Scheme 2.
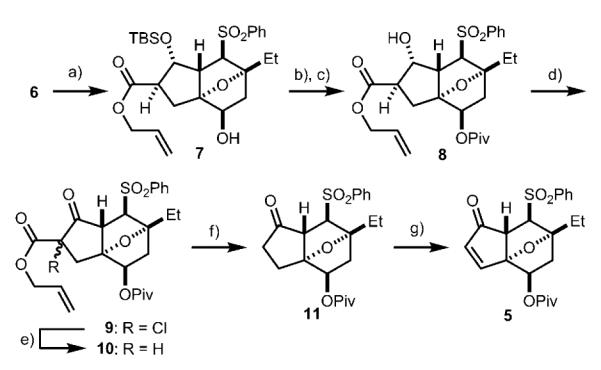
Synthesis of enone 5. a) NaBH4, allyl alcohol, −78°C, 5 min; then acetone, 23°C, 15 min, 89 %; b) PivCl, pyridine, CH2Cl2, 40°C, 24 h; c) TBAF, THF, 23°C, 16 h, 91 % over two steps; d) DMSO, (COCl)2, −78°C, 30 min; then Et3N, 23°C, 30 min; e) Zn dust, AcOH, 23°C, 2 h, 96 % over two steps; f) [PdCl2(PPh3)2], nBu3SnH, AcOH, toluene, 0°C, 30 min; then 110°C, 30 min, 85 %; g) LHMDS, THF, −78°C, 30 min; then PhSeBr, −78°C, 5 min; then H2O2,CH2Cl2/THF 1:1, 0°C, 4 h, 73 %. Piv = trimethylacetyl, TBAF = tetrabutylammonium fluoride, THF = tetrahydrofuran, DMSO = dimethyl sulfoxide, LHMDS = lithium bis(trimethylsilyl)amide.
