Abstract
The present investigation was undertaken to evaluate the healing efficacy of lyophilized aqueous leaf extract of Sea buckthorn (Hippophae rhamnoides L., family Elaeagnaceae) (SBT) and to explore its possible mechanism of action on experimental burn wounds in rats. The SBT extract, at various concentrations, was applied topically, twice daily for 7 days. Treatment with silver sulfadiazine (SSD) ointment was used as reference control. The most effective concentration of the extract was found to be 5.0% (w/w) for burn wound healing and this was further used for detailed study. The SBT-treated group showed faster reduction in wound area in comparison with control and SSD-treated groups. The topical application of SBT increased collagen synthesis and stabilization at the wound site, as evidenced by increase in hydroxyproline, hexosamine levels and up-regulated expression of collagen type-III. The histological examinations and matrix metalloproteinases (MMP-2 and -9) expression also confirmed the healing efficacy of SBT leaf extract. Furthermore, there was significant increase in levels of endogenous enzymatic and non-enzymatic antioxidants and decrease in lipid peroxide levels in SBT-treated burn wound granulation tissue. The SBT also promoted angiogenesis as evidenced by an in vitro chick chorioallantoic membrane model and in vivo up-regulated vascular endothelial growth factor (VEGF) expression. The SBT leaf extract had no cytotoxic effect on BHK-21 cell line. In conclusion, SBT aqueous leaf extract possesses significant healing potential in burn wounds and has a positive influence on the different phases of wound repair.
1. Introduction
The skin is one of the largest organs in the body that performs numerous vital functions including fluid homeostasis, thermoregulation, immunologic, neurosensory and metabolic functions. The skin also provides primary protection against infection by acting as a physical barrier. When this barrier is damaged, pathogens have a direct route to infiltrate the body, potentially resulting in infection. Cutaneous wound repair consists of an orderly progression of events that establish the integrity of the damaged tissue. The sequence of events that repairs the damage is categorized into three overlapping phases: inflammation, proliferation and tissue remodeling. The normal healing process can be impeded at any step along its path by a variety of factors that can contribute to impaired healing [1]. Impaired wound healing may be a consequence of pathologic states associated with diabetes, immune disorders, ischemia, venous stasis and injuries such as burn, frost-bite and gun-shot wounds [2].
Burns are one of the most common and devastating forms of trauma. Healing impairment in burn injury is characterized by increased free-radicals-mediated damage, delayed granulation tissue formation, reduced angiogenesis and decreased collagen reorganization leading to chronic wound healing. Burn management entails significant duration of hospital stay, expensive medication, multiple operative procedures and prolonged period of rehabilitation [3]. Topical anti-bacterial agents and disinfectants are good in protecting against infection, but the occurrence of allergic reactions and skin irritations to these agents reduces the rate of skin regeneration and increases the recovery time [4, 5]. However, recombinant growth factors and tissue-engineered wound dressings are highly expensive and beyond the reach of most of the patients in the economically developing and underdeveloped countries.
In alternative and complementary systems of medicines such as Ayurveda, Siddha, Amchi, Chinese and aromatherapy, plants are being used to combat several disease and pathological conditions. Herbal products seem to possess moderate efficacy with no or less toxicity and are less expensive as compared with synthetic drugs. Many plants and plants-derived products have been shown to possess potent wound-healing activity [2, 6–8]. Hippophae rhamnoides L. subspecies turkestanica (family Elaeagnaceae) commonly known as Sea buckthorn (SBT) is a branched and thorny nitrogen-fixing deciduous shrub, native to Europe and Asia. The plant has been used extensively in oriental traditional system of medicine for treatment of asthma, skin diseases, gastric ulcers and lung disorders. All parts of the plant are considered to be rich source of a large number of bioactive substances like flavonoids (isorhamnetin, quercetin, myricetin, kaempferol and their glycoside compounds), carotenoids (α, β, δ-carotene, lycopene), vitamins (C, E, K), tannins, triterpenes, glycerides of palmitic, stearic and oleic acids and some essential amino acids [9, 10]. SBT has been found to possess significant anti-oxidative, anti-microbial, anti-inflammatory, immunomodulatory, radio-protective, adaptogenic and tissue regenerative properties [9, 11–15]. The aim of the present study was to investigate the healing efficacy of lyophilized aqueous SBT leaf extract on full-thickness burn wounds in rats and dissect its possible mechanism of action.
2. Methods
2.1. Experimental Animals
Male Sprague-Dawley rats, each weighing 180 ± 20 g, from the animal colony of the DIPAS, Delhi, were used for this study. The animals were maintained under controlled environment in the Institute's animal house at 25 ± 1°C and 12-h light-dark cycle. The experiments were performed in accordance with the regulations specified by the Institute's Animal Ethical Committee and conform to the national guidelines on the care and use of laboratory animals, India.
2.2. Collection and Extraction of Plant Material
The leaves of SBT were collected from the hilly regions (at an altitude of 2500–4000 m) of the North-West Himalayas (the region lies between latitude 32–36° North and longitude 76–79° East) in the month of September 2006, where the plant grows widely under natural conditions. Plant material (Voucher specimen SBTL-2006) was characterized by Dr O. P. Chaurasia, an ethanobotanist at the Defence Institute of High Altitude Research (DIHAR), Leh, India.
Fresh leaves of SBT were cleaned and washed thoroughly with water and re-washed with distilled water. Washed fresh leaves were dried under shade in a clean, dust-free environment. The aqueous extract was prepared by soaking powdered dry leaves in distilled water (1 : 5 w/v) at room temperature (25 ± 1°C). After 24 h, the supernatant was decanted and the residue re-soaked in fresh distilled water. The process was repeated four times for complete extraction. The supernatants were pooled, filtered through muslin cloth and centrifuged at 5000 g, 4°C. The supernatants obtained after centrifugation were frozen at −20°C and then lyophilized in Heto lyophilizer (HITOSICC, Heto-Holten A/S, Denmark). Lyophilized powder of the SBT leaf aqueous extract was stored at −20°C in an airtight plastic container until further use (yield 13.7% w/w). The high-performance liquid chromatography (HPLC) fingerprinting of each batch of the extract was carried out and maintained throughout the experiment, to avoid batch-to-batch variation.
2.3. MTT Assay for Cytotoxicity
In this study, the [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] MTT assay was used to assess cytotoxicity caused by the SBT leaf extract. BHK-21 cell line was purchased from the National Center for Cell Science (NCCS), Pune, India. The cells were maintained at 37°C in an incubator with a humidified atmosphere of 5% CO2 and cultured in Glasgow's; minimum essential medium (GMEM) containing 10% heat-inactivated fetal calf serum (FCS), streptomycin (100 μg mL−1) and neomycin sulfate (50 μg mL−1). The BHK-21 cells were seeded on a 24-well plate at 1 × 105 cells mL−1. Sixteen hours after plating, cells were treated with various concentrations of the SBT leaf extract (25, 50, 100, 200, 400 μg mL−1) for 24 h. MTT stock solution (100 μL; 5 mg mL−1) was then added and incubated for 4 h. The formazan crystals generated in each well were dissolved in 1 mL of acidified isopropanol and read spectrophotometrically at 570 nm [8].
2.4. In Vitro Chick Chorioallantoic Membrane Model
The chick chorioallantoic membrane (CAM) model was used to assess the angiogenic activity of SBT extract as described by Lobb et al. [16]. Nine-day-old fertilized chick eggs were selected and a small window of 1.0 cm2 made in the shell. The window was opened and a sterile disk of methylcellulose loaded with SBT extract was placed at the junction of two large vessels. The window was resealed by tape and the eggs were incubated at 37°C in a well-humidified chamber. After 72 h, eggs were opened and new blood vessel formation was observed and compared with the control eggs containing disks without SBT extract. The photographic images of CAM model were taken for quantitative morphometric analysis of the density of blood capillaries in terms of number of red pixel per unit areas using National Institutes of Health Image J software (v1.38) and AngioQunat software.
2.5. Burn Wound Model
The animals were anesthetized by intra-peritoneal (i.p.) injection of thiopentone (25 mg kg−1), the dorsal surface of the rat was shaved, and the underlying skin was cleaned with 70% ethanol. Full-thickness burn wound was created by using an aluminum metal rod (diameter 1.5 cm, melting point 660°C) heated to 85°C. The temperature of the metal rod was monitored with a fabricated digital computerized multimeter. Hot rod was exposed on the shaved area of the rat for 20 s, resting on its own weight of 30 g. No additional pressure was applied on the hand-leaded metal rod. Single burn wound was created on dorsal part of each rat. After 24 h, dead tissues were excised using sterile surgical blade [17]. Animals were allowed to recover from anesthesia and housed individually in sterile cages.
2.6. Experimental Design
In the preliminary screening, dose-response study of the aqueous leaf extract of SBT was performed to find out the optimal concentration. Different doses of the extract (2.5, 5.0, 7.5 and 10.0%; w/w) prepared in soft white petroleum jelly (S.D. Fine Chem, India) were applied topically and twice daily for 7 days in rats. Silver sulfadiazine (SSD) cream USP, 1.0% w/w (Ranbaxy Laboratories Ltd., Delhi, India), was used for treating reference controls. Control group received the vehicle alone in an identical manner. The most effective concentration of the SBT extract was found to be 5.0% for burn wound healing and this was further used for detailed study.
2.6.1. Pro-Healing Parameters
The wound surface area was traced on a transparent paper and measured planimetrically [2]. The granulation tissue excised on eighth postwounding day was used to analyze the biochemical parameters: hydroxyproline, hexosamine and total protein contents [18–20].
2.6.2. Antioxidant Analysis
A 10% homogenate of granulation tissue was made in 0.15 M KCl containing 5 mM EDTA. After homogenization, samples were sonicated and an aliquot was withdrawn for the estimation of reduced glutathione (GSH) [21]. In the remaining homogenate, triton X-100 was added at 0.1% (v/v). Samples were incubated at 4°C for 2.5 h. After incubation, samples were centrifuged at 4226 g, and the supernatant was used for the estimation of superoxide dismutase, catalase, glutathione-S-transferase activities and vitamin C content [22–25]. Malondialdehyde (MDA), a marker for lipid peroxidation, was measured by the method of Ohkawa et al. [26]. Protein in the tissue samples was determined by the method of Lowry et al. [20].
2.6.3. Histological Studies and Morphometric Analysis
The granulation tissues were preserved in 10% neutral formalin. The tissue sections of 6-μm thickness were stained with hematoxylin and eosin and observed for histological changes under light microscope. Masson's trichome staining for collagen was also performed on paraffin sections, followed by photomicrography. All morphometric parameters were recorded with Image Analyzer (Olympus Microscope BX61) by using image analyzing computer program (Image-Pro Plus 6.2). All histological sections were assessed through the center of the wounds to obtain maximum wound diameter. The measurements were taken three times by examining the slides in random sequence, blinded to treatment. The thickness of the newly formed epidermis was measured at 1-mm interval and the mean was calculated. The blood vessel density was evaluated by taking average number of cells in six high-power fields (60X objective), midway in the wound bed.
2.6.4. Gelatin Zymography
Matrix metalloproteinases (MMPs) expression was studied in the granulation tissues by gelatin zymography assay [17]. Granulation tissue was homogenized with Tris buffer (saline 0.9%, Tris 0.05 M, Triton X-100 0.25% and CaCl2 0.02 M) and centrifuged at 4226 g for 30 min. Tissue homogenate (50 μg) was subjected to 10% SDS-PAGE containing 0.1% SDS and 1 g L−1 gelatin under non-reducing conditions without prior boiling. After electrophoresis, gels were washed in 2.5% Triton X-100 for 30 min to remove SDS and allow protein to renature and gels were then subsequently immersed in activity buffer (50 mM Tris-HCl, 5 mM CaCl2, 0.2 M NaCl, 0.02% NaN3) for 16 h at 37°C. The gels were then stained with 0.25% Coomassie brilliant blue (CBR-250) in methanol, acetic acid and water (4 : 1 : 5) followed by destaining with methanol, acetic acid and water (4 : 1 : 5). Enzymatic activities were detected as clear bands of gelatin lysis against a blue background [17].
2.6.5. SDS-PAGE and Western Immunoblotting
Wound tissues were chopped into small pieces and collected in Tris buffer (50 mM, pH 6.8) containing protease inhibitors, phenyl methyl sulfonyl fluoride (PMSF) and aprotonin (Sigma, St Louis, MO, USA) at 10 and 2 μg mL−1, respectively. Tissues were homogenized in Polytron homogenizer (PT 3100, Kinematica AG, Littau-Lucerne, Switzerland) with four strokes of 15 s each in ice bath. After homogenization, the samples were spun at 3000 g at 4°C and the total protein in the supernatant was measured according to the method of Lowry et al. [20].
Analysis of total protein in the tissue samples was done by PAGE in 4% (v/v) stacking and 10% (v/v) separation polyacrylamide gels in the presence of SDS, followed by staining with CBR-250. The homogenized tissue samples mixed in a sample buffer containing 1% SDS, 2% 2-mercaptoethanol and 10% glycerol were heat reduced in a boiling water bath, whereas the gel and running buffer contained 0.1 and 0.2% SDS, respectively. The molecular weight was determined using standard protein markers (broad range 200–6.9 kDa, Bio-Rad, Hercules, CA, USA) [17].
After electrophoretic separation of tissue proteins by SDS-PAGE, the proteins were electro-transferred on to a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked for 1 h in Tris-buffered saline with 0.1% Tween-20 (TBST, pH 7.5) containing 5% milk protein. After incubation with rabbit polyclonal primary antibody (VEGF, Santa Cruz Biotechnology, CA, USA) and mouse monoclonal primary antibody (Collagen type-III, Sigma) for 2 h, the blots were washed extensively with TBST. Primary antibodies were revealed via incubation with alkaline phosphatase-conjugated secondary antibody, goat-anti-rabbit and goat-anti-mouse (Santa Cruz Biotechnology), respectively for 1 h. The blots then were developed with 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (BCIP/NBT) liquid substrate system (Sigma) [17].
2.7. Statistical Analysis
Data are expressed as mean ± SE, and statistical significance between experimental and control values was analyzed by one way ANOVA followed by Dunnett's test using Graph Pad Prism 2.01 (Graph Pad Software Inc., La Jolla, CA, USA). A P-value <.05 was considered statistically significant.
3. Results
3.1. In Vitro Cytotoxicity Assay
No cytotoxic effect was observed on BHK-21 cells, when incubated under control conditions up to 400 μg mL−1 of SBT aqueous leaf extract.
3.2. Enhanced Angiogenesis Revealed by CAM Model
In the in vitro CAM model for angiogenesis, methylcellulose disks containing 40, 80, 160, or 240 μg SBT leaf extract were observed for new blood vessel formation. All the concentrations promoted angiogenesis but the maximum effect was observed with disk containing 80 μg extract. Quantitative measurement of the CAM assay showed that relative density of arteries in the SBT treated eggs was much higher compared with eggs containing disks without the extract (Figure 1) (Supplementary Figure S1).
Figure 1.
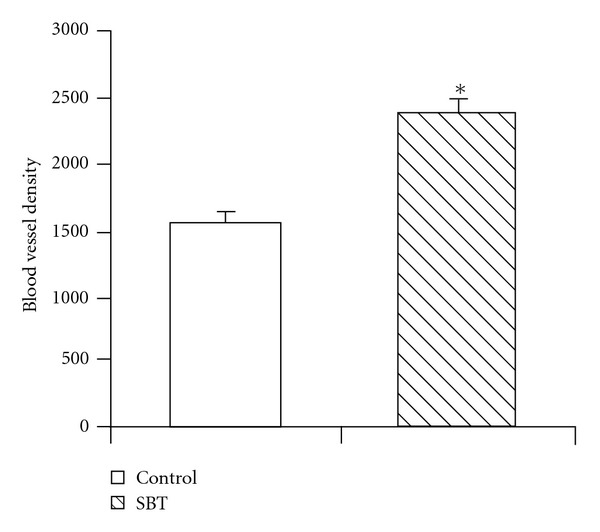
Density of blood vessels in in vitro CAM assays of chick eggs (12-days-old) for neovascularization. Control eggs were loaded with sterile methylcellulose disks and experimental eggs were treated with 80 μg SBT leaf extract impregnated in methylcellulose disks. Blood vessels were analyzed in terms of number of red pixel per unit area using NIH Image J software (v1.38) and AngioQuant software. Values are mean ± SE of six eggs. *P < .05 compared with control untreated eggs.
3.3. Wound-Healing Potential
Visual inspection of the wounds treated with SBT extract showed no evidence of wound bleeding, exudates, pus or wound inflammation at any time, and all wounds healed without incident. However, untreated burn wounds showed edema reflecting persistent inflammation, whereas wounds treated with SBT showed reduced or no edema. In general, the skin around the burns was soft and appeared normal except in the SSD group where it was dry.
3.3.1. Augmented Pro-Healing Parameters
In dose–response study, it was observed that 5.0% (w/w) SBT leaf extract was most effective concentration for wound-healing activity. Table 1 depicts the effect of topical application of various concentrations of SBT leaf extract on wound area reduction at different time intervals. The animals treated with 5.0% SBT extract showed significant faster reduction in wound area on day 4 (113.17 versus 138.5 mm2 in the untreated group) and day 8 (50.33 versus 90.83 mm2 in the untreated group) postwounding (Table 1). Levels of hydroxyproline, hexosamine, and total protein were also significantly increased in the rats treated with 5.0% SBT extract (Table 2).
Table 1.
Effect of topical application of SBT lyophilized aqueous leaf extract on wound area contraction (mm2).
| Post wounding day | Burn control | SBT aqueous leaf extract | SSD | |||
|---|---|---|---|---|---|---|
| 2.5% (w/w) | 5.0% (w/w) | 7.5% (w/w) | 10.0% (w/w) | |||
| Day 0 | 176.83 ± 0.87 | 177.67 ± 1.26 | 178.33 ± 0.99 | 179.17 ± 0.60 | 177.33 ± 0.99 | 176.5 ± 1.80 |
| Day 4 | 138.50 ± 4.60 (22) | 124.50 ± 2.79 (30) | 113.17 ± 3.48* (37) | 121.33 ± 3.57* (32) | 133.00 ± 3.17 (25) | 128.83 ± 5.28 (27) |
| Day 8 | 90.83 ± 2.63 (49) | 71.67 ± 3.68* (60) | 50.33 ± 3.84* (71) | 63.33 ± 3.79*(65) | 79.00 ± 3.48 (55) | 72.00 ± 4.33* (59) |
Values are mean ± SE; n = 6; Numbers in parenthesis indicate percentage of wound contraction.
*P < .05 compared with burn control.
Table 2.
Effect of topical application of SBT lyophilized aqueous leaf extract for 7 days on various biochemical parameters in granulation tissue.
| Group | Burn control | SBT aqueous leaf extract | SSD | |||
|---|---|---|---|---|---|---|
| 2.5% w/w | 5.0% w/w | 7.5% w/w | 10.0% w/w | |||
| Hydroxyproline (mg g−1 tissue wt.) | 22.80 ± 0.32 | 29.12 ± 0.70* | 29.96 ± 0.82* | 26.72 ± 0.73* | 23.19 ± 0.80 | 25.73 ± 0.67* |
| Hexosamine (mg g−1 tissue wt.) | 0.50 ± 0.04 | 0.58 ± 0.03 | 0.71 ± 0.04* | 0.62 ± 0.02* | 0.60 ± 0.03 | 0.53 ± 0.03 |
| Protein (mg g−1 tissue wt.) | 88.74 ± 4.18 | 115.63 ± 7.40* | 120.87 ± 7.77* | 109.11 ± 3.17* | 97.51 ± 3.61 | 109.78 ± 4.16* |
Value are mean ± SE; n = 6.
*P < .05 compared with burn control.
3.3.2. Antioxidant Status
Table 3 shows the antioxidants and MDA levels in the granulation tissue of control and SBT leaf extract (5.0%) treated burn wounds. There was significant increase in levels of enzymatic and non-enzymatic antioxidants such as glutathione (29%), superoxide dismutase (29%), catalase (23%), glutathione-S-transferase (20%) and vitamin C (46%), whereas the MDA level was significantly reduced (26%) in SBT treated burn wounds.
Table 3.
Effect of topical application of SBT lyophilized aqueous leaf extract (5.0% w/w) for 7 days on the levels of antioxidants and lipid peroxide (in terms of MDA) in granulation tissue.
| Parameters | Burn control | SBT leaf extract |
|---|---|---|
| Glutathione (μg mg−1 protein) | 1.68 ± 0.07 | 2.16 ± 0.09* |
| Vitamin C (μg mg−1 protein) | 2.46 ± 0.37 | 3.60 ± 0.27* |
| Superoxide dismutase (U mg−1 protein) | 1.25 ± 0.07 | 1.61 ± 0.06* |
| Catalase (U mg−1 protein) | 8.18 ± 0.35 | 10.03 ± 0.46* |
| Glutathione-S-transferase (U mg−1 protein) | 2.04 ± 0.08 | 2.45 ± 0.15* |
| MDA (nmol mg−1 protein) | 2.41 ± 0.12 | 1.79 ± 0.21* |
Values are mean ± SE; n = 6.
*P < .05 compared with burn control.
3.3.3. Histological and Morphometric Examinations
On the day 8, the SBT treated group showed more advanced re-epithelialization and layering with continuous basement membrane in addition to a better organization of the collagen bundles. The SBT leaf extract and SSD treated animals showed reduced congestion, edema and polymorphonuclear leukocytes infiltration. The control group showed a distinct space between old and new regenerating layers of epithelium. The histological studies showed an overall early recovery and regeneration in the SBT treated group when compared with control group (Figure 2) (Supplementary Figure S2). The morphometric analyses of the histological sections showed that the SBT treatment resulted in increased blood-vessel density and thicker epidermis when compared with untreated burn wounds (Table 4). Furthermore, Masson's trichome staining showed uniform, compact and regularly arranged collagen fibers in the wound tissue of SBT treated rats, whereas untreated burn wounds had less compact and irregularly arranged collagen fibers (Figure 3) (Supplementary Figure S3).
Figure 2.
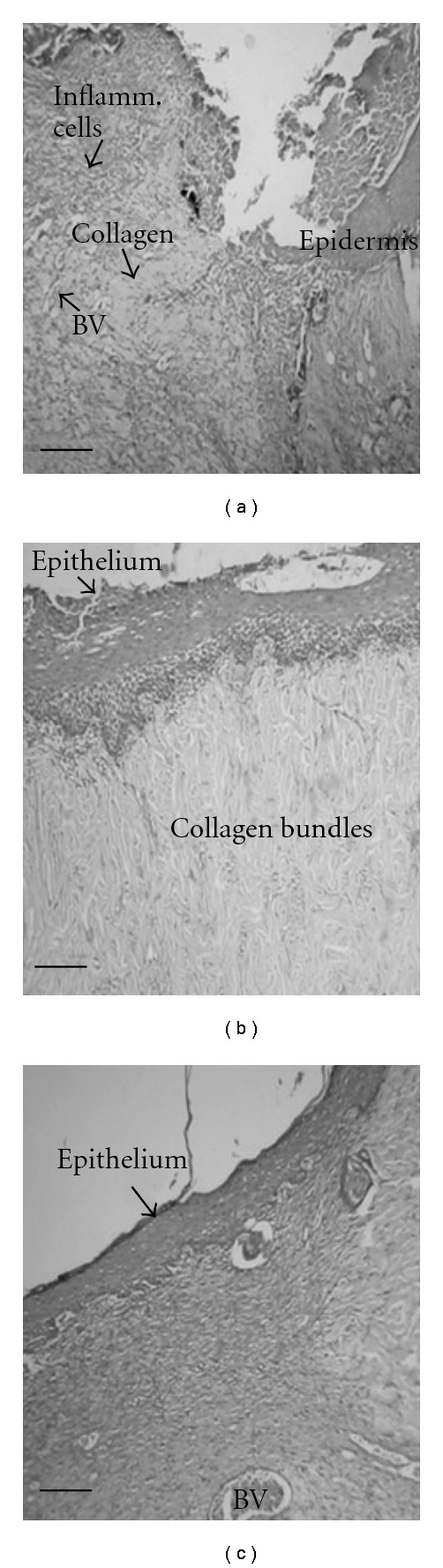
Histopathological changes on eighth post-wounding day in skin wound section of (a) control rats showing non-epithelialized wound surface with the presence of inflammatory cells, (b) SBT leaf extract (5.0%) and (c) SSD treated rats showing wound surface with well-organized thick epithelium. Collagen alignment is well developed in SBT leaf extract treated burn wounds. Scale bar, 100 μm.
Table 4.
Effect of topical application of SBT lyophilized aqueous leaf extract (5.0% w/w) for 7 days on epidermal thickness and blood vessel density.
| Burn control | SBT leaf extract | SSD | |
|---|---|---|---|
| Epidermal thickness (μm) | 100.33 ± 5.86 | 149.83 ± 5.9* | 147.83 ± 3.56* |
| Blood vessel density | 1244.17 ± 32.91 | 2188.50 ± 119.60* | 1278.50 ± 39.82 |
Values are mean ± SE; n = 6.
*P < .05 compared with burn control.
Figure 3.
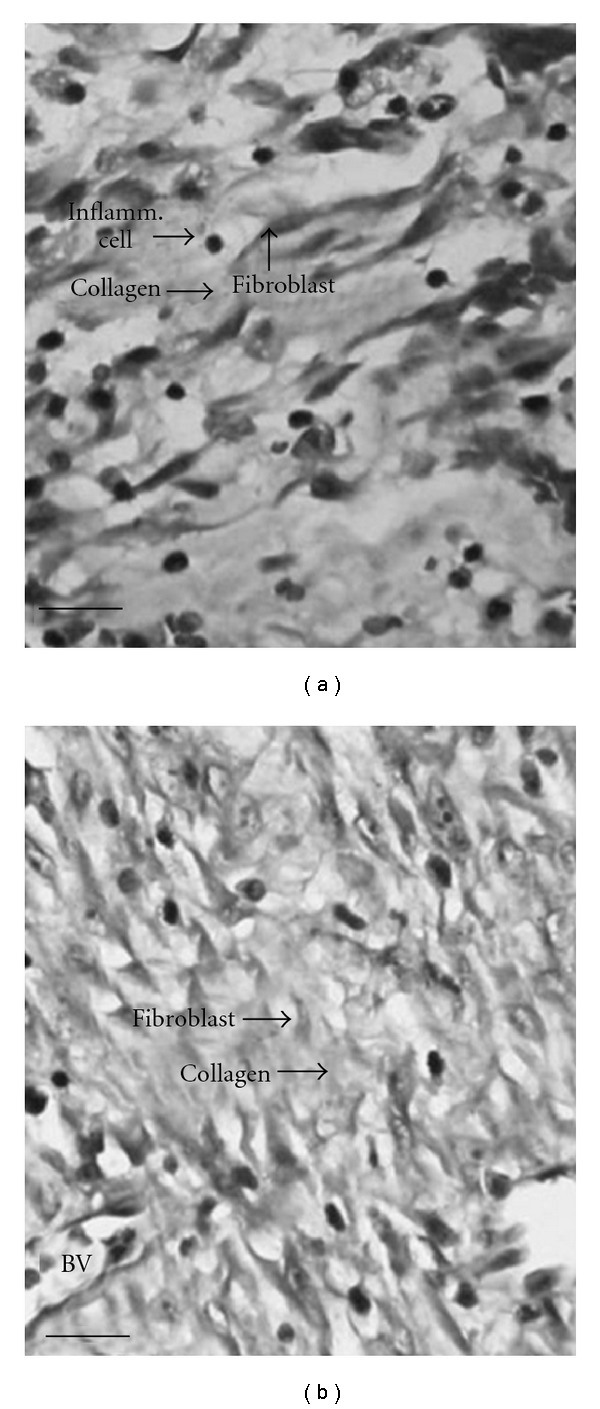
Masson's trichome staining for collagen on eighth-day postwounding in skin wound section of (a) control rats showing less and irregularly arranged collagen, (b) SBT leaf extract (5.0%) treated rats showing compact and well-aligned collagen fibers. Scale bar, 20 μm.
3.3.4. Enhanced Expression of MMPs
Gelatin zymography analysis of the granulation tissue after 4 and 7 days of topical treatment with SBT leaf extract showed increased expression of both MMP-2 and -9 in comparison with the control burn wounds (Figure 4(a)).
Figure 4.
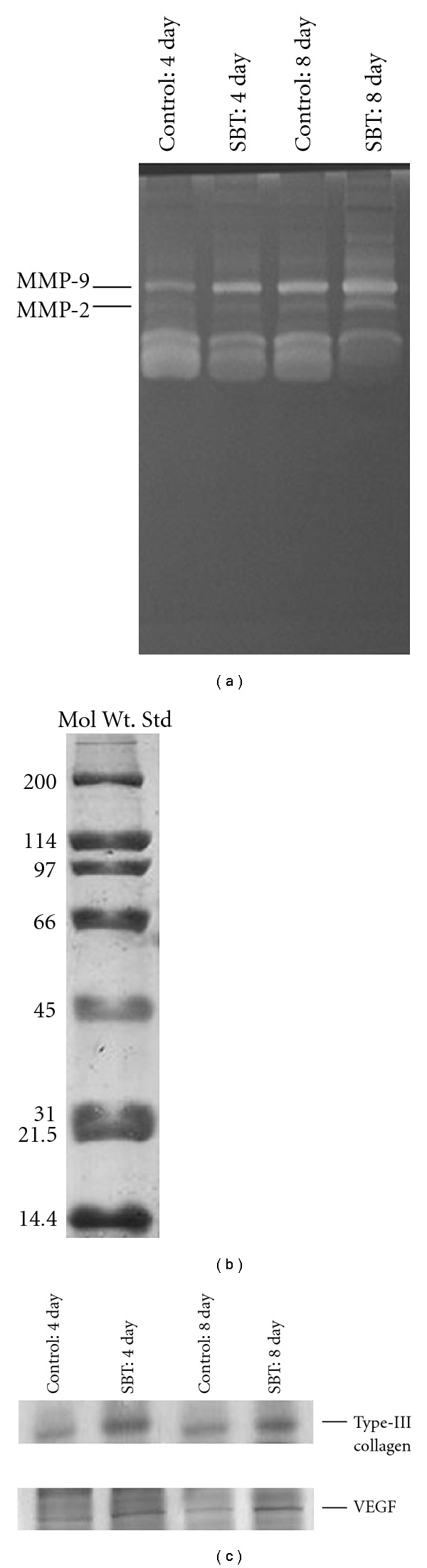
(a) Matrix metalloproteinase expression by gelatin zymography (10% SDS-PAGE, 1 g L−1 gelatin) in SBT leaf extract (5.0%) treated and untreated burn wound tissue of experimental rats after 4 and 7 days of treatment. (b) Expression of standard marker proteins (200–6.9 kDa, BIO RAD, 10% SDS–PAGE). (c) VEGF and collagen type-III analyzed by western blot in SBT leaf extract (5.0%) treated and untreated wound tissue of experimental rats after 4 and 7 days of topical treatment.
3.3.5. Up-Regulation of VEGF and Collagen Type-III
SDS-PAGE analysis of granulation tissue on fourth and eighth day of treatment with SBT leaf extract in experimental rats showed differential expression of some proteins as compared with the control burn wounds. Western blot analysis showed an up-regulated expression of VEGF and collagen type-III in SBT treated wounds as compared with untreated control burn wounds (Figure 4(c)).
4. Discussion
The results of the present study indicated that the aqueous extract of SBT leaf promotes wound healing in experimental burn wounds. This was demonstrated by a significant increase in the rate of wound contraction, hydroxyproline, hexosamine and protein contents. The increased wound contraction in SBT treated rats might be due to an enhanced activity of fibroblasts in regenerated wound tissue. Myofibroblasts are believed to play a key role in wound contraction by exerting tension on the surrounding extracellular matrix (ECM) and secreting ECM proteins such as collagen to stabilize the contraction. Collagen is a major protein of ECM and component that ultimately contributes to wound strength [1]. The enhanced levels of hydroxyproline and hexosamine in SBT treated burn wounds probably provided the strength to the regenerated wound tissue. We observed enhanced expression of collagen type-III in SBT treated burn wounds as compared with untreated control group. Collagen type-III is a predominant form of collagen in the early stages of wound healing that helps in providing strength to the provisional ECM [1].
MMPs are key players in every phase of the healing process, that is, eliminate damaged protein, destroy the provisional ECM, facilitate migration to the center of the wound, remodel the granulation tissue, probably control angiogenesis and also regulate the activity of some growth factors [27]. Increased expression of MMP-2 and -9 in SBT treated experimental rats suggested that SBT played an important role in remodeling of the ECM.
Angiogenesis is a critical component of wound healing. Delayed or aberrant revascularization at the wound sites contributes to the etiology of chronic wounds [2]. The SBT treatment promoted angiogenesis in both in vivo and in vitro models as indicated by histological studies and new vessel formation in CAM model, which might be due to enhanced expression of VEGF in regenerated tissue. VEGF is an important pro-angiogenic cytokine and improves angiogenesis during wound healing by stimulating the migration and proliferation of endothelial cells through the ECM [28]. Furthermore, it has been reported that SBT extract significantly increases blood flow to the surrounding skin of the burn wounds and can enhance wound healing [29].
Free radicals are generated at the site of injury, which are known to impair the healing process by causing damage to cellular membranes, nucleotides, proteins and lipids [3]. In the present study, the MDA levels were found to be significantly decreased after SBT extract treatment, suggesting decreased oxidative injury, which could be due to increased quenching or scavenging of oxygen free radicals by the elevated levels of enzymatic and non-enzymatic antioxidants. The use of antioxidants has been shown to promote wound healing process [30]. The SBT leaf extract was found to be rich in flavonoids (catechin, rutin, quercetin, kaempferol and isorhamnetin) [10]. Flavonoids are efficient antioxidants, capable of scavenging free radical species and reported to play an important role in augmenting the wound-healing process [31–33]. Presence of flavonoids could be one of the factors contributing to wound-healing potential of SBT leaf extract.
SSD undoubtedly is a very efficient anti-bacterial agent; however, it is reported to have an inhibitory effect on the proliferation of fibroblasts and keratinocytes that may impair the wound-healing process [5]. In the present study, it was observed that prolonged application of SSD resulted in the excessive dryness of the regenerated and normal skin surrounding the burn area. However, the SBT treated wounds had softer surrounding skin and satisfactory regenerated wound tissue.
In conclusion, as illustrated in Figure 5, the present study suggests that lyophilized aqueous extract of SBT leaves possesses a significant wound-healing activity in full-thickness burn wounds. The topical treatment with SBT leaf extract augmented endogenous antioxidants and prevented the free-radical-mediated tissue injury. It also played an important role in angiogenesis, ECM deposition and remodeling phase of wound healing.
Figure 5.
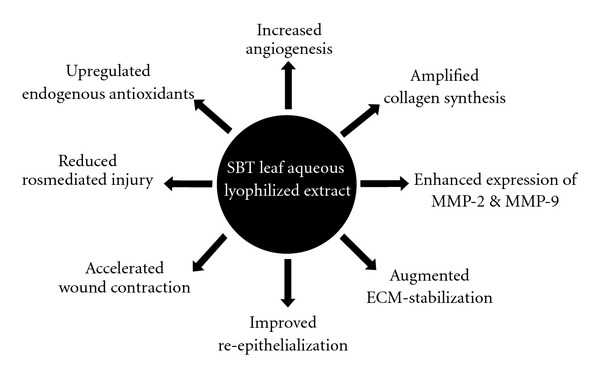
The schematic diagram showing the possible effect of the SBT leaf extract in promoting wound-healing activity.
Supplementary Data
Supplementary Data are available at ECAM online.
Funding
Defence Research and Development Organization, Delhi, India; Senior Research Fellowship from Council of Scientific and Industrial Research (CSIR), New Delhi, India (to N.K.U.).
Supplementary Material
Figure S1: Relative density of arteries in the SBT treated eggs was much higher compared with eggs containing disks without the extract.
Figure S2: The histological studies showed an overall early recovery and regeneration in the SBT treated group when compared with control group.
Figure S3: Masson's trichome staining showed uniform, compact and regularly arranged collagen fibers in the wound tissue of SBT treated rats.
Acknowledgments
The authors are thankful to Director, Defence Institute of Physiology and Allied Sciences, Delhi, India, for his keen interest and providing support to the study.
References
- 1.Singer AJ, Clark RAF. Cutaneous wound healing. The New England Journal of Medicine. 1999;341(10):738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 2.Gupta A, Upadhyay NK, Sawhney RC, Kumar R. A poly-herbal formulation accelerates normal and impaired diabetic wound healing. Wound Repair and Regeneration. 2008;16(6):784–790. doi: 10.1111/j.1524-475X.2008.00431.x. [DOI] [PubMed] [Google Scholar]
- 3.Arturson G. Pathophysiology of the burn wound and pharmacological treatment. The Rudi Hermans Lecture, 1995. Burns. 1996;22(4):255–274. doi: 10.1016/0305-4179(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 4.Thomas GW, Rael LT, Bar-Or R, et al. Mechanisms of delayed wound healing by commonly used antiseptics. Journal of Trauma. 2009;66(1):82–90. doi: 10.1097/TA.0b013e31818b146d. [DOI] [PubMed] [Google Scholar]
- 5.Lee A-RC, Leem H, Lee J, Park KC. Reversal of silver sulfadiazine-impaired wound healing by epidermal growth factor. Biomaterials. 2005;26(22):4670–4676. doi: 10.1016/j.biomaterials.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 6.Shetty S, Udupa S, Udupa L. Evaluation of antioxidant and wound healing effects of alcoholic and aqueous extract of Ocimum sanctum Linn in rats. Evidence-Based Complementary and Alternative Medicine. 2008;5(1):95–101. doi: 10.1093/ecam/nem004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nayak BS, Sandiford S, Maxwell A. Evaluation of the wound-healing activity of ethanolic extract of Morinda citrifolia L. leaf. Evidence-Based Complementary and Alternative Medicine. 2009;6(3):351–356. doi: 10.1093/ecam/nem127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phan TT, Hughes MA, Cherry GW. Enhanced proliferation of fibroblasts and endothelial cells treated with an extract of the leaves of Chromolaena odorata (Eupolin), an herbal remedy for treating wounds. Plastic and Reconstructive Surgery. 1998;101(3):756–765. doi: 10.1097/00006534-199803000-00027. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A, Kumar R, Pal K, Banerjee PK, Sawhney RC. A preclinical study of the effects of seabuckthorn (Hippophae rhamnoides L.) leaf extract on cutaneous wound healing in albino rats. International Journal of Lower Extremity Wounds. 2005;4(2):88–92. doi: 10.1177/1534734605277401. [DOI] [PubMed] [Google Scholar]
- 10.Zu Y, Li C, Fu Y, Zhao C. Simultaneous determination of catechin, rutin, quercetin kaempferol and isorhamnetin in the extract of sea buckthorn (Hippophae rhamnoides L.) leaves by RP-HPLC with DAD. Journal of Pharmaceutical and Biomedical Analysis. 2006;41(3):714–719. doi: 10.1016/j.jpba.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 11.Negi PS, Chauhan AS, Sadia GA, Rohinishree YS, Ramteke RS. Antioxidant and antibacterial activities of various seabuckthorn (Hippophae rhamnoides L.) seed extracts. Food Chemistry. 2005;92(1):119–124. [Google Scholar]
- 12.Ganju L, Padwad Y, Singh R, et al. Anti-inflammatory activity of Seabuckthorn (Hippophae rhamnoides) leaves. International Immunopharmacology. 2005;5(12):1675–1684. doi: 10.1016/j.intimp.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Geetha S, Sai Ram M, Singh V, Ilavazhagan G, Sawhney RC. Anti-oxidant and immunomodulatory properties of seabuckthorn (Hippophae rhamnoides)—an in vitro study. Journal of Ethnopharmacology. 2002;79(3):373–378. doi: 10.1016/s0378-8741(01)00406-8. [DOI] [PubMed] [Google Scholar]
- 14.Chawla R, Arora R, Singh S, et al. Radioprotective and antioxidant activity of fractionated extracts of berries of Hippophae rhamnoides . Journal of Medicinal Food. 2007;10(1):101–109. doi: 10.1089/jmf.2006.007. [DOI] [PubMed] [Google Scholar]
- 15.Saggu S, Divekar HM, Gupta V, Sawhney RC, Banerjee PK, Kumar R. Adaptogenic and safety evaluation of seabuckthorn (Hippophae rhamnoides) leaf extract: a dose dependent study. Food and Chemical Toxicology. 2007;45(4):609–617. doi: 10.1016/j.fct.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Lobb RR, Alderman EM, Fett JW. Induction of angiogenesis by bovine brain derived class 1 heparin-binding growth factor. Biochemistry. 1985;24(19):4969–4973. doi: 10.1021/bi00340a001. [DOI] [PubMed] [Google Scholar]
- 17.Upadhyay NK, Kumar R, Mandotra SK, et al. Safety and healing efficacy of Sea buckthorn (Hippophae rhamnoides L.) seed oil on burn wounds in rats. Food and Chemical Toxicology. 2009;47(6):1146–1153. doi: 10.1016/j.fct.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Woessner JF., Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Archives of Biochemistry and Biophysics. 1961;93(2):440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 19.Elson LA, Morgan WTJ. A colorimetric method for the determination of glucosamine and chondrosamine. The Journal of Biochemistry. 1933;27:1824–1828. doi: 10.1042/bj0271824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowry OH, Rosenburgh NJ, Farr AL, Randell BJ. Protein measurement with folin phenol reagent. The Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- 21.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. The Journal of Laboratory and Clinical Medicine. 1963;61:882–888. [PubMed] [Google Scholar]
- 22.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. European Journal of Biochemistry. 1974;47(3):469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 23.Aebi H. Catalase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York, NU, USA: Academic Press; 1984. pp. 673–684. [Google Scholar]
- 24.Habig WH, Pabst MJ, Jakoby WB. Glutathione S transferases. The first enzymatic step in mercapturic acid formation. Journal of Biological Chemistry. 1974;249(22):7130–7139. [PubMed] [Google Scholar]
- 25.Rae JH. Chemical determination of ascorbic acid, dehydroascorbic acid and diketogluconic acids. In: Glick Q, editor. Methods of Biochemical Analysis. New York, NY, USA: Interscience Publishers; 1984. pp. 115–139. [Google Scholar]
- 26.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 27.Inkinen K, Turakainen H, Wolff H, Ravanti L, Kähäri V-M, Ahonen J. Expression and activity of matrix metalloproteinase-2 and -9 in experimental granulation tissue. APMIS. 2000;108(5):318–328. doi: 10.1034/j.1600-0463.2000.d01-63.x. [DOI] [PubMed] [Google Scholar]
- 28.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. Journal of Molecular Medicine. 1999;77(7):527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- 29.Seven B, Varoglu E, Aktas O, et al. Hippophae rhamnoides L. and dexpanthenol-bepanthene on blood flow after experimental skin burns in rats using 133Xe clearance technique. Hellenic Journal of Nuclear Medicine. 2009;12(1):55–58. [PubMed] [Google Scholar]
- 30.Martin A. The use of antioxidants in healing. Dermatologic Surgery. 1996;22(2):156–160. doi: 10.1111/j.1524-4725.1996.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 31.Bao M, Lou Y. Flavonoids from seabuckthorn protect endothelial cells (EA.hy926) from oxidized low-density lipoprotein induced injuries via regulation of LOX-1 and eNOS expression. Journal of Cardiovascular Pharmacology. 2006;48(1):834–841. doi: 10.1097/01.fjc.0000232064.64837.67. [DOI] [PubMed] [Google Scholar]
- 32.Kim H, Kawazoe T, Han D-W, et al. Enhanced wound healing by an epigallocatechin gallate-incorporated collagen sponge in diabetic mice. Wound Repair and Regeneration. 2008;16(5):714–720. doi: 10.1111/j.1524-475X.2008.00422.x. [DOI] [PubMed] [Google Scholar]
- 33.Gomathi K, Gopinath D, Ahmed MR, Jayakumar R. Quercetin incorporated collagen matrices for dermal wound healing processes in rat. Biomaterials. 2003;24(16):2767–2772. doi: 10.1016/s0142-9612(03)00059-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Relative density of arteries in the SBT treated eggs was much higher compared with eggs containing disks without the extract.
Figure S2: The histological studies showed an overall early recovery and regeneration in the SBT treated group when compared with control group.
Figure S3: Masson's trichome staining showed uniform, compact and regularly arranged collagen fibers in the wound tissue of SBT treated rats.


