Abstract
Functional neuroimaging studies indicate that a primary marker of specific reading disability (RD) is reduced activation of left hemisphere (LH) posterior regions during performance of reading tasks. However, the severity of this disruption, and the extent to which these LH systems might be available for reading under any circumstances is unclear at present. Experiment 1 examined the cortical effects of stimulus manipulations (frequency, imageability, consistency) that have known facilitative effects on reading performance for both nonimpaired (NI) and RD readers. Experiment 2 examined stimulus repetition, another facilitative variable, in an additional sample of adolescent NI and RD readers. For NI readers, factors that made words easier to process were associated with relatively reduced activation. For RD readers, facilitative factors resulted in increased activation in these same reading-related sites, suggesting that the LH reading circuitry in adolescent RD appears to be poorly trained but not wholly disrupted.
Converging evidence from functional neuroimaging studies indicates that a primary neurobiological marker of specific reading disability (RD) is reduced activation of left hemisphere (LH) posterior regions relative to activation levels for non-impaired (NI) readers during tasks that make demands on language and printed word processing. Together with the failure to reliably engage LH temporoparietal and occipitotemporal regions, RD readers tend also to show heightened activation of right hemisphere (RH) posterior and bilateral frontal regions (see Pugh et al., 2000a; Sarkari et al., 2002 for reviews); the tendency to hyper-engage these regions may serve to compensate for deficient linguistic processing in the left hemisphere. Although this RD profile appears to be reasonably stable across different ages, tasks, and languages (Paulesu et al., 2001), the question still remains as to the severity of this LH disruption (Pugh et al., 2000b). Evidence from recent intervention studies suggests that compromised LH systems appear to be responsive to intensive training in young RD populations (Simos et al., 2002; Shaywitz et al., 2004; Temple et al., 2003). That is, many LH regions that are critically involved in reading and are not activated during reading tasks in young RD readers prior to an intervention period show increased activation after intervention. However, the extent to which these LH systems are available for reading in older children whose reading difficulties have persisted is less studied. Recently, Cao et al. (2006) contrasted NI and RD children in a rhyming task with easy vs. hard trials. The often-observed diminished activation of key LH regions in RD was obtained only on hard trials suggesting that stimulus difficulty is an important variable in making group contrasts. Hoeft et al. (2007) used reading age (RA) and chronological age (CA) controls to assess performance effects on group contrasts, and concluded that hyperactivation in frontal areas in RD is experience and skill-related while hypoactivation in RD at LH posterior regions (particularly LH temporoparietal sites) is more fundamental to the syndrome (indeed, structural imaging reinforced this conclusion; with reliably reduced grey matter volume in temporoparietal areas in RD).
The current experiments were designed to examine learning and difficulty effects in NI and RD more directly by focusing more on within-group learning differences as a window on latent functionality. Addressing this issue is of high priority for predicting likely neurobiological and behavioral outcomes of systematic reading intervention and remediation in older children. As a first step, it is critical that we begin to examine whether adolescent RD readers demonstrate reading-related functionality in these LH systems under any conditions. The current study examines the cortical effects of stimulus manipulations that have known facilitative effects on word reading latencies and accuracy in adolescent RD readers. In Experiment 1, we focus specifically on the ways in which stimulus familiarity (frequency) and semantic features (imageability) modulate processing of words that vary with regard to complexity of orthographic-to-phonological mappings (consistency). This first experiment will provide a window into how these top down, semantic, facilitative features are able to affect the altered neural circuitry for reading in RD readers. In Experiment 2 we examine stimulus repetition (a highly salient facilitative variable in RD performance) to characterize online learning processes in RD readers. By examining online learning we can begin to move beyond first generation questions of where in the brain activation levels differ in these groups in general, to a more systems-level account of the ways in which readers with very different skill levels modulate activation patterns in the context of learning. We predict that this more dynamic approach will provide a better model for clinical contrast in general than one that simply looks for regional differences in a more static manner.
Experiment 1
One of the most often used indices of the influence of phonology on printed word naming is the spelling-to-sound consistency effect. This refers to the finding that identification is faster and more accurate for words that have consistent (1:1) correspondences between the orthographic body and phonological rime (e.g., -ill only corresponds to /Il/ as in pill, mill) than for inconsistent words that have multiple body-rime correspondences (e.g., -int corresponds to /Int/ as in mint and /aInt/ as in pint). Behavioral studies have shown that frequency moderates this effect such that consistency effects are most robust for low-frequency words (Jared, McRae, & Seidenberg, 1990; Seidenberg, Waters, Barnes, & Tanenhaus, 1984). Further work by Strain and his colleagues demonstrated that the typically obtained interaction of consistency and frequency during printed word naming tasks is modulated by a semantic variable, imageability (Strain et al., 1995, 2002). Consistency effects were observed primarily on words that are both low-frequency and low-imageable; consistency effects for high-imageable low-frequency words were either attenuated or not significant across experiments. These findings reveal that semantics can attenuate the difficulties associated with reading words that have inconsistent orthographic-to-phonological mappings.
Research on phonology and lexico-semantics in RD readers has shown that they are particularly challenged by spelling-to-sound inconsistent words and particularly benefited by frequency and imageability. That is, RD readers demonstrate amplified consistency effects relative to NI readers (Bruck, 1992) and greater advantages than skilled readers for high frequency words relative to low frequency words, in both for accuracy and latency (Shaywitz et al., 2003). Moreover, with regard to the top-down influence of semantics (Strain et al., 1995, 2002) poor readers show even greater benefit from imageability than skilled readers on the processing of difficult-to-decode inconsistent words (Strain & Herdman, 1999). Thus, for all reading levels, performance on difficult-to-decode inconsistent words is facilitated when tokens are high frequency and/or highly imageable, but this effect is amplified for poor readers. In summary, these semantic factors can, at least to some degree, offset problematic phonological assembly skills in RD readers. Identification of the neurobiological correlates of this type of top-down modulation in RD will allow us to address changing activation profiles as demands on core processes are systematically manipulated, in order to assess potential functionality in LH reading-related systems in RD.
Experiment 1 examined go/no-go naming responses for words while manipulating frequency, consistency, and imageability. Go/no-go naming (in which participants are required to name the token if it is a real English word but not if it is a pseudoword) was chosen because this overt naming paradigm strongly engaging phonological processing, while also accentuating the influences of lexico-semantics (Frost et al., 2005).
We predict that our behavioral findings will follow those of previous studies: consistency effects will be greater for RD relative to NI readers, and frequency and/or imageability will facilitate naming latencies and accuracy on difficult-to-decode inconsistent words for all participants, but with a much larger effect in RD. With respect to anticipated brain activation patterns, for NI readers, previous research indicates that stimuli that are easier to process should be associated with reduced BOLD signal at reading-related regions in the LH, reflecting increased processing efficiency within these regions (Frost et al., 2005; Katz et al., 2005; Poldrack & Gabrieli, 2001; Sandak et al., 2004). For RD readers in this age range, the predictions are less clear. As we stated earlier, adolescent RD, for whom altered reading circuitry has been established, may exhibit complete dysfunctionality in LH systems for reading. If so, we would anticipate that words that are easier to process will be associated only with modulated activation of the compensatory RH and frontal circuitry. However, if LH posterior regions are less stable but not wholly dysfunctional, we would predict words that are easier to process should reveal greater engagement of reading-related LH posterior regions for RD readers. That is, whereas inconsistent words in general should be associated with diminished LH posterior responses in RD, the facilitative influences of frequency and imageability might be associated with increased LH posterior responsiveness (and possibly with reduced reliance on RH posterior and bilateral anterior compensatory circuits).
Method
Participants
Forty-four native English speakers (27 males, 17 females) ranging from 11.0 years in age to 19.0 years participated in exchange for payment; 24 NI and 20 RD. NI readers had averaged standard scores > 100 (mean = 116) on a composite of the following three assessments: 1) Woodcock Johnson basic reading (mean = 115) Woodcock Johnson spelling (mean = 124), and 3) TOWRE total word reading (mean = 108) measures. Twenty met our criteria for Reading Disability of averaged standard scores < 90 on a composite of these reading tests (Woodcock Johnson basic reading (mean = 90) Woodcock Johnson spelling (mean = 87), and 3) TOWRE total word reading (mean = 75) measures), and/or averaged standard score < 100 with RD history. Groups did not differ on age (RD = 14.94; NI = 15.32, t<1) or WASI performance IQ (RD = 105; NI = 109, t < 1). All reported normal or corrected-to-normal vision and no history of known neurological impairments. The experiment was conducted with the understanding and the written consent of each participant and all procedures were approved by the Yale University Institutional Review Board.
Stimuli and Design
Word stimuli represented the crossing of frequency (low, high), imageability (low, high), and spelling-to-sound consistency (consistent, inconsistent) with 40 words per condition, yielding a total of 320 “go” trials. Non-word trials were made up of 80 pseudowords matched on factors including length, bigram frequency, and initial phoneme served as “no-go” trials. Because we were unable to obtain naming latencies in the MRI scanner, behavioral data were collected in a separate session. In order to assess the behavioral profiles for each group we asked participants to return to the lab on a day subsequent to fMRI scanning and perform the go/no go task with the same materials in order to record behavioral data. Thirty (16 NI, 14 RD) subjects returned for the out of scanner session (fMRI analyses include the full cohort of 44).
Procedure
For the fMRI session, a go/no-go naming paradigm was employed in a block design. Each 20 sec experimental block consisted of five 4 sec trials in which participants were presented with a letter string for 1 sec that either made a real English word or not and were instructed to name it aloud if it was a real English word (“go trial”) and to do nothing if it was not a word (“no-go trial”). The proportion of go and no-go trials was equivalent across conditions. During baseline blocks participants passively viewed displays of intermixed hash marks and asterisks.
fMRI Image Acquisition and Analysis
Functional imaging runs consisted of eight 20-second experimental blocks (one for each stimulus condition) of four word trials and one pseudoword trial, and five 20-second baseline blocks. A total of 1,300 full-brain functional images were acquired across 10 imaging runs; 100 images for each experimental condition and 500 images in the baseline condition. Each subject received the same pseudorandom order of runs. Order of activation block types was counterbalanced across runs.
Functional imaging was performed on GE Signa 1.5 Tesla and Siemens Sonata 1.5 Tesla MR systems. Participants' heads were immobilized within a circularly polarized head coil using a neck support, foam wedges and a restraining band drawn tightly around the forehead. Prior to functional imaging, 20 axial-oblique anatomic images (TE, 11 ms; TR, 500 ms; FOV, 200 mm; 6 mm slice thickness, no gap; 256 ×256 × 2 NEX) were prescribed parallel to the intercommissural line based on sagittal localizer images (TE, 11; TR, 600 ms; FOV, 240 mm; 23 slices, 5 mm slice thickness, no gap; 256 × 256 × 1 NEX). Activation images were collected using single shot, gradient echo, echo planar acquisitions (flip angle, 80 degrees; TE, 50 ms; TR, 2000 ms; FOV, 200 mm; 6 mm slice thickness, no gap; 64 × 64 × 1 NEX) at the same 20 slice locations used for anatomic images.
Functional images were first sinc-interpolated to correct for slice acquisition time, corrected for motion (Friston et al., 1995), and spatially smoothed with a Gaussian filter of size 3.125 mm FWHM. For each subject, an affine transformation to the standardized space defined by the Montreal Neurological Institute (MNI) was obtained using BioImagesuite (Papademetris et al 2003; www.bioimagesuite.org), mapping between the subject-space T1 anatomic and the MNI-space “Colin” brain (available at http://www.bic.mni.mcgill.ca). Prior to across-subjects analysis, this transformation was applied to the single-subject activation maps, with trilinear interpolation, into 2 mm isotropic MNI space. For each subject and voxel, linear regression was used to compare the mean signal during each experimental condition to the baseline condition, and these differences were converted to standardized activation values by dividing them by the square root of the error mean-square for the model. Across subjects these values were entered into a mixed-model or repeated measures ANOVA (Kirk, 1982; Woods, 1996; Holmes et al., 1998) with planned comparisons for main effects, frequency, imageability, and consistency, and their interactions, conducted on a voxel-wise basis.
Region of interest coordinates were defined by peak activation sites within the group by stimulus difficulty interaction analysis (see below). From this analysis we identified seven LH regions that 1) have been previously implicated in reading (c.f., Posner et al., 1999; Pugh et al., 2000a, 2000b; Price 2000) and 2) showed a significant group by stimulus difficulty interaction. Table 1 presents the MNI coordinates and significance levels for peak activation as well as the volume for the following regions: LH fusiform/occipitotemporal (OT), middle temporal gyrus (MTG), thalamus (THAL), superior temporal gyrus (STG), insula (INS), inferior frontal gyrus (IFG), and supramarginal gyrus (SMG). RH regions were examined based on previous studies implicating RD differences and the presence of a group by stimulus difficulty interaction (Pugh et al., 2000a, 2000b).
Table 1.
Regions of Interest (ROIs) showing Reader Group × Stimulus Difficulty interaction.
| Region | X | Y | Z | p-value | Volume (mm3) |
|---|---|---|---|---|---|
| L. Occipito- temporal/Fusiform | −48 | −46 | −18 | 0.0031 | 184 |
| L. Middle Temporal | −44 | −48 | −6 | 0.0004 | 304 |
| L. Thalamus | −18 | −30 | −2 | 0.0056 | 248 |
| L. Superior Temporal | −44 | −44 | 10 | 0.0002 | 384 |
| L. Insula | −38 | 0 | 4 | 0.0003 | 408 |
| L. Inferior Frontal | −42 | 6 | 24 | 0.0007 | 664 |
| L. Supramarginal | −50 | −34 | 32 | 0.0001 | 320 |
Results
Behavioral analysis
Separate 2×2×2×2 mixed factors analyses of variance (ANOVA) were conducted on latencies to correct responses and on errors. Frequency (low/high), Imageability (low/high) and Consistency (consistent/inconsistent) served as within-subjects factors and Reader Group (NI/RD) served as a between-subjects factor. Overall, naming latencies were slower, F(1,28) = 13.05, p < .01, and less accurate, F(1,28) = 8.15, p < .01, for RD relative to NI participants. The group by imageability interaction was marginal for naming latency, F(1,28) = 3.46, p = .07, and significant for accuracy, F(1,28) = 6.91, p < .05, revealing heightened effects of imageability (faster and more accurate responses for high imageable relative to low imageable words) in RD readers. There were also reliable group by frequency interactions on both naming latency, F(1,28) = 12.27, p < .01, and accuracy, F(1,28) = 6.44, p < .05, indicating heightened effects of frequency (faster and more accurate responses for high frequency relative to low frequency words) in RD. These interactions were further qualified by a 3-way interaction of Group X Frequency X Imageability on accuracy, F(1,28) = 4.81, p< .05 such that frequency effects were greater for low imageable relative to high imageable words and this difference was greater for RD readers (3%) than for NI readers (.4%). A reliable 4-way interaction of Group X Frequency X Imageability X Consistency was also observed, F(1,28) = 7.01, p < .05 (driven by a maximum drop in proportion correct to 0.86 for RD on low frequency, low imageable, inconsistent words, as anticipated from previous research).
Given that both frequency and imageability had a heightened facilitative influence on naming inconsistent words for RD participants, a targeted stimulus difficulty analysis of extreme conditions contrasting low frequency/low imageable/inconsistent words (henceforth LF-LI-INC) with high frequency/high imageable/inconsistent words (HF-HI-INC) was performed in order to compare the reader groups on difficult-to-decode words that differ systematically with regard to familiarity and semantic features. This extreme contrast allows us to directly examine purported top down effects on problematic decoding in RD with maximal power. A group interaction was obtained for both accuracy F(1,28) = 5.87, p < .025 and latencies F(1,28) = 8.73, p < .01, and (shown in Figure 1A and 1B, respectively). As predicted, whereas both groups were faster and more accurate on HF-HI-INC than on LF-LI-INC words, this advantage was amplified for RD participants.
Figure 1.
Mean percent error (1A) and reaction time (1B) for NI and RD readers on the contrast of low frequency, low imageable, inconsistent (LF-LI-INC) vs. high-frequency, high-imageable, inconsistent (HF-HI-INC) words.
fMRI Analysis
Naming engaged a broad bi-hemispheric circuitry in general, and overall, activation during the go/no-go task (collapsed across stimulus type) was reliably higher in a large number of regions for NI, relative to RD, participants, as seen in previous studies (see Figure 2A). Of more acute interest in the current experiment however, is how group differences in activation were qualified by stimulus characteristics.
Figure 2.

Omnibus group differences indicate regions where activation for NI is greater than RD (yellow/red) or where RD is greater than NI (blue/purple) (2A). Group difference on the contrast of low frequency, low imageable, inconsistent (LF-LI-INC) vs. high-frequency, high-imageable, inconsistent (HF-HI-INC) words (2B). In yellow/red are those regions where NI show decreases in activation for HF-HI-INC words relative to LF-LI-INC words and RD showed increases. Images are presented at a univariate threshold of p < 0.01, corrected for mapwise false discovery rate (FDR; Genovese et al 2002). Images from top to bottom correspond to the following position along the Z-axis in MNI space: +34, +26, +18, +12, +4, +0, −6, and −20, respectively with the left hemisphere on the right side of the images.
Stimulus difficulty
The behavioral data indicate that RD readers benefited from high frequency and high imageability when reading difficult-to-decode inconsistent words. Therefore, to examine the maximum benefit from our stimulus manipulations, we conducted an analysis of extreme conditions (LF-LI-INC vs. HF-HI-INC words) that paralleled the analysis conducted on the behavioral data. Regions showing this group by stimulus difficulty interaction are shown in Figure 2B. For NI, the easier HF-HI-INC items were associated with relatively reduced activation at virtually all of these regions. For RD, by contrast, these easier words were associated primarily with heightened activation at key, LH, reading-related regions.
Regions of Interest
To more fully investigate stimulus-qualified reader group interactions across the set of a priori defined regions previously implicated as reading-relevant, we conducted region of interest (ROI) analyses. We isolated those clusters of voxels in these regions that were associated with reliable a Group X Stimulus Difficulty (LF-LI-INC vs. HF-HI-INC) interaction (see Figure 2B and Table 1). A striking pattern was observed at several regions, particularly temporoparietal areas, including STG and SMG (see Figure 3A) For NI, the easier, HF-HI-INC words were associated with reduced activation at every ROI, whereas for RD, the opposite pattern was observed (increased activation for easier tokens). Thus, while activation of key LH regions was low for inconsistent words in general for RD readers, when these difficult-to-decode words were of both high frequency and high imageability, activation levels increased modestly.
Figure 3.
Standardized activation values for the reader group by stimulus difficulty contrasts of LF-LI-INC vs. HF-HI-INC words in the seven LH regions of interest: OT/Fusiform (3A); MTG (3B); thalamus (3C); STG (3D); insula (3E); IFG (3F), and SMG (3G).
Discussion
The findings from Experiment 1 suggest latent functionality in LH regions in RD including IFG and STG: RD readers increased engagement of the LH reading systems for easier stimuli. However, two important points should be noted with respect to whether activation in these reading systems may be ‘normalized’. First, despite reliable increases in major reading-related areas for HF-HI-INC stimuli relative to LF-LI-INC stimuli (see Figure 2B), activation levels were still relatively weak for RD readers compared with typical levels for NI readers. Second, the commonly seen RD compensatory response in RH posterior and bilateral IFG was still evident even on easy tokens (higher activation for RD readers), implying limits on normalization of response for this type of manipulation.
Experiment 2
In order to further examine the limits on normalization of function we employed a second experimental manipulation - repetition. The frequency-related activation effects in Experiment 1 indirectly suggest that number of exposures is a critical variable in increased LH responses in RD. In Experiment 2, we conduct a direct test of this notion by manipulating the number of exposures to a given token in the short term with a repetition paradigm (Katz et al., 2005; Poldrak & Gabrieli, 2001). In addition to being one of the strongest behavioral manipulations, repetition allows us to more precisely examine learning-dependent brain activation changes, because we directly control short-term experience in both groups by manipulating how frequently a token is encountered. By employing an animacy judgment (living/nonliving) using a button press, we also measure latency and accuracy, which allows for a more precise comparison of coordinated behavioral and neurobiological changes in NI and RD groups than in Experiment 1. The goal of this repetition manipulation was to bring RD readers to a point of over-learning for repeated tokens (Adams, 1990) in order to examine whether LH systems are robustly engaged with higher learning levels.
Method
Participants
Thirty native English speakers (17 males, 13 females) ranging from 9 years in age to 20 years (mean = 13) participated in exchange for payment; 16 NI and 14 RD. Of the sixteen NI readers, fourteen averaged standard scores > 100 on the composite of the reading tests described in Experiment 1 (overall mean = 115, Woodcock Johnson basic reading (mean = 113) Woodcock Johnson spelling (mean = 119), and 3) TOWRE total word reading (mean = 112) measures)1. Fourteen participants met our criteria for Reading Disability with averaged standard scores < 90 on a composite of these reading tests, and/or averaged standard score < 100 with RD history (overall mean = 86, Woodcock Johnson basic reading (mean = 88) Woodcock Johnson spelling (mean = 88), and 3) TOWRE total word reading (mean = 80)). Groups did not differ on age (RD = 12.89; NI = 13.84, t<1.5) or WASI performance IQ (RD = 108; NI = 111), t < 1). All reported normal or corrected-to-normal vision and no history of known neurological impairments. The experiment was conducted with the understanding and the written consent of each participant and all procedures were approved by the Yale University Institutional Review Board.
Stimuli and Design
Two hundred and eight mid-frequency nouns were selected for the study. All words were four or five letters in length and all had regular spelling-to-sound mappings. Sixty-five percent of the items were “non-living” and 35 percent were “living”. Repeated and novel conditions were matched for mean frequency, length in letters, and proportion of living/non-living trials within each imaging run. Two lists were created for counterbalancing purposes such that a subset of the novel words in the first list served as repeated words in the second list and vice versa. Participants were randomly assigned to one of the two lists.
Procedure
Eight functional imaging runs in an event-related animacy judgment (living/non-living) paradigm employed (a) interleaved acquisition to increase the effective sampling rate of the hemodynamic response (Josephs et al., 1997), (b) multiple randomized or “jittered” trial durations (4–7 sec) (Miezen et al., 2000), and (c) and 6 “null” trials (Friston et al., 1999) to improve our estimate of baseline activation. Each 6:18 minute run consisted of 56 trials in which 6 words were presented 6 times in a pseudorandom fashion with 20 intermixed tokens serving as unrepeated control words. All participants completed at least 6 runs, for a minimum of 216 repetition trials and 120 novel trials across runs. On each trial a word appeared in the center of screen for 2500 msec and participants were instructed to indicate, as quickly as possible, whether the word came from the category of living or non-living objects via a right hand button press. Participants pressed buttons on a response pad with the middle finger for “living” responses and the index finger for “non-living” responses. In-scanner behavioral measures (i.e., reaction time and accuracy) were collected for all subjects using PsyScope (Cohen, MacWhinney, Flatt, & Provost, 1993). Response timing started at the onset of the stimulus presentation and continued until the end of the trial. Participants received 16 practice trials before functional scanning began in order to familiarize themselves with the task and setup.
fMRI Image Acquisition and Analysis
Image acquisition and pre-processing was conducted as described in Experiment 1 except that high-resolution anatomical images were obtained for 3D reconstruction (sagittal MPRAGE acquisition, FA 45°; TE, 4.66 ms; TR, 2000 ms; FOV, 25.6 × 25.6 cm; 1 mm slice thickness, no gap; 256 × 256 × 1 NEX; 28 slices total). For each subject, a nonlinear transformation was then obtained using BioImageSuite (Papademetris et al 2003; www.bioimagesuite.org), mapping between the subject-space high-resolution anatomic and the standard brain space defined by the MNI “Colin” brain. Prior to across-subjects analysis, this transformation was applied to the single-subject activation maps (described below), with trilinear interpolation, into 2 mm isotropic MNI space.
For single-subject event-related analysis, a regression-based method was utilized, allowing for direct estimation of the hemodynamic response for each trial type, at each voxel separately, without prior specification of a reference function (Miezin et al., 2000). Parameters from this regression model were then used to uniquely estimate the mean response for each condition from −3 to +15 seconds relative to stimulus onset. Subject activation maps were then created for each condition using the regression estimates to calculate the mean difference in activity for an activation period (3–8 seconds post trial onset) relative to a baseline period (0–3 seconds prior to trial onset). Linear contrasts for effects of interest, including the evoked response of each trial-type, simple subtractions among trial-types, main effects, and interactions, were applied to these regression estimates to obtain contrast images for each subject. Across subjects, each voxel in these contrast images was tested versus zero with an F-test, implementing a mixed-model or repeated measures ANOVA (Holmes & Friston, 1998; Kirk, 1982; Woods, 1996).
Region of interest coordinates were defined by peak activation sites within a group by linear trend analysis performed to isolate voxels where there was a linear trend across the six presentations that differed by reader group. From this analysis we identified sites in the seven LH regions that have been previously implicated in reading (c.f., Posner et al., 1999; Pugh et al., 2000a, 2000b; Price 2000) and were examined in Experiment 1. For the thalamus, STG, and SMG, including voxels that passed the threshold of p<.01 FDR corrected yielded a volume of less than 100 mm3; thus we adjusted the threshold to include voxels that passed p<.05 FDR to obtain a more stable descriptor of activation of these regions. MNI coordinates and significance levels for peak activation as well as the volume for the seven regions are presented in Table 2. RH regions were examined based on previous studies implicating RD differences and the presence of a group by linear trend interaction (Pugh et al., 2000a, 2000b).
Table 2.
Regions of Interest (ROIs) showing Reader Group × Linear Trend interaction.
| Region | X | Y | Z | p-value | Volume (mm3) |
|---|---|---|---|---|---|
| L. Occipito-temporal/Fusiform | −46 | −44 | −20 | 0.0002 | 112 |
| L. Middle Temporal | −58 | −34 | −6 | 0.0002 | 880 |
| Thalamus | −26 | −32 | 8 | 0.0024 | 216 |
| L. Superior Temporal | −66 | −14 | 10 | 0.0002 | 432 |
| L. Insula | −44 | 2 | −2 | 0.0011 | 248 |
| L. Inferior Frontal | −56 | 18 | 24 | 0.0002 | 216 |
| L. Supramarginal | −34 | −64 | 56 | 0.0042 | 560 |
Results
Behavioral analysis
Separate ANOVAs were performed on both accuracy and latencies to correct responses. Reading group was the sole between subjects factor and repetition was the within subject factor. Due to the relatively small number of stimuli in each exposure condition, we collapsed the six exposures into three periods: early (first and second exposures) middle (third and fourth exposures) and late (fifth and sixth exposures). This three level coding is employed for both behavioral and fMRI region of interest analyses. Accuracy analyses (Figure 4A) revealed a main effect of reader group (F(1,28) = 4.61, p < .05), a marginal effect of repetition (p < .10), and no interaction. Latency analyses shown in Figure 4B revealed main effects of reading group (F(1,28) = 7.01, p < .05) and repetition (F(2,56 = 36.6, p < .001). The interaction of reading group by repetition was not significant (F<1). These data indicate that effects of repetition were facilitative as expected and were of similar magnitude for both NI and RD groups. A general advantage for NI was observed but performance in RD was nonetheless quite good on this task.
Figure 4.
Mean percent error (1A) and reaction time (1B) for NI and RD readers for early, middle, and late exposures to words.
fMRI Analysis
The main effect analysis for group differences revealed the commonly seen underactivation of wide numbers of regions in RD relative to NI readers (see Figure 5A). The reading group by linear repetition comparison revealed reading group differences in the direction of the repetition effect in a number of regions including LH OT, MTG, thalamus, STG, insula, IFG, and SMG (see Figure 5B). These interactions, shown in more detail in the following ROI analyses section, indicate differential effects of repetition on activation for the two groups in these regions. On early increased activation for NI relative to RD is seen at multiple regions including LH OT, STG, insula, IFG, MFG cerebellum and thalamus along with RH sites including OT, IFG, and MTG. On late trials the differences are more circumscribed; limited to OT, extrastriate, and LH insula. Heightened RH activation for RD is apparent at OT and MTG sites, and LH prefrontal increases in RD are seen. Critically, activation differences in several reading-related sites such as STG and IFG are no longer apparent on late trials.
Figure 5.
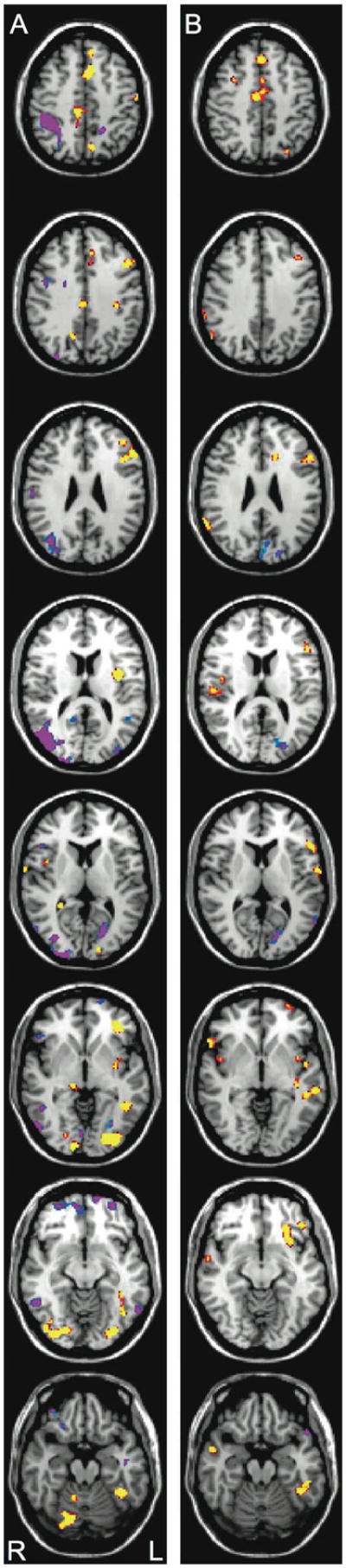
The group difference on un-repeated words (5A) shows regions where activation for NI is greater than RD (yellow/red) or where RD is greater than NI (blue/purple) (p<.001, FDR corrected). The group by linear repetition interaction shows regions where NI decrease activation across the six exposures to a word (yellow/red) and RD showed increased activation across exposures (p<.01, FDR corrected). Images from top to bottom correspond to the following position along the Z-axis in MNI space: +46, +36, +26, +14, +8, −4, −12, −12, and −20, respectively with the left hemisphere on the right side of the images
Regions of Interest
Figure 6 presents the activation levels for each of the seven ROIs described earlier at each exposure period for the two reader groups. The pattern in most of these regions is such that, for NI readers, reduction in activation from early to middle to late was seen, while for RD the opposite pattern was observed. Indeed, for the RD readers, each region exhibits significant activation on late trials (5th and 6th exposures combined). Note that in SMG, STG and Insula, a shift from deactivation to activation in RD is seen by the middle trials, while in OT the shift occurs by the late trials. Moreover, in most regions we observed activation decreases for NI but with some sustained activity even on late trials, while IFG shows de-activation on late trials. This is consistent with our previous study (Katz et al., 2005) suggesting the pre-motor activation is eliminated in silent reading tasks with multiple repetitions (and increased efficiency) for skilled readers. Of note is the activation pattern in MTG that is uniformly low for NI but increases dramatically across repetitions in RD.
Figure 6.
Activation values for the reader group by linear repetition interaction in the seven left hemisphere regions of interest: OT/Fusiform (3A); MTG (3B); thalamus (3C); STG (3D); insula (3E); IFG (3F), and SMG (3G).
Discussion
The findings from Experiment 2 demonstrate that repetition has a similar, facilitative effect on reaction time and accuracy for both NI and RD readers, but an opposite effect on BOLD activation. In crucial LH regions, RD readers show the often-reported deactivation early but show reliable increases with between three and six exposures whereas, NI readers demonstrated continued reduction in activation with increased exposure. The current findings reinforce our conclusion from Experiment 1 that the LH systems in RD are poorly tuned but can respond when processing words is made easier. Of note, while there are still some residual differences in LH regions such as the insula, and slightly elevated RH response in posterior ventral areas for RD even with multiple exposures, the overall activation in several LH regions appears to be robust and not reliably different as performance improves.
The similar pattern of findings in the two experiments suggests that these learning-related increases in RD generalize to both overt naming tasks (Experiment 1) and silent reading tasks (Experiment 2). Note that the regions of maximum activation tend to differ somewhat within the broadly defined regions of interest but this is not surprising given the differing demands of naming and silent lexical access. In order to identify those voxels which showed reliable group interactions with the stimulus manipulations in both experiments, presumably task invariant reading sites, we conducted an intersect analysis (Hadjikhani and Roland, 1998), which identified those voxels for NI that reduced activation across stimulus difficulty (Experiment 1) repetition (Experiment 2) and increased activation across stimulus difficulty and repetition for RD. As seen in Figure 7 overlapping sites are found at IFG, insula, STG, and MTG that show this pattern in each experiment. Thus, despite rather varied response demands, a core set of areas in LH cortex showed opposite activation changes in NI and RD as a function of stimulus difficulty.
Figure 7.
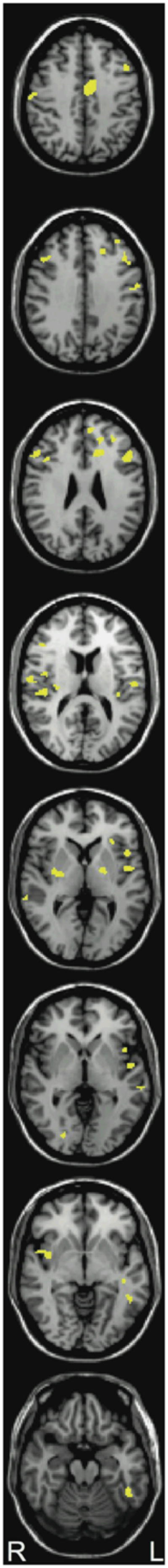
Intersect analysis showing voxels that showed a reader group by stimulus difficulty effect and a reader group by repetition effect (p <.05 FDR corrected in each; conjoint threshold of p<.0025). Images from top to bottom correspond to the following position along the Z-axis in MNI space: +42, +34, +26, +14, +2, −2, −6, and −20, respectively with the left hemisphere on the right side of the images.
General Discussion
The current findings suggest a degree of latent functionality in LH neurocircuitry for reading in RD readers. The behavioral results from Experiment 1 (on both naming latencies and accuracy) demonstrate that factors such as frequency and imageability enhance performance for RD and NI readers on difficult-to-decode (inconsistent) words. Similarly, repetition of tokens in Experiment 2 facilitated processing on repeated tokens for both groups of readers. This work extends recent studies examining stimulus difficulty and effort effects on activation differences (Cao et al., 2006; Hoeft et al., 2007), by demonstrating significant increases in neural activity in LH systems for adolescent RD as a consequence of learning and experience.
More striking was the differential pattern of brain activation across both experiments. In Experiment 1, the often-reported group differences in activation (lower BOLD signal across the LH reading-related circuitry in RD relative to NI) were qualified by stimulus difficulty. Specifically, on the most difficult words (LF-LI-INC), RD readers demonstrated reduced activation relative to NI across almost all reading-relevant zones; indeed, for these items no reliable activation was seen in key temporoparietal sites including STG and SMG. For easier stimulus types (e.g., HF-HI-INC words), RD readers demonstrated increased activation relative to the hardest stimuli at these same LH regions, suggesting that these cortical networks are poorly trained in RD but not wholly unavailable during reading performance (Pugh et al., 2000a). Although NI readers showed stable activation of these regions for all stimulus types, when compared to RD readers the NI group showed the opposite pattern of modulation for easier-to-process words with relatively reduced activation apparent across the LH reading circuit -- presumably reflecting increased processing efficiency (Katz et al., 2005; Poldrack & Gabrieli, 2001; Sandak et al., 2004). Although the data indicate that RD readers increased engagement of the LH reading systems for easier stimuli, Experiment 1was not definitive regarding whether activation in these reading systems may be ‘normalized’. Despite modest increases in major reading-related areas for HF-HI-INC stimuli (see Figure 2B), activation levels were still relatively weak for RD readers compared with typical levels for NI readers. Moreover, the commonly seen RD compensatory response in RH posterior and bilateral IFG was evident even on easy tokens, implying limits on normalization of response.
Experiment 2 provided clear evidence for a shift from deactivation to robust activation of most key LH regions with repetition in RD readers. We can conclude from these experiments that many important reading-related regions are capable of engaging in print processing for stimuli that are made easier to process either through repetition-induced learning or increased top down support from imageability or frequency manipulations.
Given that phonologically-sensitive subregions of the LH (i.e., IFG, SMG, STG) are generally found to be under-engaged in RD (including in the current study for more difficult stimuli), the increased responsiveness in RD for high frequency/high imageable inconsistent tokens relative to low frequency/low imageable inconsistent tokens in naming at each of the key phonologically-tuned areas (Pugh et al., 2006) may reflect increased communication or resonance with semantically-tuned networks; that is, stabilization of a poorly tuned phonological coding system via support from non-phonological systems. Experiment 2 is empirically straightforward with regard to increased activation in phonologically tuned regions, but whether this reflects semantic reinforcement (given that the task involves animacy judgment), orthographic/phonological reinforcement, or both of these things is not fully answerable. One possible answer is suggested by the pattern of activation in MTG (see Figure 6), a region strongly associated with lexical-semantic processing (Price, 2000; Pugh et al., 2000). In the MTG NI readers showed minimal and unchanging activation while RD demonstrated initial deactivation and then robust activation by middle to late trials. This might reflect differential sensitivity to semantic support in RD readers. In general though, we see that relatively poorly-tuned phonologically-sensitive regions are positively affected by ameliorative factors for RD in both Experiments 1 and 2.
The current results suggest a neurobiological learning curve wherein NI and RD readers start at very different points on an inverted-U shaped relationship between learning and neural activation. Neuroimaging studies of perceptual and motor skill learning in non-impaired populations have demonstrated that initial skill acquisition (unskilled performance) is associated with increased activation in task-specific cortical areas, whereas continued practice of an acquired skill tends to be associated with task-specific decreases in activation in the same cortical regions. (e.g., Katz et al., 2005; Poldrack & Gabrieli, 2001). Other studies of skilled reading have also shown different patterns of learning-related changes in brain activation as a function of item familiarity. For example, Henson et al. (2002) found that repetition of familiar real words was associated with decreases in cortical activation, whereas repetition of (initially unfamiliar) pseudowords was associated with increased activation in the same regions. Thus, this learning-curve hypothesis suggests that whether learning is associated with increased or decreased activation depends upon the degree to which processing is over-learned and automatic (how far along the learning curve it is). With regard to developmental trajectories our cross sectional studies have suggested that beginning readers show low activation of regions such as the putative VWFA but increase with experience (Shaywitz et al., 2002). However, once these systems are in place, increased routinization for stimuli (such as the repetition effect in the current Experiment 2) results in drops in activation (thus a non-monotonic relation between activation and experience in nonimpaired readers).
In the current study RD readers appear to start at a very low point on this curve but repetition and learning result in increases. This does not necessarily imply a simple developmental delay account of specific reading disability, since these regions are clearly less than ideally organized even after many years of reading experience in our adolescent RD readers, and this suggests some degree of compromise in the neural circuitry. But the robust activation seen after multiple exposures in Experiment 2 does lend itself to speculation that these systems are trainable (indeed, it will be critical in future studies to push these systems further to test limits on learning effects).
One question that the data from Experiment 2 raise, is why the initial (first exposure) activation response in LH is so low given that RD readers have certainly seen the words used in this experiment thousands of times. The most straightforward hypothesis, and one that would point to a very specific learning problem in RD, is that these readers fail to consolidate the learning experience into a longer term neural changes in processing and organization. Thus, the system might be available for processing but fails to demonstrate savings with longer term modulation of connections. If this turns out to be the case (for all or some subtypes of RD), then this would shift focus away from exclusive focus on simple mapping deficits toward more systematic investigation of the mechanisms of explicit or implicit learning. Of course, there is nonetheless some indication of neural consolidation in Experiment 1, where both frequency and imageability (a semantic variable) were associated with a heightened LH response even without local repetition. However, the increases for HF-HI-INC tokens in that experiment were modest. In any event, the mechanisms underlying what appears to be a possible consolidation deficit in RD will require us to explore a new line of dynamic learning paradigms to measure long term learning under varied training conditions (e.g., Sandak et al., 2004).
The current results along with our previous work that has shown non-uniformity in hemodynamic effects of learning as a function of stimulus type (Katz et al., 2005; Sandak et al., 2004a) have some important methodological and design implications as well. These findings reveal the importance of controlling stimulus factors in order to derive a more precise understanding of brain/behavior relations in RD. Although most published reports show lower LH posterior activation in RD, the results of these experiments indicate that the extent of this difference is dependant on stimulus difficulty. From a design consideration, we argue that dynamic designs which parametrically examine the ways in which learning modulates relative activation across distributed systems in NI will allow for the development of a more detailed theory of the neurobiological mechanisms of reading, and can provide a framework for examining systems level differences in RD. Indeed, it seems plausible that in searching for biomarkers that are diagnostic in this condition, response patterning to learning tasks may prove more substantive than context variable absolute activation levels and group differences.
The current results provide some constraint on the sorts of hypotheses we entertain regarding neurobiological mechanisms in RD. Whatever the biologic mechanism (or mechanisms) that engender risk for RD, this mechanism must be of the sort that results in a neurocircuitry that is relatively disrupted in general, but is nonetheless not so fundamentally compromised that a more typical reading response cannot be induced. Indeed, recent intervention studies with at-risk or RD children indicate increased engagement of all these LH areas following intensive remediation (Shaywitz et al., 2004, Temple et al., 2003, Simos et al., 2002).
Various biological accounts have been proposed to explain this LH dysfunction, including a suggestion of a higher numbers of cortical dysplasias or ectopias (Galaburda, 1992), reduced myelination in white matter tracts (Klingberg, et al., 2000) connecting anterior and posterior language zones, or abnormalities in grey matter development (Miller et al., 2003). Obviously, the current findings do not directly assess any of these speculations but they do suggest a clear biologic constraint: the systems are weakened but not wholly dysfunctional in even severe older RD readers. The general notion of a “developmental lesion” at critical LH systems in reading disability (c.f., Eden and Zeffiro, 1998) would appear to be inconsistent with data suggesting functional activation of these systems under certain conditions (see Pugh et al., 2000a for similar conclusions with functional connectivity analyses). Speculatively, these findings seem most consistent with accounts that posit “noisy” or unstable neural systems (Sperling et al., 2005).
Summary
In Experiment 1 effects of imageability and frequency on behavioral performance in NI and RD participants were similar. Both groups show facilitation but, as predicted, the benefit was larger for RD. Effects of imageability and frequency on brain activation in phonologically-tuned subsystems in NI and RD were wholly dissimilar. For NI readers, easier words were associated with relatively reduced activation. For RD readers, easier words resulted in increased activation. Experiment 2 replicated this pattern with a simple and direct manipulation of on-line learning through stimulus repetition. Thus, the phonologically-tuned subsystems in adolescent RD readers appear to be poorly trained but not wholly disrupted. Further studies will be required to test the limits on learning in these LH systems in RD.
Acknowledgments
Supplementary materials, including characteristics of the word stimuli, complete reaction time and accuracy data, and additional contrasts maps are available from the first author. This study is supported by NICHD Grant HD01994 to Haskins Laboratories and NICHD Grants HD40411, HD 048830 to Kenneth R. Pugh. We thank Gina DellaPorta, Kelley Delaney, Eleanor Tejada, and Priya Pugh for behavioral assessment and Hedy Serofin and Teri Hickey for help with imaging participants.
Footnotes
Two of the sixteen readers were unavailable for testing, but their overall performance on the in-scanner task was 97% and 98% correct and they had no history of reading difficulties.
References
- Adams MJ. Beginning to read: thinking and learning about print. A Bradford Book, the MIT Press; 1990. [Google Scholar]
- Bruck M. Persistence of dyslexics' phonological deficits. Developmental Psychology. 1992;28:874–886. [Google Scholar]
- Cao F, Bitan T, Chou TL, Burman DD, Booth JR. Deficient orthographic and phonological representations in developmental dyslexics revealed by brain activation patterns. Journal of Child Psychology and Psychiatry. 40:1041–1050. doi: 10.1111/j.1469-7610.2006.01684.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: A new graphic interactive environment for designing psychology experiments. Behavioral Research Methods, Instruments, and Computers. 1993;25:257–271. [Google Scholar]
- Eden GF, Zeffiro TA. Neural systems affected in developmental dyslexia revealed by functional neuroimaging. Neuron. 1998;21:279–82. doi: 10.1016/s0896-6273(00)80537-1. [DOI] [PubMed] [Google Scholar]
- Frost SJ, Mencl WE, Sandak R, Moore DL, Rueckl J, Katz L, Fulbright RK, Pugh KR. An fMRI study of the trade-off between semantics and phonology in reading aloud. Neuroreport. 2005;16:621–624. doi: 10.1097/00001756-200504250-00021. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RSJ. Spatial registration and normalization of images. Human Brain Mapping. 1995;2:165–189. [Google Scholar]
- Galaburda AM. Neurology of developmental dyslexia. Current Opinion in Neurology and Neurosurgery. 1992;5:71–76. [PubMed] [Google Scholar]
- Hadjikhani N, Roland PE. Cross-modal transfer of information between the tactile and the visual representations in the human brain: A positron emission tomographic study. Journal of Neuroscience. 1998;18:1072–84. doi: 10.1523/JNEUROSCI.18-03-01072.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Price CJ, Rugg MD, Turner R, Friston KJ. Detecting latency differences in event-related BOLD responses: Application to words versus pseudowords and initial versus repeated face presentations. NeuroImage. 2002;15:83–97. doi: 10.1006/nimg.2001.0940. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor-Hill H, Martindale JL, McMillon G, Kolchugina G, Black JM, Faizi A, Deutsch GK, Siok WT, Reiss AL, Whitfield-Gabrieli S, Gabrieli JDE. Functional and morphometric brain dissociation between dyslexia and reading ability. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4234–4239. doi: 10.1073/pnas.0609399104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ. Generalizability, random effects, and population inference. NeuroImage. 1998;7:S34. [Google Scholar]
- Jared D, McRae K, Seidenberg MS. The basis of consistency effects in word naming. Journal of Memory & Language. 1990;29:687–715. [Google Scholar]
- Katz L, Lee CH, Tabor W, Frost SJ, Mencl WE, Sandak R, Rueckl JG, Pugh KR. Effects of printed word repetition in lexical decision and naming on behavior and brain Activation. Neuropsychologia. 2005;43:2068–2083. doi: 10.1016/j.neuropsychologia.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Experimental design: Procedures for the social sciences. Belmont, CA: Wadsworth; 1982. [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JD, Moseley ME, Poldrack RA. Microstructure of temporo-parietal white matter as a basis for reading ability: evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;5:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: Effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. NeuroImage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Miller CJ, Sanchez J, Hynd GW. Neurological correlates of reading disabilities. In: Swanson HL, Harris KR, Graham S, editors. Handbook of learning disabilities. New York: Guilford Press; 2003. [Google Scholar]
- Papademetris X, Jackowski AP, Schultz RT, Staib LH, Duncan JS. Computing 3D non-rigid brain registrations using extended robust point matching for composite multisubject fMRI analysis. In: Ellis RE, Peters TM, editors. Medical Image Computing and Computer Assisted Intervention. Berlin: Springer-Verlag; 2003. pp. 788–795. [Google Scholar]
- Paulesu E, Demonet J-F, Fazio F, McCrory E, Chanoine V, Brunswick N, Cappa SF, Cossu G, Habib M, Frith CD, Frith U. Dyslexia: Cultural diversity and biological unity. Science. 2001;291:2165–2167. doi: 10.1126/science.1057179. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Gabrieli JDE. Characterizing the neural mechanisms of skill learning and repetition priming: evidence from mirror reading. Brain. 2001;124:67–82. doi: 10.1093/brain/124.1.67. [DOI] [PubMed] [Google Scholar]
- Posner MI, Abdullaev YG, McCandliss BD, Sereno SC. Anatomy, circuitry and plasticity of word reading. In: Everatt J, editor. Reading and Dyslexia: Visual and attentional processes. London and New York: Routledge; 1999. pp. 137–162. [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. Journal of Anatomy. 2000;197:335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Shaywitz SA, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Shankweiler DP, Katz L, Fletcher JM, Gore JC. Cerebral organization of component processes in reading. Brain. 1996;119:1221–1238. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Mencl WE, Jenner AR, Katz L, Frost SJ, Lee JR, Shaywitz SE, Shaywitz BA. Functional neuroimaging studies of reading and reading disability (developmental dyslexia) Mental Retardation & Developmental Disabilities Research Reviews. 2000a;6:207–213. doi: 10.1002/1098-2779(2000)6:3<207::AID-MRDD8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Pugh K, Mencl WE, Shaywitz BA, Shaywitz SE, Fulbright RK, Skudlarski P, Constable RT, Marchione K, Jenner AR, Shankweiler DP, Katz L, Fletcher J, Lacadie C, Gore JC. The angular gyrus in developmental dyslexia: Task-specific differences in functional connectivity in posterior cortex. Psychological Science. 2000b;11:51–56. doi: 10.1111/1467-9280.00214. [DOI] [PubMed] [Google Scholar]
- Sandak R, Mencl WE, Frost S, Pugh KR. The neurobiological basis of skilled and impaired reading: Recent findings and new directions. Scientific Studies of Reading. 2004b;8:273–292. [Google Scholar]
- Sandak R, Mencl WE, Frost SJ, Rueckl JG, Katz L, Moore D, Mason SM, Fulbright RK, Constable RT, Pugh KR. The neurobiology of adaptive learning in reading: A contrast of different training conditions. Cognitive, Affective, & Behavioral Neuroscience. 2004a;4:67–88. doi: 10.3758/cabn.4.1.67. [DOI] [PubMed] [Google Scholar]
- Sarkari S, Simos PG, Fletcher JM, Castillo EM, Breier JI, Papanicolaou AC. The emergence and treatment of developmental reading disability: Contributions of functional brain imaging. Seminars in Pediatric Neurology. 2002;9:227–236. doi: 10.1053/spen.2002.35506. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Shaywitz SE, Pugh KR, Mencl WE, Fulbright RK, Constable RT, Skudlarski P, Jenner A, Fletcher JM, Marchione KM, Shankweiler D, Katz L, Lacadie C, Gore JC. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biological Psychology. 2002;52:101–110. doi: 10.1016/s0006-3223(02)01365-3. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Pugh KR, Holohan JM, Marchione KE, Fletcher JM, Lyon GR, Gore JC. Neural systems for compensation and persistence: Young adult outcome of childhood reading disability. Biological Psychiatry. 2003;54:25–33. doi: 10.1016/s0006-3223(02)01836-x. [DOI] [PubMed] [Google Scholar]
- Shaywitz B, Shaywitz S, Blachman B, Pugh KR, Fulbright R, Skudlarski P, Mencl WE, Constable T, Holohan J, Marchione K, Fletcher J, Lyon R, Gore J. Development of left occipitotemporal systems for skilled reading following a phonologically based intervention in children. Biological Psychiatry. 2004;55:926–933. doi: 10.1016/j.biopsych.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Simos PG, Fletcher JM, Bergman E, Breier JI, Foorman BR, Castillo EM, Davis RN, Fitzgerald M, Papanicolaou AC. Dyslexia-specific brain activation profile becomes normal following successful remedial training. Neurology. 2002;58:1203–1213. doi: 10.1212/wnl.58.8.1203. [DOI] [PubMed] [Google Scholar]
- Seidenberg MS, Waters GS, Barnes M, Tanenhaus MK. When does irregular spelling or pronunciation influence word recognition? Journal of Verbal Learning and Verbal Behavior. 1984;23:383–404. [Google Scholar]
- Sperling AJ, Lu Z, Manis FR, Seidenberg MS. Deficits in perceptual noise exclusion in developmental dyslexia. Nature Neuroscience. 2005;8:862–863. doi: 10.1038/nn1474. [DOI] [PubMed] [Google Scholar]
- Strain E, Herdman CM. Imageability effects in word naming: An individual differences analysis. Canadian Journal of Experimental Psychology. 1999;53:347–359. doi: 10.1037/h0087322. [DOI] [PubMed] [Google Scholar]
- Strain E, Patterson K, Seidenberg MS. Semantic effects in single-word naming. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;21:1140–1154. doi: 10.1037//0278-7393.21.5.1140. [DOI] [PubMed] [Google Scholar]
- Strain E, Patterson K, Seidenberg MS. Theories of word naming interact with spelling-sound consistency. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2002;28:207–214. doi: 10.1037/0278-7393.28.1.207. [DOI] [PubMed] [Google Scholar]
- Temple E, Deutsch GK, Poldrack RA, Miller SL, Tallal P, Merzenich MM, Gabrieli JDE. Neural deficits in children with dyslexia ameliorated by behavioral remediation: Evidence from functional MRI. Proceedings of the National Academy of Sciences. 2003;100:2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RP. Modeling for intergroup comparisons of imaging data. NeuroImage. 1996;4:S84–S94. doi: 10.1006/nimg.1996.0058. [DOI] [PubMed] [Google Scholar]






