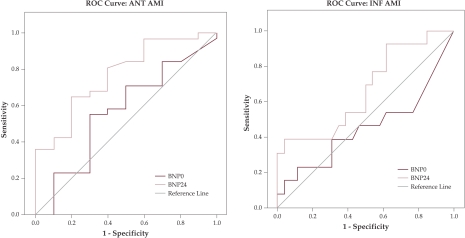
ROC curve analysis showed that BNP measurements on admission and at 24 hours after revascularization have no predictive value for diastolic LV dysfunction neighter in anteior nor in inferior AMI patients (figure 4). Also these measurements have no predictive value for systolic LV dysfunction in inferior AMI patients (figure 5 (b)). Only BNP levels at 24 hours after revascularization can predict systolic LV dysfunction in anterior AMI patients with a 90.3% sensitivity and a 60% false positive rate (1-specificity) at a cutt off value of 90pg/ml (figure 5 (a)). ❑
Figure 4. ROC curve for BNP predicting diastolic LV dysfunction.
Receiver operating characteristic (ROC) curve analysis for B-type Natriuretic peptide (BNP) levels on admission (0)and at 24 hours (24) after revascularization therapy as predictor for diastolic LV dysfunction; (a) ANT. AMI = anterior acute myocardial infarction; (b) INF AMI = inferior acute myocardial infarction
Figure 5. ROC curve for BNP predicting systolic LV dysfunction.
Receiver operating characteristic (ROC) curve analysis for B-type Natriuretic peptide (BNP) levels on admission (0) and at 24 hours (24) after revascularization therapy as predictor for systolic LV dysfunction; (a) ANT AMI = anterior acute myocardial infarction; (b) INF AMI = inferior acute myocardial infarction
DISCUSSION
In this study we have demonstrated that the early BNP measurement provides important information regarding systolic LV dysfunction in STEMI with anterior location patients undergoing revascularization.
Elevated BNP after AMI identifies patients at risk of adverse left ventricular remodeling, chronic left ventricular dysfunction and congestive heart failure (23).
It is well known that BNP levels are correlated with age, renal function, intracardiac pressures and ejection fraction (17). The majority of patients enrolled in this study were of masculin gender and of rather young age. Renal impairement defined by creatinine clearance ≤ 30ml/min was used as an exclusion criterion. More then that, we've used Killip class I STEMI as a inclusion criterion in order to ensure that at baseline patients were similar regarding LV filling pressure due to prior disease and the consequent BNP levels dynamics could be interpreted as a consequence of LV filling pressure variation secondary to the reperfusion outcome. Thus we/ve minimalised the factors that could bias our data.
This study provides support for the use of BNP as a screening tool for systolic LV dysfunction in anterior AMI patients at a threshold of 90pg/ml. This threshold is very similar to that established for the diagnostic of chronic heart failure (100 pg/ml) in a recent prospective study (24). Therefore, routine treatment, even without clinical LV dysfunction, with after load reducing agents or other agents that may improve infarct healing or ventricular remodeling after AMI may be particularly beneficial in STEMI patients with early elevated BNP serum levels.
At this moment there is no management strategy based on early elevated BNP levels for STEMI patients. Additional studies are needed to identify novel therapies that may reduce the risk associated with increased BNP levels in STEMI patients.
Limitation to this study should be considered. First, this study had a relatively small number of patients, so data should be interpreted with caution. Second, the lack of LV function data is an objective limitation of our study.
CONCLUSIONS
-
1.
Early measurement of BNP levels may allow early prediction of anterior AMI patients at risk of developing systolic LV dysfunction after revascularization therapy.
-
2.
An elevated plasma concentration of BNP (>90 pg/ml) at 24 hours after revascularization in anterior AMI patients predicts systolic LV dysfunction with 90.3% sensitivity and a 60% false positive rate. ❑
ACKNOLEDGENENTS
This study was part of National Commitee for Heigher Education Scientific Research (CNCSIS) GRANT number 22 / 2006, a type A project.
We are grateful to the staff of the Cardiology Department, Emergengy Clinical Hospital Bucharest, for their support.
References
- 1.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 2.Piper HM, GarciaDorado D, Ovize M. A fresh look at reperfusion injury. Cardiovasc Res. 1998;38:291–300. doi: 10.1016/s0008-6363(98)00033-9. [DOI] [PubMed] [Google Scholar]
- 3.Wiese S, Breyer T, Dragu A, et al. Gene expression of brain natriuretic peptide in isolated atrial and ventricular human myocardium: influence of angiotensin II and diastolic fiber length. Circulation. 2000;102:3074–3079. doi: 10.1161/01.cir.102.25.3074. [DOI] [PubMed] [Google Scholar]
- 4.Yasue H, Yoshimura M, Sumida H, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparation with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90:195–203. doi: 10.1161/01.cir.90.1.195. [DOI] [PubMed] [Google Scholar]
- 5.Yoshimura M, Yasue H, Okumura K, et al. Different secretion patterns of atrial natriuretic peptide and brain natriuretic peptide in patients with congestive heart failure. Circulation. 1993;87:464–469. doi: 10.1161/01.cir.87.2.464. [DOI] [PubMed] [Google Scholar]
- 6.Stein BC, Levin RI. Natriuretic peptides: physiology, therapeutic potential and risk stratification in ischemic heart disease. Am. Heart J. 1998;135:914–923. doi: 10.1016/s0002-8703(98)70054-7. [DOI] [PubMed] [Google Scholar]
- 7.Omland T, Aakvaag A, Vik-Mo H. Plasma cardiac natriuretic peptide determination as a screening test for the detection of patients with mild left ventricular impairment. Heart. 1996;76:232–237. doi: 10.1136/hrt.76.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dao Q, Krishnaswamy P, Kazanegra R, et al. Utility of B-type natriuretic peptide in the diagnosis of congestive heart failure in an urgent-care setting. J Am Coll Cardiol. 2001;37:379–385. doi: 10.1016/s0735-1097(00)01156-6. [DOI] [PubMed] [Google Scholar]
- 9.Maisel A. B-type natriuretic peptide levels: diagnostic and prognostic in congestive heart failure: what's next? Circulation. 2002;105:2328–2331. doi: 10.1161/01.cir.0000019121.91548.c2. [DOI] [PubMed] [Google Scholar]
- 10.Omland T, Aakvaag A, Bonarjee VV, et al. Plasma brain natriuretic peptide as an indicator of left netricular systolic function and long-term survival after acute myocardial infarction. Comparison with plasma atrial natriuretic peptide and N-terminal proatrial natriuretic peptide. Circulation. 1996;93:1963–1969. doi: 10.1161/01.cir.93.11.1963. [DOI] [PubMed] [Google Scholar]
- 11.Hall C, Cannon CP, Forman S, et al. Prognosis value of N-terminal proatrial natriuretic factor plasma levels measured within the first 12 hours after myocardial infarction. Thrombolysis in Myocardial Infarction (TIMI) II Investigators. J Am Coll Cardiol. 1995;26:1452–1456. doi: 10.1016/0735-1097(95)00342-8. [DOI] [PubMed] [Google Scholar]
- 12.Morrow DA, de Lemos JA, Sabatine MS, et al. Evaluation of B-type natriuretic peptide for risk assessment in unstable angina/non-ST elevation myocardial infarction. J Am Coll Cardiol. 2003;41:1264–1272. doi: 10.1016/s0735-1097(03)00168-2. [DOI] [PubMed] [Google Scholar]
- 13.de Lemos JA, Morrow DA, Bentley JH, et al. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001;345:1014–1021. doi: 10.1056/NEJMoa011053. [DOI] [PubMed] [Google Scholar]
- 14.Omland T, Persson A, Ng L, et al. N-terminal pro-B-type natriuretic peptide and long-term mortality in acute coronary syndromes. Circulation. 2002;106:2913–2918. doi: 10.1161/01.cir.0000041661.63285.ae. [DOI] [PubMed] [Google Scholar]
- 15.Omland T, de Lemos JA, Morrow DA, et al. Prognostic value of N-terminal pro-atrial and pro-brain natriuretic peptide in patients with acute coronary syndromes. Am J Cardiol. 2002;89:463–465. doi: 10.1016/s0002-9149(01)02271-8. [DOI] [PubMed] [Google Scholar]
- 16.Jernberg T, Stridsberg M, Venge P, et al. N-terminal pro brain natriuretic peptide on admission for early risk stratification of patients with chest pain and no ST-segment elevation. J Am Coll Cardiol. 2002;40:437–445. doi: 10.1016/s0735-1097(02)01986-1. [DOI] [PubMed] [Google Scholar]
- 17.James SK, Lindaht B, Siegbalm A, et al. N-terminal pro-brain natriuretic peptide and other risk markers for the separate prediction of mortality and subsequent myocardial infarction in patients with unstable coronary artery disease: a Global Utilization of Strategies To Open occluded arteries (GUSTO) IV substudy. Circulation. 2003;108:275–281. doi: 10.1161/01.CIR.0000079170.10579.DC. [DOI] [PubMed] [Google Scholar]
- 18.Mega JL, Morrow DA, de Lemos JA, et al. B-type natriuretic peptide at presentation and prognosis in patients with ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2004;44:335–339. doi: 10.1016/j.jacc.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki S, Yashimura M, Nakayama M, et al. Plasma level of B-type natriuretic peptide as a prognostic marker after acute myocardial infarction. Circulation. 2004;110:1387–1391. doi: 10.1161/01.CIR.0000141295.60857.30. [DOI] [PubMed] [Google Scholar]
- 20.Galvani M, Ottani F, Oltrona L, et al. N-terminal pro-brain natriuretic peptide on admission has prognostic value across the whole spectrum of acute coronary syndromes. Circulation. 2004;110:128–134. doi: 10.1161/01.CIR.0000134480.06723.D8. [DOI] [PubMed] [Google Scholar]
- 21.Tateishi J, Masutani M, Ohyanagy M, et al. Transient increase in plasma brain natriuretic peptide after percutaneous transluminal coronary angioplasty. Clin Cardiol. 2000;23:776–780. doi: 10.1002/clc.4960231016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyriakides ZS, Markianos M, Michailis L, et al. Brain natriuretic peptide increases acutely and much more prominently than atrial natriuretic peptide during coronary angioplasty. Clin Cardiol. 2000;23:285–288. doi: 10.1002/clc.4960230412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Lamos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet. 2003;362:316–322. doi: 10.1016/S0140-6736(03)13976-1. [DOI] [PubMed] [Google Scholar]
- 24.Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]