Abstract
Type 2 diabetes is associated with higher fracture risk. Diabetes-related conditions may account for this risk. Cardiovascular Health Study participants (N = 5641; 42.0% men; 15.5% black; 72.8±5.6 years) were followed 10.9 ± 4.6 years. Diabetes was defined as hypoglycemic medication use or fasting glucose (FG) ≥126 mg/dL. Peripheral artery disease (PAD) was defined as ankle-arm index <0.9. Incident hip fractures were from medical records. Crude hip fracture rates (/1000 person-years) were higher for diabetic vs. non-diabetic participants with BMI <25 (13.6, 95% CI: 8.9–20.2 versus 11.4, 95% CI: 10.1–12.9) and BMI ≥25 to <30 (8.3, 95% CI: 5.7–11.9 versus 6.6, 95% CI: 5.6–7.7), but similar for BMI ≥30. Adjusting for BMI, sex, race, and age, diabetes was related to fractures (HR = 1.34; 95% CI: 1.01–1.78). PAD (HR = 1.25 (95% CI: 0.92–1.57)) and longer walk time (HR = 1.07 (95% CI: 1.04–1.10)) modified the fracture risk in diabetes (HR = 1.17 (95% CI: 0.87–1.57)). Diabetes was associated with higher hip fracture risk after adjusting for BMI though this association was modified by diabetes-related conditions.
1. Introduction
Type 2 diabetic adults have an approximately 40–70% increased fracture risk [1, 2] compared to non-diabetic adults although the mechanism for this is not well established. Recent studies found fracture risk is elevated not just in the older diabetic adults, but also for middle-aged type 2 diabetic adults [3–6]. The risk appears to be equivalent for both diabetic men and women [7], suggesting an increased risk in both sexes. Importantly, in older diabetic adults, their generally higher bone mineral density (BMD) does not protect them from fracture and they have a higher fracture rate at an equivalent BMD to non-diabetic adults, which may potentially be due to their burden of diabetic complications [8, 9]. Although the higher weight of many type 2 diabetic patients is likely the main contributor to of their overall higher BMD [10], at an equivalent body size to a non-diabetic older adult, type 2 diabetic patients are more likely to fracture [9]. Therefore, other factors in type 2 diabetes besides BMD and obesity are likely contributing to the higher fracture rates. Identifying factors contributing to the higher fracture rate in diabetes may ultimately lead to preventative efforts for fracture in this high-risk population.
Hyperglycemia itself may not directly account for the increased fracture risk in diabetes [1, 2] and impaired fasting glucose (IFG), or prediabetes, was not associated with higher fracture risk in older adults [9]. Emerging evidence suggests that clinical and subclinical alterations in peripheral nerve function [11], vascular function [12], and kidney function [13–15] are related to lower BMD, bone loss, or fracture in a dose-response manner. These complications could contribute to the higher fracture risk in diabetes. The objectives of the current study are to determine if type 2 diabetes or IFG are independently associated with a higher risk for hip fractures for older white and black men and women and if diabetes-related conditions contribute to the risk of hip fracture in older type 2 diabetic adults.
2. Subjects, Materials, and Methods
2.1. Study Population
The CHS is a prospective, multicenter, cohort study of risk factors for cardiovascular disease in older community-dwelling adults. The study methods were previously described in detail [16]. In 1989-1990 (white cohort) and in 1992-1993 (black cohort), a total of 5888 noninstitutionalized, ambulatory men and women 65 years or older were enrolled from Medicare eligibility lists in four US communities (Pittsburgh, PA; Hagerstown, MD; Sacramento, CA; Winston-Salem, NC). Each center's institutional review committee approved the study and all participants gave informed consent prior to exams. Mean age at enrollment was 73 years (range: 65–100); 58% were women and 16% were black. Participants underwent an extensive baseline evaluation, including standardized clinical examinations, laboratory assessments, physical and cognitive functioning assessments, and questionnaires on medical history, health status, and risk factors, components of which were repeated at annual clinic visits through 1998/99. In 2005/06, the entire surviving CHS cohort was re-recruited to reevaluate physical and cognitive functioning for the CHS All Stars examination, an ancillary study to reassess functional status (median age 85, range 77–102; 66.5% female; 16.6% black) [17]. Phone followup for health outcomes was conducted every six months throughout the study and through the present time. Participants with hip fracture concurrent to motor vehicle accidents (N = 9) or pathologic fracture (N = 9), missing fasting glucose (N = 64), missing or noncompliant fasting status (N = 159), or missing information on hypoglycemic medications (N = 6) were excluded, resulting in 5,641 participants available for analyses.
2.2. Hip Fractures
Information regarding hospitalizations was collected every 6 months from participants. To ensure completeness and verify the accuracy of the self-reported data, Medicare claims data were also used to identify any hospitalizations. Incident hip fractures were ascertained from a comprehensive review of hospitalization records through June 30, 2005, using International Classification of Diseases, Ninth Revision codes (ICD-9 codes 820.xx). Only the first hospitalization for hip fracture was considered and fractures were excluded if they were the result of excessive trauma (e.g., motor vehicle accident, ICD-9 E810-E825) or a pathologic condition (e.g., cancer, ICD-9 733.1). Among 5641 participants, 541 incident hip fractures were identified over 10.9 ± 4.6 years of followup.
2.3. Diabetes
Baseline and incident diabetes status were defined based on medication information collected annually by medications inventories through 1998-1999, and fasting glucose (≥8 hours) measured on blood samples drawn in 1989-90, 1992-93, and 1996-97. Diabetes at study entry was defined as hypoglycemic medication use or a fasting glucose ≥126 mg/dL (≥7.0 mmol/L). Impaired fasting glucose (IFG) at study entry was defined as ≥100 mg/dL (≥6.1 mmol/L) but <126 mg/dL (<7.0 mmol/L) and no use of insulin or oral hypoglycemic medications. Participants were considered to have incident diabetes if either of the following conditions were met during the period after enrollment through a 1998-99 assessment: (1) any use of insulin or oral hypoglycemic medications or (2) fasting glucose ≥126 mg/dL. Detailed methods regarding blood draw, sample storage, quality assurance, and assay performance were previously described [18]. At baseline, of the 918 participants with prevalent diabetes, 361 were taking oral hypoglycemic medications only, 128 were taking insulin only, 11 were on both insulin and oral hypoglycemic medications, 417 were not taking medications, and 1 was missing medication information.
2.4. Body Composition and Physical Function
Body weight was measured using a calibrated balance beam scale and height was measured with a wall-mounted stadiometer. Body mass index (BMI) was calculated in kg/m2. Waist circumference (cm) was measured at the umbilicus. Physical function was evaluated by the time in seconds to walk 15 feet in a corridor from a standing start. Participants self-reported a history of frequent falls in the past year.
2.5. Ankle-Arm Index
Subclinical peripheral arterial disease (PAD) was defined as ankle-arm index <0.9 (AAI), measured as the ratio of the ankle systolic blood pressure to the arm systolic blood pressure, using a standard protocol [19]. After a five minute rest lying on an examination table, a standard arm blood pressure cuff was used to measure systolic blood pressure in the right arm and then each ankle. After palpating the brachial and posterior tibial arteries and applying ultrasound gel, a Doppler stethoscope and standard mercury manometer were used to measure systolic blood pressure in the right brachial artery and each posterior tibial artery in rapid succession. The lower value of either the left or right AAI was used to classify subclinical PAD.
2.6. Additional Covariates
Serum creatinine was measured using the Kodak Ektachem 700 Analyzer (Eastman Kodak, Rochester, NY), a colorimetric method. Kidney disease was defined as creatinine ≥1.5 mg/dL in men and ≥1.3 mg/dL in women [20] or an estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2 [21]. Fasting serum insulin level was measured by solid-phase immunoassay (Diagnostic Products Corp, Los Angeles, CA). Health histories collected at baseline included self-reported weight at age 50 years, current smoking, alcohol consumption, vision problems (unable to see to drive, to watch TV, or to recognize someone across a room with or without glasses) and clinical cardiovascular (CVD) disease (myocardial infarction, angina, congestive heart failure, claudication, stroke, transient ischemic attack). From the medication inventory, oral estrogen use was obtained [22], and participants were asked if they took any calcium supplements one or more times per week. Physical activity was calculated in kcal/week from total self-reported activities, excluding household chores, from the Minnesota Leisure Time Activities questionnaire [23].
2.7. Statistical Analyses
Univariate differences between DM, IFG, and normoglycemic groups were evaluated by chi-square tests for categorical covariates, by t-tests for normal continuous covariates, and by Mann-Whitney nonparametric tests for nonnormal continuous covariates, in men and women separately. Crude hip fracture rates (/1000 person-years) were determined for normal FG, IFG, and DM groups and also stratified by BMI categories of <25, ≥25 to <30, and >30. Cox proportional hazards models were used to estimate the relative risk (hazard ratio) of hip fracture associated with glycemic status. The participants' entry time into the analysis corresponded to their study enrollment date with time-at-risk until the earliest of incident hip fracture or censoring at death, loss-to-followup, or the last day of adjudicated followup (June 30, 2005). Multivariable models were adjusted for age, sex, race, BMI, diabetes, subclinical PAD (AAI < 0.9), and other potential confounders and mediators listed in the “Additional Covariates” section above. Sex did not satisfy the proportionality assumption due to an interaction of time with sex and therefore models were internally stratified for sex. Covariates described above in the Methods were retained in the final model if they attenuated the risk estimate by 10% or more or had a P-value < 0.10. Covariates correlated at r ≥ 0.50 were not entered simultaneously, but one was entered separately and then the other separately at the same step of the model, for example, BMI and waist circumference (r = 0.81); BMI and weight at age 50 (r = 0.50). For example, BMI was entered in the final regression model instead of waist circumference given the high correlation and the stronger relationship of BMI to hip fracture. Other covariates were not highly correlated, for example, smoking and subclinical PAD (r = 0.09) and smoking and alcohol use (r = 0.05). Potential effect modification of race, sex, BMI, and subclinical PAD with both DM and IFG were evaluated through multiplicative interaction terms with likelihood ratio tests. Stratified analyses by sex were performed for certain models a priori. Osteoporosis medication including bisphosphonates, calcitonin, and raloxifene, and thiazolidinediones (TZDs) were not used at the 1989-1990 baseline in any participants. However, a sensitivity analyses was performed which excluded 236 participants that took these medications during followup. Because incident diabetes in the normoglycemic and impaired fasting glucose groups may have modified the risk estimates for the diabetic group, a separate sensitivity analysis was done by excluding incident diabetes cases from these two groups. A sensitivity analysis was also done by excluding participants with AAI > 1.4, since this may represent vascular calcification and vessel stiffness. Analyses were performed using Stata software, version 10.0 (Stata Corp., College Station, Texas).
3. Results
Participants with DM or IFG were more likely men (49.3% and 46.4% versus 36.4%; P < 0.001) than those with normal FG (Table 1). Participants with DM and IFG had a higher weight, a higher BMI, a greater self-reported weight at age 50 years, a higher waist circumference, a higher fasting insulin level, a lower eGFR (men only), lower physical activity, and were less likely to use oral estrogen or calcium supplements (women only) compared to those with normal FG. Additionally, participants with DM were more likely to be black, less likely to be a current drinker, had slower completion of the measured walk, and had more diabetes-related conditions (vision problems, subclinical PAD, prevalent CVD, and renal insufficiency) than those with normal FG.
Table 1.
Baseline descriptive characteristics by glycemic status for 5,641 women and men in the CHS.
| Women (n = 3254) | Men (n = 2387) | |||||
|---|---|---|---|---|---|---|
| DM (n = 465) | IFG (n = 1144) | No IFG or DM (n = 1645) | DM (n = 453) | IFG (n = 991) | No IFG or DM (n = 943) | |
| Black race | 139 (29.9%)† | 162 (14.2%) | 248 (15.1%) | 84 (18.5%) | 100 (10.1%)‡ | 142 (15.1%) |
| Age (years) | 72.7 (±5.7) | 72.6 (±5.5) | 72.4 (±5.3) | 72.9 (±5.2) | 73.2 (±5.6) | 73.5 (±6.1) |
| Current smoker | 50 (10.8%) | 149 (13.0%) | 213 (13.0%) | 42 (9.3%) | 105 (10.6%) | 118 (12.5%) |
| Current drinker | 122 (26.3%)† | 535 (46.9%) | 777 (47.4%) | 189 (41.8%)† | 623 (63.2%) | 572 (60.9%) |
| Oral estrogen use | 20 (4.3%)† | 102 (8.9%)† | 270 (16.4%) | NA | NA | NA |
| Calcium supplement use | 73 (15.9%)† | 273 (24.1%)† | 513 (31.5%) | 35 (7.8%) | 95 (9.7%) | 92 (9.9%) |
| Height (cm) | 159.4 (±6.3) | 158.9 (±6.4) | 158.7 (±6.1) | 173.6 (±6.8) | 172.9 (±6.6) | 173.0 (±6.5) |
| Weight (lbs) | 165.2 (±32.3)† | 154.4 (±31.2)† | 141.6 (±28.3) | 184.2 (±31.2)† | 177.2 (±26.8)† | 167.1 (±24.8) |
| BMI (kg/m2) | 29.5 (±5.4)† | 27.7 (±5.2)† | 25.5 (±4.8) | 27.7 (±4.3)† | 26.9 (±3.7)† | 25.3 (±3.3) |
| Waist circumference (cm) | 100.4 (±14.1)† | 94.1 (±14.1)† | 88.4 (±13.5) | 101.2 (±11.2)† | 98.8 (±10.0)† | 94.6 (±9.5) |
| Weight at age 50 (lbs) | 154.7 (±28.7)† | 141.2 (±23.1)† | 135.3 (±22.1) | 184.3 (±29.7)† | 172.6 (±24.1)† | 167.1 (±21.5) |
| Fasting insulin (IU/mL) | 21 (14,31)∗† | 14 (11,19)∗† | 11 (8,14)* | 17 (12,28)∗† | 14 (10,19)∗† | 11 (8,14)* |
| Walk time (s to walk 15 ft) | 6.7 (±2.7)† | 5.9 (±2.1) | 5.9 (±2.3) | 6.0 (±3.1)‡ | 5.5 (±2.0) | 5.5 (±2.2) |
| Physical activity (kcal/wk)* | 210 (0,749)∗† | 385 (28,1094)∗‡ | 495 (79,1193)* | 856 (230,1920)∗‡ | 917 (306,2166)∗‡ | 1088 (405,2407)* |
| Frequent falls | 33 (7.1%)† | 38 (3.3%) | 72 (4.4%) | 16 (3.6%) | 13 (1.3%) | 23 (2.5%) |
| Vision problem | 53 (12.4%)‡ | 83 (7.7%) | 117 (7.6%) | 18 (4.0%) | 50 (5.1%) | 55 (6.0%) |
| AAI < 0.90 | 89 (20.1%)† | 123 (11.1%) | 180 (11.2%) | 102 (23.2%)† | 129 (13.2%) | 112 (12.0%) |
| Prevalent CVD | 151 (32.5%)† | 237 (20.7%)‡ | 285 (17.3%) | 189 (41.7%)† | 285 (28.8%) | 290 (30.8%) |
| High creatinine (≥1.5 mg/dL men or ≥1.3 mg/dL women) | 37 (8.2%)‡ | 52 (4.6%) | 70 (4.3%) | 55 (12.2%) | 108 (10.9%) | 95 (10.1%) |
| eGFR <60 mL/min/ 1.73 m2 | 99 (21.9%) | 245 (21.4%) | 333 (20.2%) | 118 (26.5%)‡ | 245 (24.7%)‡ | 196 (20.8%) |
Data are means (±standard deviations) or proportions unless otherwise indicated.
*Data are medians (25th percentile, 75th percentile).
† P value <0.001. ‡ P value <0.05.
Crude hip fracture rates (/1000 person-years) were 9.1 (95% CI: 8.1–10.3) for normal FG, 7.1 (95% CI: 6.1–8.2) for IFG, and 7.7 (95% CI: 6.0–9.8) for DM. Crude hip fracture rates were higher for diabetic compared to non-diabetic participants with BMI <25 (13.4, 95% CI: 8.9–20.2 versus 10.6, 95% CI: 8.5–13.4 IFG and 11.8, 95% CI: 10.1–13.7 normal FG) and BMI ≥25 to <30 (8.3, 95% CI: 5.7–11.9 versus 6.3, 95% CI: 5.0–8.0 IFG and 6.8, 95% CI: 5.4–8.5 normal FG), but were similar for BMI ≥30 (4.1, 95% CI: 2.4–7.0 versus 4.3, 95% CI: 2.9–6.4 IFG and 5.5, 95% CI: 3.5–8.7 normal FG) (Figure 1). The percentage of diabetic participants with fractures was 10.6% (23/218) for BMI <25, 7.5% (29/385) for BMI ≥25 to <30, and 4.2% (13/313) for BMI ≥30. The percentage of non-diabetic participants with fractures was 12.4% (243/1964) for BMI <25, 7.6% (149/1958) for BMI ≥25 to <30, and 5.6% (44/788) for BMI ≥30.
Figure 1.
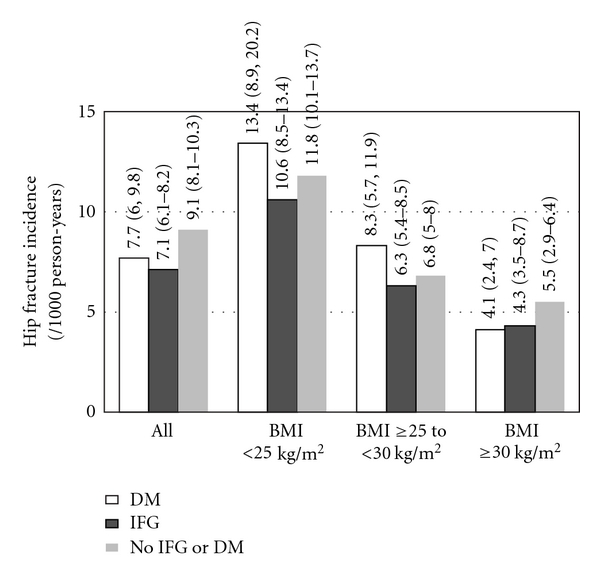
Crude incident hip fracture rate (/1000 person-years) by BMI category for diabetes mellitus, IFG, and normal FG.
Diabetes was not significantly related to fractures in unadjusted models or models adjusted for sex, race, and age. The addition of BMI to the models adjusted for sex, race, and age increased the risk of hip fracture in DM participants (HR = 1.34; 95% CI: 1.01–1.78; Table 2). This indicated that the DM participants were at higher incidence fracture risk at an equivalent BMI to participants with normal glycemia. IFG was slightly protective for fracture in models minimally adjusted for age, sex, and race though this association was eliminated after adjusting for BMI and IFG was not shown to increase the risk of hip fracture (HR = 0.91; 95% CI: 0.75–1.11) in fully adjusted models. These results may indicate that higher BMI overall in the IFG group is protective for fracture but once BMI is adjusted for in the models, fracture risk is similar at an equivalent BMI to participants with normal glycemia. Excluding participants with incident diabetes (N = 103 from the normoglycemic group and N = 327 from the IFG group), did not appreciably change these estimates for DM (HR = 1.32; 95% CI: 1.00–1.76) or IFG (HR = 0.93; 95% CI: 0.76–1.15). Estimates were similar for diabetic women (HR = 1.35; 95% CI: 0.96–1.91) and men (HR = 1.31; 95% CI: 0.80–2.13), though lower statistical power likely made the results nonsignificant for these stratified analyses. IFG was not shown to increase the risk of hip fracture in separate analyses for women (HR = 0.94; 95% CI: 0.74–1.18) or men (HR = 0.86; 95% CI: 0.59–1.26). There were no interactions of race, sex, BMI, or subclinical PAD with glycemic status.
Table 2.
Association of impaired fasting glucose and diabetes mellitus with incident hip fracture.
| No IFG or DM | IFG | Diabetes | |
|---|---|---|---|
| Overall (n) | 2588 | 2135 | 918 |
| No. of hip fractures | 269 | 169 | 65 |
| Person-years | 29,462 | 23,851 | 8,428 |
|
| |||
| Multivariable HR (95% CI) | |||
|
| |||
| Model 1: Age-sex-race adjusted | 1.0 (ref.) | 0.79 (0.65–0.96) | 1.05 (0.80–1.39) |
| Model 2: Model 1 + BMI | 1.0 (ref.) | 0.91 (0.75–1.11) | 1.34 (1.01–1.77) |
| Model 3: Model 2 + AAI < 0.9 | 1.0 (ref.) | 0.92 (0.75–1.12) | 1.25 (0.93–1.67) |
Addition of subclinical PAD (ankle-arm index <0.9) to the model reduced the HR for DM (1.25 (0.94–1.68)), rendering the association nonsignificant, and low AAI was significantly related to hip fracture (1.31 (1.01–1.71)). Excluding participants with AAI >1.4 (N = 67) did not change these results. Additional adjustment for current smoking, current drinking and 15 ft walk time further decreased the HR for DM (1.17 (0.87–1.58)), though each to a lesser degree than PAD, and decreased the HR for subclinical PAD (1.20 (0.92–1.57)) (Table 3). A longer time to complete the measured walk was significantly related to hip fracture incidence (1.07 (1.04–1.10)) and further reduced the HR for DM after the addition of current smoking and drinking to the models. Results did not change after excluding 236 participants taking osteoporosis medication during the followup period, including bisphosphonates, calcitonin, raloxifene, and thiazolidinediones (TZDs).
Table 3.
Final model for association of impaired fasting glucose and Diabetes Mellitus with incident hip fracture.*
| HR | 95% CI | |
|---|---|---|
| DM | 1.17 | 0.87–1.57 |
| IFG | 0.93 | 0.76–1.13 |
| AAI, <0.9 | 1.20 | 0.92–1.57 |
| BMI, kg/m2 | 0.93 | 0.91–0.95 |
| Time for walk, s | 1.07 | 1.04–1.10 |
*Models were internally stratified for sex and adjusted for age, race, current smoking, and current alcohol use. Clinical cardiovascular disease, use of oral estrogen, use of calcium supplements, renal insufficiency (either creatinine ≥1.5 mg/dL in men/≥1.3 mg/dL in women or eGFR <60 mL/min/1.73 m2), fasting insulin level, physical activity, history of falls, and vision problems were not included in the final model since they did not attenuate the HR for diabetes and were not significantly related to hip fracture.
4. Discussion
Our results indicate that diabetic participants with similar BMI as non-diabetic participants were more likely to fracture, particularly in the normal and overweight BMI categories. DM was associated with 34% higher hip fracture risk after adjusting for higher BMI in diabetic participants, consistent with previous estimates [1, 2]. One possibility for this observation is that sicker participants are losing weight due to advanced illnesses. Higher BMD loss among older diabetic women from the Health ABC Study was partly due to their greater weight loss over time [24]. The higher risk of fracture in the diabetic participants was partially modified by adding subclinical PAD to the models, which accounted for a reduction in the risk of fracture and made the primary association nonsignificant. This finding is important because although there is much evidence for the higher risk of fracture with DM [1–9, 25, 26], less is known about the factors that underlie this increased risk. Nearly all PAD in the elderly is subclinical, with 98% asymptomatic [19]. Although subclinical PAD is underappreciated clinically [27], it is preventable [28]. Although our overall attenuation with subclinical PAD was modest, our results, taken with previous findings of clinical and subclinical vascular disease associated with osteoporosis [12, 29–32], suggest that subclinical PAD may be a contributor to fractures in older diabetic populations. In the Health ABC Study the higher risk of fracture in diabetes persisted after adjustment for subclinical PAD [9], though this population was likely healthier at baseline than participants in our study. The association of vascular disease and osteoporosis may be the result of shared risk factors for which we adjusted for in the analyses (e.g., age, smoking) or shared biologic pathways (e.g., inflammatory cytokines, endogenous sex hormones) [33]. Alternatively, vascular disease may reduce blood flow to the lower extremities, including the hip, and modify bone metabolism to lead to osteoporosis.
Additional possible explanations for higher fracture risk in older adults with diabetes may be disease complications due to a long disease duration [1, 2, 34, 35], and/or specific complications such as impaired vision [25, 35] or neurologic impairments (e.g., neuropathy, stroke) [9, 25, 26, 36]. Further support exists from previous type 1 diabetes studies, summarized by Strotmeyer et al. [37], which indicate that lower BMD is associated with type 1 diabetic complications of nephropathy, neuropathy, and retinopathy. One reason for our lack of association between IFG and hip fracture may be that it is not the higher glucose levels per se that increase fracture risk but rather the diabetes-related conditions. Previous studies, including several meta-analyses, have also failed to find an association of IFG and fracture [1, 2, 9]. Falling and impaired motor abilities are more common in diabetic adults [9, 25, 26, 38, 39], and physical performance accounted for some of the increased risk for hip fractures in several cohorts of older adults [40, 41]. Our results indicated a slight modification of the higher fracture rate in DM once accounting for the slower walk time in DM participants.
Our study was a large established cohort of black and white men and women followed for over a decade with an excellent ascertainment of hip fracture. However, we did not have a measure of BMD. Participants with higher BMI likely had a higher BMD as well, given the strong correlation usually observed between these two measures. A similar cohort which adjusted for the generally higher BMD observed in older diabetic adults found that diabetic participants with similar BMD as non-diabetic participants were more likely to be at risk for incident fractures and adding BMD to the models actually increased the strength of the association of fracture with DM [9]. We did not have measures of diabetes duration or of peripheral nerve function. Poor peripheral nerve function may increase the risk for falls [42] and has been related to lower bone mineral density [11, 37]. Additionally, we did not assess vitamin D deficiency, which is associated with osteoporosis and has been linked to diabetes and vascular disease [43]. TZDs, although associated with fracture in diabetic women [44], were introduced late in our study followup period and our results were unaffected when we excluded participants using TZDs during the followup period for the fracture outcome. Furthermore, the use of these medications were likely not prevalent enough to account for the relationship of DM and fracture and past studies indicating higher fracture risk in type 2 diabetic adults were conducted prior to the introduction and widespread use of TZD medication [45]. Although we were not able to fully address this issue, hypoglycemic medication use is important to evaluate in the context of diabetes and fracture and it is critical to recognize the potential for confounding by indication. Finally, older diabetic adults with more severe disease may have been less likely to participate in our study and this may have made our risk estimates for fracture more conservative.
Likely diabetes-related conditions account for a portion of increased fracture risk and potentially BMD changes; however, these have not been comprehensively evaluated. Our results indicated that subclinical PAD and slower walking time be related to a part of the higher risk for hip fracture in older diabetic adults. PAD is preventable and treatable [27, 28] and clinical PAD was recently linked to a three-time higher risk of hip fracture [46]. Other diabetes-related complications may be important as well and should be investigated for their role in fractures in older diabetic adults. Poor physical function in older diabetic adults could also be improved through physical therapy or exercise interventions. Potentially, preventing diabetes-related conditions in older diabetic adults may reduce fractures in this population.
Conflict of Interests
The authors report no conflicts of interest.
Acknowledgments
The authors express their gratitude to the CHS participants and Ms. Michelle Utz-Kiley for assistance with manuscript preparation. The research reported in this article was supported by contracts N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, and grant HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through AG-023629, AG-15928, AG-20098, and AG-027058 from the National Institute on Aging (NIA), and the American Diabetes Association 1-04-JF-46 (ESS). A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. Dr. Newman was also supported by the University of Pittsburgh Claude. D. Pepper Older Americans Independence Center P30-AG-024827. A. Kamineni was supported by an unrestricted educational grant from Amgen to support analyses related to diabetes in CHS. Statistical analyses were performed by A. Kamineni, Group Health Research Institute, Seattle, WA. E. S. Strotmeyer, A.B. Newman and A. Kamineni had full access to the data at all times.
References
- 1.Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporosis International. 2007;18(4):427–444. doi: 10.1007/s00198-006-0253-4. [DOI] [PubMed] [Google Scholar]
- 2.Janghorbani M, Van Dam RM, Willett WC, Hu FB. Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. American Journal of Epidemiology. 2007;166(5):495–505. doi: 10.1093/aje/kwm106. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed LA, Joakimsen RM, Berntsen GK, Fønnebø V, Schirmer H. Diabetes mellitus and the risk of non-vertebral fractures: the Tromso study. Osteoporosis International. 2006;17(5):495–500. doi: 10.1007/s00198-005-0013-x. [DOI] [PubMed] [Google Scholar]
- 4.Janghorbani M, Feskanich D, Willett WC, Hu F. Prospective study of diabetes and risk of hip fracture: the nurses’ health study. Diabetes Care. 2006;29(7):1573–1578. doi: 10.2337/dc06-0440. [DOI] [PubMed] [Google Scholar]
- 5.Holmberg AH, Johnell O, Nilsson PM, Nilsson J, Berglund G, Åkesson K. Risk factors for fragility fracture in middle age. A prospective population-based study of 33,000 men and women. Osteoporosis International. 2006;17(7):1065–1077. doi: 10.1007/s00198-006-0137-7. [DOI] [PubMed] [Google Scholar]
- 6.Holmberg AH, Johnell O, Nilsson J, Åkesson K, Nilsson PM, Berglund G. Erratum to "Risk factors for fragility fracture in middle age. A prospective population-based study of 33,000 men and women". Osteoporosis International. 2006;17(11, article 1704) doi: 10.1007/s00198-006-0137-7. [DOI] [PubMed] [Google Scholar]
- 7.Lipscombe LL, Jamal SA, Booth GL, Hawker GA. The risk of hip fractures in older individuals with diabetes: a population-based study. Diabetes Care. 2007;30(4):835–841. doi: 10.2337/dc06-1851. [DOI] [PubMed] [Google Scholar]
- 8.Bonds DE, Larson JC, Schwartz AV, et al. Risk of fracture in women with type 2 diabetes: the women’s health initiative observational study. Journal of Clinical Endocrinology and Metabolism. 2006;91(9):3404–3410. doi: 10.1210/jc.2006-0614. [DOI] [PubMed] [Google Scholar]
- 9.Strotmeyer ES, Cauley JA, Schwartz AV, et al. Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the health, aging, and body composition study. Archives of Internal Medicine. 2005;165(14):1612–1617. doi: 10.1001/archinte.165.14.1612. [DOI] [PubMed] [Google Scholar]
- 10.Strotmeyer ES, Cauley JA, Schwartz AV, et al. Diabetes is associated independently of body composition with BMD and bone volume in older white and black men and women: the health, aging, and body composition study. Journal of Bone and Mineral Research. 2004;19(7):1084–1091. doi: 10.1359/JBMR.040311. [DOI] [PubMed] [Google Scholar]
- 11.Strotmeyer ES, Cauley JA, Schwartz AV, et al. Reduced peripheral nerve function is related to lower hip BMD and calcaneal QUS in older white and black adults: the Health, Aging, and Body Composition Study. Journal of Bone and Mineral Research. 2006;21(11):1803–1810. doi: 10.1359/jbmr.060725. [DOI] [PubMed] [Google Scholar]
- 12.Farhat GN, Strotmeyer ES, Newman AB, et al. Volumetric and areal bone mineral density measures are associated with cardiovascular disease in older men and women: the health, aging, and body composition study. Calcified Tissue International. 2006;79(2):102–111. doi: 10.1007/s00223-006-0052-0. [DOI] [PubMed] [Google Scholar]
- 13.Fried LF, Shlipak MG, Stehman-Breen C, et al. Kidney function predicts the rate of bone loss in older individuals: the Cardiovascular Health Study. Journals of Gerontology—Series A Biological Sciences and Medical Sciences. 2006;61(7):743–748. doi: 10.1093/gerona/61.7.743. [DOI] [PubMed] [Google Scholar]
- 14.Fried LF, Biggs ML, Shlipak MG, et al. Association of kidney function with incident hip fracture in older adults. Journal of the American Society of Nephrology. 2007;18(1):282–286. doi: 10.1681/ASN.2006050546. [DOI] [PubMed] [Google Scholar]
- 15.Ensrud KE, Lui L-Y, Taylor BC, et al. Renal function and risk of hip and vertebral fractures in older women. Archives of Internal Medicine. 2007;167(2):133–139. doi: 10.1001/archinte.167.2.133. [DOI] [PubMed] [Google Scholar]
- 16.Fried LP, Borhani NO, Enright P, et al. The cardiovascular health study: design and rationale. Annals of Epidemiology. 1991;1(3):263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 17.Newman AB, Arnold AM, Sachs MC, et al. Long-term function in an older cohort—the cardiovascular health study all stars study. Journal of the American Geriatrics Society. 2009;57(3):432–440. doi: 10.1111/j.1532-5415.2008.02152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clinical Chemistry. 1995;41(2):264–270. [PubMed] [Google Scholar]
- 19.Newman AB, Siscovick DS, Manolio TA, et al. Ankle-arm index as a marker of atherosclerosis in the cardiovascular health study. Circulation. 1993;88(3):837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 20.Shlipak MG, Fried LF, Crump C, et al. Cardiovascular disease risk status in elderly persons with renal insufficiency. Kidney International. 2002;62(3):997–1004. doi: 10.1046/j.1523-1755.2002.00522.x. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: improving Global Outcomes (KDIGO) Kidney International. 2005;67(6):2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 22.Psaty BM, Lee M, Savage PJ, et al. Assessing the use of medications in the elderly: mmethods and initial experience in the cardiovascular health study. Journal of Clinical Epidemiology. 1992;45(6):683–692. doi: 10.1016/0895-4356(92)90143-b. [DOI] [PubMed] [Google Scholar]
- 23.Taylor HL, Jacobs DR, Schucker B. A questionnaire for the assessment of leisure time physical activities. Journal of Chronic Diseases. 1978;31(12):741–755. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz AV, Sellmeyer DE, Strotmeyer ES, et al. Diabetes and bone loss at the hip in older black and white adults. Journal of Bone and Mineral Research. 2005;20(4):596–603. doi: 10.1359/JBMR.041219. [DOI] [PubMed] [Google Scholar]
- 25.Forsén L, Meyer HE, Midthjell K, Edna TH. Diabetes mellitus and the incidence of hip fracture: results from the Nord-Trondelag health survey. Diabetologia. 1999;42(8):920–925. doi: 10.1007/s001250051248. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz AV, Sellmeyer DE, Ensrud KE, et al. Older women with diabetes have an increased risk of fracture: a prospective study. Journal of Clinical Endocrinology and Metabolism. 2001;86(1):32–38. doi: 10.1210/jcem.86.1.7139. [DOI] [PubMed] [Google Scholar]
- 27.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286(11):1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 28.Hankey GJ, Norman PE, Eikelboom JW. Medical treatment of peripheral arterial disease. JAMA. 2006;295(5):547–553. doi: 10.1001/jama.295.5.547. [DOI] [PubMed] [Google Scholar]
- 29.van der Klift M, Pols HAP, Hak AE, Witteman JCM, Hofman A, De Laet CEDH. Bone mineral density and the risk of peripheral arterial disease: the Rotterdam Study. Calcified Tissue International. 2002;70(6):443–449. doi: 10.1007/s00223-001-2076-9. [DOI] [PubMed] [Google Scholar]
- 30.Wong SYS, Kwok T, Woo J, et al. Bone mineral density and the risk of peripheral arterial disease in men and women: results from Mr. and Ms Os, Hong Kong. Osteoporosis International. 2005;16(12):1933–1938. doi: 10.1007/s00198-005-1968-3. [DOI] [PubMed] [Google Scholar]
- 31.Jørgensen L, Joakimsen O, Berntsen GKR, Heuch I, Jacobsen BK. Low bone mineral density is related to echogenic carotid artery plaques: a population-based study. American Journal of Epidemiology. 2004;160(6):549–556. doi: 10.1093/aje/kwh252. [DOI] [PubMed] [Google Scholar]
- 32.Kiel DP, Kauppila LI, Cupples LA, Hannan MT, O’Donnell CJ, Wilson PWF. Bone loss and the progression of abdominal aortic calcification over 25 year period: the Framingham Heart Study. Calcified Tissue International. 2001;68(5):271–276. doi: 10.1007/BF02390833. [DOI] [PubMed] [Google Scholar]
- 33.Baldini V, Mastropasqua M, Francucci CM, D’Erasmo E. Cardiovascular disease and osteoporosis. Journal of Endocrinological investigation. 2005;28(10):69–72. [PubMed] [Google Scholar]
- 34.Nicodemus KK, Folsom AR. Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women. Diabetes Care. 2001;24(7):1192–1197. doi: 10.2337/diacare.24.7.1192. [DOI] [PubMed] [Google Scholar]
- 35.Ivers RQ, Cumming RG, Mltchell P, Peduto AJ. Diabetes and risk of fracture: the blue mountains eye study. Diabetes Care. 2001;24(7):1198–1203. doi: 10.2337/diacare.24.7.1198. [DOI] [PubMed] [Google Scholar]
- 36.Melton LJ, Leibson CL, Achenbach SJ, Therneau TM, Khosla S. Fracture risk in type 2 diabetes: update of a population-based study. Journal of Bone and Mineral Research. 2008;23(8):1334–1342. doi: 10.1359/JBMR.080323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strotmeyer ES, Cauley JA, Orchard TJ, Steenkiste AR, Dorman JS. Middle-aged premenopausal women with type 1 diabetes have lower bone mineral density and calcaneal quantitative ultrasound than nondiabetic women. Diabetes Care. 2006;29(2):306–311. doi: 10.2337/diacare.29.02.06.dc05-1353. [DOI] [PubMed] [Google Scholar]
- 38.Ottenbacher KJ, Ostir GV, Kristen Peek M, Goodwin JS, Markides KS. Diabetes mellitus as a risk factor for hip fracture in Mexican American older adults. Journals of Gerontology—Series A Biological Sciences and Medical Sciences. 2002;57(10):M648–M653. doi: 10.1093/gerona/57.10.m648. [DOI] [PubMed] [Google Scholar]
- 39.Wallace C, Reiber GE, LeMaster J, et al. Incidence of falls, risk factors for falls, and fall-related fractures in individuals with diabetes and a prior foot ulcer. Diabetes Care. 2002;25(11):1983–1986. doi: 10.2337/diacare.25.11.1983. [DOI] [PubMed] [Google Scholar]
- 40.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. The New England Journal of Medicine. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 41.Cawthon PM, Fullman RL, Marshall L, et al. Physical performance and risk of hip fractures in older men. Journal of Bone and Mineral Research. 2008;23(7):1037–1044. doi: 10.1359/JBMR.080227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richardson JK, Ashton-Miller JA. Peripheral neuropathy: an often-overlooked cause of falls in the elderly. Postgraduate Medicine. 1996;99(6):161–172. [PubMed] [Google Scholar]
- 43.Holick MF. Diabetes and the vitamin D connection. Current Diabetes Reports. 2008;8(5):393–398. doi: 10.1007/s11892-008-0068-0. [DOI] [PubMed] [Google Scholar]
- 44.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. The New England Journal of Medicine. 2006;355(23):2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 45.Strotmeyer ES, Schwartz AV, Newman AB. Thiazolidinediones and the risk of nontraumatic fractures in patients with diabetes: in reply. Archives of Internal Medicine. 2006;166(9, article 1043) doi: 10.1001/archinte.166.9.1043. [DOI] [PubMed] [Google Scholar]
- 46.Sennerby U, Melhus H, Gedeborg R, et al. Cardiovascular diseases and risk of hip fracture. JAMA. 2009;302(15):1666–1673. doi: 10.1001/jama.2009.1463. [DOI] [PubMed] [Google Scholar]


