Abstract
Recent studies of the sea urchin embryo have elucidated the mechanisms that localize and pattern its nervous system. These studies have revealed the presence of two overlapping regions of neurogenic potential at the beginning of embryogenesis, each of which becomes progressively restricted by separate, yet linked, signals, including Wnt and subsequently Nodal and BMP. These signals act to specify and localize the embryonic neural fields – the anterior neuroectoderm and the more posterior ciliary band neuroectoderm – during development. Here, we review these conserved nervous system patterning signals and consider how the relationships between them might have changed during deuterostome evolution.
Keywords: Wnt, TGFβ, Nodal, BMP, Dorsal-ventral axis, Anterior-posterior axis, Lefty, Chordin, Six3, FoxQ2, Ciliary band, Apical organ, Ectoderm patterning, Neural development, Neural patterning
Introduction
Rapid progress has been made in the last five years in understanding the mechanisms that govern ectoderm, and hence nervous system, patterning in sea urchin embryos. These findings have revealed that a fascinating cascade of Wnt-dependent pathways functions during sea urchin development to regulate Nodal and bone morphogenetic protein (BMP) signaling. Together, these restrict the neurogenic capacity that is initially present throughout the entire embryo to specific locations, generating first neuroectoderm at the anterior end of the embryo [the anterior neuroectoderm (ANE), see Glossary, Box 1] and then, within and along a band of ciliated ectoderm, the ciliary band ectoderm (the CBE, see Glossary, Box 1). These neuroectoderm territories are established at different times during development by molecularly distinct signaling mechanisms in sea urchin embryos and neural patterning occurs in the absence of any complex tissue layer movements or interactions, factors that facilitate a clearer understanding of how embryonic nervous systems are shaped by these signals. Importantly, because the sea urchin shares a common ancestor with the chordates, analyses of the mechanisms that pattern the sea urchin nervous system can reveal the shared and derived features of chordate neural patterning, providing us with insights into the evolution of deuterostome (see Glossary, Box 1) nervous systems.
Box 1. Glossary
Aboral ectoderm. Ectoderm on the dorsal side of the embryo and a small region anterior to the anus on the ventral side that together form a squamous epithelium.
Anterior neuroectoderm (ANE). Ectoderm that is derived from the animal pole region of the egg, has the potential to produce nerves, and is restricted by canonical Wnt signaling-dependent processes to the anterior end of the embryo.
Ascidians. A class of urochordates, also known as sea squirts, that are marine filter-feeders.
Bilaterians. Organisms with bilateral symmetry that arose from pre-bilaterians and include all the remaining animal phyla divided into two major groups: the protostomes and deuterostomes.
Centralized nervous system. A system of nerves that is restricted to specific regions of ectoderm, such as the dorsal nerve cords of vertebrates and ventral nerve cords of arthropods, or the ANE and CBE of sea urchin embryos.
Cephalochordates. One of the three chordate subphyla, along with vertebrates and urochordates. A modern representative is amphioxus, or lancelet.
Ciliary band ectoderm (CBE). Ectoderm that has the potential to produce nerves and is restricted by TGFβ signaling to a narrow band of cells (CB) between ventral and dorsal ectoderm.
Cnidarians. A sister group to the bilateria. Includes corals, sea anemones, jellyfish and hydroids.
Ctenophores. Commonly known as comb jellies, a pre-bilaterian phylum of marine animals that use groups of cilia (`combs') to swim.
Diffuse nervous system. A system of nerves that is distributed throughout the ectoderm and can form a network of interconnected neurons, such as is found in pre-bilaterians.
Deuterostome. Organisms in which the blastopore becomes the anus and a second opening forms the mouth; coeloms develop as hollow outgrowths of the gut. Deuterostomes mentioned here include echinoderms (sea urchins), hemichordates (Saccoglossus kowalevskii, Ptychodera flava), cephalochordates (amphioxus), urochordates (ascidians) and vertebrates (fish, frog, chick, mouse).
Hemichordates. A phylum of marine deuterostomes, considered to be a sister group of the echinoderms.
Pre-bilateria. Organisms with radial symmetry; extant members include cnidarians, ctenophores and sponges.
Protostomes. Organisms in which the blastopore becomes the mouth and coeloms develop from solid masses of mesodermal cells. Protostomes mentioned here include ecdysozoans (flies) and lophotrochozoans (polychaete annelids and snails).
Urbilaterian. Refers to the hypothetical last common ancestor of bilaterians.
The organization of the nervous system is a defining feature of an animal body plan. Vertebrates and flies possess a centralized nervous system (see Glossary, Box 1), whereas a more primitive and diffuse nervous system (see Glossary, Box 1) is characteristic
of pre-bilaterians (see Glossary, Box 1), including cnidarians (see Glossary, Box 1) (Marlow et al., 2009) and ctenophores (see Glossary, Box 1). Transforming growth factor β (TGFβ) signaling centralizes the nervous system along the dorsal side in vertebrates and along the ventral side in flies, suggesting that the common bilaterian (see Glossary, Box 1) ancestor had a centralized system that was sculpted by the same patterning mechanism (Arendt et al., 2008; Denes et al., 2007; Mizutani and Bier, 2008).
The fact that nervous systems are localized to either the dorsal (as in vertebrates) or ventral (as in flies) side of embryos, with the position of the mouth defining the ventral side, sparked an interesting and strongly debated hypothesis that the dorsal-ventral (DV) axis inverted during evolution (De Robertis and Sasai, 1996; Gerhart, 2000), which led investigators to examine evolutionarily more basal organisms, such as ascidians and the hemichordates (see Glossary, Box 1). Attention was focused on the Saccoglossus kowalevskii hemichordate embryo, which was considered to represent a good basal model for chordate nervous system patterning because its anterior-posterior (AP) gene expression patterns are the same as those observed in chordate embryos (Lowe et al., 2003). However, the nervous system of Saccoglossus embryos is, in part, diffuse rather than centralized, and nerve development was found to be insensitive to changes in BMP signaling, in contrast to other organisms (Lowe et al., 2006). Although part of the nervous system of the Saccoglossus embryo is distributed throughout the epidermis, other components are localized along both the dorsal and the ventral midlines (Bullock and Horridge, 1965; Knight-Jones, 1952; Nomaksteinsky et al., 2009). These localization patterns are also found in the nervous system of another enteropneust hemichordate adult, Ptychodera flava, as judged by the expression patterns of several neural marker genes (Nomaksteinsky et al., 2009). However, very little is understood about the molecular mechanisms that pattern the nervous systems of hemichordate embryos.
By contrast, a great deal of progress has been made in recent years in elucidating the mechanisms that pattern the neural and non-neural ectoderm territories in embryos of the sea urchin, which represents another basal deuterostome. Although a sister phylum of the hemichordates, the echinoderms have routinely been excluded from studies of the origins of the chordate nervous system, largely because their adult body plans lack apparent AP polarity and are thought to be highly derived. The nervous system of echinoderms has also been said to be diffuse throughout development. However, sea urchin embryos clearly have AP as well as DV polarity, with nerves organized into highly restricted patterns within the ectoderm, as is the case for centralized nervous systems (Fig. 1). Importantly, the creation of these localized neuroectoderm domains is now understood at a mechanistic level, owing to recent functional studies.
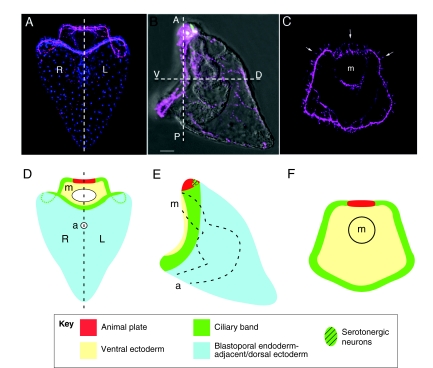
Fig. 1.
The organization of the sea urchin embryo nervous system. (A-C) Sea urchin larvae showing the position of neurons (magenta) and serotonergic neurons (green). (A) Ventral/posterior view. Bilateral symmetry is indicated by the dashed line; the left (L) and right (R) sides of the larva are marked. Cell nuclei are labeled in blue. (B) Lateral view indicating the embryonic AP and DV axes (see Box 2 for explanation). (C) Ventral view, indicating the position of the mouth (m). Several examples of cell bodies are indicated (arrows). Scale bar: 20 μm. (D-F) Schematic representations of the larvae shown in A-C, highlighting regions of the ectoderm and the positions of the anterior neuroectoderm containing the animal plate (red), the ciliary band neuroectoderm (green), mouth (m) and anus (a).
Here, we review the signals and mechanisms that act to specify and localize the anterior and ciliary band neural ectoderm fields (the ANE and the CBE) during sea urchin embryo development. We also compare these neural patterning mechanisms with those used by vertebrates, highlighting the shared features and signaling pathways utilized. Finally, based on these comparisons, we discuss the evolution of these neural patterning mechanisms and propose that, although the same mechanisms might have been used throughout evolution, the regulatory relationships between these signaling mechanisms have changed.
The primary (AP) and secondary (DV) axes of the sea urchin embryo
Because the patterning of ectoderm into neural and non-neural fields in the sea urchin embryo is intimately connected to the initial mechanisms that control cell fate specification along the body axes, we begin with a brief review of these axes and their developmental properties. An explanation for the axial designations used in this review and a comparison of these designations with other commonly used nomenclatures for these axes are provided in Box 2.
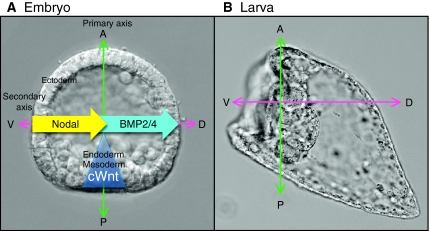
Box 2. A note on axis designations
Classically, the vertebrate egg axis has been referred to as the animal-vegetal (AV) axis; the embryo develops primarily from the animal half, whereas the vegetal half contains most of the yolk. However, the primary axis of an early vertebrate (e.g. Xenopus) embryo does not align with the egg AV axis and is instead referred to as the anterior-posterior (AP) axis, with the head marking the anterior and the blastopore marking the posterior. By contrast, in sea urchins the blastopore forms in the region corresponding to the egg vegetal pole, leading most investigators to use AV for both the egg and embryonic primary axes. Because the early molecular mechanisms that pattern sea urchin embryos along this axis (A; canonical Wnt, blue triangle) are similar to those operating along the vertebrate AP axis, in this review we refer to the sea urchin embryonic axis as primary or AP. The most commonly used designation for the orthogonal, secondary axis of the sea urchin embryo has been oral-aboral. However, again because the mechanisms used for patterning this axis (A; Nodal, yellow arrow; BMP2/4, blue arrow) are similar to those that pattern the DV axes of other deuterostomes, we refer to this axis as dorsal-ventral (DV), with the dorsal and ventral surfaces corresponding approximately to the aboral and oral tissue territories. As the embryo develops to the pluteus larva stage, the gut bends to form the mouth and skeletogenesis supports expansion of the dorsal ectoderm, creating the longest dimension of the larva, which has been referred to as the AP axis. However, this axis does not align with the earlier signaling axes (B; green and pink arrows) that establish the body plan and the organization of the embryonic nervous system. Therefore, here we use only the early AP and DV axial designations to compare the mechanisms that control the positioning of the nervous systems in sea urchin and vertebrate embryos.
The ectoderm, endoderm and mesoderm are arrayed along the AP axis
The developmental capacities of the animal and vegetal halves of the fertilized sea urchin egg differ, implying that the animal-vegetal (AV) axis is established during oogenesis; isolated animal halves become permanent blastulae in which many neurons develop, whereas the vegetal halves often form complete embryos (Hörstadius, 1973; Wikramanayake and Klein, 1997; Yaguchi et al., 2006). The egg AV axis corresponds to the primary axis of the embryo, which extends from the animal pole of the embryo to the blastopore (see Box 2), which is the prospective anus of the larva. The prospective ectoderm, endoderm and mesoderm are arrayed along this axis (see Box 2). The mouth forms as a secondary opening which, along with bilateral symmetry (Fig. 1A), is a hallmark feature of deuterostomes.
Posterior signals are required for mesoderm and endoderm specification
In the last decade, a paradigm has emerged that early canonical Wnt signaling establishes AP polarity in bilaterians and marks the site of gastrulation (Petersen and Reddien, 2009). In sea urchin embryos, canonical Wnt signaling also plays a role in specifying mesoderm and endoderm fates in blastomeres derived from the vegetal regions of the egg and, based on this, the vegetal pole of the sea urchin embryo can be considered analogous to the posterior of vertebrate embryos (see Box 2).
In sea urchins, canonical Wnt signaling is first detectable as a wave of nuclear β-catenin that begins at the 16-cell stage in the most posterior cells, which are called the micromeres, and passes during the next two cleavage stages through concentric tiers of progressively less posterior cells that will form mesoderm, endoderm and a small part of the ectoderm (Logan et al., 1999; Wikramanayake et al., 1998) (Fig. 2A). Micromeres are sufficient to induce endomesoderm because, when transplanted to the anterior end, they induce these tissues in presumptive ectoderm (Hörstadius, 1973; Minokawa and Amemiya, 1999; Ransick and Davidson, 1995). Four known micromere signals, all of which depend on canonical Wnt signaling, are known to exist. These include Delta, an activator of the Notch signaling pathway (Sherwood and McClay, 2001; Sweet et al., 2002), ActivinB (Sethi et al., 2009), Wnt8 (Minokawa et al., 2005; Smith et al., 2008; Smith et al., 2007; Wikramanayake et al., 2004) and an undefined fourth signal that downregulates the transcription factor SoxB1, which is a negative regulator of canonical Wnt signaling in mesoderm tissues (Oliveri et al., 2003; Sethi et al., 2009). The most upstream component of the canonical Wnt signaling pathway to be identified thus far is Dishevelled (Dsh), which is highly concentrated in vegetal egg cortex (Byrum et al., 2009; Leonard and Ettensohn, 2007; Weitzel et al., 2004). Elucidation of the importance of canonical Wnt signaling in endomesoderm development and gastrulation led to large-scale screens to identify and construct the gene regulatory networks controlling these processes (Davidson, 2009; Davidson et al., 2002; Oliveri et al., 2008).
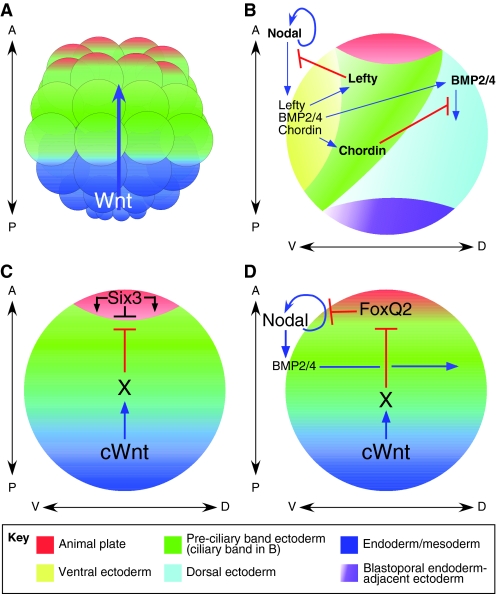
Fig. 2.
Signals that specify cell fate along the sea urchin developmental axes. (A) Beginning at the 16-cell stage, a wave of canonical Wnt signaling (blue arrow) passes through successive tiers to specify mesoderm and endoderm during subsequent cleavages. Anterior neuroectoderm (ANE) fate is eliminated from the remainder of the ectoderm, much of which has a pre-ciliary band neuroectoderm (CBE) fate. (B) TGFβ signaling specifies ectodermal fates along the DV axis, beginning with Nodal, which specifies ventral ectoderm. Nodal is necessary for the subsequent expression of BMP2/4, which specifies dorsal ectoderm and ectoderm adjacent to the blastopore endoderm. Nodal upregulates its own expression and induces the expression of Lefty (a Nodal antagonist) and Chordin (a BMP antagonist), which together protect the ciliary band (green strip) from the epidermis-promoting influences of Nodal and BMP2/4. (C) Wnt-dependent processes determine the anterior position of the ANE (red) through an unknown intermediate process `X'. Mutual antagonism between canonical Wnt-dependent and Six3-dependent processes is thought to determine the border of the ANE. (D) Canonical Wnt signals support the elimination of FoxQ2 from the ectoderm except at the anterior end of the embryo, probably through the same intermediate process `X' as in C. This allows Nodal signaling to increase through autoregulation to levels sufficient to initiate DV axis patterning. cWnt, canonical Wnt.
The secondary (DV) axis is patterned by Nodal and BMP2/4
The zygotic expression of Nodal, a member of the TGFβ superfamily, is both necessary and sufficient to specify ventral structures and to initiate cell fate specification along the secondary (DV) axis of sea urchin embryos (Duboc et al., 2004). During late cleavage stages, nodal transcription is restricted to the presumptive ventral ectoderm (see Box 2), where it upregulates its own expression and that of four key genes – bmp2/4, chordin, lefty and gsc – all of which play major roles in cell fate specification and patterning along the secondary axis (Angerer et al., 2001; Angerer et al., 2000; Bradham et al., 2009; Duboc et al., 2004; Lapraz et al., 2009; Saudemont et al., 2010) (Fig. 2B). The mechanism that controls the spatially restricted expression of nodal is not yet completely understood, but might be linked to a maternal redox gradient along the secondary axis (Coffman et al., 2004). In the unfertilized egg, mitochondria are more abundant on the future ventral side, and when they are artificially transplanted to the prospective dorsal side the secondary axis often is reversed (Coffman et al., 2009). In addition, the redox-sensitive kinase p38 is required for secondary axis polarity (Bradham and McClay, 2006) and cis-regulatory studies have identified several cis elements within the nodal promoter that may bind transcription factors, the orthologs of which are known to be redox sensitive (Nam et al., 2007; Range et al., 2007). However, redox-sensitive activation of a transcription factor required for nodal transcription in the sea urchin embryo has not yet been demonstrated. Two other ubiquitous factors required to initiate nodal expression are Univin, a member of the growth differentiation factor (GDF) subfamily of TGFβs, and the transcription factor SoxB1 (Range et al., 2007). As in other organisms, strong autoregulation of nodal is thought to amplify a small initial asymmetry to lock down its localized expression, which is then maintained by Lefty, a direct Nodal target and Nodal signaling antagonist (Duboc et al., 2008; Meno et al., 1996; Thisse and Thisse, 1999). Consequently, Nodal function is
confined to within several cell diameters of its initial expression site. In the sea urchin embryo this is in ectoderm on the ventral side (Yaguchi et al., 2007), restricted there presumably because Lefty diffuses further than Nodal and effectively interferes with Nodal autoregulation (Bolouri and Davidson, 2009; Duboc et al., 2008) (Fig. 2B).
BMP2/4, another member of the TGFβ superfamily, is functionally polarized along the secondary axis of sea urchins, as in all other bilaterians (Lapraz et al., 2009). It is transcribed only on the ventral side of embryos under the control of Nodal, as is the TGFβ antagonist Chordin (Angerer et al., 2000; Bradham et al., 2009; Duboc and Lepage, 2006; Duboc et al., 2004). However, BMP2/4 functions to specify aboral ectoderm (see Glossary, Box 1) on the opposite side of the embryo (Angerer et al., 2000; Bradham et al., 2009; Duboc et al., 2004; Lapraz et al., 2009). BMP2/4 has been shown to be part of a relay mechanism from Nodal to the dorsal side, as cell-autonomous activation of the Nodal signaling pathway in only one blastomere of an 8-cell embryo results in the transcription of bmp2/4, which is sufficient to rescue patterning along the entire secondary axis (Lapraz et al., 2009). BMP2/4 signaling is blocked on the ventral side, where it is inhibited by Chordin (Lapraz et al., 2009; Saudemont et al., 2010), and moves to the dorsal side, possibly aided by Chordin, as has been shown in other embryos (Shimmi et al., 2005).
Positioning the ANE and the CBE
Canonical Wnt signals not only specify mesoderm and endoderm tissues along the AP axis and promote gastrulation at the posterior end of the embryo, but they are also required to restrict the domain of ANE to the opposite end of the embryo. The regulatory protein signature of the sea urchin ANE is similar to that of vertebrate anterior neuroectoderm. After ANE restriction, most of the remaining ectoderm retains a regulatory state that can support the development of posterior neuroectoderm. However, this neuroectoderm is subsequently restricted to a narrow strip of cells (the ciliary band or CB) as the CBE. The expression of Hnf6, the earliest known CBE marker gene, indicates that the CB is specified by the end of the mesenchyme blastula stage at the border between ventral and dorsal ectoderm tissues, which are diverted to an epidermal fate by Nodal and BMP2/4, respectively, as described above.
Canonical Wnt-dependent processes position the ANE at the animal pole
When canonical Wnt signaling in sea urchin embryos is blocked, either by removing the posterior half of the cleavage-stage embryo or by inhibiting β-catenin nuclearization, a remarkable phenotype is produced: the embryos consist almost entirely of ANE that contains many serotonergic neurons, a neural cell type that in normal three-day embryos develops only in the ANE (Yaguchi et al., 2006). This observation suggested that Wnt signaling represses ANE specification in posterior ectoderm and led to genome-wide screens for candidate ANE regulatory genes that are repressed, rather than activated, by canonical Wnt signaling in this ectoderm (Wei et al., 2006; Wei et al., 2009). This resulted in the discovery that the transcription factor Six3 is necessary to drive ANE fate and is sufficient to greatly expand this domain throughout the embryo (Wei et al., 2009). The transcription of six3 and other early ANE factors is initially activated broadly in the cleavage-stage embryo, presumably by maternally encoded factors, and is subsequently repressed by canonical Wnt-dependent mechanisms (Wei et al., 2009; Yaguchi et al., 2008) at the early blastula stage in all regions except the ANE (Fig. 2C). Six3 is required for the formation of all ANE nerves and also for the expression of a large number of downstream early ANE regulatory genes, many of which have orthologs that are expressed in the vertebrate forebrain (Wei et al., 2009). Thus, much of the regulatory apparatus of the sea urchin embryo ANE corresponds to that of the anterior ectoderm of higher vertebrates, further supporting the view that the sea urchin primary axis is functionally analogous to the AP axes of other embryos. Furthermore, like the vertebrate forebrain, the ANE expresses several Six3-dependent orthologs of known canonical Wnt antagonists, and Six3 misexpression can suppress Wnt ligand transcription (Wei et al., 2009). Thus, canonical Wnt signaling and Six3 affect ANE development in reciprocal ways: misexpression of stabilized β-catenin or loss of Six3 eliminates nerves, whereas misexpression of Six3 or loss of canonical Wnt signaling greatly expands the ANE and increases the number of nerves (Wei et al., 2009; Yaguchi et al., 2006). These studies suggest that the balance between Six3-dependent and canonical Wnt-dependent processes regulates the size of the ANE domain (Fig. 2C).
Nodal and BMP2/4 signaling position the CBE
The CB is specified as a 4- to 5-cell-wide strip of cells that is carved out, in part, by converting regions of the dorsal and ventral ectoderm to an epidermal fate (Duboc et al., 2008; Duboc et al., 2004; Lapraz et al., 2009; Saudemont et al., 2010). The earliest molecular evidence of CB formation is the expression of the transcription factor Hnf6 (or onecut) within the presumptive CB during mesenchyme blastula stages (Otim et al., 2004; Poustka et al., 2004), several hours after Nodal and BMP2/4 signaling have begun, as indicated by phospho-Smad2/3 and phospho-Smad1/5 accumulation, respectively (Bergeron et al., 2010; Lapraz et al., 2009; Yaguchi et al., 2007). When Nodal (and consequently BMP2/4) signals are eliminated, the expression domain of Hnf6 and those of many other genes that are normally restricted to the CB expand into the presumptive ventral and dorsal ectoderm (Bradham et al., 2009; Duboc et al., 2004; Yaguchi et al., 2010a; Yaguchi et al., 2006; Saudemont et al., 2010), suggesting that the regulatory state in the absence of TGFβ signaling supports a CB-like fate, as originally proposed by Lepage and colleagues (Duboc et al., 2004). Similarly, when only BMP2/4 signaling is eliminated, the expression of CBE markers expands into the dorsal ectoderm, but not into the ventral ectoderm where Nodal signaling remains active (Lapraz et al., 2009; Yaguchi et al., 2010a; Saudemont et al., 2010). The altered expression of CBE markers in response to these perturbations is not observed in the most posterior ectoderm adjacent to the blastopore (Saudemont et al., 2010; Yaguchi et al., 2010a). Because this region of ectoderm derives from posterior blastomeres, in which canonical Wnt signaling is active during cleavage stages, it is likely to be regulated, at least in part, by distinct signals.
The Nodal and BMP signaling antagonists Lefty and Chordin are thought to prevent conversion of the CBE to epidermal fates. In Lefty morphants, Nodal expression expands, ventralizing the embryo and eliminating the CBE except in the ANE (Duboc et al., 2008; Saudemont et al., 2010; Yaguchi et al., 2010a). Conversely, in Lefty-misexpressing embryos, the CBE is radialized, as is also observed in Nodal morphants. In Chordin morphants, the secondary axis patterning mechanism is also compromised because elevated, ectopic BMP2/4 signaling on the ventral side interferes with Nodal expression, causing an expanded CBE phenotype (Lapraz et al., 2009; Saudemont et al., 2010). By contrast, in Chordin-misexpressing embryos, as in embryos lacking BMP signaling (Lapraz et al., 2009; Yaguchi et al., 2010a), the CBE expands to the dorsal side of the embryo. Thus, Nodal and BMP inhibit CBE development, whereas Lefty and Chordin support it by inhibiting the inhibitors of CBE development. An interesting, but yet unanswered, question is how these diffusible signals and antagonists precisely control the position and the width of the CB in the normal embryo.
In summary, the ANE is established first at the animal pole during cleavage stages by a balance of opposing canonical Wnt-dependent and Six3-dependent processes, whereas the CB is established later at the border between the ventral and dorsal ectoderm by factors that antagonize Nodal and BMP signaling. In the sea urchin embryo these territories are created at separate times and places by distinct mechanisms – properties that have facilitated dissection of the mechanisms driving the development of each.
Coordination of ANE and CBE positioning mechanisms
The canonical Wnt signaling mechanism that positions the ANE is distinct from the TGFβ-based mechanism that positions the CBE, but these mechanisms are connected in a very interesting way. When canonical Wnt signaling is blocked, secondary axis polarity is also lost. As shown previously (Yaguchi et al., 2008), the loss of this polarity results from a disruption in Nodal autoregulatory amplification, a key process operating at the top of the secondary axis regulatory system. Surprisingly, this blockade requires FoxQ2, a transcription factor involved in ANE development (Fig. 2D), which is initially expressed throughout the anterior half of the cleavage-stage embryo and is then progressively restricted to the ANE. In embryos lacking canonical Wnt signaling, FoxQ2 continues to be expressed ectopically throughout the expanded ANE. If it is also removed from these embryos, nodal expression and secondary patterning are restored, allowing the formation of a CB (Yaguchi et al., 2008). This double-repression mechanism of canonical Wnt signaling downregulating FoxQ2, which suppresses nodal, ensures that, in the absence of any embryonic signaling, the ANE fate is downregulated in posterior ectoderm precursors before they are subject to secondary axis patterning. In the normal embryo, FoxQ2 retains its ability to suppress Nodal autoregulation in the ANE, thereby helping to maintain its neuroectodermal fate.
Nervous system development within the ANE and CBE territories
The specification of the localized neuroectoderm territories, the ANE and CBE, is completed in the blastula stages (18-27 hours). Neuronal precursors are present during late blastula and gastrula stages and appear to differentiate and become functional in early larval stages (55-96 hours). As in flies and vertebrates, the regulation of neuronal differentiation within the sea urchin ANE and CBE territories involves Delta-Notch-mediated lateral inhibition (Wei et al., 2011). Not all of the cell types within the ANE and CBE have been defined. The ANE contains serotonergic and non-serotonergic neurons that express Synaptotagmin B and some cells that produce long, immotile cilia (Hörstadius, 1939), which might have sensory function, whereas the CBE is predominantly composed of specialized ciliated cells and functions as a swimming and feeding organ (Strathmann, 1975).
Nerves within the ANE
The ANE territory is currently thought to consist of two parts: a central disk called the animal plate and a surrounding torus of cells. The first nerves to form are the serotonergic neurons, which appear after gastrulation is complete (Bisgrove and Burke, 1986; Yaguchi et al., 2000) (Fig. 3A, hatched green ovals). Serotonergic neurons are restricted to the dorsal margin of the animal plate as a consequence of Nodal-mediated suppression of their development on the ventral side (Yaguchi et al., 2007) (Fig. 2B, Fig. 3B). Later, additional non-serotonergic nerves expressing Synaptotagmin B (Burke et al., 2006) appear around the animal plate and form axons and dendrites.
Fig. 3.
The structure of neuroectodermal territories. Ventral/posterior (A) and lateral (B) views of a three-day old sea urchin larva. At this stage, the ANE is composed of the animal plate (red) and a surrounding torus of cells. Within the animal plate are cells with long, immotile cilia that constitute the apical tuft (black lines in B). Serotonergic neurons (hatched green ovals) develop at the dorsal edge of the animal plate. Neural precursors in the region of the ciliary band are indicated by pink ovals. The positions of the mouth (m) and anus (a) are indicated.
The expression of ANE marker genes during blastula stages indicates that the anterior ectoderm is already molecularly patterned during gastrulation. At the mesenchyme blastula/early gastrula stage, six3 transcripts accumulate primarily at the edge of the animal plate where serotonergic and other non-serotonergic neurons will develop, whereas foxQ2 is expressed in the central animal plate region. Six3 is necessary for development of the thickened columnar epithelial structure of the ANE and for the development of all neurons that develop there (Wei et al., 2009). FoxQ2 is also necessary for serotonergic neuron development, as well as for the expression of the transcription factor Nk2.1 (Yaguchi et al., 2008), which in turn controls the expression of a novel, ankryn repeat-containing protein (AnkAT) that is required for the formation of apical tuft cilia (Dunn et al., 2007; Yaguchi et al., 2010b). At the late mesenchyme blastula stage, cells expressing delta appear in the animal plate (Lapraz et al., 2009; Rottinger et al., 2006; Saudemont et al., 2010; Walton et al., 2006) and neuron number is controlled by lateral inhibition (Wei et al., 2011). All animal plate cells express Hnf6 (Poustka et al., 2004; Yaguchi et al., 2006) and neuronal β3-tubulin (Duboc et al., 2004; Casano et al., 1996). Thus, the ANE is specified by Six3-dependent regulatory factors and behaves like a neural epithelium, with many cells having neural potential.
Nerves within the CB
The CBE also contains scattered delta-expressing cells and cells that express orthologs of genes that encode neural markers in other embryos (Saudemont et al., 2010). Several groups of cells first appear during gastrulation near the CB, beneath the ventral ectoderm and the lateral sections of the CB, inside the dorsal ectoderm (Fig. 3A). Subsequently, ∼40-50 regularly spaced neural cell bodies connected by processes form a highly structured chain along the ventral side of the CB (Fig. 1C, Fig. 3B). Axons of these neurons are either bundled in the central tract or they project towards and beneath the dorsal ectoderm (Nakajima et al., 2004) (Fig. 1, Fig. 3B). How these CB nerves connect to each other or to nerves in the ANE is unknown. In addition, how the early CB-associated neurons respond to TGFβ signals is unclear, but in the absence of these signals the scattered nerves that develop within the expanded CBE do not form interconnecting bundled axonal tracts (Yaguchi et al., 2010a). In addition, Nodal and BMP signals might play a later role in regulating neural identity, as has been found in insects and vertebrates (Mizutani et al., 2006; Rusten et al., 2002).
The distribution of nerves within the CBE is controlled by the positioning of the CBE, as shown by Nodal and BMP2/4 perturbations that alter the position, size and shape of the CBE (Bradham et al., 2009; Lapraz et al., 2009; Saudemont et al., 2010; Yaguchi et al., 2010a). The observation that ectopic neurons produced under a variety of experimental conditions [e.g. in Nodal morphants or following inhibition of BMP signaling (Saudemont et al., 2010; Yaguchi et al., 2010a)] remain largely confined to the CBE strongly suggests that the CBE provides an environment conducive to neural differentiation. When the Nodal or BMP2/4 signaling pathways are blocked by interfering with components that function cell-autonomously (e.g. their receptors and Smads), the distribution, but not the number, of nerves changes in proportion to the size of the CBE. This implies that TGFβ signaling primarily controls the size and position of the CBE, which has neurogenic potential, but not the number of nerves that develop within it. In addition, TGFβ signaling appears to regulate the structure and connectivity of CB neurons (Yaguchi et al., 2010a). These signaling mechanisms appear to be the same as those used by chordate embryos to shape their neuroectoderm territories.
A four-step model for nervous system development in sea urchin embryos
Based on the studies discussed above, a four-step model for patterning the nervous system of sea urchin embryos is presented in Fig. 4 and is summarized below.
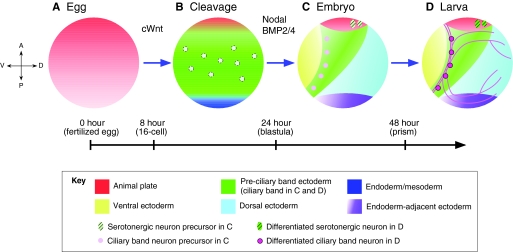
Fig. 4.
A four-step model for specification and organization of the sea urchin embryo nervous system. (A) In the first step, an anterior neuroectoderm regulatory state (red) is present throughout the egg and much of the embryo during early cleavage stages. (B) In the second step, which occurs during very early blastula stages, this state is eliminated by canonical Wnt (cWnt)-dependent signals from all but the anterior neuroectoderm, revealing a ciliary band-like neuroectoderm (green) that contains scattered neural precursors (light pink circles). (C) In the third step, which occurs during the mesenchyme blastula/early gastrula stages, Nodal and BMP2/4 signals convert ventral and dorsal ectoderm to non-neural ectoderm except in the anterior neuroectoderm (red) and ciliary band (green), which are protected from these signals. (D) During the fourth and final step, by which point the embryo has transitioned into a larva, CBE and ANE neural progenitors differentiate. The timeline indicates hours post-fertilization and embryonic stages.
First, zygotic gene expression establishes a pre-ANE gene regulatory state throughout the embryonic ectoderm. Initially, the early ectoderm displays an ANE bias established by the early broad zygotic transcription of six3 and other genes in the ANE gene regulatory network. Second, canonical Wnt-mediated signaling from posterior blastomeres downregulates the expression of six3, foxQ2 and other members of the ANE regulatory network, progressively restricting the expression of these genes to the definitive ANE domain. An additional consequence is that FoxQ2 is removed from all but the anterior ectoderm, allowing nodal autoregulation to occur. Third, Nodal upregulation activates the secondary axial patterning mechanism and, together with BMP, suppresses neurogenesis in the ventral and dorsal ectoderm, thus respecifying these tissues as epidermis. A strip of cells between these regions retains the early neuroectoderm specification of the CBE, presumably through the activities of Lefty and Chordin, which suppress Nodal and BMP2/4 function in this region. Fourth, nerves develop in the ANE and CBE, regions that are protected from canonical Wnt and TGFβ signals.
The two themes of this model are that the embryo starts with two regulatory layers of broad overlapping neurogenic potential (the ANE and the CBE), each of which is sequentially downregulated by successive and distinct signals. Protection from signaling appears to be the principal mechanism that results in restricted regions that differentiate as neuroepithelia.
Deuterostome neural system patterning: shared mechanisms
The pre-signaling neuroectodermal regulatory states of early embryonic cells and the signaling mechanisms that specify neural versus non-neural fates in the sea urchin are remarkably similar to those observed in other deuterostome embryos. The highly ordered localized arrangement of nerves in sea urchin embryos strongly reinforces the recent findings that hemichordate nervous systems are more centralized (Nomaksteinsky et al., 2009) than previously thought (Lowe et al., 2003). Centralization mechanisms also exist in the protostome (see Glossary, Box 1) polychaete annelid Platynereis dumerilii (Denes et al., 2007), supporting their existence in the urbilaterian ancestor (see Glossary, Box 1). Below, we highlight the mechanisms of neural patterning that are shared among deuterostomes.
Patterning of the nervous system along the AP axis of deuterostomes employs an ancient Wnt-based set of signals
Embryonic cells become anterior neuroectoderm unless instructed otherwise
In the absence of all known zygotic signaling (i.e. in the absence of canonical Wnt), virtually the entire sea urchin embryo assumes an ANE fate (Yaguchi et al., 2006). Similarly, in Xenopus embryos, when early signals that promote non-neural ectodermal fates are eliminated, the entire embryo expresses Sox2 (Reversade and De Robertis, 2005; Reversade et al., 2005), a transcription factor that supports the neural precursor state in mouse embryos (Bylund et al., 2003; Graham et al., 2003). Other anterior neural markers are expressed in the early medial epiblast cells of chick embryos (reviewed by Wilson and Houart, 2004), in the epiblast of mouse embryos (reviewed by Levine and Brivanlou, 2007) and in mouse embryonic stem cells in the absence of signals that promote non-neural ectodermal fates (Tropepe et al., 2001; Vallier et al., 2004). Thus, it is likely that in vertebrates, as in sea urchin embryos, ANE fate is not induced but rather is driven by early maternal regulatory factors.
Wnt signaling defines the posterior pole and restricts the ANE to the opposite pole
As discussed recently by others (Niehrs, 2010; Petersen and Reddien, 2009), canonical Wnt signaling is an ancient function that operates at the posterior end of nearly all bilaterian embryos. Similarly, in sea urchin embryos a gradient of nuclear β-catenin emanates from posterior blastomeres during cleavage stages and is necessary to specify mesoderm and endoderm (Logan et al., 1999; Wikramanayake et al., 1998). At the opposite end of vertebrate and sea urchin embryos, the tissues that develop are of anterior character. Whereas suppression of canonical Wnt signaling anteriorizes all three germ layers in vertebrates, this process is known thus far to affect only the ectoderm in sea urchin embryos, as blocking canonical Wnt completely abrogates endomesoderm development. Thus, since the ectoderm at the anterior end of sea urchin embryos has many properties of anterior neuroectoderm, and as canonical Wnt signaling functions at the opposite end in these embryos, the primary axis of the sea urchin resembles the AP axis of other embryos.
The canonical Wnt-dependent remodeling of a broad neuroectoderm territory is an ancient function. In sea urchin embryos, it restricts FoxQ2 and the apical tuft, which depends on FoxQ2 (Yaguchi et al., 2010b), to the anterior end of the embryo. The same canonical Wnt-mediated restriction has recently been described in Saccoglossus (Darras et al., 2011). FoxQ2 is also expressed at the anterior end of the embryo of the cephalochordate (see Glossary, Box 1) amphioxus (Branchiostoma floridae) (Yu et al., 2002), raising the possibility that a similar mechanism also operates in these organisms. Amazingly, this process also occurs in the hydrozoan cnidarian Clytia hemisphaerica: FoxQ2 is expressed where the apical tuft forms, at the pole opposite to canonical Wnt signaling (Momose et al., 2008), which is where gastrulation occurs (Wikramanayake et al., 2003). Further, as in sea urchin embryos, the size of the FoxQ2 expression domain in Clytia is regulated by canonical Wnt signaling (Momose et al., 2008). This indicates that canonical Wnt-restricted anterior expression of FoxQ2 predates the cnidarian-bilaterian split.
The canonical Wnt-dependent restriction of expression of other genes, such as six3, to the ANE of sea urchin embryos or to the anterior neural plate and forebrain of vertebrate embryos is conserved (Braun et al., 2003; Lagutin et al., 2003; Wei et al., 2009). Furthermore, an AP gradient of nuclear β-catenin has been demonstrated in the presumptive Xenopus neural plate ectoderm during gastrulation, around the time when the AP and DV axes begin to separate (Kiecker and Niehrs, 2001). Thus, anterior neuroectoderm fate requires low levels of canonical Wnt signaling in both sea urchin and vertebrate embryos.
Roles of canonical Wnt and TGFβ signaling in positioning anterior neuroectoderm
In the sea urchin embryo, canonical Wnt-mediated suppression of ANE fate occurs in non-ANE ectoderm during cleavage stages by a mechanism that has not yet been defined. It requires neither Nodal nor BMP2/4, nor their antagonists (Wei et al., 2009). When canonical Wnt signaling is blocked, the entire embryo assumes ANE fate. By contrast, in vertebrate embryos, canonical Wnt signaling results in localized production of BMP signaling antagonists, which protect the neuroectoderm regulatory state in the dorsal ectoderm. Thus, when canonical Wnt signaling is blocked in vertebrate embryos, they completely lack neuroectoderm and are strongly ventralized (Heasman et al., 2000). In addition, canonical Wnt signal activity posteriorizes the neuroectoderm except where it is protected by Wnt antagonists (reviewed by Yamaguchi, 2001; Houart et al., 2002). These two processes, canonical Wnt-dependent creation of a neural plate by antagonizing BMP signaling and posteriorization by high levels of canonical Wnt activity in posterior regions of the neural plate, occur during gastrulation. If both BMP signaling and canonical Wnt signaling are blocked, then the underlying neuroectoderm regulatory state can be detected and it is anterior in character, as posteriorizing signals are lacking (see Reversade et al., 2005). Thus, in both vertebrate and sea urchin embryos, there is a basal, probably maternally driven, ANE regulatory state that is restricted to anterior ectoderm regions by canonical Wnt signaling. Where the differences lie is in the regulatory relationships between canonical Wnt and TGFβ signaling activities. In sea urchin embryos, the production of Nodal and BMP2/4 (and their respective antagonists) depends on canonical Wnt signaling, whereas in the vertebrate embryo this is only the case for the BMP antagonists. Furthermore, in sea urchin embryos, the creation of the more posterior CBE via TGFβ signaling depends on the prior restriction of the ANE to the anterior end of the embryo, whereas in vertebrate embryos these are independent events that may occur at approximately the same time. Thus, the development of non-neural and neural ectoderm territories in sea urchin and vertebrate embryos make use of the same signaling pathways, but these are deployed differently as a result of the different regulatory linkages among them.
Some regulatory properties of the sea urchin embryo ANE are conserved
The ANE regulatory properties of the sea urchin embryo are similar to those described for mouse embryos. In both mouse and sea urchins, Six3 functions near the top of the ANE regulatory networks (Lagutin et al., 2003; Lavado et al., 2008; Wei et al., 2009). Furthermore, many of the same transcription factors, including, Rx, Achaete-Scute, Zic2, Ebf3, Fez, Nkx2.1, SoxC and the Notch ligand Delta (Wei et al., 2009) are expressed in the ANE of both mouse and sea urchin embryos. Mutual antagonism between Six3 and canonical Wnt signals operates in both systems. The sea urchin ANE is likely to be protected from canonical Wnt signals by Six3-dependent Wnt antagonists, such as Dkk1, just as the zebrafish telencephalon is protected by a secreted frizzled-related protein, a Wnt antagonist called Tlc (Houart et al., 2002).
Many of the transcription factors expressed in the sea urchin embryo ANE (Kenny et al., 2003; Wei et al., 2009) are also expressed in the ectodermal layer of the cnidarian Nematostella vectensis [e.g. six3, hbn, rx, otx, soxb1, soxb2 (Marlow et al., 2009)]. Some of these are expressed in patterns similar to those observed in the sea urchin embryo ANE, consistent with the intriguing possibility that the cnidarian ectoderm is similar to the sea urchin ANE. Both regions resist respecification by canonical Wnt signaling and contain many neurons. An additional provocative hypothesis suggests that most of the body of Hydra, another cnidarian, gave rise to the heads of deuterostomes and protostomes (Meinhardt, 2002). This shared and ancient state of early ectoderm might thus be part of the default state of early, pre-signaling ectoderm in vertebrate embryos as well.
Nervous system positioning along the DV axes of deuterostomes relies on TGFβ signals
Much of the ectoderm adopts a CBE fate unless instructed otherwise by Nodal and BMP2/4
Nodal and BMP2/4 carve out the CB by diverting ectoderm on the ventral and dorsal sides of the embryo to a non-neural epidermal fate. This process shares some properties with neuroectoderm patterning mechanisms along the DV axes of other bilaterian embryos. It operates along an axis orthogonal to the canonical Wnt system, which specifies cell fates along the AP axis. Although nodal, lefty, bmp2/4 and chordin are all transcribed on the ventral side of the sea urchin embryo, BMP2/4 signaling occurs only on the side opposite to that of nodal and chordin expression, as is seen in vertebrate and arthropod embryos. As discussed above, most of the widespread latent bias toward neuroectoderm and neural structures in vertebrate embryos is suppressed by early BMP signaling and maintained where antagonistic activities prevent this signaling. In addition, fibroblast growth factor (FGF) signaling and inhibition of canonical Wnt signaling promote neural development by reducing the half-life of the BMP effector protein Smad (Fuentealba et al., 2007), and, in Xenopus embryos, this is required in addition to secreted antagonists for neural development (Pera et al., 2003). Although it is clear that downregulation of BMP2/4 signals is important for neuroectoderm development in the sea urchin embryo, whether FGF signaling also plays a role in this process is not yet known.
Although antagonism of BMP signaling is considered to be the primary mechanism that prevents ectoderm from differentiating as epidermis in higher deuterostomes, Nodal signaling also overrides an early neuroectoderm regulatory state. In Xenopus, continued suppression of effectors downstream of both Nodal and BMP signaling is required for neural induction (Chang and Harland, 2007), and, in zebrafish and mice, neural fates have been shown to emerge in presumptive endomesoderm cells in the absence Nodal signaling (Camus et al., 2006; Feldman et al., 2000; Schier and Talbot, 2001).
DV axis patterning mechanisms are connected to different upstream polarizing mechanisms
Although the antagonism of TGFβ signaling is employed in positioning the nervous system in virtually all cases that have been examined, this process is connected to distinctly different initial polarizing mechanisms, and the timing and manner in which these mechanisms are utilized are highly variable. For example, DV axial specification in Xenopus is provided by the sperm entry point and by cytoplasmic rotation (Moon and Kimelman, 1998). In flies, it depends on a gradient of Dorsal activity controlled by follicle cell activities (Steward and Govind, 1993), whereas in sea urchins redox asymmetry is thought to be involved (Coffman et al., 2009; Coffman et al., 2004; Steward and Govind, 1993). The sea urchin embryo provides an especially clear example of that connection because both the establishment of the nervous system and the specification of cell fates along the secondary axis can be directly traced to a single event: the activation of Nodal in the presumptive ventral ectoderm, possibly dependent on the mitochondrial gradient in the egg. The fact that Nodal controls the expression of BMP2/4, and that Nodal and BMP2/4 then play repressive roles in positioning the nervous system, make Nodal a key regulator of this process in the sea urchin embryo.
Patterns of Nodal expression during evolution
The nodal gene appeared sometime after the cnidarian-bilaterian split in the common ancestor of protostomes and deuterostomes, as it is expressed in echinoderms and in snails (Grande and Patel, 2009). In snails, it functions to specify right-sidedness, assuming the mouth is ventral. This, along with the fact that BMP2/4 signaling in sea urchins and BMP2/4 expression in hemichordates is dorsal (Lowe et al., 2006), suggest that the positioning of major parts of the nervous system in basal deuterostomes should be ventral. This is not the case in sea urchin embryos, largely because Nodal specifies that region as non-neural ventral ectoderm. During deuterostome evolution the position of Nodal signaling and its relative importance in nervous system positioning have changed. In vertebrate embryos, the primary function of Nodal is in specifying mesoderm and endoderm rather than ectoderm, whereas in the cephalochordate amphioxus (Onai et al., 2010; Yu et al., 2007) it supports dorsal/anterior fates in both mesoderm and ectoderm (Onai et al., 2010). Although the wide variations in the patterns of Nodal expression in the ectoderm among deuterostomes make it impossible to clearly identify ancestral versus derived traits, studies on Nodal function in sea urchin embryos demonstrate that it plays a crucial role in determining non-neural fates and, as a consequence, the organization of the nervous system.
Conclusions
In sea urchin embryos, the positioning of the ANE and the CBE within a broad neuroectoderm territory is controlled by successive primary and secondary axis patterning mechanisms. The regulatory links between these signaling mechanisms allow their functions to be separated in time – Nodal expression does not become established until the ANE is restricted to the anterior end of the embryo by canonical Wnt-dependent processes, and BMP2/4 expression does not begin without Nodal. By contrast, the regulatory scheme in vertebrate embryos compresses these events temporally and spatially, in part because BMP expression initially occurs broadly and independently of canonical Wnt and Nodal. As a result, the positioning of the nervous system dorsally in higher deuterostomes or ventrally in protostomes depends heavily on the localized production of activities that suppress BMP signaling. Although the signals and their antagonists are the same, the regulatory connections between the Wnt and BMP pathways are different in the embryos of sea urchins and vertebrates, yielding distinct neural versus non-neural patterns. The neural patterning mechanisms in the common ancestor of echinoderms and other deuterostomes cannot be determined, but a clearer understanding of the evolution of extant deuterostomes can be obtained by detailed comparisons of diverse embryos. Importantly, it is becoming evident that to understand their role in neural patterning we must not simply determine the expression patterns of Wnt and TGFβ signaling pathway components, but also test their regulatory relationships, as has been done in the sea urchin embryo.
Acknowledgments
We thank Adi Sethi, Diane Adams and Ryan Range for discussions and especially Zheng Wei for experimental and bioinformatic contributions. Support for this work was provided by the Division of Intramural Research in the National Institute for Dental and Craniofacial Research, National Institutes of Health (L.M.A. and R.C.A.), Special Coordination Funds for Promoting Science and Technology of the Ministry of Education, Culture, Sports, Science and Technology of the Japanese Government (MEXT), by a Grant-in Aid for Young Scientists (Start-up) (S.Y.) and NSERC (R.D.B.). Deposited in PMC for release after 12 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- Angerer L. M., Oleksyn D. W., Logan C. Y., McClay D. R., Dale L., Angerer R. C. (2000). A BMP pathway regulates cell fate allocation along the sea urchin animal-vegetal embryonic axis. Development 127, 1105-1114 [DOI] [PubMed] [Google Scholar]
- Angerer L. M., Oleksyn D. W., Levine A. M., Li X., Klein W. H., Angerer R. C. (2001). Sea urchin goosecoid function links fate specification along the animal-vegetal and oral-aboral embryonic axes. Development 128, 4393-4404 [DOI] [PubMed] [Google Scholar]
- Arendt D., Denes A. S., Jekely G., Tessmar-Raible K. (2008). The evolution of nervous system centralization. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 1523-1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron K. F., Xu X., Brandhorst B. P. (2010). Oral-aboral patterning and gastrulation of sea urchin embryos depend on sulfated glycosaminoglycans. Mech. Dev. 128, 71-89 [DOI] [PubMed] [Google Scholar]
- Bisgrove B. W., Burke R. D. (1986). Development of serotongic neurons in embryos of the sea urchin, Strongylocentrotus purpuratus. Dev. Growth Differ. 28, 569-574 [DOI] [PubMed] [Google Scholar]
- Bolouri H., Davidson E. H. (2009). The gene regulatory network basis of the `community effect', and analysis of a sea urchin embryo example. Dev. Biol. 340, 170-178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradham C. A., McClay D. R. (2006). p38 MAPK is essential for secondary axis specification and patterning in sea urchin embryos. Development 133, 21-32 [DOI] [PubMed] [Google Scholar]
- Bradham C. A., Oikonomou C., Kuhn A., Core A. B., Modell J. W., McClay D. R., Poustka A. J. (2009). Chordin is required for neural but not axial development in sea urchin embryos. Dev. Biol. 328, 221-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M. M., Etheridge A., Bernard A., Robertson C. P., Roelink H. (2003). Wnt signaling is required at distinct stages of development for the induction of the posterior forebrain. Development 130, 5579-5587 [DOI] [PubMed] [Google Scholar]
- Bullock T., Horridge G. (1965). Hemichordata. Structure and Function of the Nervous System of Invertebrates, pp. 1567-1577 San Francisco: W. H. Freeman; [Google Scholar]
- Burke R. D., Osborne L., Wang D., Murabe N., Yaguchi S., Nakajima Y. (2006). Neuron-specific expression of a synaptotagmin gene in the sea urchin Strongylocentrotus purpuratus. J. Comp. Neurol. 496, 244-251 [DOI] [PubMed] [Google Scholar]
- Bylund M., Andersson E., Novitch B. G., Muhr J. (2003). Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat. Neurosci. 6, 1162-1168 [DOI] [PubMed] [Google Scholar]
- Byrum C. A., Xu R., Bince J. M., McClay D. R., Wikramanayake A. H. (2009). Blocking Dishevelled signaling in the noncanonical Wnt pathway in sea urchins disrupts endoderm formation and spiculogenesis, but not secondary mesoderm formation. Dev. Dyn. 238, 1649-1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camus A., Perea-Gomez A., Moreau A., Collignon J. (2006). Absence of Nodal signaling promotes precocious neural differentiation in the mouse embryo. Dev. Biol. 295, 743-755 [DOI] [PubMed] [Google Scholar]
- Casano C., Ragusa M., Cutrera M., Costa S., Gianguzza F. (1996). Spatial expression of alpha and beta tubulin genes in the late embryogenesis of the sea urchin Paracentrotus lividus. Int. J. Dev. Biol. 40, 1033-1041 [PubMed] [Google Scholar]
- Chang C., Harland R. M. (2007). Neural induction requires continued suppression of both Smad1 and Smad2 signals during gastrulation. Development 134, 3861-3872 [DOI] [PubMed] [Google Scholar]
- Coffman J. A., McCarthy J. J., Dickey-Sims C., Robertson A. J. (2004). Oral-aboral axis specification in the sea urchin embryo. II. Mitochondrial distribution and redox state contribute to establishing polarity in Strongylocentrotus purpuratus. Dev. Biol. 273, 160-171 [DOI] [PubMed] [Google Scholar]
- Coffman J. A., Coluccio A., Planchart A., Robertson A. J. (2009). Oral-aboral axis specification in the sea urchin embryo III. Role of mitochondrial redox signaling via H2O2. Dev. Biol. 330, 123-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darras S., Gerhart J., Terasaki M., Kirschner M., Lowe C. J. (2011). {beta}-Catenin specifies the endomesoderm and defines the posterior organizer of the hemichordate Saccoglossus kowalevskii. Development 138, 959-970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson E. H. (2009). Network design principles from the sea urchin embryo. Curr. Opin. Genet. Dev. 19, 535-540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson E. H., Rast J. P., Oliveri P., Ransick A., Calestani C., Yuh C. H., Minokawa T., Amore G., Hinman V., Arenas-Mena C., et al. (2002). A provisional regulatory gene network for specification of endomesoderm in the sea urchin embryo. Dev. Biol. 246, 162-190 [DOI] [PubMed] [Google Scholar]
- De Robertis E. M., Sasai Y. (1996). A common plan for dorsoventral patterning in Bilateria. Nature 380, 37-40 [DOI] [PubMed] [Google Scholar]
- Denes A. S., Jekely G., Steinmetz P. R., Raible F., Snyman H., Prud'homme B., Ferrier D. E., Balavoine G., Arendt D. (2007). Molecular architecture of annelid nerve cord supports common origin of nervous system centralization in bilateria. Cell 129, 277-288 [DOI] [PubMed] [Google Scholar]
- Duboc V., Lepage T. (2006). A conserved role for the nodal signaling pathway in the establishment of dorso-ventral and left-right axes in deuterostomes. J. Exp. Zool. B Mol. Dev. Evol. 310, 41-53 [DOI] [PubMed] [Google Scholar]
- Duboc V., Rottinger E., Besnardeau L., Lepage T. (2004). Nodal and BMP2/4 signaling organizes the oral-aboral axis of the sea urchin embryo. Dev. Cell 6, 397-410 [DOI] [PubMed] [Google Scholar]
- Duboc V., Lapraz F., Besnardeau L., Lepage T. (2008). Lefty acts as an essential modulator of Nodal activity during sea urchin oral-aboral axis formation. Dev. Biol. 320, 49-59 [DOI] [PubMed] [Google Scholar]
- Dunn E. F., Moy V. N., Angerer L. M., Angerer R. C., Morris R. L., Peterson K. J. (2007). Molecular paleoecology: using gene regulatory analysis to address the origins of complex life cycles in the late Precambrian. Evol. Dev. 9, 10-24 [DOI] [PubMed] [Google Scholar]
- Feldman B., Dougan S. T., Schier A. F., Talbot W. S. (2000). Nodal-related signals establish mesendodermal fate and trunk neural identity in zebrafish. Curr. Biol. 10, 531-534 [DOI] [PubMed] [Google Scholar]
- Fuentealba L. C., Eivers E., Ikeda A., Hurtado C., Kuroda H., Pera E. M., De Robertis E. M. (2007). Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell 131, 980-993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart J. (2000). Inversion of the chordate body axis: are there alternatives? Proc. Natl. Acad. Sci. USA 97, 4445-4448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham V., Khudyakov J., Ellis P., Pevny L. (2003). SOX2 functions to maintain neural progenitor identity. Neuron 39, 749-765 [DOI] [PubMed] [Google Scholar]
- Grande C., Patel N. H. (2009). Nodal signalling is involved in left-right asymmetry in snails. Nature 457, 1007-1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman J., Kofron M., Wylie C. (2000). Beta-catenin signaling activity dissected in the early Xenopus embryo: a novel antisense approach. Dev. Biol. 222, 124-134 [DOI] [PubMed] [Google Scholar]
- Hörstadius S. (1939). The mechanics of sea urchin development, studied by operative methods. Biol. Rev. Camb. Philos. Soc. 14, 48 [Google Scholar]
- Hörstadius S. (1973). Experimental Embryology of Echinoderms. Oxford: Clarendon Press; [Google Scholar]
- Houart C., Caneparo L., Heisenberg C., Barth K., Take-Uchi M., Wilson S. (2002). Establishment of the telencephalon during gastrulation by local antagonism of Wnt signaling. Neuron 35, 255-265 [DOI] [PubMed] [Google Scholar]
- Kenny A. P., Oleksyn D. W., Newman L. A., Angerer R. C., Angerer L. M. (2003). Tight regulation of SpSoxB factors is required for patterning and morphogenesis in sea urchin embryos. Dev. Biol. 261, 412-425 [DOI] [PubMed] [Google Scholar]
- Kiecker C., Niehrs C. (2001). A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development 128, 4189-4201 [DOI] [PubMed] [Google Scholar]
- Knight-Jones E. (1952). On the nervous system of Saccoglossus cambrensis (Enteropneusta). Philos. Trans. R. Soc. Lond. B Biol. Sci. 236, 315-354 [Google Scholar]
- Lagutin O. V., Zhu C. C., Kobayashi D., Topczewski J., Shimamura K., Puelles L., Russell H. R., McKinnon P. J., Solnica-Krezel L., Oliver G. (2003). Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes Dev. 17, 368-379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapraz F., Besnardeau L., Lepage T. (2009). Patterning of the dorsal-ventral axis in echinoderms: insights into the evolution of the BMP-chordin signaling network. PLoS Biol. 7, e1000248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavado A., Lagutin O. V., Oliver G. (2008). Six3 inactivation causes progressive caudalization and aberrant patterning of the mammalian diencephalon. Development 135, 441-450 [DOI] [PubMed] [Google Scholar]
- Leonard J. D., Ettensohn C. A. (2007). Analysis of dishevelled localization and function in the early sea urchin embryo. Dev. Biol. 306, 50-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A. J., Brivanlou A. H. (2007). Proposal of a model of mammalian neural induction. Dev. Biol. 308, 247-256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan C. Y., Miller J. R., Ferkowicz M. J., McClay D. R. (1999). Nuclear beta-catenin is required to specify vegetal cell fates in the sea urchin embryo. Development 126, 345-357 [DOI] [PubMed] [Google Scholar]
- Lowe C. J., Wu M., Salic A., Evans L., Lander E., Stange-Thomann N., Gruber C. E., Gerhart J., Kirschner M. (2003). Anteroposterior patterning in hemichordates and the origins of the chordate nervous system. Cell 113, 853-865 [DOI] [PubMed] [Google Scholar]
- Lowe C. J., Terasaki M., Wu M., Freeman R. M., Jr, Runft L., Kwan K., Haigo S., Aronowicz J., Lander E., Gruber C., et al. (2006). Dorsoventral patterning in hemichordates: insights into early chordate evolution. PLoS Biol. 4, e291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow H. Q., Srivastava M., Matus D. Q., Rokhsar D., Martindale M. Q. (2009). Anatomy and development of the nervous system of Nematostella vectensis, an anthozoan cnidarian. Dev. Neurobiol. 69, 235-254 [DOI] [PubMed] [Google Scholar]
- Meinhardt H. (2002). The radial-symmetric hydra and the evolution of the bilateral body plan: an old body became a young brain. BioEssays 24, 185-191 [DOI] [PubMed] [Google Scholar]
- Meno C., Saijoh Y., Fujii H., Ikeda M., Yokoyama T., Yokoyama M., Toyoda Y., Hamada H. (1996). Left-right asymmetric expression of the TGF beta-family member lefty in mouse embryos. Nature 381, 151-155 [DOI] [PubMed] [Google Scholar]
- Minokawa T., Amemiya S. (1999). Timing of the potential of micromere-descendants in echinoid embryos to induce endoderm differentiation of mesomere-descendants. Dev. Growth Differ. 41, 535-547 [DOI] [PubMed] [Google Scholar]
- Minokawa T., Wikramanayake A. H., Davidson E. H. (2005). cis-Regulatory inputs of the wnt8 gene in the sea urchin endomesoderm network. Dev. Biol. 288, 545-558 [DOI] [PubMed] [Google Scholar]
- Mizutani C. M., Bier E. (2008). EvoD/Vo: the origins of BMP signaling in the neuroectoderm. Nat. Rev. Genet. 9, 663-677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani C. M., Meyer N., Roelink H., Bier E. (2006). Threshold-dependent BMP-mediated repression: a model for a conserved mechanism that patterns the neuroectoderm. PLoS Biol. 4, e313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momose T., Derelle R., Houliston E. (2008). A maternally localised Wnt ligand required for axial patterning in the cnidarian Clytia hemisphaerica. Development 135, 2105-2113 [DOI] [PubMed] [Google Scholar]
- Moon R. T., Kimelman D. (1998). From cortical rotation to organizer gene expression: toward a molecular explanation of axis specification in Xenopus. BioEssays 20, 536-545 [DOI] [PubMed] [Google Scholar]
- Nakajima Y., Kaneko H., Murray G., Burke R. D. (2004). Divergent patterns of neural development in larval echinoids and asteroids. Evol. Dev. 6, 95-104 [DOI] [PubMed] [Google Scholar]
- Nam J., Su Y. H., Lee P. Y., Robertson A. J., Coffman J. A., Davidson E. H. (2007). Cis-regulatory control of the nodal gene, initiator of the sea urchin oral ectoderm gene network. Dev. Biol. 306, 860-869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niehrs C. (2010). On growth and form: a Cartesian coordinate system of Wnt and BMP signaling specifies bilaterian body axes. Development 137, 845-857 [DOI] [PubMed] [Google Scholar]
- Nomaksteinsky M., Rottinger E., Dufour H. D., Chettouh Z., Lowe C. J., Martindale M. Q., Brunet J. F. (2009). Centralization of the deuterostome nervous system predates chordates. Curr. Biol. 19, 1264-1269 [DOI] [PubMed] [Google Scholar]
- Oliveri P., Davidson E. H., McClay D. R. (2003). Activation of pmar1 controls specification of micromeres in the sea urchin embryo. Dev. Biol. 258, 32-43 [DOI] [PubMed] [Google Scholar]
- Oliveri P., Tu Q., Davidson E. H. (2008). Global regulatory logic for specification of an embryonic cell lineage. Proc. Natl. Acad. Sci. USA 105, 5955-5962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onai T., Yu J. K., Blitz I. L., Cho K. W., Holland L. Z. (2010). Opposing Nodal/Vg1 and BMP signals mediate axial patterning in embryos of the basal chordate amphioxus. Dev. Biol. 344, 377-389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otim O., Amore G., Minokawa T., McClay D. R., Davidson E. H. (2004). SpHnf6, a transcription factor that executes multiple functions in sea urchin embryogenesis. Dev. Biol. 273, 226-243 [DOI] [PubMed] [Google Scholar]
- Pera E. M., Ikeda A., Eivers E., De Robertis E. M. (2003). Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes Dev. 17, 3023-3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C. P., Reddien P. W. (2009). Wnt signaling and the polarity of the primary body axis. Cell 139, 1056-1068 [DOI] [PubMed] [Google Scholar]
- Poustka A. J., Kuhn A., Radosavljevic V., Wellenreuther R., Lehrach H., Panopoulou G. (2004). On the origin of the chordate central nervous system: expression of onecut in the sea urchin embryo. Evol. Dev. 6, 227-236 [DOI] [PubMed] [Google Scholar]
- Range R., Lapraz F., Quirin M., Marro S., Besnardeau L., Lepage T. (2007). Cis-regulatory analysis of nodal and maternal control of dorsal-ventral axis formation by Univin, a TGF-{beta} related to Vg1. Development 134, 3649-3664 [DOI] [PubMed] [Google Scholar]
- Ransick A., Davidson E. H. (1995). Micromeres are required for normal vegetal plate specification in sea urchin embryos. Development 121, 3215-3222 [DOI] [PubMed] [Google Scholar]
- Reversade B., De Robertis E. M. (2005). Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell 123, 1147-1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reversade B., Kuroda H., Lee H., Mays A., De Robertis E. M. (2005). Depletion of Bmp2, Bmp4, Bmp7 and Spemann organizer signals induces massive brain formation in Xenopus embryos. Development 132, 3381-3392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottinger E., Croce J., Lhomond G., Besnardeau L., Gache C., Lepage T. (2006). Nemo-like kinase (NLK) acts downstream of Notch/Delta signalling to downregulate TCF during mesoderm induction in the sea urchin embryo. Development 133, 4341-4353 [DOI] [PubMed] [Google Scholar]
- Rusten T. E., Cantera R., Kafatos F. C., Barrio R. (2002). The role of TGF beta signaling in the formation of the dorsal nervous system is conserved between Drosophila and chordates. Development 129, 3575-3584 [DOI] [PubMed] [Google Scholar]
- Saudemont A., Emmanuel H., Mekpoh F., Bessodes N., Quirin M., Lapraz F., Duboc V., Rottinger E., Range R., Oisel A., et al. (2010). Gene regulatory network analysis in an echinoderm reveals ancestral regulatory circuits regulating ectoderm patterning and morphogenesis downstream of Nodal and BMP2/4. PLoS Genet. 6, e1001259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier A. F., Talbot W. S. (2001). Nodal signaling and the zebrafish organizer. Int. J. Dev. Biol. 45, 289-297 [PubMed] [Google Scholar]
- Sethi A. J., Angerer R. C., Angerer L. M. (2009). Gene regulatory network interactions in sea urchin endomesoderm induction. PLoS Biol. 7, e1000029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood D. R., McClay D. R. (2001). LvNotch signaling plays a dual role in regulating the position of the ectoderm-endoderm boundary in the sea urchin embryo. Development 128, 2221-2232 [DOI] [PubMed] [Google Scholar]
- Shimmi O., Umulis D., Othmer H., O'Connor M. B. (2005). Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo. Cell 120, 873-886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J., Theodoris C., Davidson E. H. (2007). A gene regulatory network subcircuit drives a dynamic pattern of gene expression. Science 318, 794-797 [DOI] [PubMed] [Google Scholar]
- Smith J., Kraemer E., Liu H., Theodoris C., Davidson E. (2008). A spatially dynamic cohort of regulatory genes in the endomesodermal gene network of the sea urchin embryo. Dev. Biol. 313, 863-875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward R., Govind S. (1993). Dorsal-ventral polarity in the Drosophila embryo. Curr. Opin. Genet. Dev. 3, 556-561 [DOI] [PubMed] [Google Scholar]
- Strathmann R. (1975). Larval feeding in echinoderms. Am. Zool. 15, 717-730 [Google Scholar]
- Sweet H. C., Gehring M., Ettensohn C. A. (2002). LvDelta is a mesoderm-inducing signal in the sea urchin embryo and can endow blastomeres with organizer-like properties. Development 129, 1945-1955 [DOI] [PubMed] [Google Scholar]
- Thisse C., Thisse B. (1999). Antivin, a novel and divergent member of the TGFbeta superfamily, negatively regulates mesoderm induction. Development 126, 229-240 [DOI] [PubMed] [Google Scholar]
- Tropepe V., Hitoshi S., Sirard C., Mak T. W., Rossant J., van der Kooy D. (2001). Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron 30, 65-78 [DOI] [PubMed] [Google Scholar]
- Vallier L., Reynolds D., Pedersen R. A. (2004). Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev. Biol. 275, 403-421 [DOI] [PubMed] [Google Scholar]
- Walton K. D., Croce J. C., Glenn T. D., Wu S. Y., McClay D. R. (2006). Genomics and expression profiles of the Hedgehog and Notch signaling pathways in sea urchin development. Dev. Biol. 300, 153-164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z., Angerer R. C., Angerer L. M. (2006). A database of mRNA expression patterns for the sea urchin embryo. Dev. Biol. 300, 476-484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z., Yaguchi J., Yaguchi S., Angerer R. C., Angerer L. M. (2009). The sea urchin animal pole domain is a Six3-dependent neurogenic patterning center. Development 136, 1179-1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z., Angerer R. C., Angerer L. M. (2011). Direct development of neurons within foregut endoderm of sea urchin embryos. Proc. Natl. Acad. Sci. USA 108, 9143-9147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzel H. E., Illies M. R., Byrum C. A., Xu R., Wikramanayake A. H., Ettensohn C. A. (2004). Differential stability of {beta}-catenin along the animal-vegetal axis of the sea urchin embryo mediated by dishevelled. Development 131, 2947-2956 [DOI] [PubMed] [Google Scholar]
- Wikramanayake A. H., Klein W. H. (1997). Multiple signaling events specify ectoderm and pattern the oral-aboral axis in the sea urchin embryo. Development 124, 13-20 [DOI] [PubMed] [Google Scholar]
- Wikramanayake A. H., Huang L., Klein W. H. (1998). beta-Catenin is essential for patterning the maternally specified animal-vegetal axis in the sea urchin embryo. Proc. Natl. Acad. Sci. USA 95, 9343-9348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikramanayake A. H., Hong M., Lee P. N., Pang K., Byrum C. A., Bince J. M., Xu R., Martindale M. Q. (2003). An ancient role for nuclear beta-catenin in the evolution of axial polarity and germ layer segregation. Nature 426, 446-450 [DOI] [PubMed] [Google Scholar]
- Wikramanayake A. H., Peterson R., Chen J., Huang L., Bince J. M., McClay D. R., Klein W. H. (2004). Nuclear beta-catenin-dependent Wnt8 signaling in vegetal cells of the early sea urchin embryo regulates gastrulation and differentiation of endoderm and mesodermal cell lineages. Genesis 39, 194-205 [DOI] [PubMed] [Google Scholar]
- Wilson S. W., Houart C. (2004). Early steps in the development of the forebrain. Dev. Cell 6, 167-181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaguchi S., Kanoh K., Amemiya S., Katow H. (2000). Initial analysis of immunochemical cell surface properties, location and formation of the serotonergic apical ganglion in sea urchin embryos. Dev. Growth Differ. 42, 479-488 [DOI] [PubMed] [Google Scholar]
- Yaguchi S., Yaguchi J., Burke R. D. (2006). Specification of ectoderm restricts the size of the animal plate and patterns neurogenesis in sea urchin embryos. Development 133, 2337-2346 [DOI] [PubMed] [Google Scholar]
- Yaguchi S., Yaguchi J., Burke R. D. (2007). Sp-Smad2/3 mediates patterning of neurogenic ectoderm by nodal in the sea urchin embryo. Dev. Biol. 302, 494-503 [DOI] [PubMed] [Google Scholar]
- Yaguchi S., Yaguchi J., Angerer R. C., Angerer L. M. (2008). A Wnt-FoxQ2-nodal pathway links primary and secondary axis specification in sea urchin embryos. Dev. Cell 14, 97-107 [DOI] [PubMed] [Google Scholar]
- Yaguchi S., Yaguchi J., Angerer R. C., Angerer L. M., Burke R. D. (2010a). TGFβ signaling positions the ciliary band and patterns neurons in the sea urchin embryo. Dev. Biol. 347, 71-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaguchi S., Yaguchi J., Wei Z., Shiba K., Angerer L. M., Inaba K. (2010b). ankAT-1 is a novel gene mediating the apical tuft formation in the sea urchin embryo. Dev. Biol. 348, 67-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T. P. (2001). Heads or tails: Wnts and anterior-posterior patterning. Curr. Biol. 11, R713-R724 [DOI] [PubMed] [Google Scholar]
- Yu J. K., Holland L. Z., Holland N. D. (2002). An amphioxus nodal gene (AmphiNodal) with early symmetrical expression in the organizer and mesoderm and later asymmetrical expression associated with left-right axis formation. Evol. Dev. 4, 418-425 [DOI] [PubMed] [Google Scholar]
- Yu J. K., Satou Y., Holland N. D., Shin I. T., Kohara Y., Satoh N., Bronner-Fraser M., Holland L. Z. (2007). Axial patterning in cephalochordates and the evolution of the organizer. Nature 445, 613-617 [DOI] [PubMed] [Google Scholar]