Abstract
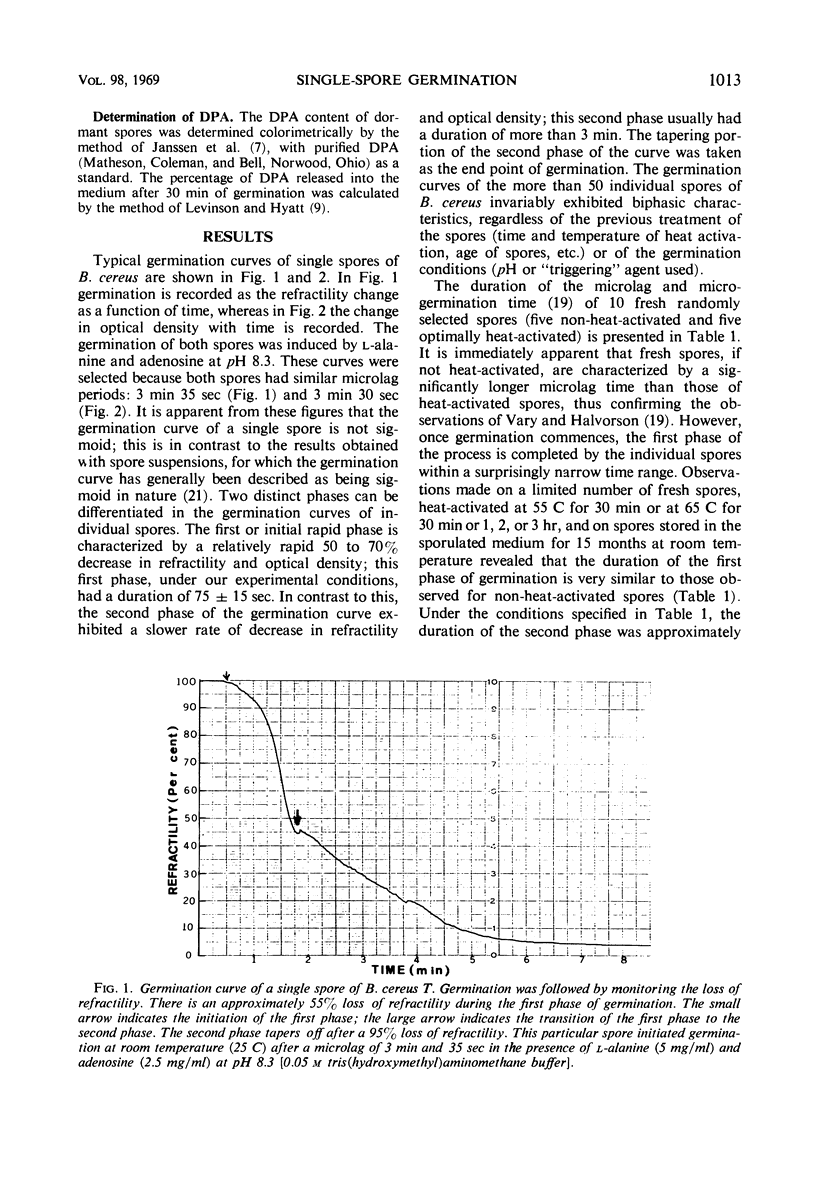
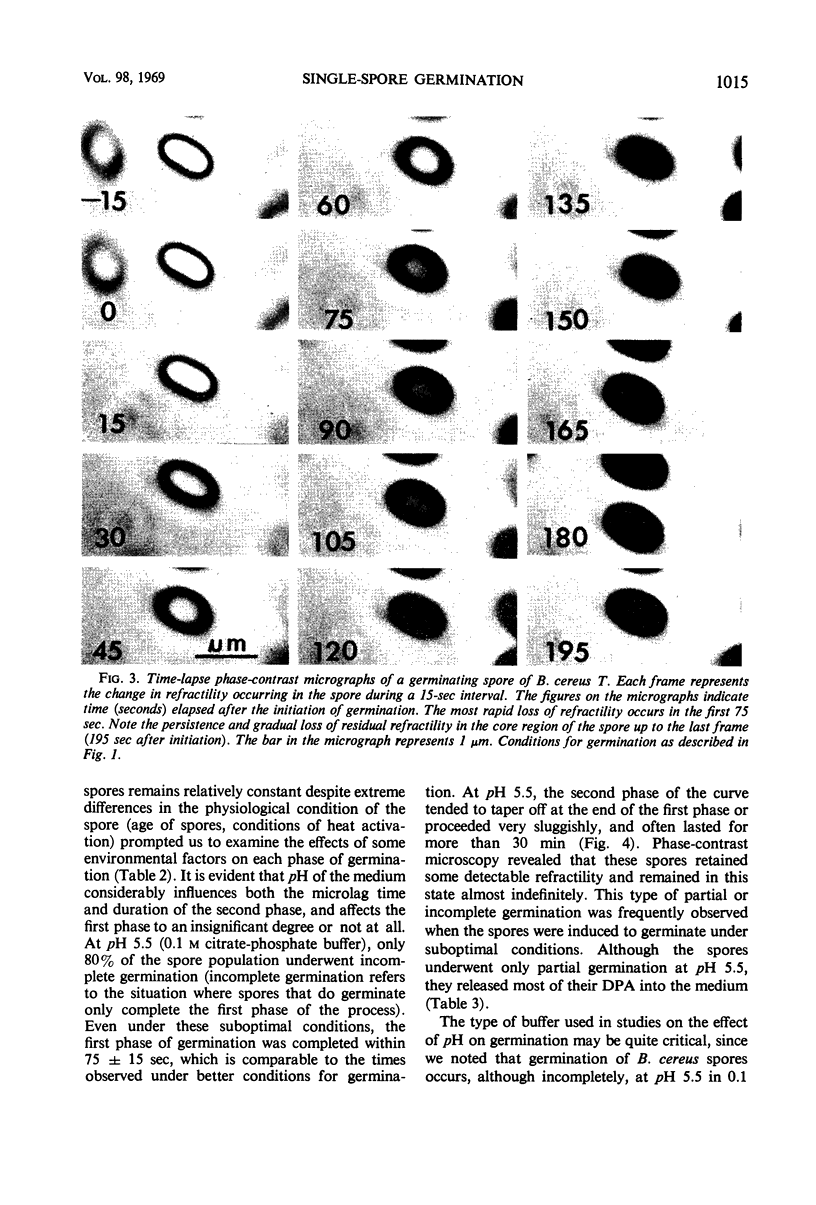
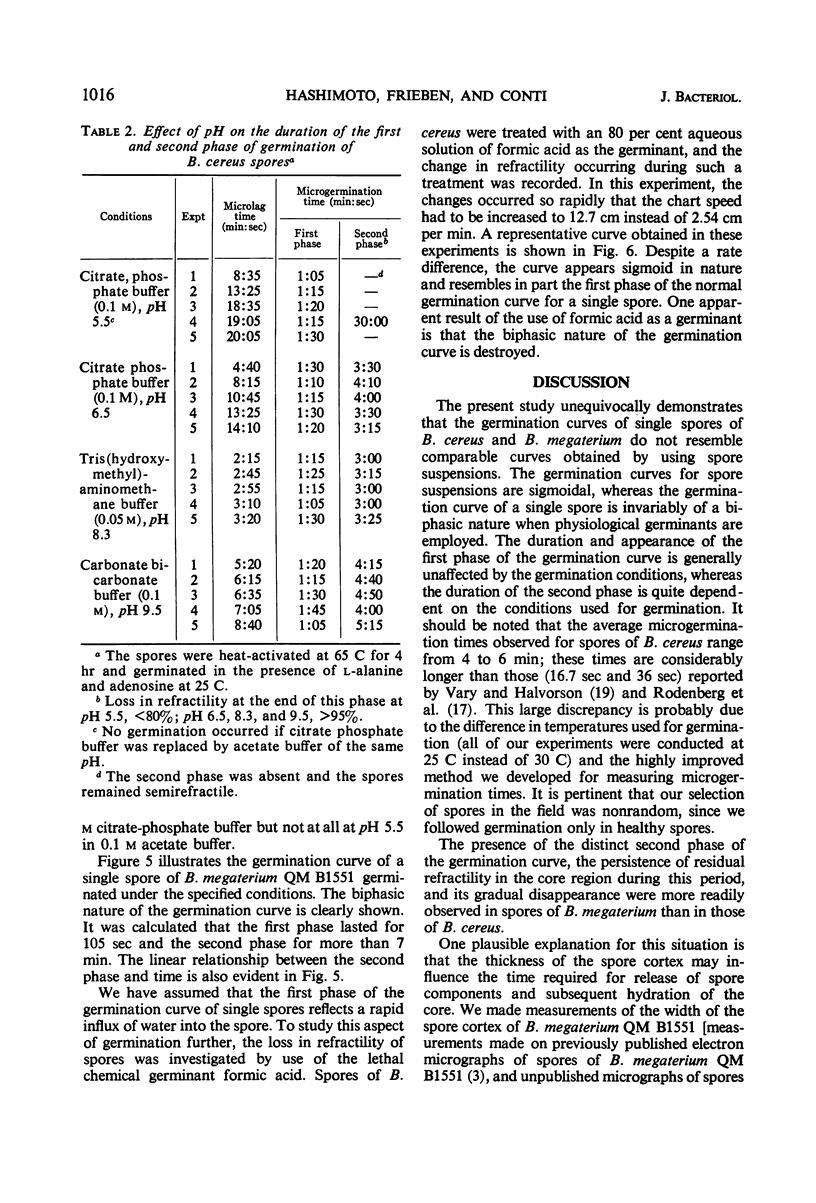
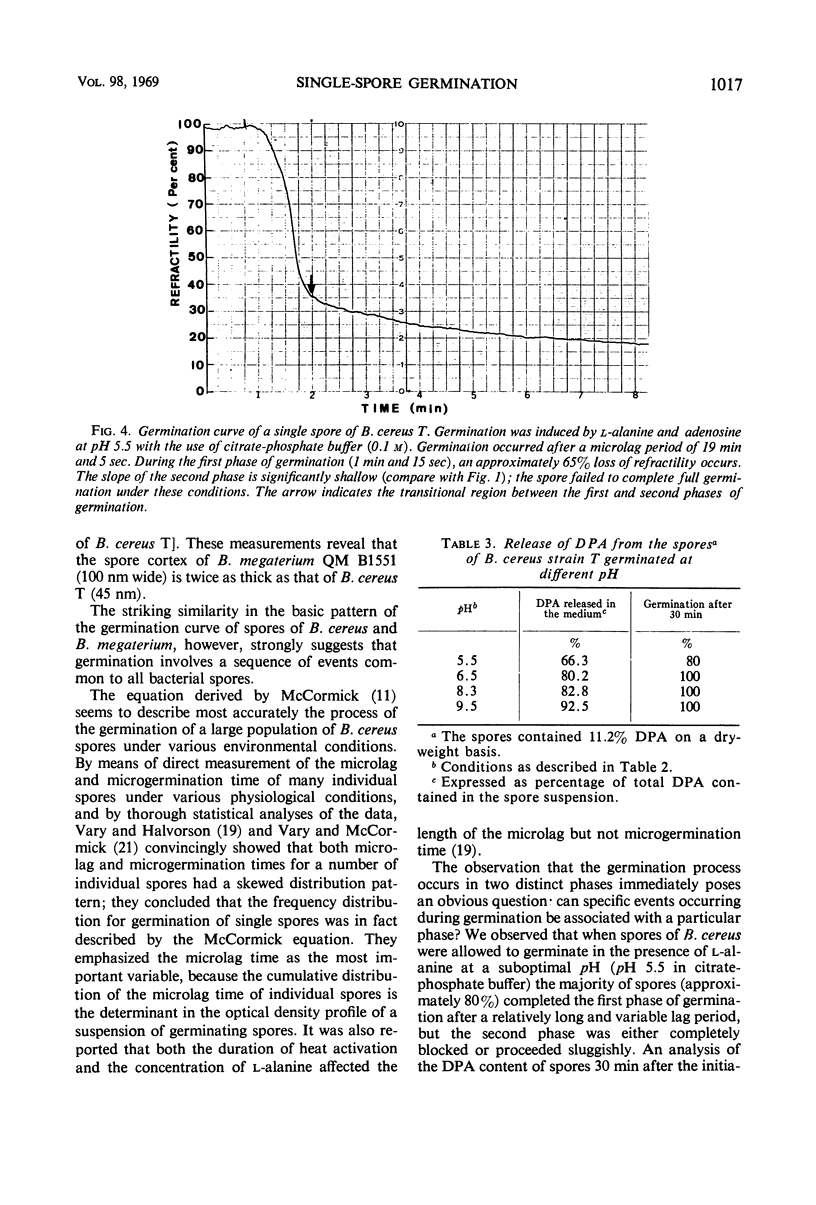
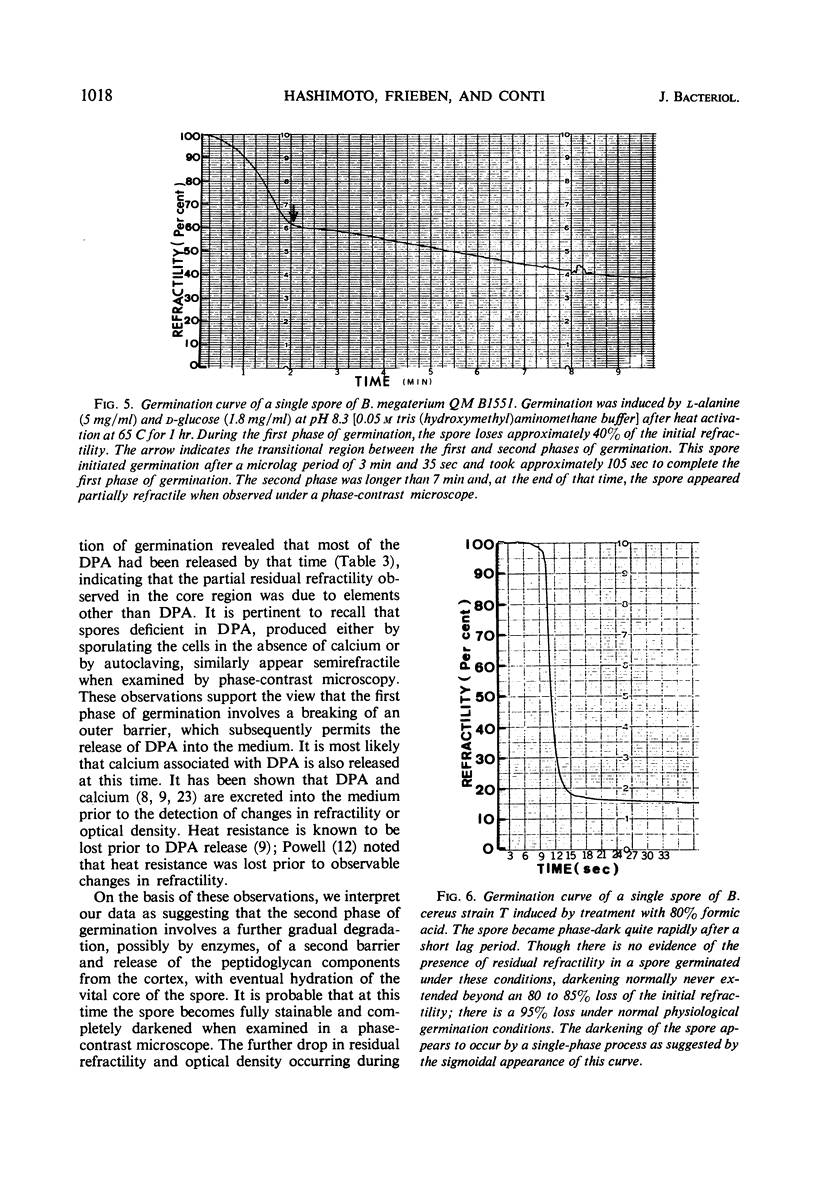
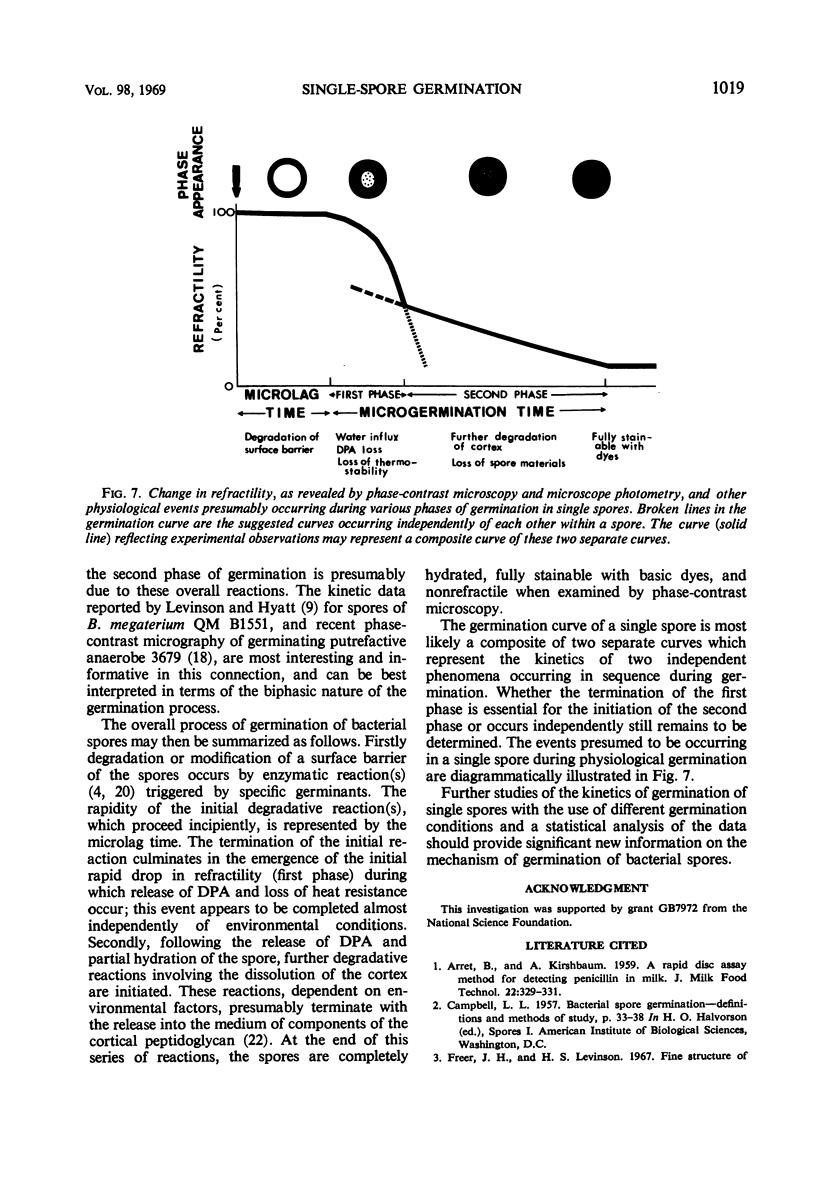
Changes in refractility and optical density occurring in individual spores of Bacillus cereus T and B. megaterium QM B1551 during germination were investigated by use of a Zeiss microscope photometer. The curves revealed that the germination process in single spores had two distinct phases; an initial rapid phase was followed by a second slower phase. Under the experimental condition employed, the first phase of germination of B. cereus spores lasted for approximately 75 ± 15 sec, whereas the second phase lasted for 3 to 4.5 min. In B. megaterium spores, the first phase was observed to last for approximately 2 min and the second phase for more than 7 min. The duration of the second phase was dependent on conditions employed for germination. The kinetics of the first phase were strikingly similar under all conditions of physiological germination. Time-lapse phase-contrast microscopy of germinating spores also revealed the biphasic nature of germination. It was postulated that the first phase represents changes induced by an initial partial hydration of the spore and release into the medium of dipicolinic acid, whereas the second phase reflects degradation of the cortex and hydration of the core.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HASHIMOTO T., BLACK S. H., GERHARDT P. Development of fine structure, thermostability, and dipicolinate during sporogenesis in a bacillus. Can J Microbiol. 1960 Apr;6:203–212. doi: 10.1139/m60-022. [DOI] [PubMed] [Google Scholar]
- Halvorson H. O., Vary J. C., Steinberg W. Developmental changes during the formation and breaking of the dormant state in bacteria. Annu Rev Microbiol. 1966;20:169–188. doi: 10.1146/annurev.mi.20.100166.001125. [DOI] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- KEYNAN A., HALVORSON H. O. Calcium dipicolinic acid-induced germination of Bacillus cereus spores. J Bacteriol. 1962 Jan;83:100–105. doi: 10.1128/jb.83.1.100-105.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson H. S., Hyatt M. T., Holmes P. K. Transition of bacterial spores into vegetative cells. Trans N Y Acad Sci. 1967 Nov;30(1):81–98. doi: 10.1111/j.2164-0947.1967.tb02454.x. [DOI] [PubMed] [Google Scholar]
- Levinson H. S., Hyatt M. T. Sequence of events during Bacillus megaterim spore germination. J Bacteriol. 1966 May;91(5):1811–1818. doi: 10.1128/jb.91.5.1811-1818.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCORMICK N. G. KINETICS OF SPORE GERMINATION. J Bacteriol. 1965 May;89:1180–1185. doi: 10.1128/jb.89.5.1180-1185.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWELL J. F. Factors affecting the germination of thick suspensions of bacillus subtilis spores in L-alanine solution. J Gen Microbiol. 1950 Sep;4(3):330–338. doi: 10.1099/00221287-4-3-330. [DOI] [PubMed] [Google Scholar]
- POWELL J. F. Isolation of dipicolinic acid (pyridine-2:6-dicarboxylic acid) from spores of Bacillus megatherium. Biochem J. 1953 May;54(2):210–211. doi: 10.1042/bj0540210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWELL J. F., STRANGE R. E. Biochemical changes occurring during the germination of bacterial spores. Biochem J. 1953 May;54(2):205–209. doi: 10.1042/bj0540205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PULVERTAFT R. J. V., HAYNES J. A. Adenosine and spore germination; phase-contrast studies. J Gen Microbiol. 1951 Oct;5(4):657–663. doi: 10.1099/00221287-5-4-657. [DOI] [PubMed] [Google Scholar]
- Rodenberg S., Steinberg W., Piper J., Nickerson K., Vary J., Epstein R., Halvorson H. O. Relationship between protein and ribonucleic acid synthesis during outgrowth of spores of Bacillus cereus. J Bacteriol. 1968 Aug;96(2):492–500. doi: 10.1128/jb.96.2.492-500.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara M., Frank H. A. Sequence of events during germination of putrefactive anaerobe 3679 spores. J Bacteriol. 1967 Sep;94(3):506–511. doi: 10.1128/jb.94.3.506-511.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARY J. C., HALVORSON H. O. KINETICS OF GERMINATION OF BACILLUS SPORES. J Bacteriol. 1965 May;89:1340–1347. doi: 10.1128/jb.89.5.1340-1347.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vary J. C., Halvorson H. O. Initiation of bacterial spore germination. J Bacteriol. 1968 Apr;95(4):1327–1334. doi: 10.1128/jb.95.4.1327-1334.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARTH A. D., OHYE D. F., MURRELL W. G. Location and composition of spore mucopeptide in Bacillus species. J Cell Biol. 1963 Mar;16:593–609. doi: 10.1083/jcb.16.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOESE C., MOROWITZ H. J. Kinetics of the release of dipicolinic acid from spores of Bacillus subtilis. J Bacteriol. 1958 Jul;76(1):81–83. doi: 10.1128/jb.76.1.81-83.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]