Abstract
Although patients treated with HIV protease inhibitor (PI) containing regimens manifest increases in naïve T cell number, it is unclear whether this is due to reduction in viral replication or a direct drug effect. We questioned whether Nelfinavir monotherapy directly impacted naïve T-cell number in HIV-negative individuals. HIV-negative volunteers received Nelfinavir, 1250 mg orally, BID for 3 weeks, and T-cell receptor recombination excision circles (TREC) content in peripheral blood were assessed. Whereas TREC copies did not change over 3 weeks in untreated controls, TREC copies/copies CCR5 increased following Nelfinavir monotherapy in 8 patients (p<0.02), and did not change in 7 patients (p=NS). Those patients who responded were younger than those who did not with a median age of 55 years for responders and 71 years for non-responders (p<0.03). The increase in TREC was most pronounced in those patients less than 40-years old (p<0.01). Moreover, the patients who did not increase TREC levels were more likely to have suffered a medical illness previously shown to reduce thymic function. In HIV-negative patients, monotherapy with the HIV PI Nelfinavir for 21 days increases TREC-positive naïve T cell number, particularly in individuals who are healthy and young.
Keywords: HIV protease inhibitors, TREC, Age, Nelfinavir
2. INTRODUCTION
Highly active antiretroviral therapy (HAART) has altered the course of Human Immunodeficiency Virus (HIV) infection. Treatment with a combination of nucleoside analogs and protease inhibitors result in profound inhibition of HIV replication, as well as quantitative and qualitative improvements in host immune function. T-cell counts increase to varying degrees in patients on HAART therapy. The increase in CD4+ T cells may result from homeostatic expansion of existing T cells, as well as production of new T cells from the thymus. Several groups have reported that HIV-infected individuals receiving HAART produce thymic-derived naïve T cells, as determined by T-cell receptor (TCR) excision circles (TREC) (1–4). However, it remains unclear whether HAART increases thymic T-cell output by reducing the pathogenic effects of HIV on the thymus or by a direct drug effect on the thymus.
A recent report demonstrated an increase in TREC-positive cells from the peripheral blood of HIV-negative health-care workers who received Nelfinavir, Zidovudine, and Lamivudine HAART for 28 days for post-HIV-exposure prophylaxis (PEP) (5), raising the possibility that HAART may directly impact thymic output.
Increasing evidence suggests that HIV protease inhibitors have intrinsic immunomodulatory activity. These effects include inhibition of proteasome function (6), anti-tumor effects in models of Kaposi's sarcoma (7), reduced cellular activation associated with inhibited NF-κB activation (8), direct inhibition of calpain (9), matrix metalloprotinease (7), and aspartyl proteases produce by PCP (10), and Candida (11), as well as anti-apoptotic effects (12). These effects also occur in vivo, since mice implanted with Kaposi's sarcoma and treated with Ritonavir experience significant tumor regression, and NOD/SCID mice implanted with adult T-cell leukemia cells experience reduced tumor growth in the presence of Ritonavir (8, 13). In addition, treatment of mice with Nelfinavir/Ritonavir reduces mortality due to polymicrobial sepsis (14), Fas-induced hepatitis (15), SEB/D-gal induced shock (15) and reduces neuronal injury due to cerebral ischemia (15). Therefore, HIV PIs have immunomodulatory effects in vivo during disease conditions unrelated to HIV.
A principal function of the thymus is to produce naïve T cells, which can be recognized in the periphery by the presence of T-cell receptor excision circles (TREC) (16). Upon entry to the thymus, bone-marrow derived CD34+ progenitors interact with the thymic stroma and undergo a series of maturation events, which require both activation, as well as the selective induction of apoptosis (17). Since HIV PIs might impact both activation, as well as apoptosis, we hypothesized that the effect of HAART on naïve T-cell number might be due to the PI component of such therapy. Since increases in naïve T-cell numbers might occur through expansion of existing peripheral naïve cells or by de novo thymic production, there is no phenotypic marker that can be used to selectively identify recent thymic emigrant T cells (18). Instead, we specifically assessed the impact of Nelfinavir on thymic-derived naïve T-cell numbers in the peripheral blood in healthy volunteers by quantitating TREC/CCR5 copy levels (19).
3. MATERIALS AND METHODS
Following approval by the Mayo Institutional Review Board, peripheral blood was sampled from 46 healthy blood donors older than 18 years of age for baseline TREC analysis. For the separate Nelfinavir trial, 15 HIV-negative individuals with no active illness and older than 18 years of age were given Nelfinavir, 1250 mg orally, twice a day for 21 days. Blood for TREC analysis was obtained before therapy and on days 3, 5, 7, 14, and 21 after starting therapy. At each blood draw, side-effects were monitored and recorded from each subject. Severity of diarrhea was graded as: Grade 0 - None; Grade 1-increase of 4 stools/day over pretreatment; Grade 2-increase of 4–6 stools/day, or nocturnal stools; Grade 3-increase of 7 stools/day or incontinence, or dehydration episode requiring parenteral support; Grade 4- episode of hemodynamic collapse requiring intensive care.
3.1. TREC analysis
DNA was isolated from peripheral blood mononuclear cells (PBMC) using an QIAamp Blood Mini DNA Kit (Qiagen, Valencia, CA) and assessed for signal joint (sj) TREC content relative to genomic CCR5 copies by real-time PCR in a spectroflourometric thermal cycler (ABI PRISM 7700 or 7900, PE Applied Biosystems, Foster City, CA). SjTREC reactions contained 12.5pmol primers (forward CACATCCCTTTCAACCATGCT), reverse GCCAGCTGCAGGGTTTAGG) 3pmoles TaqMan probe (FAM-ACACCTCTGGTTTTTGTAAAGGTGCCCACTTAMRA), 1× TaqMan Universal PCR Master Mix (PE Applied Biosystems, Foster City, CA), and 50 to 400ng of template DNA in a total volume of 25 μl. CCR5 reactions contained 12.5 pmol primers (forward GTGTCAAGTCCAATCTATGACATCAA, and reverse GCCTGCGATTTGCTTCACA), 3pmoles TaqMan probe (FAM-TATTATACATCGGAGCCCTGCCAAAAAATCATAMRA), 1× TaqMan Universal PCR Master Mix, and 50- 400 ng of template of genomic DNA in a total volume of 25μl. Thermal cycling conditions consisted of a 2-minute incubation at 50°C and an initial denaturation/activation step at 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute in a microplate format. For each plate of reactions, standard curves were generated with 1–100,000 copies of human sjTREC plasmid (provided by Dr. D. Douek, NIH) and human CCR5 plasmid (cloned by RTPCR into pCI) in order to calculate copies of TREC versus CCR5.
3.2. Statistical analysis
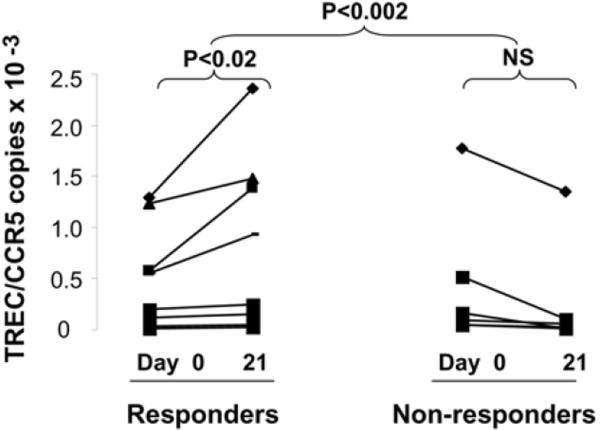
Experiments from every figure were performed in duplicate and repeated at least twice. All measurements are presented as means and standard deviations with statistical comparisons made between day 21 and baseline TREC/CCR5 measurements using the Student's t test for paired observations. For Figure 2, change in baseline to day 21 TREC/CCR5 levels were stratified according to subjects age with 2 subjects <40, 2 subjects 40–65, and 11 subjects >65 years old.
Figure 2.
Nelfinavir therapy increases TREC levels in a subset of healthy adults.
4. RESULTS
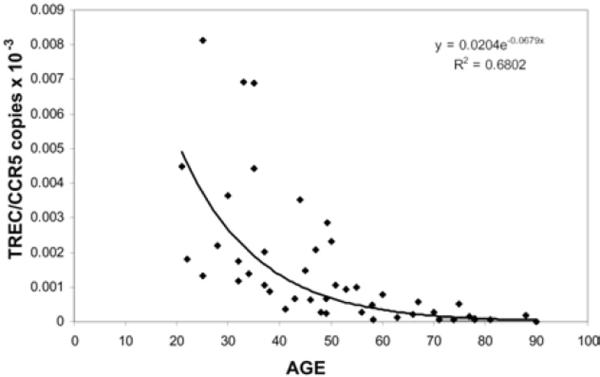
TREC levels were analyzed in 46 volunteers, ages 22–95 years old. Consistent with previous findings (20), TREC level decreased with advancing subject age (R2= 0.6802) (Figure 1). Fifteen HIV-negative adults, ages 22–95, were treated with Nelfinavir, 1250 mg orally, twice a day for 21 days. Nelfinavir monotherapy was clinically well tolerated; six (40%) of the subjects experienced Grade 1 or 2 diarrhea, and all subjects completed the 21 days of therapy. Compliance was good in all patients, as assessed by self-reporting and pill counts at the time of each blood draw.
Figure 1.
TREC levels decrease with advancing age. Baseline TREC/CCR5 copies were assessed from the peripheral blood of healthy adults. The results are plotted according to subject age.
In order to establish if Nelfinavir monotherapy increased naïve T cells after 21 days of treatment in adult subjects, a paired analysis of change in TREC levels from baseline to days 3, 5, 7, 14, and 21 were determined. In our previous report, TREC/CCR5 levels on T cells did not alter over 21 days in an untreated control group of two HIV-negative adults (5). No significant change in TREC levels occurred below baseline (day 0) and days 3, 5, 7, or 14. After 21 days of therapy, although there was no change between baseline and day 21 TREC/CCR5 levels when the entire cohort was analyzed, it was apparent that some subjects responded to Nelfinavir therapy with increasing TREC/CCR5 copy levels. Since no patient in our control group had any increase in TREC levels over a 3-week period, we analyzed volunteers who increased TREC/CCR5 levels separately (5). Eight volunteers (53% of total) responded to Nelfinavir therapy with increases in TREC numbers from baseline to day 21, with a median increase in TRECs of 1.2 copies TREC/CCR5 (p<0.02). Conversely, 7 volunteers (47% of total) did not increase TREC numbers following 21 days of Nelfinavir (Figure 2).
Although only half of the subjects increased TREC/CCR5 levels after Nelfinavir therapy, sub-group analysis demonstrated an age-related association. The average increase in TREC levels for subjects less than 40 was 1.2 TREC/CCR5 copies, for subjects 40–65 was 0.80 TREC/CCR5 copies, and the average change in TREC levels for subjects greater than 65 was 0.89 TREC/CCR5 copies after Nelfinavir therapy. The median age of the responders was 55 years (range 23–90), whereas, the mean age of the non-responders was 71 years (range 37–88) (p<0.03), suggesting that age, and consequently, thymic reserve might determine thymic response to Nelfinavir.
5. DISCUSSION
Our results confirm that the normal process of aging results in impaired thymopoiesis (Figure 1) that may predispose to infections and cancers (20–23). Moreover, aging-associated impairments in thymopoiesis, and/or thymic reserve, appear to influence thymic response to Nelfinavir therapy (Figure 2). Younger compared to older individuals responded more robustly to Nelfinavir, consistent with observations that aging is associated with reduced thymic stroma, size, function, and output (24).
The overlap in ages between Nelfinavir responders and non-responders is likely reflective of aging-independent causes of early thymic atrophy, including puberty, pregnancy, stress, exercise and trauma, as well as disease states including cancers, Diabetes, and Parkinson's disease [reviewed in (24)]. Of note, five of the seven non-responders had prior or chronic illness that may have decreased their thymic reserve, including prostate cancer, non-small cell lung cancer, Diabetes mellitus, and Parkinson's disease (25). In contrast, only one of eight of the responders had a similar illness (Table 1). Increased understanding of thymic biology has led to cytokine and hormone-based approaches to reversing thymic involution in animal models using agents such as IL-7 (26), GH (27), IGF-1 (28). Recognizing that HIV PI may also impact thymic function, offers new insights into potential therapies aimed at restoring thymic function in vivo. Moreover, the changes observed in this study confirm and highlight the direct immunologic effects of HIV PI therapy.
Table 1.
Characteristics of HIV-negative subjects treated with Nelfinavir for 21 days
| Subject | Age | Cancer | Chronic Disease | Delta TREC (×10−3) |
|---|---|---|---|---|
| 1 | 67 | Breast | +0.30 | |
| 2 | 88 | +0.20 | ||
| 3 | 71 | Parkinson's Disease | −0.30 | |
| 4 | 75 | −0.15 | ||
| 5 | 77 | Prostate | −0.12 | |
| 6 | 78 | Diabetes Mellitus | −0.10 | |
| 7 | 81 | Parkinson's Disease | −0.12 | |
| 8 | 90 | +0.20 | ||
| 9 | 78 | Lung | −0.11 | |
| 10 | 63 | +0.34 | ||
| 11 | 74 | +0.14 | ||
| 12 | 36 | −0.70 | ||
| 13 | 26 | +0.30 | ||
| 14 | 23 | +1.1 | ||
| 15 | 33 | +0.8 |
Delta TREC levels represent day 21 TREC levels (end of therapy) – baseline TREC levels (before therapy)
ACKNOWLEDGEMENTS
SRV is supported by a grant from the Mayo Program in Translational Immunovirology and Biodefense and a Kogod Award in Aging from the Mayo Foundation. ADB is supported by NIH RO1 AI62261, RO1 AI40384 and a Burroughs Wellcome award ID#1005160. SRV and ADB have filed a patent related to off label use of HIV protease inhibitors for immunomodulatory effects. Trial registration: Clinicaltrials.gov registry number: NCT00346619
Abbreviatons
- HAART
Highly Active Antiretroviral Therapy
- HIV
Human Immunodeficiency Virus
- PBMC
Peripheral Blood Mononuclear Cells
- PEP
Post-HIV-exposure Prophylaxis
- sj
Signal Joint
- TCR
T-cell receptor
- TREC
T-cell Receptor Excision Circles
REFERENCES
- 1.Teixeira L, Valdez H, McCune JM, Koup RA, Badley AD, Hellerstein MK, Napolitano LA, Douek DC, Mbisa G, Deeks S, Harris JM, Barbour JD, Gross BH, Francis IR, Halvorsen R, Asaad R, Lederman MM. Poor CD4 T cell restoration after suppression of HIV-1 replication may reflect lower thymic function. Aids. 2001;15:1749–56. doi: 10.1097/00002030-200109280-00002. [DOI] [PubMed] [Google Scholar]
- 2.Ruiz-Mateos E, de la Rosa R, Franco JM, Martinez-Moya M, Rubio A, Soriano N, Sanchez-Quijano A, Lissen E, Leal M. Endogenous IL-7 is associated with increased thymic volume in adult HIV-infected patients under highly active antiretroviral therapy. Aids. 2003;17:947–54. doi: 10.1097/00002030-200305020-00002. [DOI] [PubMed] [Google Scholar]
- 3.Ometto L, De Forni D, Patiri F, Trouplin V, Mammano F, Giacomet V, Giaquinto C, Douek D, Koup R, De Rossi A. Immune reconstitution in HIV-1-infected children on antiretroviral therapy: role of thymic output and viral fitness. Aids. 2002;16:839–49. doi: 10.1097/00002030-200204120-00003. [DOI] [PubMed] [Google Scholar]
- 4.De Rossi A, Walker AS, Klein N, De Forni D, King D, Gibb DM. Increased thymic output after initiation of antiretroviral therapy in human immunodeficiency virus type 1-infected children in the Paediatric European Network for Treatment of AIDS (PENTA) 5 Trial. J Infect Dis. 2002;186:312–20. doi: 10.1086/341657. [DOI] [PubMed] [Google Scholar]
- 5.Graham DB, Bell MP, Huntoon CJ, Weaver JG, Hawley N, Badley AD, McKean DJ. Increased thymic output in HIV-negative patients after antiretroviral therapy. Aids. 2005;19:1467–72. doi: 10.1097/01.aids.0000182520.69159.8a. [DOI] [PubMed] [Google Scholar]
- 6.Andre P, Groettrup M, Klenerman P, de Giuli R, Booth BL, Jr., Cerundolo V, Bonneville M, Jotereau F, Zinkernagel RM, Lotteau V. An inhibitor of HIV-1 protease modulates proteasome activity, antigen presentation, and T cell responses. Proc Natl Acad Sci USA. 1998;95:13120–4. doi: 10.1073/pnas.95.22.13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sgadari C, Barillari G, Toschi E, Carlei D, Bacigalupo I, Baccarini S, Palladino C, Leone P, Bugarini R, Malavasi L, Cafaro A, Falchi M, Valdembri D, Rezza G, Bussolino F, Monini P, Ensoli B. HIV protease inhibitors are potent anti-angiogenic molecules and promote regression of Kaposi sarcoma. Nat Med. 2002;8:225–32. doi: 10.1038/nm0302-225. [DOI] [PubMed] [Google Scholar]
- 8.Dewan MZ, Uchihara JN, Terashima K, Honda M, Sata T, Ito M, Fujii N, Uozumi K, Tsukasaki K, Tomonaga M, Kubuki Y, Okayama A, Toi M, Mori N, Yamamoto N. Efficient intervention of growth and infiltration of primary adult T-cell leukemia cells by an HIV protease inhibitor, ritonavir. Blood. 2006;107:716–24. doi: 10.1182/blood-2005-02-0735. [DOI] [PubMed] [Google Scholar]
- 9.Ghibelli L, Mengoni F, Lichtner M, Coppola S, De Nicola M, Bergamaschi A, Mastroianni C, Vullo V. Anti-apoptotic effect of HIV protease inhibitors via direct inhibition of calpain. Biochem Pharmacol. 2003;66:1505–12. doi: 10.1016/s0006-2952(03)00505-7. [DOI] [PubMed] [Google Scholar]
- 10.Atzori C, Angeli E, Mainini A, Agostoni F, Micheli V, Cargnel A. In vitro activity of human immunodeficiency virus protease inhibitors against Pneumocystis carinii. J Infect Dis. 2000;181:1629–34. doi: 10.1086/315437. [DOI] [PubMed] [Google Scholar]
- 11.Cassone A, De Bernardis F, Torosantucci A, Tacconelli E, Tumbarello M, Cauda R. In vitro and in vivo anticandidal activity of human immunodeficiency virus protease inhibitors. J Infect Dis. 1999;180:448–53. doi: 10.1086/314871. [DOI] [PubMed] [Google Scholar]
- 12.Phenix BN, Lum JJ, Nie Z, Sanchez-Dardon J, Badley AD. Antiapoptotic mechanism of HIV protease inhibitors: preventing mitochondrial transmembrane potential loss. Blood. 2001;98:1078–85. doi: 10.1182/blood.v98.4.1078. [DOI] [PubMed] [Google Scholar]
- 13.Pati S, Pelser CB, Dufraine J, Bryant JL, Reitz MS, Jr., Weichold FF. Antitumorigenic effects of HIV protease inhibitor ritonavir: inhibition of Kaposi sarcoma. Blood. 2002;99:3771–9. doi: 10.1182/blood.v99.10.3771. [DOI] [PubMed] [Google Scholar]
- 14.Weaver JG, Rouse MS, Steckelberg JM, Badley AD. Improved survival in experimental sepsis with an orally administered inhibitor of apoptosis. Faseb J. 2004;18:1185–91. doi: 10.1096/fj.03-1230com. [DOI] [PubMed] [Google Scholar]
- 15.Weaver JG, Tarze A, Moffat TC, Lebras M, Deniaud A, Brenner C, Bren GD, Morin MY, Phenix BN, Dong L, Jiang SX, Sim VL, Zurakowski B, Lallier J, Hardin H, Wettstein P, van Heeswijk RP, Douen A, Kroemer RT, Hou ST, Bennett SA, Lynch DH, Kroemer G, Badley AD. Inhibition of adenine nucleotide translocator pore function and protection against apoptosis in vivo by an HIV protease inhibitor. J Clin Invest. 2005;115:1828–38. doi: 10.1172/JCI22954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kong F, Chen CH, Cooper MD. Thymic function can be accurately monitored by the level of recent T cell emigrants in the circulation. Immunity. 1998;8:97–104. doi: 10.1016/s1074-7613(00)80462-8. [DOI] [PubMed] [Google Scholar]
- 17.Zediak VP, Bhandoola A. Aging and T cell development: interplay between progenitors and their environment. Semin Immunol. 2005;17:337–46. doi: 10.1016/j.smim.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Al-Harthi L, Marchetti G, Steffens CM, Poulin J, Sekaly R, Landay A. Detection of T cell receptor circles (TRECs) as biomarkers for de novo T cell synthesis using a quantitative polymerase chain reaction-enzyme linked immunosorbent assay (PCR-ELISA) J Immunol Methods. 2000;237:187–97. doi: 10.1016/s0022-1759(00)00136-8. [DOI] [PubMed] [Google Scholar]
- 19.Douek DC, McFarland RD, Keiser PH, Gage EA, Massey JM, Haynes BF, Polis MA, Haase AT, Feinberg MB, Sullivan JL, Jamieson BD, Zack JA, Picker LJ, Koup RA. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–5. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 20.Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–52. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 21.Dare R, Sykes PJ, Morley AA, Brisco MJ. Effect of age on the repertoire of cytotoxic memory (CD8+CD45RO+) T cells in peripheral blood: the use of rearranged T cell receptor gamma genes as clonal markers. J Immunol Methods. 2006;308:1–12. doi: 10.1016/j.jim.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 22.Hadrup SR, Strindhall J, Kollgaard T, Seremet T, Johansson B, Pawelec G, thor Straten P, Wikby A. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. 2006;176:2645–53. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- 23.Goronzy JJ, Weyand CM. T cell development and receptor diversity during aging. Curr Opin Immunol. 2005;17:468–75. doi: 10.1016/j.coi.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 24.Taub DD, Longo DL. Insights into thymic aging and regeneration. Immunol Rev. 2005;205:72–93. doi: 10.1111/j.0105-2896.2005.00275.x. [DOI] [PubMed] [Google Scholar]
- 25.Baba Y, Kuroiwa A, Uitti RJ, Wszolek ZK, Yamada T. Alterations of T-lymphocyte populations in Parkinson disease. Parkinsonism Relat Disord. 2005;11:493–8. doi: 10.1016/j.parkreldis.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Phillips JA, Brondstetter TI, English CA, Lee HE, Virts EL, Thoman ML. IL-7 gene therapy in aging restores early thymopoiesis without reversing involution. J Immunol. 2004;173:4867–74. doi: 10.4049/jimmunol.173.8.4867. [DOI] [PubMed] [Google Scholar]
- 27.Murphy WJ, Durum SK, Longo DL. Role of neuroendocrine hormones in murine T cell development. Growth hormone exerts thymopoietic effects in vivo. J Immunol. 1992;149:3851–7. [PubMed] [Google Scholar]
- 28.Montecino-Rodriguez E, Clark R, Dorshkind K. Effects of insulin-like growth factor administration and bone marrow transplantation on thymopoiesis in aged mice. Endocrinology. 1998;139:4120–6. doi: 10.1210/endo.139.10.6263. [DOI] [PubMed] [Google Scholar]