Abstract
Hepatitis C virus (HCV) is presently the leading indication for liver transplantation in Western countries. Treatment for HCV infection includes a combination of pegylated interferon and ribavirin, which produces highly variable response rates. This reflects the lack of information regarding the roles of host and viral components during viral pathogenesis. Vital processes regulated by the liver, including metabolism, lipid homeostasis, cellular proliferation, and the immune response, are known to be systematically dysregulated as a result of persistent HCV infection. Nuclear receptors and their ligands are recognized as indispensable regulators of liver homeostasis. Pathways mediated by the nuclear receptor superfamily have been shown to be profoundly disrupted during HCV infection, leading to an increased importance in elucidating the exact nature of this complex relationship. Expanded understanding of the role of nuclear receptors in HCV infection may therefore be an essential step in the search for a more universally effective treatment.
Keywords: hepatitis, liver, steatosis, retinoic acid, retinoic acid receptor, autophagy
1. Introduction
Hepatitis C virus (HCV) is a critical global health problem, with over 170 million infected worldwide. After primary infection, 70% of patients develop chronic infection, and 5–20% progress to liver cirrhosis within 20 years ("NIH Consensus Statement on Management of Hepatitis C: 2002," 2002). There are known risk factors that contribute to development of cirrhosis, including alcohol consumption, insulin resistance, and hepatic steatosis; however, the specific molecular mechanisms involved in HCV disease progression are not well understood.
The nuclear receptor superfamily constitutes a group of ligand-activated transcription factors that play critical roles in such diverse biological processes as xenobiotic metabolism, endobiotic homeostasis, proliferation, differentiation, inflammation, and cell death. It is hypothesized that dysregulation of nuclear receptor-mediated signaling contributes to development of pathological conditions associated with chronic HCV infection. Nuclear receptors have historically been attractive drug targets, and drugs that modulate nuclear receptor activity are among the most prescribed pharmaceuticals on the market (Pearce, 2004). Nuclear receptors have the potential to act as therapeutic targets for HCV-associated pathological changes. This review focuses on the relationship between HCV and nuclear receptors.
1.1 HCV
HCV is an enveloped positive-strand RNA virus, with 6 major genotypes and more than 100 subtypes (Nguyen, 2005). The high degree of variability reflects the lack of fidelity of the viral RNA-dependent RNA polymerase. The viral genome consists of a 5’ noncoding region followed by an open reading frame coding structural and nonstructural proteins, followed by a 3’ noncoding region that is required for replication. The translation product is a 3,000 amino acid polyprotein that is cleaved by viral and cellular proteases into individual proteins (Joyce, 2010). The primary target of HCV in vivo is the hepatocyte. Viral entry involves viral envelope proteins E1 and E2, cell surface receptors CD81, scavenger receptor class B type 1, low-density lipoprotein receptor (LDL-R), and cell surface heparan proteoglycans (Barth, 2006) as well as the more recently identified co-receptors claudin and human occludin (Evans, 2007; Ploss, 2009a). The virus is endocytosed, the envelope disintegrates in the cytoplasm and subsequently translation takes place using both viral and host machinery and the ribosome in the endoplasmic reticulum (ER) to translate the viral RNA into a polyprotein chain. Host and viral proteases cleave the polyprotein into 10 proteins that catalyze viral RNA replication and provide for the assembly of new viral particles (Dubuisson, 2008; Joyce, 2010). Lipid droplets are also essential components of viral replication (Miyanari, 2007). Virus maturation is complete when the encapsulated viral particle is enveloped by a lipid layer as it exits the ER (Pawlotsky, 2007). HCV hijacks host very low-density lipoprotein (VLDL) processing machinery, including microsomal transfer protein and apolipoprotein B, to facilitate viral exit (Gastaminza, 2008). It is clear that HCV is closely associated with cellular lipid homeostasis that is regulated by nuclear receptor-mediated pathways (Figure 1). Thus, the action of nuclear receptors can influence HCV disease progression. Potential mechanisms are discussed below.
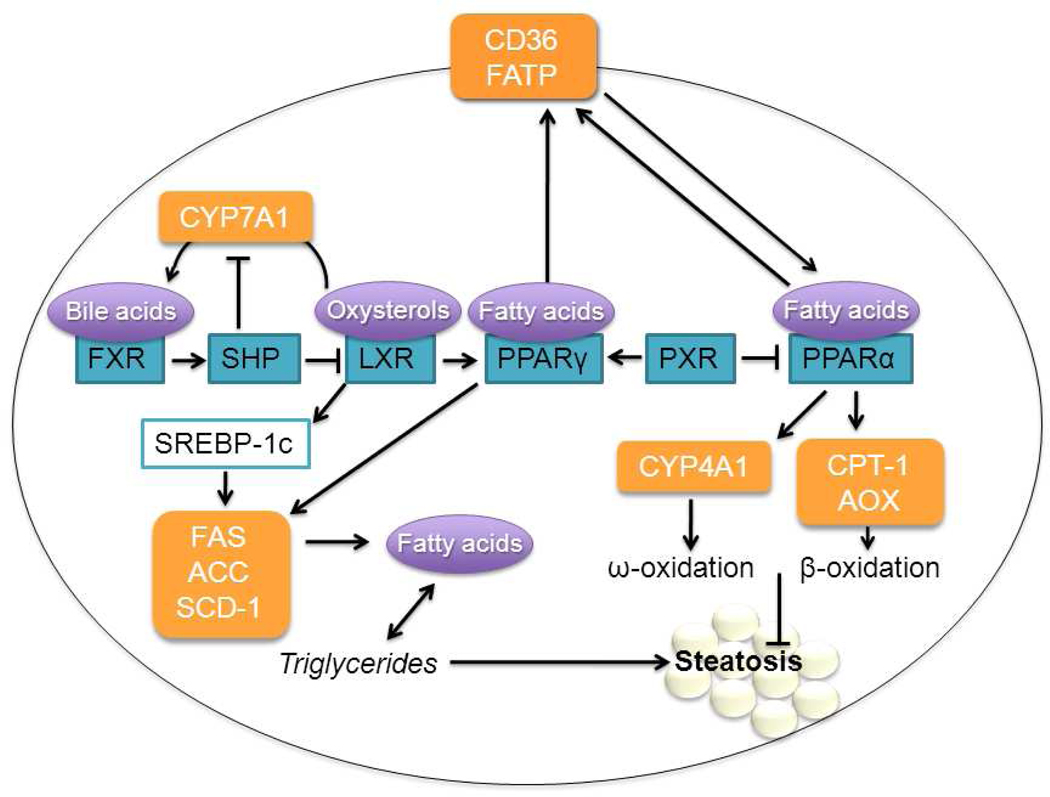
Figure 1. Nuclear receptor control of lipid homeostasis in the hepatocyte.
PPAR-controlled fatty acid transporters CD36 and FATP import fatty acids into the hepatocyte (Motojima, 1998). These fatty acids can then be stored as triglycerides or undergo ω- or β-oxidation, principally controlled by PPARα (Reddy, 2001). Inhibition of the PPARα pathway can lead to steatosis. LXR induces lipogenic transcription factor SREBP-1c, which acts by up regulating lipogenic genes including FAS, ACC, and SCD-1 (Lima-Cabello, 2010). PPARγ also induces these genes (Gavrilova, 2003; S. Yu, 2003). PXR promotes lipogenesis through inhibition of PPARα and activation of PPARγ (Zhou, 2006). FXR inhibits lipogenesis through SHP induction, which blocks LXR. SHP also inhibits CYP7A1, the rate-limiting enzyme for the formation of bile acids (Gadaleta, 2010). ACC, acyl-coA carboxylase; AOX, acyl-coA oxidase ;CPT-1, carnitine palmitoyl transferase 1; CYP4A1, cytochrome P450 4A1; CYP7A1, cholesterol 7α-hydroxylase; FAS, fatty acid synthase; FATP, fatty acid transport protein; FXR, farnesoid X receptor; LXR, liver X receptor; PPAR, peroxisome proliferator activated receptor; PXR, pregnane X receptor; SCD-1, stearyl-coA dehydrogenase; SHP, small heterodimer partner; SREBP-1c, steroid regulatory element-binding protein.
In addition to lipid metabolism, HCV disrupts numerous other cellular processes. HCV can hinder the immune response through direct interference with the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway, inhibition of antiviral genes such as 2’5’ oligoadenylate synthetase and protein kinase RNA-activated, attenuation of interferon sensitizing genes by induction of IL-8, and interference with T-cell response (Burke, 2010; Gale, 2005). Pegylated interferon (IFN) in combination with ribavirin is the current treatment standard for HCV infection. However, there is only a 50% response rate in patients infected with genotype 1 virus, which is the most common genotype in Western populations (Fowell, 2010). Chronic hepatitis C infection typically progresses slowly without symptoms; until recently, many patients typically presented with advanced disease. Disease progression is characterized by hepatic inflammation and often steatosis, leading to fibrosis, cirrhosis, and in some cases, hepatocellular carcinoma (HCC). HCV infection causes chronic hepatic inflammation, which leads to the release of stellate-cell activating cytokine transforming growth factor β (TGFβ). Activated hepatic stellate cells (HSCs) promote extracellular matrix deposition and fibrogenesis (Feld, 2006). Virus-mediated insulin resistance can also cause HSC activation; genotypes 1 and 3 are associated with insulin resistance and fat accumulation, respectively (Douglas, 2009). HCV proteins can also induce apoptosis via increased oxidative stress (Abdalla, 2005; Okuda, 2002). Eventually, regenerating hepatocytes become surrounded by fibrotic tissue and form the nodules characteristic of cirrhosis. Thus, HCV creates a hepatic microenvironment favorable to the development of HCC, while also acting through other pro-oncogenic pathways.
Research has continually been hampered by the lack of a suitable infectious model, as the natural species tropism of HCV is limited to humans and chimpanzees (Ploss, 2009b). Recently, there has been a great deal of progress, including the establishment of HCV replicons, an infectious culture model, and small animal models expressing the HCV core protein (Brass, 2007). There have also been recent developments in human liver chimeric mice capable of propagating HCV (Bissig, 2010; Mercer, 2001; Washburn, 2011). While these models have been extremely helpful, there are still areas of concern. Viral titer may be different in rodent and human models, and there is a question of the cytopathicity of the core protein to the mouse hepatocyte (Chayama, 2011; Pasquinelli, 1997). There is an urgent need to develop additional models to study the pathogenesis of HCV.
1.2 Nuclear Receptors
Nuclear receptors are classified into three families based on their DNA- and ligand-binding properties. The most well characterized group is the endocrine receptors, including the glucocorticoid receptor. The second class is made up of the orphan nuclear receptors, which are structurally related, but lack known natural ligands. The last group and the focus of this review are the adopted orphan nuclear receptors, originally part of the orphan nuclear receptor class until the discovery of their physiological ligands. Members of this group include the lipid-responsive peroxisome proliferator activated receptors (PPARs), the oxysterol receptor liver X receptor (LXR), the bile acid receptor farnesoid X receptor (FXR), the xenobiotic receptors pregnane X receptor (PXR) and constitutive androstane receptor (CAR), the retinoic acid receptor (RAR), and the retinoid x receptor (RXR) (K. Wang, 2008). These receptors are structurally conserved, each containing an N-terminal regulatory domain that functions independently of ligand, a DNA-binding domain, a hinge domain to determine intracellular localization, a ligand binding domain and a C-terminal domain containing a variable sequence unique to the individual nuclear receptor (Lee, 2007). Please see recent reviews for the roles of these receptors (Bushue, 2010; Glass, 2010; Gyamfi, 2010; McKenna, 2009; K. Wang, 2008).
1.3 Nuclear Receptors and HCV
Nuclear receptors are master regulators of lipid homeostasis, inflammation, and endobiotic metabolism, processes intricately involved in HCV infection and progression. HCV-mediated dysregulation of these processes influences the replicative efficiency of the virus (Chang, 2007; Fujita, 2006; Morbitzer, 2005; Pazienza, 2010; Watashi, 2003). Because nuclear receptors regulate processes essential to the progression of chronic hepatitis C (Figure 2), it is important to understand the interaction between HCV and nuclear receptor-mediated signaling pathways (Blackham, 2010; Woodhouse, 2010). A comprehensive understanding of this relationship will elucidate the potential of nuclear receptors as prospective drug targets.
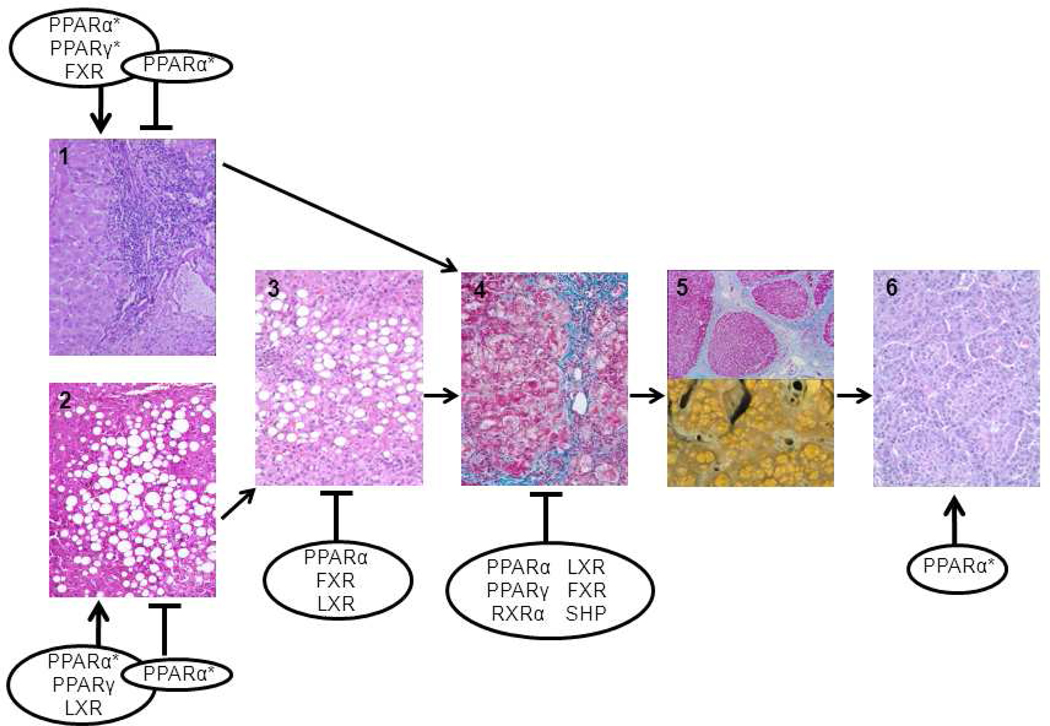
Figure 2. Nuclear receptors and the pathogenesis of HCV.
Nuclear receptor-mediated pathways influence the progression of HCV-induced chronic hepatitis. HCV infection can lead to (1) inflammation, (2) steatosis, (3) steatohepatitis, (4) fibrosis, (5) cirrhosis, and (6) HCC. Depending on the model used, conflicting results were obtained. For example, PPARα can either promote or prohibit viral replication (Lyn, 2009; Nishimura-Sakurai, 2010; Rakic, 2006; Tanaka, 2008a; Tanaka, 2008b). Some of the findings have only been found in animal or cell line models (*). Please see Table 1 for details. Depending on the stage of the disease and the cell type involved, activation of nuclear receptors can either inhibit or promote the pathogenic process. For example, activation of PPARγ and LXR favors steatosis (Moriishi, 2007; Yasui, 2010) but inhibits fibrosis (Beaven, 2010; Zhao, 2006), and activation of FXR favors viral replication (Chang, 2007) but may have anti-inflammatory and anti-fibrotic effects (Fiorucci, 2004; Fiorucci, 2005; Zhang, 2009). This complicated scheme makes treatment challenging and stage-specific treatment strategies may have to be considered. FXR, farnesoid X receptor; LXR, liver X receptor; PPAR, peroxisome proliferator activated receptor; RXR, retinoid X receptor; SHP, small heterodimer partner.
2. PPARs
PPARs play a crucial role in the maintenance of hepatic lipid and glucose homeostasis, the inflammatory process, cell differentiation, and proliferation. Because of this diversity, PPARs have been the subject of intense scrutiny. PPARs are natural targets for HCV-related study because of their ubiquitous nature in the liver and involvement in processes known to be dysregulated by HCV.
2.1 PPARα
PPARα is the major regulator of the hepatic fatty acid metabolism pathway. Defects in PPARα signaling can lead to reduced energy metabolism and increased lipid accumulation, eventually resulting in steatosis and steatohepatitis. PPARα exerts direct transcriptional control over genes involved in fatty acid transport, uptake, and oxidation. Its downstream targets include carnitine palmitoyl transferase 1 (CPT-1), acyl-CoA oxidase (AOX), and fatty acid transporters CD36, fatty acid transport protein (FATP), and liver fatty acid binding protein (LFABP) (Motojima, 1998; Poirier, 2001; Reddy, 2001). Closely related is its mediation of the inflammatory response, as fatty acid derivatives such as leukotriene B4 capable of inducing inflammation are degraded through PPARα-activated pathways. PPARα−/− mice display prolonged inflammatory response (Devchand, 1996), and activation of PPARα leads to decreased levels of acute phase response proteins C-reactive protein, fibrinogen, and serum amyloid A (Gervois, 2004; Kleemann, 2003; Maison, 2002) as well as adhesion molecules (Marx, 1999).
Human studies show impaired PPARα activity and decreased CPT-1 expression in the livers of chronic hepatitis C patients (Cheng, 2005; Dharancy, 2005). CPT-1 is a key β-oxidation gene involved in the transport of long-chain fatty acids across the mitochondrial membrane. Consequently, CPT-1 and PPARα mRNA and protein levels are significantly decreased in steatotic hepatitis C-infected livers compared with non-steatotic livers (Yasui, 2010). It is likely that HCV core protein is the culprit, as it contains RNA binding domains capable of suppressing the transcriptional activity of PPARα (Dharancy, 2005). This type of interaction has been shown for other viral protein-nuclear receptor partners (RXRα-HCV, HNF4α-HBV, RXRα-HBV) (H. Tang, 2001; Tsutsumi, 2002).
Conversely, studies in HCV core transgenic mice found expression of core protein is associated with PPARα activation (Tanaka, 2008a; Tanaka, 2008b). The core serves as a co-activator and nuclear stabilizer of PPARα, and may transactivate PPARα through ERK1/2 activation and p38 MAPK phosphorylation (Tanaka, 2008b). Although seemingly counterintuitive, PPARα up regulates genes involved in the generation of reactive oxygen species (ROS) through activation and induction of AOX and cytochrome P450 4A1. This causes increased β and ω oxidation, respectively, which can damage the mitochondrial membrane, impairing β oxidation and leading to fatty acid accumulation in hepatocytes (Tanaka, 2008a; Tanaka, 2008b). PPARα also increases expression of fatty acid transporters, promoting fatty acid influx and leading to further PPARα activation by acting as PPAR ligands (Tanaka, 2008a; Tanaka, 2008b). This helps explain the role of PPARα in HCV-induced steatosis in the animal model. Human liver, however, expresses less than 1/10 the PPARα mRNA and functional DNA binding capacity of mouse liver, suggesting that PPARα signaling may be significantly more robust in mice (Gonzalez, 2008). This may explain species differences in the carcinogenic properties of PPARα. An animal model that over expresses HCV core protein in humanized PPARα mice (Q. Yang, 2008) may help to address the species difference.
PPARα also has anti-inflammatory effects; activation results in decreased levels of intercellular adhesion molecules (I-CAMs) and vascular cell adhesion molecules (V-CAMs), which decreases inflammatory cell infiltration into the mouse liver (Ip, 2004). PPARα down regulation observed in humans may also exacerbate HCV-induced inflammation (Dharancy, 2005; Li, 2010). For example, the HCV core protein negatively regulates the inhibitory effect of PPARα in nuclear factor kappa B (NFκB) activity (Li, 2010) and thus activates NFκB. PPARα-mediated pathways also play a role in liver fibrosis. PPARα activation reduces hepatic fibrosis in a rat model by enhancing catalase expression and activity, resulting in reduced oxidative stress and less HSC activation, and thus reduced liver injury (Toyama, 2004).
PPARα is also involved in the development of HCV-related HCC in animal models. PPARα+/+/HCV core transgenic mice develop HCC at a rate of about 30% higher than PPARα+/−/HCV core or PPARα−/−/HCV core transgenic mice (Koike, 2009; Tanaka, 2008b). This may be due to the involvement of PPARα in ROS generation that subsequently leads to increases in oxidative DNA damage, predisposing hepatocytes to malignant transformation. PPARα activation in mice also leads to increases in cell division by altering cyclin and cyclin-dependent kinase (CDK) expression without subsequent increases in apoptosis (Tanaka, 2008b). Though activation of PPARα in rodents causes hepatomegaly and liver cancer, carcinogenic effects of PPARα have not been noted in humans.
Conflicting evidence has also emerged concerning the role of PPARα in HCV replication upon study of its ligands. It has been shown that both PPARα agonists and antagonists inhibit HCV replication (Nishimura-Sakurai, 2010; Rakic, 2006). Bezafibrate, a PPARα activator, is widely used to treat hyperlipidemia by reducing serum LDL, VLDL, and triglycerides. Fibrates may decrease HCV RNA titers in patients who were previously unresponsive to IFN therapy (Fujita, 2006; Fujita, 2004). This effect is attributable to its reduction of HCV RNA bound to LDL. It is also possible that PPARα-mediated suppression of NFκB is involved in HCV repression (Fujita, 2006). The repressive effects of an agonist are logical due to the anti-inflammatory as well as anti-lipogenic properties of PPARα. However, PPARα antagonists also make sense given the environment needed for viral replication. HCV replication takes place in membranous ER-derived complexes that associate with lipid droplets. HCV core induces changes in lipid metabolism (Adinolfi, 2001; Kapadia, 2005) as well as the formation and redistribution these droplets (Boulant, 2008; Miyanari, 2007). PPARα antagonist 2-chloro-5-nitro-N-(pyridyl) benzamide causes hyperlipidemia and consequent disruption in the membranous structures and in the composition of lipid droplets (notably an increase in triglyceride content) in Huh7 cells. This causes changes in the localization of HCV RNA and disruption of the replication complex (Lyn, 2009; Rakic, 2006). It is also likely that the dysregulation of critical lipid metabolizing and transfer genes regulated by PPARα is involved in the observed repression. The relationship between HCV and PPARs observed in different models is summarized in Table 1.
Table 1.
Relationship between HCV and PPARs
| Model | Genotype | Treatment | Effect* | Reference | |
|---|---|---|---|---|---|
| Cell line |
Vector | ||||
| HepG2 | pEF352neo expressing HCV core protein | 1b | PPARα↓ | (Cheng et al., 2005; Dharancy et al., 2005) | |
| Huh7 | pFK-I389neo/NS3-3′/5.1 subgenomic replicon | 1b, 2a | PPARα agonists clofibrate, fenofibrate | HCV↓ after treatment | (Nishimura-Sakurai et al., 2010) |
| pFK-I389neo/NS3-3′/5.1 subgenomic replicon | 1b | PPARα antagonist BA# | HCV↓ after treatment | (Rakic et al., 2006) | |
| pFK-I389neo/NS3-3′/5.1 subgenomic replicon | 1a, 3a | PPARα antagonist BA | HCV↓ after treatment | (Lyn et al., 2009) | |
| pFK-I389neo/NS3-3′/5.1 subgenomic replicon | 1b | PPARα antagonist BA | HCV↓ after treatment | (Rakic et al., 2006) | |
| pRep-Feo replicon | 1b | PPARγ↑ | (Kim et al., 2009) | ||
| pIRES2-EGFP expressing HCV 3a core protein | 3a | PPARγ↓ | (Pazienza et al., 2010) | ||
| pIRES2-EGFP expressing HCV 3a or 1b core protein | 1a, 3a | PPARγ↓ | (Pazienza et al., 2007) | ||
| Human | HCV status (patient sample size) | ||||
| HCV− (40) HCV+ (46) |
1, 2, 3, 4 | PPARα↓ in HCV+ patients | (Cheng et al., 2005; Dharancy et al., 2005) | ||
| HCV+ steatosis− (57) HCV+ steatosis+ (43) |
1, 2 | PPARα↓ in steatosis patients | (Yasui et al., 2010) | ||
| HCV+ treatment (6) | 1b, 2a | PPARα agonist benzafibrate | HCV↓ by PPARα agonist | (Fujita et al., 2004) | |
| HCV+ no treatment (15) HCV+ treatment (15) |
1b, 2a, 2b | PPARα agonist benzafibrate | HCV↓ by PPARα agonist | (Fujita et al., 2006) | |
| Normal (23) NAFLD+ HCV− (43) HCV+ (44) |
1 | PPARγ↑ in HCV+ patients | (Lima-Cabello et al., 2010) | ||
| HCV+ Peg-IFNα2b/ribavirin (49) HCV+ Peg-IFNα2b/ribavirin + treatment (48) |
4 | PPARγ agonist pioglitazone | HCV↓ by PPARγ agonist | (Khattab et al., 2010) | |
| Mouse | Genetic background | ||||
| C57BL/6N HCV core transgenic | 1b | PPARα↑ | (Tanaka et al., 2008b) | ||
| C57BL/6N HCV core transgenic | 1b | PPARα↑ | (Koike, 2009; Tanaka et al., 2008a) |
Effect: Modulation in PPAR-mediated signaling or HCV replication
BA: 2-chloro-5-nitro-N-(pyridyl) benzamide
2.2 PPARγ
PPARγ, by contrast, is the major regulator of hepatic lipogenesis and adipocyte differentiation. PPARγ mediates the development of steatosis in mouse models by up regulating critical genes in the lipogenic pathway including fatty acid synthase (FAS), acyl-coA carboxylase (ACC), and stearyl-coA deyhydrogenase-1 (SCD-1) (Gavrilova, 2003; S. Yu, 2003). It also shares control of fatty acid transporters CD36 and FATP with PPARα (Motojima, 1998). As with PPARα, there is some controversy about the interaction between PPARγ and HCV. Several cell line studies using the human HCC cell line Huh7 have linked HCV (specifically viral protein NS5A) with increased transcriptional activity of PPARγ (K. Kim, 2009; K. H. Kim, 2007) as well as increased recruitment of PPARγ coactivator 1 α (PGC1α) to the peroxisome proliferator response element (K. Kim, 2009). Increased expression of PPARγ mRNA has also been observed in human livers infected with HCV, with the highest levels in patients with HCV-associated steatosis (Lima-Cabello, 2010). PPARγ-mediated up regulation of lipogenic genes is a relatively simple mechanism for HCV-related steatosis. However, HCV genotype 3a-mediated down regulation of PPARγ in Huh7 cells has also been observed, leading to induction of suppressor of cytokine signaling 7 (SOCS-7), which is normally repressed by PPARγ, and helping the virus inhibit cytokine signaling and escape the immune system (Pazienza, 2010). SOCS-7 also plays a role in the development of insulin resistance, a well-known result of chronic HCV infection, by degrading insulin receptor substrate 1 in Huh7 cells (Pazienza, 2007; Pazienza, 2010).
PPARγ activation attenuates stellate cell activation in mice and rats (L. Yang, 2006; J. Yu, 2010). PPARγ is expressed in quiescent HSCs, and this expression is absent in active cells (Marra, 2000). Reintroducing PPARγ into HCV-activated HSCs causes a return of the activated HSC to quiescence (L. Yang, 2006; J. Yu, 2010). A possible mechanism is PPARγ-mediated inhibition of TGFβ, which causes HSC activation (Zhao, 2006). These activities demonstrate PPARγ’s role in hepatic fibrosis.
PPARγ ligands thiazolidinediones (TZDs) are in widespread use due to their insulin-sensitizing actions. Pioglitazone, a member of this class, has been used in clinical trials in combination with traditional HCV treatment with some success in improving viral response (Khattab, 2010; Overbeck, 2008). Since increasing levels of insulin resistance are associated with reduced response rates to peg-IFN plus ribavirin, increases in insulin sensitivity may be helping nonresponsive patients in these studies. TZDs also increase adiponectin levels, which are inversely correlated with HCV-induced steatosis (Petit, 2005). Adiponectin is a serum protein secreted by hepatocytes, and its levels correlate inversely with BMI, abdominal fat, and insulin resistance. Adiponectin is involved in increased insulin sensitivity by decreasing triglyceride content in the muscle and liver of mice (Yamauchi, 2001).
3. FXR
FXR is highly expressed in the liver and intestine due to its role in lipid, cholesterol, and bile acid (BA) homeostasis (Gadaleta, 2010). BAs are the products of cholesterol metabolism; they are synthesized solely by the liver, secreted into the small intestine via the gallbladder, and then reabsorbed and passed into enterohepatic circulation via the portal vein (Gadaleta, 2010). The primary BAs (cholate [CA] and chenodeoxycholate [CDCA]) are secreted into the intestinal tract, where they are broken down into secondary BAs (lithocholate [LCA] and deoxycholate [DCA]) (Gadaleta, 2010; Scholtes, 2008). Both primary and secondary BAs are natural ligands for FXR (Gadaleta, 2010). When FXR is activated by BAs, it is translocated to the nucleus and typically dimerizes with RXR (Gadaleta, 2010). FXR also dimerizes with the retinoic acid receptor (RAR) (Vavassori, 2009). The action of FXR is to regulate the expression of cholesterol 7α-hydroxylase (CYP7A1), which is the rate-limiting enzyme in cholesterol metabolism and subsequent BA formation (Gadaleta, 2010; Vavassori, 2009). FXR controls the synthesis of BAs via a negative feedback loop mediated through small heterodimer partner (SHP) that inhibits further conversion of cholesterol to BAs when cellular levels of BAs are high (Gadaleta, 2010). In addition to regulation of BA synthesis, FXR activation affects BA re-absorption, conjugation, detoxification, and transport (Gadaleta, 2010).
BAs play an important role in HCV replication in hepatocytes through modulation of FXR signaling pathways (Chang, 2007; Scholtes, 2008). BAs increase HCV RNA and protein level in GS4.1 cells, which harbor the HCV genotype 1b replicon vector (Chang, 2007). Furthermore, z-guggulsterone, an antagonist of FXR, prevents CDCA-induced HCV RNA expression (Chang, 2007). When IFNα or IFNγ and each bile acid are incubated together, bile acids significantly reduced the anti-HCV effect of IFN. BAs inhibit key innate immune system factors including IFN, protein kinase A (PKA), and STAT1 (Chang, 2007; Chang, 2004). Thus, BAs are factors for IFN non-responsiveness (Chang, 2007). Taken together, BAs and FXR play a role in HCV replication.
Activation of FXR favors viral replication, but FXR activation also has anti-fibrotic and antiinflammatory effects. FXR inhibits HSC activation by decreasing TGFβ expression. Additionally, FXR activation results in down regulation of monocyte chemotactic protein-1, keratinocyte-derived chemokine, and VCAM-1, whose encoded proteins promote infiltration of inflammatory cells (Zhang, 2009). FXR can also induce SHP expression, which suppresses the expression of extracellular matrix genes α-1 collagen, α-smooth muscle actin, and tissue inhibitor of metalloproteinase 1 and 2 (Fiorucci, 2004; Fiorucci, 2005) and thus has anti-fibrotic effects.
4. LXR
LXR has two isoforms, α and β; LXRβ is expressed in all tissue, while LXRα is expressed in the spleen, liver, adipose tissue, intestine, kidney, and lung (Zhao, 2010). For the purpose of this review, we will only discuss LXRα as it relates to liver function. Oxysterols, oxidized derivatives of cholesterol, are synthesized in the liver under conditions of excessive cellular cholesterol levels; these are natural ligands for LXRs (Gadaleta, 2010; Lima-Cabello, 2010). Fittingly, LXR is a fatty acid-activated transcription factor and sterol sensor that regulates cholesterol homeostasis and prevents damage from excessive cholesterol accumulation (Lima-Cabello, 2010). Activation of LXR stimulates reverse cholesterol transport from hepatocytes and biliary cholesterol secretion through up regulation of ABCG5/ABCG8 hepatic canalicular transporter (Gadaleta, 2010). LXR also regulates the expression of steroid regulatory element-binding protein-1c (SREBP-1c), a family member of SREBPs (Moriishi, 2007) that plays a pivotal role in maintaining lipid homeostasis (Lima-Cabello, 2010). Via LXR activation, SREBP-1c is involved in positive transcriptional control of acetyl-CoA carboxylase (ACC), FAS, and SCD-1 (Moriishi, 2007). Increase in the activity of these enzymes causes a greater production of saturated and unsaturated fatty acids and triglycerides (Moriishi, 2007). Therefore, activation of LXR and its subsequent pathways leads to an increase in lipogenesis and thus steatosis (Moriishi, 2007). It has been hypothesized that the HCV core protein-associated increase in the incidence of steatosis is mediated by the SREBP-1c pathway after activation of LXRα/RXRα (Moriishi, 2007). The localization and consequential degradation of the HCV core protein in the hepatocyte nucleus occurs in a proteasome activator PA28γ/REGγ-dependent manner (Moriishi, 2007). PA28γ/REGγ regulates the expression of host nuclear receptor protein as well as of the HCV core protein (Moriishi, 2007). PA28γ −/−/HCV Core+/+ mice show nuclear accumulation of HCV core protein. The accumulated HCV core protein in the nucleus disrupts the development of steatosis normally seen in HCV core transgenic mice (Moriishi, 2007). It was found that HCV core protein can enhance the binding of LXRα/RXRα to the LXR response element in the SREBP-1c gene, and this enhancement only occurs in the presence of PA28γ. Thus, steatosis that occurs as a result of HCV infection may be mediated by the interaction between PA28γ and nuclear receptor LXRα/RXRα (Moriishi, 2007).
Like FXR, LXR has anti-inflammatory and anti-fibrotic effects. LXR is among the most highly expressed nuclear receptors in HSCs and is involved in the repression of pro-inflammatory gene expression as well as of stellate cell activation. However, in patients with HCV infection and steatosis, LXR down regulates the immune system, including Interleukin-6 and TNFα (Lima-Cabello, 2010), which can lead to viral evasion of the immune system and increased viral propagation. In addition, LXR-null mice are more susceptible to fibrosis, and show increased expression of HSC-activation related genes (Beaven, 2010).
5. RXR and RAR
Retinoid X receptor (RXR) is unique among the adopted orphan nuclear receptors in that it can bind as a homodimer to DNA (Wan, 2000). In addition, RXR dimerizes with RAR, PPARs, LXRs, FXR, CAR, and VDR. RXR has three isoforms (α, β, and γ); 9-cis retinoic acid (RA) is the ligand for all isoforms (Chambon, 1996). The HCV core protein binds to RXRα and stabilizes the binding of RXRα/PPARα to the DNA resulting in up regulation of lipid metabolism enzymes including cellular retinol binding protein II (CRBPII) and AOX (Tsutsumi, 2002).
Retinoic acid receptor (RAR) has three isoforms (α, β, and γ), all of which are activated by all-trans RA and 9-cis RA. Heterodimers of RAR and RXR mediate the physiological action of these ligands as they pertain to cell survival, apoptosis, metabolism, and a host of other cellular functions. RAR/RXR acts as a transcriptional repressor in the absence of ligand (RA) (X. H. Tang, 2010). All-trans RA has long been known to have anti-carcinogenic properties and is currently in clinical trials for a number of cancers (Bushue, 2010). RA has been used in combination with traditional HCV treatment with some success in reducing viral load in genotype 1 patients previously nonresponsive to therapy (Bocher, 2008). A study in Huh7 cells found that RA enhances the anti-replicative effect of IFN through up regulation of type I interferon receptor (Hamamoto, 2003). Interestingly, HCV core protein sensitizes cells to RA-mediated apoptosis in human breast cancer MCF-7 cells (Watashi, 2003). This enhancement is accompanied by increased expression of tissue transglutaminase, an important mediator of RA-dependent apoptosis and an RAR target gene (Watashi, 2003). HCV core proteins also activate RARα-mediated transcription by interacting with Sp110b, a potent transcriptional corepressor of RARα (Watashi, 2003). HCV core recruits Sp110b from its normal location in the nucleus to the cytoplasm and thus abolishes its ability to repress RARα. This observation explains HCV core related enhancement of apoptosis induced by RA (Watashi, 2003). RA treatment also induces the expression of gastrointestinal glutathione peroxidase (GI-GPx), an antioxidant enzyme which counteracts cellular stress, in Huh7 cells (Morbitzer, 2005). GI-GPx is normally inhibited by HCV; probably contributing to the oxidative stress common in HCV infected cells (Morbitzer, 2005).
9-cis RA and all-trans RA suppress α-1 collagen expression by activating RXRβ and RARα, which together repress its transcription in transfected rat HSCs. This inhibition is mediated somewhat unusually by binding, along with coactivators steroid receptor coactivator-1 and growth hormone receptor interacting protein-1, to RA response elements in the α−1 collagen promoter (L. Wang, 2002). Activation of these two receptors also decreases oxidative stress (L. Wang, 2007). The combination of 9-cis and PPARγ agonist rosiglitazone has an additive effect in inhibiting TGFβ signaling and HSC proliferation and thus may slow fibrosis progression (Bruck, 2009).
6. Nuclear Receptors, Autophagy and HCV
Autophagy constitutes another important link between nuclear receptors and HCV. Autophagy is a method of self-cannibalization by which the cell degrades various cellular components either because the products of the breakdown are necessary for survival or to free itself from damaged elements (Rabinowitz, 2010). Autophagy is essential for normal tissue homeostasis. Both nuclear receptors and autophagy have regulatory metabolic roles, and it is likely that interaction between the autophagic pathway and nuclear receptors has important implications for metabolism (Rabinowitz, 2010). Indeed, there is emerging evidence that nuclear receptors may be involved in the regulation of autophagy. PXR-null mice have decreased autophagy proteins LCB-I and -II as well as Beclin-1 (K. Wang, 2010). Both PPARγ activation (Zhou, 2009) and disruption of PPARγ signaling (Jiang, 2010) induce autophagy, and our unpublished data show that RA can induce autophagy in human liver cancer cells. The relationship between autophagy and HCV is an interesting one. Given that autophagy is involved in the degradation of liver lipid droplets (lipophagy) (Singh, 2009), which are required for HCV replication, and that defects in autophagy contribute to insulin resistance (L. Yang, 2010), HCV-mediated suppression of autophagy would be logical. However, it has been shown that autophagy machinery is actually required for initiation of HCV replication (Dreux, 2009; Guevin, 2010). There are several possible mechanisms by which autophagy may facilitate viral replication. Autophagy remodels ER membranes and therefore may be involved in the formation of the membranous webs upon which HCV replicates. Autophagic proteins may be required for translation or delivery of the viral genome to the translation site (Dreux, 2009). Other research has shown that HCV indeed induces the formation of autophagosomes but inhibits their fusion with lysosomes and therefore prevents the completion of the autophagic program (Sir, 2008). Thus, it is a possible mechanism for HCV-induced disruption of autophagic signaling and may be involved in virus-mediated insulin resistance. Knockdown of autophagy proteins in immortalized human hepatocytes inhibits HCV replication via up regulation of the interferon signaling pathway, further indicating the possibility of a commensal relationship between HCV and autophagy (Shrivastava, 2011). It is apparent that much more research is necessary to elucidate the connections between nuclear receptors, autophagy, and HCV.
6. Conclusion and Future Direction
There is sufficient evidence that HCV infection affects nuclear receptor-mediated pathways and leads to problems in hepatic metabolism. It is clear that this area of research warrants greater attention. It is becoming clear that metabolic problems may be at the root of many disease states, including cancer and Alzheimer’s. Because of their complexity, these pathways have been passed over in the past in favor of more obvious answers, but they are again coming to the forefront and may provide a way to resolve the myriad of unexplored potential connections that remain between HCV and nuclear receptors. For example, one of the target genes of LXR is the inducible degrader of the LDL receptor (IDOL) (Hong, 2010), a receptor known to be involved in viral entry. HCV core protein can enhance LXR binding to DNA and therefore may enhance the expression of IDOL, but the subsequent effect on viral entry is unknown.
Most of the research to date concerning nuclear receptors and fibrosis has been done in either cell lines or small animal models. Considering the crucial role nuclear receptors play in suppressing fibrosis, it is imperative to confirm these findings in humans. Additionally, most of the above-mentioned studies utilized a high fat diet-induced non-alcoholic fatty liver disease (NAFLD) or chemical (thioacetamide or carbon tetrachloride) model to induce fibrosis. While this provides a solid foundation, research into the interplay between nuclear receptors and HCV-specific fibrosis is necessary. There is a pressing need for studies using human tissue to validate the information generated from cell lines and animal models. Although HCV induces fibrosis through many of the same mechanisms as NAFLD, it also has unique methods of inducing inflammation, oxidative stress, and insulin resistance, all of which contribute to the development and progression of fibrosis (Sheikh, 2008). Preventing or slowing fibrosis progression would be a huge stride towards comprehensive HCV therapy. Progression from mild fibrosis to cirrhosis is an important prognostic step in the development of decompensated chronic hepatitis C. Nuclear receptors play vital roles in the prevention of fibrosis and may prove excellent therapeutic targets.
The relationship between hepatitis C and nuclear receptors is undoubtedly complex, and it is difficult to assemble the often discordant findings into a comprehensive picture. However, what emerges clearly is the profound impact of nuclear receptor-regulated pathways on the critical steps of the viral process. In-depth understanding of the interaction may prove a crucial step in the development of treatment and prevention strategies.
Acknowledgments
The authors acknowledge Drs. Ivan Damjanov and Maura O’Neil for the pathology photographs. The authors thank Dr. Chuanghong Wu for his commentary and Mr. David Johnson for editing this manuscript. We dedicate this article to the KU Liver Center Tissue Bank (http://www.kumc.edu/livercenter/liver_tissue_bank.html), which is supported by KUMC. More than 1,000 human liver specimens have been deposited in this Bank. The specimens are mostly HCV positive and made available for research use. This work is supported by NIH grant CA 53596.
Abbreviations
- ACC
acyl-coA carboxylase
- AOX
acyl-coA oxidase
- BA
bile acid
- CPT-1
carnitine palmitoyl transferase 1
- CYP7A1
cholesterol 7α-hydroxylase
- ER
endoplasmic reticulum
- FAS
fatty acid synthase
- FAT/CD36
fatty acid transporter
- FATP
fatty acid transport protein
- FXR
farnesoid X receptor
- GI-GPx
gastrointestinal glutathione peroxidase
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HSC
hepatic stellate cell
- I-CAM
intercellular adhesion molecule
- IDOL
inducible degrader of the LDL receptor
- IFN
interferon
- JAK
Janus kinase
- LDL-R
low-density lipoprotein receptor
- LFABP
liver fatty acid binding protein
- LXR
liver X receptor
- NAFLD
nonalcoholic fatty liver disease
- NFκB
nuclear factor kappa B
- PPAR
peroxisome proliferator activated receptor
- PXR
pregnane X receptor
- RA
retinoic acid
- RAR
retinoic acid receptor
- ROS
reactive oxygen species
- RXR
retinoid X receptor
- SCD-1
stearyl-coA dehydrogenase
- SHP
small heterodimer partner
- SOCS-7
suppressor of cytokine signaling 7
- SREBP
steroid regulatory element-binding protein
- STAT
signal transducer and activator of transcription
- TGFβ
transforming growth factor β
- tTGase
tissue transglutaminase
- TZD
thiazolidinedione
- V-CAM
vascular adhesion molecule
- VLDL
very low-density lipoprotein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdalla MY, Ahmad IM, Spitz DR, Schmidt WN, Britigan BE. Hepatitis C virus-core and non structural proteins lead to different effects on cellular antioxidant defenses. J Med Virol. 2005;76:489–497. doi: 10.1002/jmv.20388. [DOI] [PubMed] [Google Scholar]
- Adinolfi LE, Gambardella M, Andreana A, Tripodi MF, Utili R, Ruggiero G. Steatosis accelerates the progression of liver damage of chronic hepatitis C patients and correlates with specific HCV genotype and visceral obesity. Hepatology. 2001;33:1358–1364. doi: 10.1053/jhep.2001.24432. [DOI] [PubMed] [Google Scholar]
- Barth H, Liang TJ, Baumert TF. Hepatitis C virus entry: molecular biology and clinical implications. Hepatology. 2006;44:527–535. doi: 10.1002/hep.21321. [DOI] [PubMed] [Google Scholar]
- Beaven SW, Wroblewski K, Wang J, Hong C, Bensinger S, Tsukamoto H, Tontonoz P. Liver X Receptor Signaling is a Determinant of Stellate Cell Activation and Susceptibility to Fibrotic Liver Disease. Gastroenterology. 2010 doi: 10.1053/j.gastro.2010.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissig KD, Wieland SF, Tran P, Isogawa M, Le TT, Chisari FV, Verma IM. Human liver chimeric mice provide a model for hepatitis B and C virus infection and treatment. J Clin Invest. 2010;120:924–930. doi: 10.1172/JCI40094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackham S, Baillie A, Al-Hababi F, Remlinger K, You S, Hamatake R, McGarvey MJ. Gene expression profiling indicates the roles of host oxidative stress, apoptosis, lipid metabolism, and intracellular transport genes in the replication of hepatitis C virus. J Virol. 2010;84:5404–5414. doi: 10.1128/JVI.02529-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocher WO, Wallasch C, Hohler T, Galle PR. All-trans retinoic acid for treatment of chronic hepatitis C. Liver Int. 2008;28:347–354. doi: 10.1111/j.1478-3231.2007.01666.x. [DOI] [PubMed] [Google Scholar]
- Boulant S, Douglas MW, Moody L, Budkowska A, Targett-Adams P, McLauchlan J. Hepatitis C virus core protein induces lipid droplet redistribution in a microtubule-and dynein-dependent manner. Traffic. 2008;9:1268–1282. doi: 10.1111/j.1600-0854.2008.00767.x. [DOI] [PubMed] [Google Scholar]
- Brass V, Moradpour D, Blum HE. Hepatitis C virus infection: in vivo and in vitro models. J Viral Hepat. 2007;14 Suppl. 1:64–67. doi: 10.1111/j.1365-2893.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- Bruck R, Weiss S, Aeed H, Pines M, Halpern Z, Zvibel I. Additive inhibitory effect of experimentally induced hepatic cirrhosis by agonists of peroxisome proliferator activator receptor gamma and retinoic acid receptor. Dig Dis Sci. 2009;54:292–299. doi: 10.1007/s10620-008-0336-5. [DOI] [PubMed] [Google Scholar]
- Burke KP, Cox AL. Hepatitis C virus evasion of adaptive immune responses: a model for viral persistence. Immunol Res. 2010;47:216–227. doi: 10.1007/s12026-009-8152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushue N, Wan YJ. Retinoid Pathway and Cancer Therapeutics. Advanced Drug Delivery. 2010 doi: 10.1016/j.addr.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- Chang KO, George DW. Bile acids promote the expression of hepatitis C virus in replicon-harboring cells. J Virol. 2007;81:9633–9640. doi: 10.1128/JVI.00795-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KO, Sosnovtsev SV, Belliot G, Kim Y, Saif LJ, Green KY. Bile acids are essential for porcine enteric calicivirus replication in association with down-regulation of signal transducer and activator of transcription 1. Proc Natl Acad Sci U S A. 2004;101:8733–8738. doi: 10.1073/pnas.0401126101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chayama K, Hayes CN, Hiraga N, Abe H, Tsuge M, Imamura M. Animal model for study of human hepatitis viruses. J Gastroenterol Hepatol. 2011;26:13–18. doi: 10.1111/j.1440-1746.2010.06470.x. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Dharancy S, Malapel M, Desreumaux P. Hepatitis C virus infection down-regulates the expression of peroxisome proliferator-activated receptor alpha and carnitine palmitoyl acyl-CoA transferase 1A. World J Gastroenterol. 2005;11:7591–7596. doi: 10.3748/wjg.v11.i48.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devchand PR, Keller H, Peters JM, Vazquez M, Gonzalez FJ, Wahli W. The PPARalpha-leukotriene B4 pathway to inflammation control. Nature. 1996;384:39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- Dharancy S, Malapel M, Perlemuter G, Roskams T, Cheng Y, Dubuquoy L, Podevin P, Conti F, Canva V, Philippe D, Gambiez L, Mathurin P, Paris JC, Schoonjans K, Calmus Y, Pol S, Auwerx J, Desreumaux P. Impaired expression of the peroxisome proliferator-activated receptor alpha during hepatitis C virus infection. Gastroenterology. 2005;128:334–342. doi: 10.1053/j.gastro.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Douglas MW, George J. Molecular mechanisms of insulin resistance in chronic hepatitis C. World J Gastroenterol. 2009;15:4356–4364. doi: 10.3748/wjg.15.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreux M, Gastaminza P, Wieland SF, Chisari FV. The autophagy machinery is required to initiate hepatitis C virus replication. Proc Natl Acad Sci U S A. 2009;106:14046–14051. doi: 10.1073/pnas.0907344106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuisson J, Helle F, Cocquerel L. Early steps of the hepatitis C virus life cycle. Cell Microbiol. 2008;10:821–827. doi: 10.1111/j.1462-5822.2007.01107.x. [DOI] [PubMed] [Google Scholar]
- Evans MJ, von Hahn T, Tscherne DM, Syder AJ, Panis M, Wolk B, Hatziioannou T, McKeating JA, Bieniasz PD, Rice CM. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature. 2007;446:801–805. doi: 10.1038/nature05654. [DOI] [PubMed] [Google Scholar]
- Feld JJ, Liang TJ. Hepatitis C -- identifying patients with progressive liver injury. Hepatology. 2006;43:S194–S206. doi: 10.1002/hep.21065. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Antonelli E, Rizzo G, Renga B, Mencarelli A, Riccardi L, Orlandi S, Pellicciari R, Morelli A. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 2004;127:1497–1512. doi: 10.1053/j.gastro.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Fiorucci S, Rizzo G, Antonelli E, Renga B, Mencarelli A, Riccardi L, Orlandi S, Pruzanski M, Morelli A, Pellicciari R. A farnesoid x receptor-small heterodimer partner regulatory cascade modulates tissue metalloproteinase inhibitor-1 and matrix metalloprotease expression in hepatic stellate cells and promotes resolution of liver fibrosis. J Pharmacol Exp Ther. 2005;314:584–595. doi: 10.1124/jpet.105.084905. [DOI] [PubMed] [Google Scholar]
- Fowell AJ, Nash KL. Telaprevir: a new hope in the treatment of chronic hepatitis C? Adv Ther. 2010;27:512–522. doi: 10.1007/s12325-010-0047-0. [DOI] [PubMed] [Google Scholar]
- Fujita N, Kaito M, Kai M, Sugimoto R, Tanaka H, Horiike S, Konishi M, Iwasa M, Watanabe S, Adachi Y. Effects of bezafibrate in patients with chronic hepatitis C virus infection: combination with interferon and ribavirin. J Viral Hepat. 2006;13:441–448. doi: 10.1111/j.1365-2893.2005.00718.x. [DOI] [PubMed] [Google Scholar]
- Fujita N, Kaito M, Tanaka H, Horiike S, Adachi Y. Reduction of serum HCV RNA titer by bezafibrate therapy in patients with chronic hepatitis C. Am J Gastroenterol. 2004;99:2280. doi: 10.1111/j.1572-0241.2004.40695_3.x. [DOI] [PubMed] [Google Scholar]
- Gadaleta RM, van Mil SW, Oldenburg B, Siersema PD, Klomp LW, van Erpecum KJ. Bile acids and their nuclear receptor FXR: Relevance for hepatobiliary and gastrointestinal disease. Biochim Biophys Acta. 2010;1801:683–692. doi: 10.1016/j.bbalip.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Gale M, Jr, Foy EM. Evasion of intracellular host defence by hepatitis C virus. Nature. 2005;436:939–945. doi: 10.1038/nature04078. [DOI] [PubMed] [Google Scholar]
- Gastaminza P, Cheng G, Wieland S, Zhong J, Liao W, Chisari FV. Cellular determinants of hepatitis C virus assembly, maturation, degradation, and secretion. J Virol. 2008;82:2120–2129. doi: 10.1128/JVI.02053-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilova O, Haluzik M, Matsusue K, Cutson JJ, Johnson L, Dietz KR, Nicol CJ, Vinson C, Gonzalez FJ, Reitman ML. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem. 2003;278:34268–34276. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- Gervois P, Kleemann R, Pilon A, Percevault F, Koenig W, Staels B, Kooistra T. Global suppression of IL-6-induced acute phase response gene expression after chronic in vivo treatment with the peroxisome proliferator-activated receptor-alpha activator fenofibrate. J Biol Chem. 2004;279:16154–16160. doi: 10.1074/jbc.M400346200. [DOI] [PubMed] [Google Scholar]
- Glass CK, Saijo K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat Rev Immunol. 2010;10:365–376. doi: 10.1038/nri2748. [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ, Shah YM. PPARalpha: mechanism of species differences and hepatocarcinogenesis of peroxisome proliferators. Toxicology. 2008;246:2–8. doi: 10.1016/j.tox.2007.09.030. [DOI] [PubMed] [Google Scholar]
- Guevin C, Manna D, Belanger C, Konan KV, Mak P, Labonte P. Autophagy protein ATG5 interacts transiently with the hepatitis C virus RNA polymerase (NS5B) early during infection. Virology. 2010;405:1–7. doi: 10.1016/j.virol.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyamfi MA, Wan YJ. Pathogenesis of alcoholic liver disease: the role of nuclear receptors. Exp Biol Med (Maywood) 2010;235:547–560. doi: 10.1258/ebm.2009.009249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamoto S, Fukuda R, Ishimura N, Rumi MA, Kazumori H, Uchida Y, Kadowaki Y, Ishihara S, Kinoshita Y. 9-cis retinoic acid enhances the antiviral effect of interferon on hepatitis C virus replication through increased expression of type I interferon receptor. J Lab Clin Med. 2003;141:58–66. doi: 10.1067/mlc.2003.8. [DOI] [PubMed] [Google Scholar]
- Hong C, Duit S, Jalonen P, Out R, Scheer L, Sorrentino V, Boyadjian R, Rodenburg KW, Foley E, Korhonen L, Lindholm D, Nimpf J, van Berkel TJ, Tontonoz P, Zelcer N. The E3 ubiquitin ligase IDOL induces the degradation of the low density lipoprotein receptor family members VLDLR and ApoER2. J Biol Chem. 2010;285:19720–19726. doi: 10.1074/jbc.M110.123729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip E, Farrell G, Hall P, Robertson G, Leclercq I. Administration of the potent PPARalpha agonist, Wy-14,643, reverses nutritional fibrosis and steatohepatitis in mice. Hepatology. 2004;39:1286–1296. doi: 10.1002/hep.20170. [DOI] [PubMed] [Google Scholar]
- Jiang M, Fernandez S, Jerome WG, He Y, Yu X, Cai H, Boone B, Yi Y, Magnuson MA, Roy-Burman P, Matusik RJ, Shappell SB, Hayward SW. Disruption of PPARgamma signaling results in mouse prostatic intraepithelial neoplasia involving active autophagy. Cell Death Differ. 2010;17:469–481. doi: 10.1038/cdd.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce MA, Tyrrell DL. The cell biology of hepatitis C virus. Microbes Infect. 2010;12:263–271. doi: 10.1016/j.micinf.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Kapadia SB, Chisari FV. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc Natl Acad Sci U S A. 2005;102:2561–2566. doi: 10.1073/pnas.0409834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattab M, Emad M, Abdelaleem A, Eslam M, Atef R, Shaker Y, Hamdy L. Pioglitazone improves virological response to peginterferon alpha-2b/ribavirin combination therapy in hepatitis C genotype 4 patients with insulin resistance. Liver Int. 2010;30:447–454. doi: 10.1111/j.1478-3231.2009.02171.x. [DOI] [PubMed] [Google Scholar]
- Kim K, Kim KH, Ha E, Park JY, Sakamoto N, Cheong J. Hepatitis C virus NS5A protein increases hepatic lipid accumulation via induction of activation and expression of PPARgamma. FEBS Lett. 2009;583:2720–2726. doi: 10.1016/j.febslet.2009.07.034. [DOI] [PubMed] [Google Scholar]
- Kim KH, Hong SP, Kim K, Park MJ, Kim KJ, Cheong J. HCV core protein induces hepatic lipid accumulation by activating SREBP1 and PPARgamma. Biochem Biophys Res Commun. 2007;355:883–888. doi: 10.1016/j.bbrc.2007.02.044. [DOI] [PubMed] [Google Scholar]
- Kleemann R, Gervois PP, Verschuren L, Staels B, Princen HM, Kooistra T. Fibrates down-regulate IL-1-stimulated C-reactive protein gene expression in hepatocytes by reducing nuclear p50-NFkappa B-C/EBP-beta complex formation. Blood. 2003;101:545–551. doi: 10.1182/blood-2002-06-1762. [DOI] [PubMed] [Google Scholar]
- Koike K. Steatosis, liver injury, and hepatocarcinogenesis in hepatitis C viral infection. J Gastroenterol. 2009;44 Suppl 19:82–88. doi: 10.1007/s00535-008-2276-4. [DOI] [PubMed] [Google Scholar]
- Lee YS, Chanda D, Sim J, Park YY, Choi HS. Structure and function of the atypical orphan nuclear receptor small heterodimer partner. Int Rev Cytol. 2007;261:117–158. doi: 10.1016/S0074-7696(07)61003-1. [DOI] [PubMed] [Google Scholar]
- Li ZH, Tang QB, Wang J, Zhou L, Huang WL, Liu RY, Chen RF. Hepatitis C virus core protein induces malignant transformation of biliary epithelial cells by activating nuclear factor-kappaB pathway. J Gastroenterol Hepatol. 2010;25:1315–1320. doi: 10.1111/j.1440-1746.2009.06201.x. [DOI] [PubMed] [Google Scholar]
- Lima-Cabello E, Garcia-Mediavilla MV, Miquilena-Colina ME, Vargas-Castrillon J, Lozano-Rodriguez T, Fernandez-Bermejo M, Olcoz JL, Gonzalez-Gallego J, Garcia-Monzon C, Sanchez-Campos S. Enhanced expression of pro-inflammatory mediators and liver X-receptor-regulated lipogenic genes in non-alcoholic fatty liver disease and hepatitis C. Clin Sci (Lond) 2010;120:239–250. doi: 10.1042/CS20100387. [DOI] [PubMed] [Google Scholar]
- Lyn RK, Kennedy DC, Sagan SM, Blais DR, Rouleau Y, Pegoraro AF, Xie XS, Stolow A, Pezacki JP. Direct imaging of the disruption of hepatitis C virus replication complexes by inhibitors of lipid metabolism. Virology. 2009;394:130–142. doi: 10.1016/j.virol.2009.08.022. [DOI] [PubMed] [Google Scholar]
- Maison P, Mennen L, Sapinho D, Balkau B, Sigalas J, Chesnier MC, Eschwege E. A pharmacoepidemiological assessment of the effect of statins and fibrates on fibrinogen concentration. Atherosclerosis. 2002;160:155–160. doi: 10.1016/s0021-9150(01)00552-4. [DOI] [PubMed] [Google Scholar]
- Marra F, Efsen E, Romanelli RG, Caligiuri A, Pastacaldi S, Batignani G, Bonacchi A, Caporale R, Laffi G, Pinzani M, Gentilini P. Ligands of peroxisome proliferator-activated receptor gamma modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology. 2000;119:466–478. doi: 10.1053/gast.2000.9365. [DOI] [PubMed] [Google Scholar]
- Marx N, Sukhova GK, Collins T, Libby P, Plutzky J. PPARalpha activators inhibit cytokine-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation. 1999;99:3125–3131. doi: 10.1161/01.cir.99.24.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna NJ, Cooney AJ, DeMayo FJ, Downes M, Glass CK, Lanz RB, Lazar MA, Mangelsdorf DJ, Moore DD, Qin J, Steffen DL, Tsai MJ, Tsai SY, Yu R, Margolis RN, Evans RM, O'Malley BW. Minireview: Evolution of NURSA, the Nuclear Receptor Signaling Atlas. Mol Endocrinol. 2009;23:740–746. doi: 10.1210/me.2009-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer DF, Schiller DE, Elliott JF, Douglas DN, Hao C, Rinfret A, Addison WR, Fischer KP, Churchill TA, Lakey JR, Tyrrell DL, Kneteman NM. Hepatitis C virus replication in mice with chimeric human livers. Nat Med. 2001;7:927–933. doi: 10.1038/90968. [DOI] [PubMed] [Google Scholar]
- Miyanari Y, Atsuzawa K, Usuda N, Watashi K, Hishiki T, Zayas M, Bartenschlager R, Wakita T, Hijikata M, Shimotohno K. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol. 2007;9:1089–1097. doi: 10.1038/ncb1631. [DOI] [PubMed] [Google Scholar]
- Morbitzer M, Herget T. Expression of gastrointestinal glutathione peroxidase is inversely correlated to the presence of hepatitis C virus subgenomic RNA in human liver cells. J Biol Chem. 2005;280:8831–8841. doi: 10.1074/jbc.M413730200. [DOI] [PubMed] [Google Scholar]
- Moriishi K, Mochizuki R, Moriya K, Miyamoto H, Mori Y, Abe T, Murata S, Tanaka K, Miyamura T, Suzuki T, Koike K, Matsuura Y. Critical role of PA28gamma in hepatitis C virus-associated steatogenesis and hepatocarcinogenesis. Proc Natl Acad Sci U S A. 2007;104:1661–1666. doi: 10.1073/pnas.0607312104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motojima K, Passilly P, Peters JM, Gonzalez FJ, Latruffe N. Expression of putative fatty acid transporter genes are regulated by peroxisome proliferator-activated receptor alpha and gamma activators in a tissue- and inducer-specific manner. J Biol Chem. 1998;273:16710–16714. doi: 10.1074/jbc.273.27.16710. [DOI] [PubMed] [Google Scholar]
- Nguyen MH, Keeffe EB. Chronic hepatitis C: genotypes 4 to 9. Clin Liver Dis. 2005;9:411–426. doi: 10.1016/j.cld.2005.05.010. vi. [DOI] [PubMed] [Google Scholar]
- NIH Consensus Statement on Management of Hepatitis C: 2002. NIH Consens State Sci Statements. 2002;19:1–46. [PubMed]
- Nishimura-Sakurai Y, Sakamoto N, Mogushi K, Nagaie S, Nakagawa M, Itsui Y, Tasaka-Fujita M, Onuki-Karakama Y, Suda G, Mishima K, Yamamoto M, Ueyama M, Funaoka Y, Watanabe T, Azuma S, Sekine-Osajima Y, Kakinuma S, Tsuchiya K, Enomoto N, Tanaka H, Watanabe M. Comparison of HCV-associated gene expression and cell signaling pathways in cells with or without HCV replicon and in replicon-cured cells. J Gastroenterol. 2010;45:523–536. doi: 10.1007/s00535-009-0162-3. [DOI] [PubMed] [Google Scholar]
- Okuda M, Li K, Beard MR, Showalter LA, Scholle F, Lemon SM, Weinman SA. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- Overbeck K, Genne D, Golay A, Negro F. Pioglitazone in chronic hepatitis C not responding to pegylated interferon-alpha and ribavirin. J Hepatol. 2008;49:295–298. doi: 10.1016/j.jhep.2008.03.033. [DOI] [PubMed] [Google Scholar]
- Pasquinelli C, Shoenberger JM, Chung J, Chang KM, Guidotti LG, Selby M, Berger K, Lesniewski R, Houghton M, Chisari FV. Hepatitis C virus core and E2 protein expression in transgenic mice. Hepatology. 1997;25:719–727. doi: 10.1002/hep.510250338. [DOI] [PubMed] [Google Scholar]
- Pawlotsky JM, Chevaliez S, McHutchison JG. The hepatitis C virus life cycle as a target for new antiviral therapies. Gastroenterology. 2007;132:1979–1998. doi: 10.1053/j.gastro.2007.03.116. [DOI] [PubMed] [Google Scholar]
- Pazienza V, Clement S, Pugnale P, Conzelman S, Foti M, Mangia A, Negro F. The hepatitis C virus core protein of genotypes 3a and 1b downregulates insulin receptor substrate 1 through genotype-specific mechanisms. Hepatology. 2007;45:1164–1171. doi: 10.1002/hep.21634. [DOI] [PubMed] [Google Scholar]
- Pazienza V, Vinciguerra M, Andriulli A, Mangia A. Hepatitis C virus core protein genotype 3a increases SOCS-7 expression through PPAR-{gamma} in Huh-7 cells. J Gen Virol. 2010;91:1678–1686. doi: 10.1099/vir.0.020644-0. [DOI] [PubMed] [Google Scholar]
- Pearce KH, Iannone MA, Simmons CA, Gray JG. Discovery of novel nuclear receptor modulating ligands: an integral role for peptide interaction profiling. Drug Discov Today. 2004;9:741–751. doi: 10.1016/S1359-6446(04)03201-5. [DOI] [PubMed] [Google Scholar]
- Petit JM, Minello A, Jooste V, Bour JB, Galland F, Duvillard L, Verges B, Olsson NO, Gambert P, Hillon P. Decreased plasma adiponectin concentrations are closely related to steatosis in hepatitis C virus-infected patients. J Clin Endocrinol Metab. 2005;90:2240–2243. doi: 10.1210/jc.2004-1266. [DOI] [PubMed] [Google Scholar]
- Ploss A, Evans MJ, Gaysinskaya VA, Panis M, You H, de Jong YP, Rice CM. Human occludin is a hepatitis C virus entry factor required for infection of mouse cells. Nature. 2009a;457:882–886. doi: 10.1038/nature07684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploss A, Rice CM. Towards a small animal model for hepatitis C. EMBO Rep. 2009b;10:1220–1227. doi: 10.1038/embor.2009.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier H, Niot I, Monnot MC, Braissant O, Meunier-Durmort C, Costet P, Pineau T, Wahli W, Willson TM, Besnard P. Differential involvement of peroxisome-proliferator-activated receptors alpha and delta in fibrate and fatty-acid-mediated inductions of the gene encoding liver fatty-acid-binding protein in the liver and the small intestine. Biochem J. 2001;355:481–488. doi: 10.1042/0264-6021:3550481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic B, Sagan SM, Noestheden M, Belanger S, Nan X, Evans CL, Xie XS, Pezacki JP. Peroxisome proliferator-activated receptor alpha antagonism inhibits hepatitis C virus replication. Chem Biol. 2006;13:23–30. doi: 10.1016/j.chembiol.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Reddy JK. Nonalcoholic steatosis and steatohepatitis. III. Peroxisomal beta-oxidation, PPAR alpha, and steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1333–G1339. doi: 10.1152/ajpgi.2001.281.6.G1333. [DOI] [PubMed] [Google Scholar]
- Scholtes C, Diaz O, Icard V, Kaul A, Bartenschlager R, Lotteau V, Andre P. Enhancement of genotype 1 hepatitis C virus replication by bile acids through FXR. J Hepatol. 2008;48:192–199. doi: 10.1016/j.jhep.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Sheikh MY, Choi J, Qadri I, Friedman JE, Sanyal AJ. Hepatitis C virus infection: molecular pathways to metabolic syndrome. Hepatology. 2008;47:2127–2133. doi: 10.1002/hep.22269. [DOI] [PubMed] [Google Scholar]
- Shrivastava S, Raychoudhuri A, Steele R, Ray R, Ray RB. Knockdown of autophagy enhances the innate immune response in hepatitis C virus-infected hepatocytes. Hepatology. 2011;53:406–414. doi: 10.1002/hep.24073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sir D, Liang C, Chen WL, Jung JU, Ou JH. Perturbation of autophagic pathway by hepatitis C virus. Autophagy. 2008;4:830–831. doi: 10.4161/auto.6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Moriya K, Kiyosawa K, Koike K, Aoyama T. Hepatitis C virus core protein induces spontaneous and persistent activation of peroxisome proliferator-activated receptor alpha in transgenic mice: implications for HCV-associated hepatocarcinogenesis. Int J Cancer. 2008a;122:124–131. doi: 10.1002/ijc.23056. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Moriya K, Kiyosawa K, Koike K, Gonzalez FJ, Aoyama T. PPARalpha activation is essential for HCV core protein-induced hepatic steatosis and hepatocellular carcinoma in mice. J Clin Invest. 2008b;118:683–694. doi: 10.1172/JCI33594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H, McLachlan A. Transcriptional regulation of hepatitis B virus by nuclear hormone receptors is a critical determinant of viral tropism. Proc Natl Acad Sci U S A. 2001;98:1841–1846. doi: 10.1073/pnas.041479698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XH, Gudas LJ. Retinoids, Retinoic Acid Receptors, and Cancer. Annu Rev Pathol. 2010 doi: 10.1146/annurev-pathol-011110-130303. [DOI] [PubMed] [Google Scholar]
- Toyama T, Nakamura H, Harano Y, Yamauchi N, Morita A, Kirishima T, Minami M, Itoh Y, Okanoue T. PPARalpha ligands activate antioxidant enzymes and suppress hepatic fibrosis in rats. Biochem Biophys Res Commun. 2004;324:697–704. doi: 10.1016/j.bbrc.2004.09.110. [DOI] [PubMed] [Google Scholar]
- Tsutsumi T, Suzuki T, Shimoike T, Suzuki R, Moriya K, Shintani Y, Fujie H, Matsuura Y, Koike K, Miyamura T. Interaction of hepatitis C virus core protein with retinoid X receptor alpha modulates its transcriptional activity. Hepatology. 2002;35:937–946. doi: 10.1053/jhep.2002.32470. [DOI] [PubMed] [Google Scholar]
- Vavassori P, Mencarelli A, Renga B, Distrutti E, Fiorucci S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol. 2009;183:6251–6261. doi: 10.4049/jimmunol.0803978. [DOI] [PubMed] [Google Scholar]
- Wan YJ, An D, Cai Y, Repa JJ, Hung-Po Chen T, Flores M, Postic C, Magnuson MA, Chen J, Chien KR, French S, Mangelsdorf DJ, Sucov HM. Hepatocyte-specific mutation establishes retinoid X receptor alpha as a heterodimeric integrator of multiple physiological processes in the liver. Mol Cell Biol. 2000;20:4436–4444. doi: 10.1128/mcb.20.12.4436-4444.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Damjanov I, Wan YJ. The protective role of pregnane X receptor in lipopolysaccharide/D-galactosamine-induced acute liver injury. Lab Invest. 2010;90:257–265. doi: 10.1038/labinvest.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Wan YJ. Nuclear receptors and inflammatory diseases. Exp Biol Med (Maywood) 2008;233:496–506. doi: 10.3181/0708-MR-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Potter JJ, Rennie-Tankersley L, Novitskiy G, Sipes J, Mezey E. Effects of retinoic acid on the development of liver fibrosis produced by carbon tetrachloride in mice. Biochim Biophys Acta. 2007;1772:66–71. doi: 10.1016/j.bbadis.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Wang L, Tankersley LR, Tang M, Potter JJ, Mezey E. Regulation of the murine alpha(2)(I) collagen promoter by retinoic acid and retinoid X receptors. Arch Biochem Biophys. 2002;401:262–270. doi: 10.1016/S0003-9861(02)00058-9. [DOI] [PubMed] [Google Scholar]
- Washburn ML, Bility MT, Zhang L, Kovalev GI, Buntzman A, Frelinger JA, Barry W, Ploss A, Rice CM, Su L. A humanized mouse model to study hepatitis C virus infection, immune response, and liver disease. Gastroenterology. 2011;140:1334–1344. doi: 10.1053/j.gastro.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watashi K, Hijikata M, Tagawa A, Doi T, Marusawa H, Shimotohno K. Modulation of retinoid signaling by a cytoplasmic viral protein via sequestration of Sp110b, a potent transcriptional corepressor of retinoic acid receptor, from the nucleus. Mol Cell Biol. 2003;23:7498–7509. doi: 10.1128/MCB.23.21.7498-7509.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhouse SD, Narayan R, Latham S, Lee S, Antrobus R, Gangadharan B, Luo S, Schroth GP, Klenerman P, Zitzmann N. Transcriptome sequencing, microarray, and proteomic analyses reveal cellular and metabolic impact of hepatitis C virus infection in vitro. Hepatology. 2010;52:443–453. doi: 10.1002/hep.23733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Yang L, Chan CC, Kwon OS, Liu S, McGhee J, Stimpson SA, Chen LZ, Harrington WW, Symonds WT, Rockey DC. Regulation of peroxisome proliferator-activated receptor-gamma in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2006;291:G902–G911. doi: 10.1152/ajpgi.00124.2006. [DOI] [PubMed] [Google Scholar]
- Yang L, Li P, Fu S, Calay ES, Hotamisligil GS. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab. 2010;11:467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Nagano T, Shah Y, Cheung C, Ito S, Gonzalez FJ. The PPAR alpha-humanized mouse: a model to investigate species differences in liver toxicity mediated by PPAR alpha. Toxicol Sci. 2008;101:132–139. doi: 10.1093/toxsci/kfm206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui K, Harano Y, Mitsuyoshi H, Tsuji K, Endo M, Nakajima T, Minami M, Itoh Y, Zen Y, Nakanuma Y, Yoshikawa T, Okanoue T. Steatosis and hepatic expression of genes regulating lipid metabolism in Japanese patients infected with hepatitis C virus. J Gastroenterol. 2010;45:95–104. doi: 10.1007/s00535-009-0133-8. [DOI] [PubMed] [Google Scholar]
- Yu J, Zhang S, Chu ES, Go MY, Lau RH, Zhao J, Wu CW, Tong L, Poon TC, Sung JJ. Peroxisome proliferator-activated receptors gamma reverses hepatic nutritional fibrosis in mice and suppresses activation of hepatic stellate cells in vitro. Int J Biochem Cell Biol. 2010;42:948–957. doi: 10.1016/j.biocel.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Yu S, Matsusue K, Kashireddy P, Cao WQ, Yeldandi V, Yeldandi AV, Rao MS, Gonzalez FJ, Reddy JK. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. J Biol Chem. 2003;278:498–505. doi: 10.1074/jbc.M210062200. [DOI] [PubMed] [Google Scholar]
- Zhang S, Wang J, Liu Q, Harnish DC. Farnesoid X receptor agonist WAY-362450 attenuates liver inflammation and fibrosis in murine model of non-alcoholic steatohepatitis. J Hepatol. 2009;51:380–388. doi: 10.1016/j.jhep.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Zhao C, Chen W, Yang L, Chen L, Stimpson SA, Diehl AM. PPARgamma agonists prevent TGFbeta1/Smad3-signaling in human hepatic stellate cells. Biochem Biophys Res Commun. 2006;350:385–391. doi: 10.1016/j.bbrc.2006.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Dahlman-Wright K. Liver X receptor in cholesterol metabolism. J Endocrinol. 2010;204:233–240. doi: 10.1677/JOE-09-0271. [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhai Y, Mu Y, Gong H, Uppal H, Toma D, Ren S, Evans RM, Xie W. A novel pregnane X receptor-mediated and sterol regulatory element-binding proteinindependent lipogenic pathway. J Biol Chem. 2006;281:15013–15020. doi: 10.1074/jbc.M511116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Zhang W, Liang B, Casimiro MC, Whitaker-Menezes D, Wang M, Lisanti MP, Lanza-Jacoby S, Pestell RG, Wang C. PPARgamma activation induces autophagy in breast cancer cells. Int J Biochem Cell Biol. 2009;41:2334–2342. doi: 10.1016/j.biocel.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]