Abstract
Skeletal muscle tissue provides mechanical force for locomotion of all vertebrate animals. It is prone to damage from acute physical trauma and physiological stress. To cope with this, it possesses a tremendous capacity for rapid and effective repair that is widely held to be accomplished by the satellite cells lying between the muscle fiber plasmalemma and the basement membrane. Cell transplantation and lineage-tracing studies have demonstrated that Pax7-expressing (Pax7+) satellite cells can repair damaged muscle tissue repeatedly after several bouts of acute injury. These findings provided evidence that Pax7+ cells are muscle stem cells. However, stem cells from a variety of other origins are also reported to contribute to myofibers upon engraftment into muscles, questioning whether satellite cells are the only stem cell source for muscle regeneration. Here, we have engineered genetic ablation of Pax7+ cells to test whether there is any significant contribution to muscle regeneration after acute injury from cells other than this source. We find that such elimination of Pax7+ cells completely blocks regenerative myogenesis either following injury to the tibialis anterior (TA) muscle or after transplantation of extensor digitorum longus (EDL) muscles into nude mice. As Pax7 is specifically expressed in satellite cells, we conclude that they are essential for acute injury-induced muscle regeneration. It remains to be established whether there is any significant role for stem cells of other origins. The implications of our results for muscle stem cell-based therapy are discussed.
Keywords: Muscle regeneration, Pax7, Satellite cells, Mouse
INTRODUCTION
Since the discovery of the satellite cell lying in a groove on the external surface of the myofiber and beneath the external basal lamina (Mauro, 1961), it has been the major candidate for the source of myogenic cells for muscle repair during injury-induced regeneration. This has been supported by a growing body of evidence. Pioneering single myofiber culture experiments demonstrated that they harbor mononucleated cells, presumed to be satellite cells, that can generate new myofibers in vitro (Bischoff, 1975; Konigsberg et al., 1975). Cultured myoblasts, presumed to be derived from satellite cells, give rise to new myofibers when transplanted into minced muscles (Lipton and Schultz, 1979; Snow, 1977). Additionally, transplantation into dystrophic muscle of a single muscle fiber along with its satellite cells generates plentiful new myofibers and myofiber-associated cells (Collins et al., 2005). More directly, single cells expressing Pax7, a marker for satellite cells (Seale et al., 2000), when isolated from myofibers, manifest muscle regenerative capacity upon engraftment (Sacco et al., 2008). Lineage studies have demonstrated that Pax7 expression marks adult muscle stem cells in injury-induced regenerative myogenesis in vivo, but expression of Pax7 is needed for myogenic function only within the perinatal period (Lepper et al., 2009). However, whether Pax7+ cells are the main or the only source for myofiber regeneration is still subject to debate.
This question has come into focus over the past decade with reports not only of heterogeneity in gene expression and myogenic potential in the population of myofiber-associated satellite cells (Cerletti et al., 2008; Kuang et al., 2007) but also of contributions to muscle regeneration on transplantation of many different types of stem cell selected and characterized in vitro on the basis of surface markers (Peault et al., 2007). The first such `unorthodox' cell type was bone marrow-derived progenitors (Ferrari et al., 1998). Subsequently, several other cell types have been demonstrated to incorporate into newly formed myofibers, including skeletal muscle side population cells (Gussoni et al., 1999), mesoangioblasts (Sampaolesi et al., 2003), pericytes (Dellavalle et al., 2007), CD133 (Prom1)+ progenitors (Torrente et al., 2004) and PW1 (Peg3)+ interstitial cells (Mitchell et al., 2010). Authentication of the myogenicity of these stem/progenitor cells is based either on transplantation after fluorescent activated cell sorting (FACS) or in vitro culture. Although they might provide potential sources for cell therapy of debilitating muscular dystrophies, their relevance to normal physiological muscle repair has not yet been established.
We reasoned that elimination of Pax7+ satellite cells would distinguish the extent of their participation in acute injury-induced regenerative myogenesis from that of the totality of other cell populations. Two distinct scenarios can be envisaged: (1) full or partial restoration of the injured muscle, indicating that Pax7 negative (Pax7–) cells can substitute for satellite cells, or (2) grossly impaired or no muscle regeneration, indicating that under normal physiological conditions, satellite cells are the major or only progenitors for myofiber regeneration after severe injury. Below, we describe genetically engineered ablation of Pax7+ cells, followed by TA muscle injury and EDL muscle transplantation assays in the mouse. Our results reveal that Pax7+ cells represent a source of stem cells that is absolutely necessary for acute injury-induced muscle regeneration. This conclusion should give a fresh perspective to the contentious issue of muscle stem cell identity, as well as to stem cell-based therapies for muscular dystrophies.
MATERIALS AND METHODS
Animals
The Pax7CE allele has been described (Lepper et al., 2009). Both R26Rlacz (Soriano, 1999) reporter and R26ReGFP-DTA (Ivanova et al., 2005) mice were obtained from the Jackson Laboratory (ME, USA). NU/NU nude mice were obtained from Charles River (MA, USA). To obtain Pax7+/CE;R26R+/lacZ mice, Pax7+/CE mice were mated to R26RlacZ/lacZ mice. Pax7+/CE;R26ReGFP-DTA/lacZ mice were obtained by mating Pax7+/CE;R26R+/lacZ mice to R26ReGFP-DTA/eGFP-DTA mice. Mice were genotyped by PCR using standard protocols and primers (see Table S1 in the supplementary material). The R26RlacZ allele was used to assess efficacy of killing of Pax7+ cells after tamoxifen administration in Pax7+/CE;R26ReGFP-DTA/lacZ mice. No β-galactosidase (β-gal; product of the lacZ gene) marked cells were found in TA muscles of such Pax7+/CE;R26ReGFP-DTA/lacZ mice (by X-galactosidase reaction for β-gal activity). A total of 12 male mice (3-4 months old) were used for analysis at day 0 and day 5 (Figs 1 and 2, and see Figs S1 and S2 in the supplementary material). Additional mice used for transplantation studies are included in the section below. All in vivo mouse manipulations were approved by the Institutional Animal Care and Use Committee (IACUC) of the Carnegie Institution for Science.
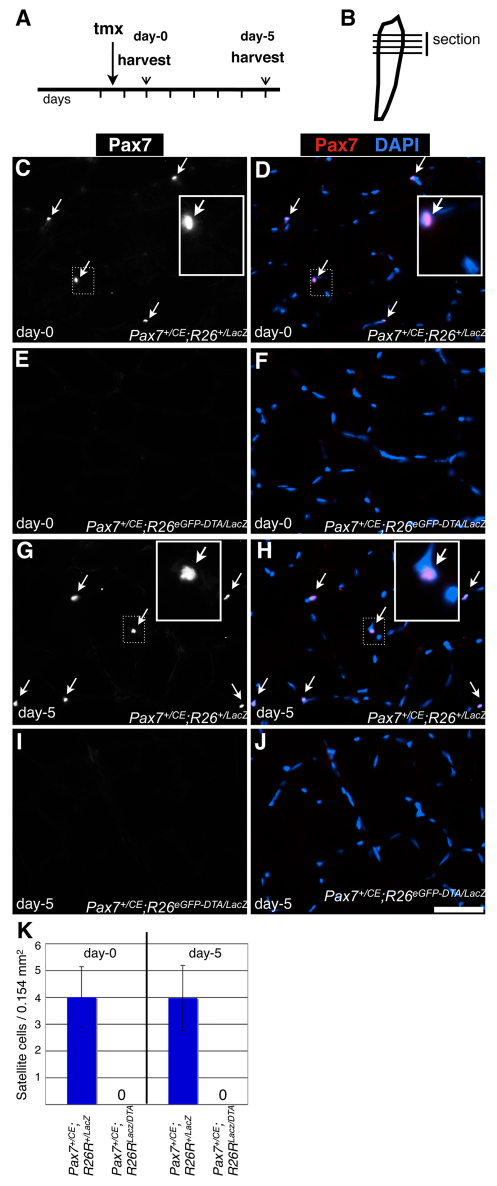
Fig. 1.
Efficient ablation of Pax7+ cells. (A) Tamoxifen (tmx) regimen and harvest scheme. Vertical lines indicate daily intervals. (B) Tibialis anterior (TA) muscle diagram. Horizontal lines indicate the orientation of sections (i.e. cross-sections) used for analysis. (C-J) Fluorescent microscopy of Pax7+/CE;R26R+/lacz (C,D,G,H) and Pax7+/CE;R26ReGFP-DTA/lacz (E,F,I,J) TA muscle cross-sections at 1.5 days after tmx administration (C-F) [referred to as day-0 as cardiotoxin (CTX)-induced injuries are performed at this time (see Fig. 2)] and 6.5 days after tamoxifen administration (G-J) [referred to as day-5 as this represents the 5-day regeneration time point (see Fig. 2)]. Pax7 is shown in white (C,E,G,I) or pseudocolored in magenta with DAPI shown in cyan (D,F,H,J). White arrows indicate satellite cells. Boxed insets show magnified views of the area indicated by a dotted white box. (K) Mean number of satellite cells at day-0 (left) and day-5 (right) per 0.154 mm2; 20 random areas/animal (n=3/genotype). Error bars represent s.d. Scale bar: 50 μm.
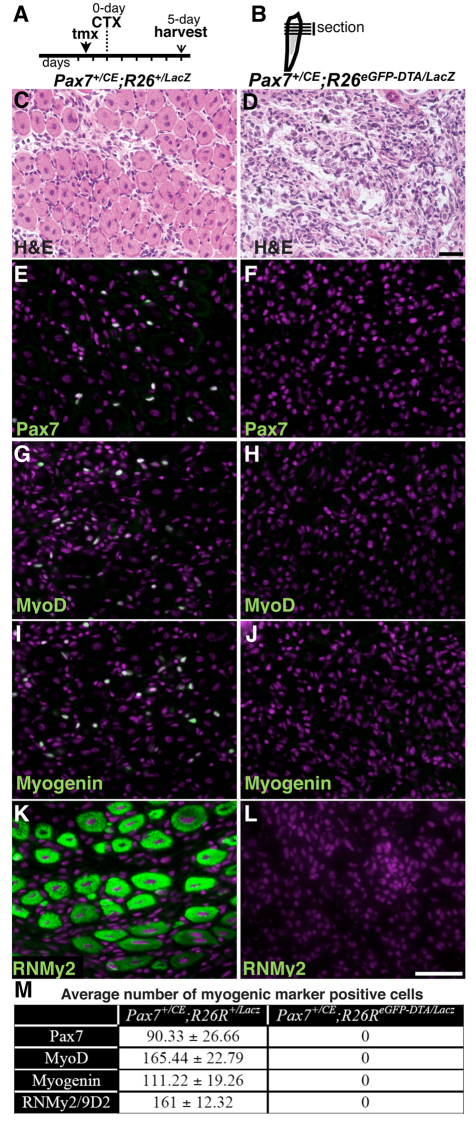
Fig. 2.
Absence of the myogenic response to acute injury in satellite cell-ablated mice. (A) Tamoxifen (tmx) and cardiotoxin (CTX) regimen and harvest scheme. Vertical lines indicate daily intervals. (B) Tibialis anterior (TA) muscle diagram with injury in gray. Horizontal lines indicate cross-sections used for analysis. (C-L) Pax7+/CE;R26R+/lacz (C,E,G,I,K) and Pax7+/CE;R26ReGFP-DTA/lacz (D,F,H,J,L) regenerated TA muscle cross-sections 5 days after CTX-induced injury. (Note: injured area is shown exclusively.) (C,D) Hematoxylin and Eosin stain. (E-L) Fluorescent microscopy of Pax7 (E,F), MyoD (G,H), myogenin (I,J) and RNMy2/9D2 (K,L). All markers are pseudocolored in green, DAPI in magenta. Scale bars: in D, 50 μm for C,D; in L, 50 μm for E-L. (M) Average numbers (±s.d.) of cells expressing the indicated myogenic markers 5 days after injury (each average is based on three random 0.154 mm2 regenerate areas per animal, n=3 per genotype).
PCR genotyping and RT-PCR
PCR genotyping and RT-PCR were performed as described previously (Lepper et al., 2009). Primer sequences are in listed in the supplementary material (see Table S1 in the supplementary material).
CreERT2 activation by tamoxifen
Tamoxifen (tmx; Sigma) was prepared as described previously (Lepper et al., 2009) and administered intraperitoneally to 3- to 4-month-old mice or NU/NU nude mice. For Pax7+ cell ablation in Pax7+/CE;R26ReGFP-DTA/lacZ mice, animals received a single 10 mg dose by intraperitoneal injection. In our hands, efficacy of satellite cell ablation via the R26ReGFP-DTA allele by this tmx regimen is equal to or better than satellite cell labeling with the R26RlacZ reporter allele using the Pax7CE allele (Lepper et al., 2009). NU/NU nude mice with Pax7+/CE;R26ReGFP-DTA/lacZ EDL muscle grafts received daily 5 mg doses by intraperitoneal injection for five consecutive days.
EdU and BrdU cell proliferation assays
EdU (5-ethyl-2′-deoxyuridine; Invitrogen) administration and detection were performed as described previously (Lepper et al., 2009). For the short-term proliferation assay, EdU was injected at 0.1 mg/30 g bodyweight on days 2, 3 and 4 after injury. BrdU (5-bromo-2′-deoxyuridine; Sigma) was administered at 0.8 mg/ml in the drinking water for eight consecutive days post-injury.
Transplantation
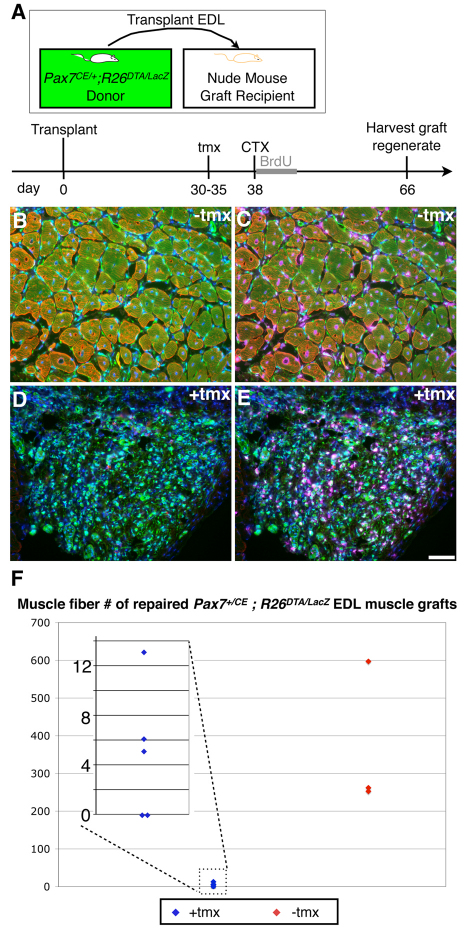
EDL muscles were used as donor muscles because their long proximal and distal tendons permit them to be removed and tied into host tendons without damaging the body of the muscle. EDL muscles, including tendons, were isolated by surgical dissection. Recipient nude mice were anaesthetized by intraperitoneal injection of Avertin (2,2,2-tribromoethanol from TCI America at 15 μl/g body weight of 20 mg/ml solution). To minimize contact with host muscles, we modified a previously published transplantation method (Grounds and Partridge, 1983) by removing all muscles from the anterior tibial compartment except the adjacent peroneal muscles whose thick epimysial covering protects against migration of unmarked satellite cells from the host into the genetically marked graft. Such mixing between donor and host satellite cells would be difficult to detect once fused into a myofiber syncytium. The proximal EDL tendons were sutured to the proximal host EDL tendon at the knee, and distal EDL tendons to the host's distal EDL tendons at the ankle. A total of 36 EDL muscles from 18 female Pax7+/CE;R26ReGFP-DTA/lacZ mice (3-4 months old) were transplanted into 36 male NU/NU recipient mice (3-4 months old; including eight preliminary trials to develop the assays). Only transplants with a robust, live GFP signal (33/36, 91.7% success rate) were processed for further analysis. The remaining 3/36 (8.3%) transplants gave no detectable GFP signal and were deemed to be technical failures, a low incidence of which is routinely seen. Seventeen transplants were performed for experiments shown in Figs 3 and 4, and eight transplants were done for experiments shown in Fig. 5 and Fig. S3 in the supplementary material.
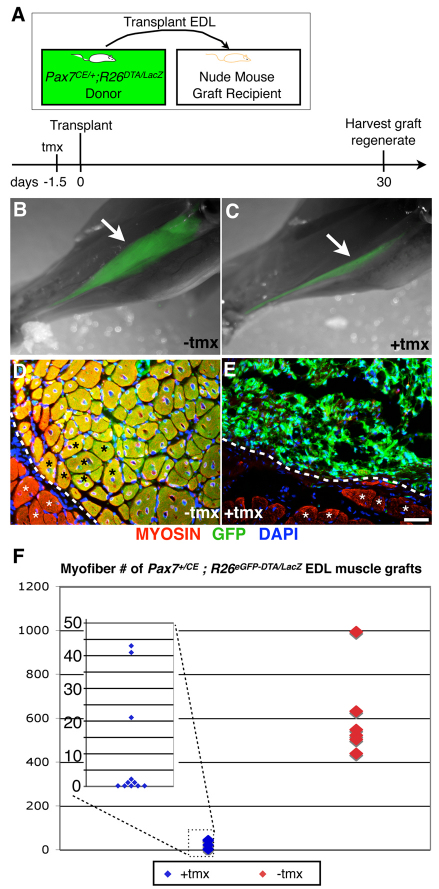
Fig. 3.
Absence of regenerative myofibers in extensor digitorum longus (EDL) muscle grafts ablated of Pax7+ cells prior to transplantation. (A) Tamoxifen (tmx) regimen, EDL transplantation and harvest scheme. (B,C) Overlays of whole-mount bright-field and GFP epi-fluorescence (green) images of lower hindlimb muscles from Pax7+/CE;R26ReGFP-DTA/lacz without (B) and with (C) exposure of the donor EDL muscle to tmx prior to transplantation. Note that EDL muscles exposed to tmx to kill Pax7+ cells prior to transplantation fail to generate robust muscle grafts. White arrows indicate grafted tissue. (D,E) Fluorescent microscopy on cross-sections of Pax7+/CE;R26ReGFP-DTA/lacz EDL grafts without (D) and with (E) tmx treatment prior to transplantation. Myosin is pseudocolored in red, GFP in green and DAPI in blue. The host-graft interface is demarcated by the white dotted line. White asterisks indicate host myofibers; black asterisks indicate graft myofibers. Scale bar: 50 μm. (F) Comparison of total myofiber numbers of EDL grafts shows a clear difference between grafts with (blue) and without (red) tamoxifen treatment prior to transplantation (P=0.0002, two-tailed Mann-Whitney U-test). As the data points for tmx-treated EDL muscles cluster closely together, all data points are shown in the magnified inset (overlapping data points are plotted next to each other, e.g. five data points for `0' myofibers).
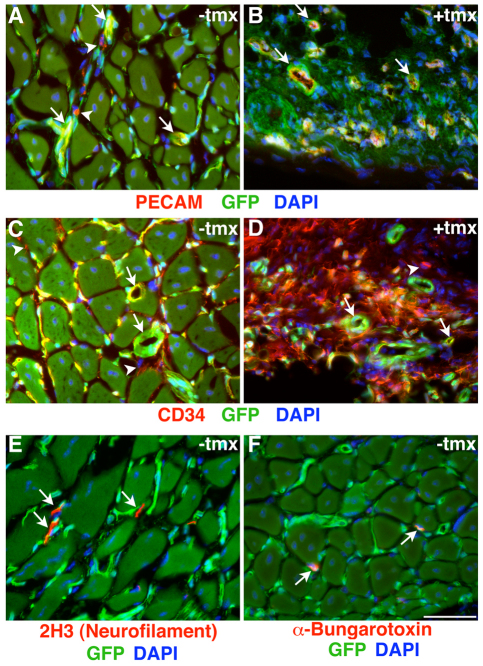
Fig. 4.
Robust vascularization of EDL muscles transplanted with or without prior ablation of Pax7+ cells and innervation of control EDL muscle grafts. (A-F) Fluorescent microscopy of Pax7+/CE;R26ReGFP-DTA/lacz EDL grafts without (A,C,E,F) and with (B,D) exposure of the donor EDL muscle to tamoxifen (tmx) prior to transplantation. (A,B) PECAM is pseudocolored in red, GFP in green and DAPI in blue. White arrows indicate PECAM+ capillaries; white arrowheads indicate GFP–/PECAM+ host infiltrating cells. (C,D) CD34 is pseudocolored in red, GFP in green and DAPI in blue. White arrows indicate CD34+ capillaries; white arrowheads indicate GFP–/CD34+ host infiltrating cells. (E) 2H3 (neurofilament)-stained neuronal axons are pseudocolored in red, GFP in green and DAPI in blue. White arrows indicate 2H3+/GFP– neuronal processes. (F) Alexa568-conjugated α-bungarotoxin-labeled motor end plates (via binding to nicotinic acetylcholine receptors) are pseudocolored in red, GFP in green and DAPI in blue. White arrows indicate bungarotoxin+/GFP– motor end plates. Scale bar: 50 μm.
Fig. 5.
Absence of muscle regeneration potential in established EDL grafts ablated of Pax7+ cells. (A) EDL transplantation, tamoxifen (tmx) treatment, injury and harvest scheme. (B-E) Fluorescent microscopy on cross-sections of established Pax7+/CE;R26ReGFP-DTA/lacz EDL grafts four weeks post-cardiotoxin (CTX)-induced injury without (B,C) and with (D,E) tmx treatment one month after transplantation prior to injury. Myosin/GFP/DAPI overlay is shown in B and D; Myosin/GFP/BrdU/DAPI overlay in C and E. Myosin is pseudocolored in red, GFP in green, DAPI in blue and BrdU in magenta. White arrows indicate BrdU+ central myonuclei. Scale bar: 50 μm. (F) Comparison of total myofiber numbers of EDL grafts four weeks after injury shows a clear difference between muscles treated with (blue) and without (red) tmx treatment prior to injury (P=0.0357, two-tailed Mann-Whitney U-test). As the data points for EDL muscle regenerates after tmx-treatment cluster closely together, all data points are shown in the magnified inset (overlapping data points are plotted next to each other, e.g. two data points for `0' myofibers).
Muscle injury with cardiotoxin
Cardiotoxin (CTX; Sigma) was prepared in PBS (10 μM) and 50 μl was injected percutaneously into TA muscles of anesthetized mice. For injury of EDL muscle grafts, NU/NU nude mice were anaesthetized by intraperitoneal Avertin injection, as above. A small incision was made in the skin to expose the grafted tissue and 15 μl of a 10 μM CTX solution (in PBS) were injected into the graft, followed by suturing the wound in the skin. Animals were kept under a warming lamp until recovery from anesthesia.
Immunofluorescence, EdU and BrdU detection, and histology
Immunostaining was performed as described previously (Lepper et al., 2009) using the following primary antibodies: anti-Pax7 [mouse monoclonal, 1:20, Developmental Studies Hybridoma Bank (DSHB)], anti-MyoD (mouse monoclonal, 1:1000, Santa Cruz), RnMy2/9D2 (mouse monoclonal, 1:30, AbCam), anti-Myogenin (F5D mouse monoclonal, 1:20, DSHB), anti-sarcomeric myosin (MF-20, mouse monoclonal, 1:20, DSHB), anti-CD34 (RAM34 rat monoclonal, 1:15, BD Biosciences), anti-CD31 (PECAM-1, rat monoclonal, 1:800, BD Biosciences), anti-GFP (rabbit monoclonal, 1:1000, Molecular Probes) and anti-neurofilament (2H3 mouse monoclonal, 1:50, DSHB). Fluorescent conjugated secondary antibodies of goat origin were used for detection: DyLight488 anti-rabbit IgG (Vector Laboratories), Alexa568 and Alexa633 anti-mouse IgG1, Alexa568, Alexa633 and Alexa647 anti-mouse IgG, and Alexa568 and Alexa633 anti-rat IgG (Molecular Probes); all were used at 1:1000.
For GFP immunofluorescence, whole-mount EDL muscle grafts were fixed in 4% paraformaldehyde/0.1 M phosphate buffer for 15 minutes on ice, then transferred to 10% sucrose in PBS and placed at 4°C with gentle agitation for overnight. Next day, the samples were transferred to 20% sucrose in PBS and placed at 4°C for overnight. Next morning, EDL muscle grafts were partially embedded in tragacanth (Sigma) on a slice of cork, dipped in OCT and flash frozen in isopentane (Sigma)/liquid nitrogen. Samples were cryosectioned at 10 μm and mounted on Superfrost Plus slides (VWR). Sectioned samples were post-fixed in 4% paraformaldehyde/0.1 M phosphate buffer for 10 minutes on ice, followed by the immunostaining procedure described previously (Lepper et al., 2009).
EdU detection was carried out using the click chemical reaction according to manufacturer's instructions using the Click-iT Kit components (Invitrogen).
For co-detection of GFP, sarcomeric myosin and BrdU by immunofluorescence, anti-GFP and anti-sarcomeric myosin (MF-20) immunostaining was performed as described above with the following modifications to the standard protocol following permeabilization. Samples were incubated in 0.3% H2O2 for 10 minutes, rinsed three times with PBS for 5 minutes each wash, and blocked using the Biotin/Avidin Blocking Kit (Vector Laboratories) according to the manufacturer's instructions. Samples were then blocked with the IgG blocking solution from the M.O.M. Kit (Vector Laboratories) following the manufacturer's instructions. Next, after anti-GFP and anti-sarcomeric myosin immunostaining, samples were fixed in 4% paraformaldehyde/0.1 M phosphate buffer for 15 minutes at room temperature and rinsed three times with PBT (PBS/0.01% Triton X-100) for 5 minutes each wash. Antigen retrieval was then performed using reagents from the BrdU In-Situ Detection Kit (BD Biosciences). Next, samples were blocked for a second time using the Biotin/Avidin Blocking Kit (Vector Laboratories) according to the manufacturer's instructions. Subsequently, samples were incubated with the BrdU In-Situ Detection Kit's biotinylated anti-BrdU antibody for one hour at room temperature. Samples were washed three times with PBT for 5 minutes each wash, and incubated with the ready-to-use Streptavidin-HRP (BrdU In-Situ Detection Kit, BD Biosciences) for 30 minutes at room temperature. Horseradish peroxidase (HRP) detection was performed using the TSA Plus Cyanine 3 System (Perkin Elmer). Samples were then washed three times in PBT for 5 minutes each wash, incubated with DAPI in PBS for 10 minutes, washed twice in PBT for 5 minutes each wash, and mounted in FluoromountG solution (Southern Biotechnology) with a coverslip (VWR).
Histology was performed using Hematoxylin and Eosin according to the manufacturer's instructions (Surgiopath).
α-Bungarotoxin
For detection of motor end plates, Alexa594-conjugated α-bungarotoxin (Molecular Probes) was applied at a 1:400 dilution (in PBS) and incubated for 30 minutes at room temperature prior to incubation with DAPI.
Image acquisition, cell quantification and statistics
Digital images of random 0.154 mm2 fields were taken under an Axioscope equipped with an AxioCam and labeled cells were counted by hand. Images were pseudocolored and superimposed using the Metamorph program. Excel was used for tabulation and statistical analysis. Numbers of fibers in grafts and regenerates subjected to different treatments were compared using the two-tailed, Mann-Whitney U-test.
RESULTS
Pax7+ cells can be eliminated by inducible expression of diphtheria toxin in vivo
To eliminate Pax7+ satellite cells, we employed genetic tools. We used the Pax7CE allele, which directs Cre-ERT2 expression, allowing tamoxifen (tmx)-inducible Pax7-specific manipulation of floxed genes (Lepper et al., 2009). Here we have used it in combination with the R26RlacZ reporter allele (Soriano, 1999) and also with the R26ReGFP-DTA allele (Ivanova et al., 2005) to express the diphtheria toxin fragment A (DTA) which mediates cell-autonomous killing. A single dose of 10 mg tmx (Fig. 1A) to Pax7+/CE;R26ReGFP-DTA/lacZ mice completely eliminated Pax7+ cells from the TA muscle within 36 hours (Fig. 1B; Fig. 1E,F; compare with Pax7+/CE;R26R+/lacZ controls in Fig. 1C,D; quantification in Fig. 1K). Consistently, neither Pax7 nor Cre transcripts was detectable by RT-PCR (see Fig. S1 in the supplementary material), demonstrating the efficacy and completeness of Pax7+ cell ablation within this 36-hour time frame. The tmx-induced activity of the Cre recombinase estrogen receptor fusion protein is rapid and evanescent (<24 hours) in the mouse (Lepper and Fan, 2010; Maes et al., 2010). Thus, any cell that activates the Pax7 gene at any time after the window of CreERT2 activity has closed would not be killed but would survive to replenish the Pax7+ satellite cell pool. No immediate replacement of Pax7+ cells from other sources was evident as they remained undetectable in TA muscles of tmx-treated Pax7+/CE;R26ReGFP-DTA/lacZ mice for at least 5 days (Fig. 1I,J; compare with Pax7+/CE;R26R+/lacZ controls in Fig. 1G,H; quantification in Fig. 1K).
Pax7+ cells are required for the initial wave of TA muscle regeneration after injury
Ablation of Pax7+ cells was accompanied by a block on regeneration in TA muscles after cardiotoxin-induced injury (see Materials and methods; Fig. 2A,B). In Pax7+/CE;R26R+/lacZ control animals, muscle regeneration was clearly evident 5 days after injury, as demonstrated by the presence of small myofibers with centrally located nuclei (Fig. 2C). By contrast, none was detected in the injured area of the TA muscle of tmx-treated Pax7+/CE;R26ReGFP-DTA/lacZ mice (Fig. 2D). Molecular characterization, with antibodies against myogenic markers Pax7, MyoD (Myod1; for activated myoblasts), myogenin (for myocytes) and RnMy2/9D2 (a monoclonal antibody against embryonic myosin heavy chain for specific identification of newly regenerated myofibers up to 6 days after injury) (Bigard et al., 1999; Lepper et al., 2009) revealed many Pax7-, MyoD-, myogenin- and RNMy2/9D2-positive cells in the injured area of TA muscles (Fig. 2E,G,J,K; quantification in Fig. 2M) in Pax7+/CE;R26R+/lacZ control animals but none in the equivalent area of tmx-treated Pax7+/CE;R26ReGFP-DTA/lacZ animals (Fig. 2F,H,J,L; quantification in Fig. 2M). Uninjured tmx-treated muscles did not show any sign of atrophy over the 5-day experiment (see Fig. S2A-C in the supplementary material), whereas injured muscles showed extensive proliferation of non-myogenic cells, probably including resident and infiltrating cells (see Fig. S2D-F in the supplementary material). These data indicate that the lack of muscle regeneration was not due to failure of general proliferative activity. As the proliferative phase of muscle repair is nearly complete by 5 days after muscle injury (McGeachie and Grounds, 1987), we conclude that failure of muscle regeneration in experimental animals is the consequence of Pax7+ cell ablation. Thus, our data demonstrate that in the absence of Pax7+ cells, there is a complete lack of myogenic differentiation during the initial wave of muscle regeneration.
EDL muscles with Pax7+ cells ablated fail to regenerate after transplantation into nude host mice
It is possible that late-acting cell sources exist that might partially restore damaged muscles. Unfortunately, long-term studies are precluded by the death of Pax7+/CE;R26ReGFP-DTA/lacZ mice 7-10 days after tmx-administration. Visual inspection and weighing of major trunk and limb muscle groups, including the TA/EDL and diaphragm muscles, revealed neither a significant reduction in mass nor changes in tissue architecture (not shown), arguing against a collapse of skeletal muscles as a cause of lethality. Interestingly, however, we found no spindle myofibers (intrafusal myofibers) in uninjured TA muscle cross-sections of Pax7+/CE;R26ReGFP-DTA/lacZ mice 5 days after tmx-administration (n=3, not shown), linking perhaps to our previous demonstration that these structures appear to be maintained by Pax7-expressing cells (Lepper et al., 2009). As spindle myofibers are crucial sensory components for the transmission of static and dynamic stretch information to the CNS, we speculate that their loss might destroy coordinated muscle activity and lead to the death of the mice. Another candidate contributor to the animals' demise is killing of Pax7-expressing cells in the CNS (see Discussion).
To circumvent this lethality for long-term muscle regeneration studies, we transplanted EDL muscles from Pax7+/CE;R26ReGFP-DTA/lacZ mice into nude hosts, which are unaffected by tmx. Transplantation induces muscle degeneration, and as such provides a crucial platform for studying post-transplantation regeneration from Pax7+ satellite cells originating exclusively from the donor muscle. Individual EDL muscles from Pax7+/CE;R26ReGFP-DTA/lacZ mice were transplanted intact (Grounds and Partridge, 1983) into the lower hindlimb of nude mice from which both EDL and TA muscles had been surgically removed (see Materials and methods; Fig. 3A). Thirty days after transplantation of EDL muscles from non-tmx-treated donors, robust regenerates were evident by whole-mount live GFP fluorescence (Fig. 3B). Hundreds of regenerated, centrally nucleated, GFP+ muscle fibers were identified in cryosections using an antibody specific for sarcomeric myosin (MF-20) (Fig. 3D; quantification in Fig. 3F). By contrast, grafts that had been exposed to tamoxifen to kill Pax7+ cells prior to transplantation were smaller in size and expressed only a weak GFP signal (Fig. 3C). Myofibers were completely absent (5/11 transplants) or very few in number (Fig. 3E; quantification in Fig. 3F). We also calculated the ablation efficacy of Pax7+ cells in the EDL muscle to be 100% [0 in tmx-treated versus 3.1±0.9 (mean±s.d.) Pax7+ cells in controls; averages from eight fields (0.154mm2/field) per sample, n=3 for each). As such, these sporadic fibers are likely to be those few that survived the transplantation, a common feature of this type of graft (Grounds and Partridge, 1983). The fact that five transplants contained no regenerative myofibers (Fig. 3E; quantification in Fig. 3F) demonstrates the need for Pax7+ cells for effective muscle regeneration after profound degeneration following transplantation.
Immunofluorescence analysis of EDL transplants revealed ample donor-derived GFP+PCAM+ (endothelial cells; Fig. 4A,B) and GFP+CD34+ (endothelial cells, stem cells, satellite cells; Fig. 4C,D) in grafts of both tmx-treated and non-treated muscles, though their distribution appeared much more organized in non-treated control EDL grafts. The different distribution patterns are likely to be influenced by the presence or absence of highly organized new myofibers. Moreover, in the non-tmx treated grafts, skeletal muscle fibers became well-innervated, as evidenced by the presence of both neuronal axons (Fig. 4E) and motor end plates (Fig. 4F). Thus, the EDL graft provides a well-vascularized and well-innervated environment conducive to proper muscle regeneration. Yet, in the absence of the donor tissue's population of Pax7+ cells, regenerative myogenesis fails. This finding indicates that neither any Pax7– cells within the grafted EDL muscle nor any host-derived cells (e.g. via the vascular system) contribute myonuclei to skeletal muscle in EDL muscle transplants.
Inducible elimination of Pax7+ cells within regenerated EDL transplants also causes a failure to produce regenerative myonuclei after secondary injury
One might conjecture that lack of vascular communication with the host immediately after transplantation coupled with the lack of Pax7+ cells in the tmx-treated EDL could deprive Pax7– graft cells of signals that they might require to enter myogenesis. Additionally, host-derived cells might not have access to the graft during this period. However, these impediments should not apply once the EDL graft has regenerated and re-established normal relationships with the host. Therefore, we induced injury by injection of cardiotoxin (CTX) into Pax7+/CE;R26ReGFP-DTA/lacZ EDL muscle grafts that had formed robust regenerates after transplantation. During the first week of this second round of regenerative myogenesis, mice were fed BrdU continuously to label proliferating myoblasts and the myonuclei to which they gave rise (Fig. 5A). The contribution from Pax7+ satellite cells to this muscle regeneration was compared in mice given no tmx and mice given tmx on 5 consecutive days (to ablate Pax7+ cells in the graft) prior to the cardiotoxin injury (see Materials and methods, Fig. 5A). Four weeks after injuring non-tmx-treated mice, many GFP+, sarcomeric myosin-positive myofibers were detected (Fig. 5B and see Fig. S3A in the supplementary material; quantification in Fig. 5F). Most of these myofibers contained central myonuclei that were positive for BrdU, demonstrating that they had formed during the CTX-induced wave of muscle regeneration (Fig. 5C and see Fig. S3B in the supplementary material). By contrast, in mice injected with tmx, there was no evidence of newly regenerated myofibers: two out of five transplants contained no fibers and the few fibers in the remaining three transplants [8±4.4 fibers (mean±s.d.), Fig. 5D and see Fig. S3C in the supplementary material; quantification in Fig. 5F] contained no BrdU+ myonuclei, strongly suggesting that they had survived CTX treatment, having been generated during the original wave of muscle regeneration following transplantation (see Fig. S3D in the supplementary material). Thus, neither host-derived cells nor Pax7– cells intrinsic to the graft tissue were able to compensate for the loss of Pax7+ cells to provide myonuclei for regenerative myogenesis. These results demonstrate that the muscle regeneration potential of established EDL muscle grafts depends entirely on its resident population of Pax7+ cells.
DISCUSSION
Pax7 expression defines the exclusive source of stem cells for skeletal muscle regeneration after acute injury
Only rarely can the expression of a single gene be used to define a tissue-specific stem cell population in its entirety. The above experiments provide evidence that acute injury-induced muscle regeneration in the hindlimb is entirely dependent on the resident Pax7+ cell population. Together with previous lineage analysis (Lepper et al., 2009), they imply that cells expressing this gene represent the only effective source of muscle stem cells in normal muscle for profound tissue restoration. Although functional muscle stem cells have been reported to arise from a variety of non-muscle origins (Dellavalle et al., 2007; Gussoni et al., 1999; Mitchell et al., 2010; Sampaolesi et al., 2003; Torrente et al., 2004), our data reveal that such unorthodox muscle stem cell populations must contain or be dependent upon Pax7+ cells.
Pax7 function versus Pax7 expression
Pax7 was first described by Seale et al. (Seale et al., 2000) as a marker of the satellite cell. As Pax7 germ line mutant mice lacked satellite cells and displayed compromised post-natal muscle growth and regeneration after acute injury (Seale et al., 2000), they suggested that Pax7 specifies satellite cell fate. Subsequently, it was found that the Pax7 mutant contains satellite cells at birth, but loses them over time by mechanisms such as improper mitosis (Kuang et al., 2006), increased apoptosis (Relaix et al., 2006) and reduced myogenic capacity (Oustanina et al., 2004). By lineage tracing, we showed that germ line mutant Pax7 cells incorporate excessively into myofibers after birth, which probably leads to failure to establish a normal satellite cell pool (Lepper et al., 2009). By contrast, inactivation of Pax7 in the adult, after the quiescent satellite cell pool has been established, does not impair injury-induced muscle regeneration (Lepper et al., 2009). None of the above studies, however, definitively excludes a contribution from non-Pax7-expressing cells to normal muscle regeneration. Here, we distinguish the requirement for `Pax7 gene function' from the requirement for `Pax7-expressing cells' in muscle regeneration induced by acute injury. To do this, we employed the classical cell ablation paradigm. Our data unequivocally demonstrate that Pax7+ cells are the exclusive source of regenerative myonuclei after acute injury. Consistent with our findings, we note that in an accompanying paper in this issue (Sambasivan et al., 2011), muscle regeneration fails when most of the Pax7+ cells are ablated by a different genetic strategy involving the local administration of DTA. It remains curious that expression of Pax7, though not functionally required, persists in adult satellite cells (Lepper et al., 2009).
Are Pax7+ cells required for muscle homeostasis?
The death of Pax7+/CE;R26ReGFP-DTA/lacZ animals 7-10 days after tmx treatment, with or without acute injury, is not entirely unexpected. Aside from the muscle lineage, Pax7 expression has not been fully characterized in other tissues in the adult. Embryonic expression of Pax7 (Jostes et al., 1990; Lepper and Fan, 2010; Mansouri et al., 1996) predicts possible expression of this gene in the adult CNS as well as in craniofacial structures developed from cephalic neural crest cells. Indeed, Pax7 expression can be detected in adult mouse brains by RT-PCR (Day et al., 2007). If any of these neuronal Pax7-expressing cells are critical for life, their elimination will result in the observed lethality. Furthermore, the observed loss of spindle myofibers in non-injured tmx-treated animals might lead directly to the animals' death by disrupting the reflex pathway that regulates muscle tone and length. In accordance with this, these mice developed a hopping gait and became slow movers prior to their death. We suggest, therefore, that lethality is due to the ablation of spindle myofibers and/or unknown essential neurons of the CNS.
In one of the accompanying manuscripts in this issue (Sambasivan et al., 2011) satellite cell ablation was mediated by a DTA-receptor knock-in allele at the Pax7 locus and local intramuscular DTA injection. Though ablation of Pax7-expressing cells was incomplete by this method, the animals' demise might be prevented by localized activity of DTA. In the manuscript by Murphy et al. (Murphy et al., 2011) using a strategy similar to ours, the animals, surprisingly, remain viable after tmx-administration. The difference in the outcome of these mice might be explained by different ablation efficiency of Pax7-expressing cells (satellite cells and neuronal cells) due to the use of distinct CreERT2 and conditional DTA alleles, as well as the route of tmx-administration.
Because we did not observe a clear reduction in muscle mass during this assay period (see Fig. S2A-C in the supplementary material), short-term skeletal muscle homeostasis does not rely on Pax7+ cells. Our previous lineage analysis further indicates that in the absence of severe myotrauma, no differentiation or fusion by Pax7+ cells is required for muscle fiber maintenance/turnover in adult mice over a 10-day window following tmx administration (Lepper et al., 2009). By contrast, a study by Nishijo et al. (Nishijo et al., 2009) suggested continuous input from Pax7+ cells to myofibers over a 4-month period. The untimely death of our experimental animals did not permit us to address whether and how loss of Pax7+ cells effects long-term homeostasis of skeletal muscle mass. Also, the EDL transplantation paradigm does not enable proper homeostasis studies because the implanted EDL does not bear the mechanical load of the removed host muscles in the anterior compartment. To address this particular issue definitively, we need a tightly regulated inducible Cre allele restricted to satellite cells to achieve complete cell ablation without lethality.
Pax7– muscle stem cell sources
Several potential stem cell sources for muscle regeneration, as defined by FACS selection of their surface markers, have been reported (Dellavalle et al., 2007; Gussoni et al., 1999; Mitchell et al., 2010; Torrente et al., 2004). When transplanted, these cells can contribute to adult myogenesis in injured or dystrophic muscle. Our data suggest that, by virtue of the isolation methods employed, some of those `marker-expression' defined stem cell sources probably contained a fraction of Pax7+ cells. Because even a single Pax7+ cell can regenerate muscle efficiently after transplantation (Sacco et al., 2008), it is likely that bulk transplants of such preparations would favor a direct contribution to muscle regeneration from such contaminating Pax7+ cells. Although Pax7–, e.g. PW1+ interstitial cells (Mitchell et al., 2010) and mesoangioblasts (Sampaolesi et al., 2003) have been shown to be myogenic on transplantation, our experimental paradigms detect no evidence of a significant contribution of such cells from endogenous sources. It remains possible that muscle repair mediated by cell transplantation of Pax7– cell sources, e.g. mesoangioblasts (Sampaolesi et al., 2003) and/or pericytes (Dellavalle et al., 2007), involves reprogramming of cells to attain myogenic potential by technical manipulation in vitro. The lack of participation of PW1+ interstitial cells might be attributed to the requirement for reprogramming by adjacent Pax7+ cells, but these re-programmable cells are reported to be lost from muscle well before the age at which we have induced muscle regeneration (Mitchell et al., 2010). Thus, the total lack of regeneration observed after Pax7+ cell ablation demonstrates a lack of emerging new functional stem cells from any source to compensate for the loss of Pax7+ cells during acute injury-induced muscle regeneration in the adult.
Our data clearly demonstrate the crucial role of Pax7+ cells in acute injury-induced muscle regeneration. We cannot exclude the possibility that in other myopathic conditions, such as chronic muscle wasting diseases, unknown signals recruit additional specialized cell populations to the myogenic fate. Though beyond the scope of this work, future pre-translational studies to test the relative contribution of Pax7+ cells in a murine Duchenne muscular dystrophy model (i.e. the Mdx mouse) should advance our understanding of the differences, if any, between basic normal biology versus pathological biology of muscle stem cells.
Therapeutic use of satellite cell versus other stem cell sources
We emphasize that our data do not diminish the potential clinical use of alternative stem cells for therapy of muscular dystrophies, which theoretically should benefit from healthy cells regardless of origin. In fact, the effectiveness of transplanted mesoangioblasts in ameliorating muscle dystrophy in a canine model, has raised considerable hope in human patients (Sampaolesi et al., 2006). Conversely, transplantation of satellite cells after culture (i.e. myoblasts) to human patients has proven ineffective, presumably owing to poor graft cell survival (Partridge, 1991). The problem of harnessing the regenerative capacity of such cells appears to be technical in nature and not insuperable. Our data, therefore, support the view that more attention should be directed at improving the survival and administration of the professional muscle stem cells, i.e. the Pax7+ satellite cells, as a route to this therapeutic dream.
Supplementary Material
Acknowledgments
We thank E. Dikovskaia and E. Siple for technical assistance and J. J. Weyers, Y. Zheng and A. Spradling for comments on the manuscript. We also thank S. Tajbakhsh and A. Galy for sharing their results and coordinating manuscript submission. The Carnegie Institution and NIH (AR060042) provided funding for this work. T.A.P. is supported by DOD/USAMRAA grants W81XWH-09-1-0599 and W81XWH-09-1-021 and by The Foundation to Eradicate Duchenne (FEDS). Deposited in PMC for release after 12 months.
Footnotes
Author contributions
C.L., T.A.P. and C.-M.F. designed and C.L. and T.A.P. conducted the research. All three authors contributed to manuscript writing.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.067595/-/DC1
References
- Bigard A. X., Janmot C., Sanchez H., Serrurier B., Pollet S., d'Albis A. (1999). Changes in myosin heavy chain profile of mature regenerated muscle with endurance training in rat. Acta Physiol. Scand. 165, 185-192 [DOI] [PubMed] [Google Scholar]
- Bischoff R. (1975). Regeneration of single skeletal muscle fibers in vitro. Anat. Rec. 182, 215-235 [DOI] [PubMed] [Google Scholar]
- Cerletti M., Jurga S., Witczak C. A., Hirshman M. F., Shadrach J. L., Goodyear L. J., Wagers A. J. (2008). Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell 134, 37-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C. A., Olsen I., Zammit P. S., Heslop L., Petrie A., Partridge T. A., Morgan J. E. (2005). Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122, 289-301 [DOI] [PubMed] [Google Scholar]
- Day K., Shefer G., Richardson J. B., Enikolopov G., Yablonka-Reuveni Z. (2007). Nestin-GFP reporter expression defines the quiescent state of skeletal muscle satellite cells. Dev. Biol. 304, 246-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellavalle A., Sampaolesi M., Tonlorenzi R., Tagliafico E., Sacchetti B., Perani L., Innocenzi A., Galvez B. G., Messina G., Morosetti R., et al. (2007). Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat. Cell Biol. 9, 255-267 [DOI] [PubMed] [Google Scholar]
- Ferrari G., Cusella-De Angelis G., Coletta M., Paolucci E., Stornaiuolo A., Cossu G., Mavilio F. (1998). Muscle regeneration by bone marrow-derived myogenic progenitors. Science 279, 1528-1530 [DOI] [PubMed] [Google Scholar]
- Grounds M. D., Partridge T. A. (1983). Isoenzyme studies of whole muscle grafts and movement of muscle precursor cells. Cell Tissue Res. 230, 677-688 [DOI] [PubMed] [Google Scholar]
- Gussoni E., Soneoka Y., Strickland C. D., Buzney E. A., Khan M. K., Flint A. F., Kunkel L. M., Mulligan R. C. (1999). Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 401, 390-394 [DOI] [PubMed] [Google Scholar]
- Ivanova A., Signore M., Caro N., Greene N. D., Copp A. J., Martinez-Barbera J. P. (2005). In vivo genetic ablation by Cre-mediated expression of diphtheria toxin fragment A. Genesis 43, 129-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jostes B., Walther C., Gruss P. (1990). The murine paired box gene, Pax7, is expressed specifically during the development of the nervous and muscular system. Mech. Dev. 33, 27-37 [DOI] [PubMed] [Google Scholar]
- Konigsberg U. R., Lipton B. H., Konigsberg I. R. (1975). The regenerative response of single mature muscle fibers isolated in vitro. Dev. Biol. 45, 260-275 [DOI] [PubMed] [Google Scholar]
- Kuang S., Charge S. B., Seale P., Huh M., Rudnicki M. A. (2006). Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J. Cell Biol. 172, 103-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S., Kuroda K., Le Grand F., Rudnicki M. A. (2007). Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129, 999-1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C., Fan C. M. (2010). Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis 48, 424-436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C., Conway S. J., Fan C. M. (2009). Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature 460, 627-631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton B. H., Schultz E. (1979). Developmental fate of skeletal muscle satellite cells. Science 205, 1292-1294 [DOI] [PubMed] [Google Scholar]
- Maes C., Kobayashi T., Selig M. K., Torrekens S., Roth S. I., Mackem S., Carmeliet G., Kronenberg H. M. (2010). Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 19, 329-344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri A., Stoykova A., Torres M., Gruss P. (1996). Dysgenesis of cephalic neural crest derivatives in Pax7–/–mutant mice. Development 122, 831-838 [DOI] [PubMed] [Google Scholar]
- Mauro A. (1961). Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 9, 493-495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachie J. K., Grounds M. D. (1987). Initiation and duration of muscle precursor replication after mild and severe injury to skeletal muscle of mice. An autoradiographic study. Cell Tissue Res. 248, 125-130 [DOI] [PubMed] [Google Scholar]
- Mitchell K. J., Pannerec A., Cadot B., Parlakian A., Besson V., Gomes E. R., Marazzi G., Sassoon D. A. (2010). Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat. Cell Biol. 12, 257-266 [DOI] [PubMed] [Google Scholar]
- Murphy M. M., Lawson J. A., Mathew S. J., Hutcheson D. A., Kardon G. (2011). Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development 138, 3625-3637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishijo K., Hosoyama T., Bjornson C. R., Schaffer B. S., Prajapati S. I., Bahadur A. N., Hansen M. S., Blandford M. C., McCleish A. T., Rubin B. P., et al. (2009). Biomarker system for studying muscle, stem cells, and cancer in vivo. FASEB J. 23, 2681-2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oustanina S., Hause G., Braun T. (2004). Pax7 directs postnatal renewal and propagation of myogenic satellite cells but not their specification. EMBO J. 23, 3430-3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge T. A. (1991). Invited review: myoblast transfer: a possible therapy for inherited myopathies? Muscle Nerve 14, 197-212 [DOI] [PubMed] [Google Scholar]
- Peault B., Rudnicki M., Torrente Y., Cossu G., Tremblay J. P., Partridge T., Gussoni E., Kunkel L. M., Huard J. (2007). Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol. Ther. 15, 867-877 [DOI] [PubMed] [Google Scholar]
- Relaix F., Montarras D., Zaffran S., Gayraud-Morel B., Rocancourt D., Tajbakhsh S., Mansouri A., Cumano A., Buckingham M. (2006). Pax3 and Pax7 have distinct and overlapping functions in adult muscle progenitor cells. J. Cell Biol. 172, 91-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco A., Doyonnas R., Kraft P., Vitorovic S., Blau H. M. (2008). Self-renewal and expansion of single transplanted muscle stem cells. Nature 456, 502-506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambasivan R., Yao R., Kissenpfennig A., Van Wittenberghe L., Paldi A., Gayraud-Morel B., Guenou H., Malissen B., Tajbakhsh S., Galy A. (2011). Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 138, 3647-3656 [DOI] [PubMed] [Google Scholar]
- Sampaolesi M., Torrente Y., Innocenzi A., Tonlorenzi R., D'Antona G., Pellegrino M. A., Barresi R., Bresolin N., De Angelis M. G., Campbell K. P., et al. (2003). Cell therapy of alpha-sarcoglycan null dystrophic mice through intra-arterial delivery of mesoangioblasts. Science 301, 487-492 [DOI] [PubMed] [Google Scholar]
- Sampaolesi M., Blot S., D'Antona G., Granger N., Tonlorenzi R., Innocenzi A., Mognol P., Thibaud J. L., Galvez B. G., Barthelemy I., et al. (2006). Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature 444, 574-579 [DOI] [PubMed] [Google Scholar]
- Seale P., Sabourin L. A., Girgis-Gabardo A., Mansouri A., Gruss P., Rudnicki M. A. (2000). Pax7 is required for the specification of myogenic satellite cells. Cell 102, 777-786 [DOI] [PubMed] [Google Scholar]
- Snow M. H. (1977). Myogenic cell formation in regenerating rat skeletal muscle injured by mincing. I. A fine structural study. Anat. Rec. 188, 181-199 [DOI] [PubMed] [Google Scholar]
- Soriano P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70-71 [DOI] [PubMed] [Google Scholar]
- Torrente Y., Belicchi M., Sampaolesi M., Pisati F., Meregalli M., D'Antona G., Tonlorenzi R., Porretti L., Gavina M., Mamchaoui K., et al. (2004). Human circulating AC133(+) stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle. J. Clin. Invest. 114, 182-195 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.