Abstract
Corynebacterium glutamicum is a member of a group of taxonomically related glutamate-excreting bacteria which utilize glucose both by the Embden-Meyerhof and the pentose phosphate pathways, the latter sequence accounting for 10 to 38% of the glucose metabolized. Some of the properties of glucose-6-phosphate dehydrogenase in crude extracts of C. glutamicum were studied. The enzyme was rapidly inactivated by dilution in tris (hydroxymethyl)aminomethane-hydrochloride buffer. This inactivation was prevented by the presence of 0.45 m NaCl. Mg++ was required for enzyme activity, but Mn++, Ca++, Sr++, and Ba++ were equally effective. Growth of the organism under differing conditions did not markedly affect the specific activity of the enzyme. A generally applicable method for detecting colonies deficient in glucose-6-phosphate dehydrogenase was developed. Mutants so obtained were found to be auxotrophic for tryptophan. Upon reversion of the tryptophan requirement, the revertants still retained the property of glucose-6-phosphate dehydrogenase deficiency. Neither the mutants nor the revertants could grow as rapidly as the parent culture in glucose, in gluconate, or in a complex medium.
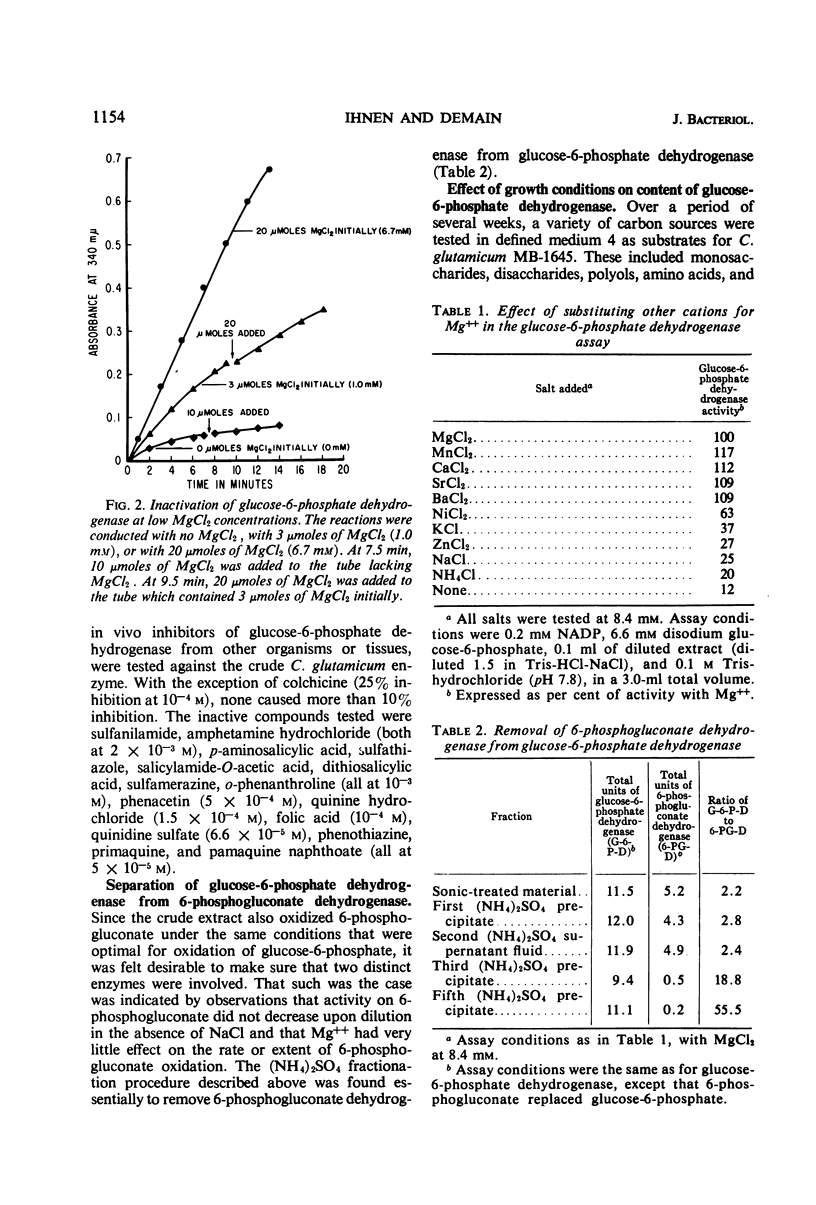
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COCHRANE V. W., PECK H. D., Jr, HARRISON A. The metabolism of species of Streptomyces. VII. The hexosemonophosphate shunt and associated reactions. J Bacteriol. 1953 Jul;66(1):17–23. doi: 10.1128/jb.66.1.17-23.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE LEY J. The hexose monophosphate oxidative route in Aerobacter cloacae. Enzymologia. 1957 Jan 30;18(1):33–46. [PubMed] [Google Scholar]
- FRAENKEL D. G., HORECKER B. L. PATHWAYS OF D-GLUCOSE METABOLISM IN SALMONELLA TYPHINMURIUM. A STUDY OF A MUTANT LACKING PHOSPHOGLUCOSE ISOMERASE. J Biol Chem. 1964 Sep;239:2765–2771. [PubMed] [Google Scholar]
- Fraenkel D. G. Selection of Escherichia coli mutants lacking glucose-6-phosphate dehydrogenase or gluconate-6-phosphate dehydrogenase. J Bacteriol. 1968 Apr;95(4):1267–1271. doi: 10.1128/jb.95.4.1267-1271.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gancedo C., Gancedo J. M., Sols A. Metabolite repression of fructose 1,6-diphosphatase in yeast. Biochem Biophys Res Commun. 1967 Mar 9;26(5):528–531. doi: 10.1016/0006-291x(67)90096-4. [DOI] [PubMed] [Google Scholar]
- HORECKER B. L. PATHWAYS OF CARBOHYDRATE METABOLISM AND THEIR PHYSIOLOGICAL SIGNIFICANCE. J Chem Educ. 1965 May;42:244–253. doi: 10.1021/ed042p244. [DOI] [PubMed] [Google Scholar]
- Horecker B. L. Glucose-6-phosphate dehydrogenase, the pentose phosphate cycle, and its place in carbohydrate metabolism. Am J Clin Pathol. 1967 Mar;47(3):271–281. doi: 10.1093/ajcp/47.3.271. [DOI] [PubMed] [Google Scholar]
- Hsie A. W., Rickenberg H. V. Catabolite repression in Escherichia coli: the role of glucose 6-phosphate. Biochem Biophys Res Commun. 1967 Nov 17;29(3):303–310. doi: 10.1016/0006-291x(67)90453-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MCNAIR SCOTT D. B. The oxidative pathway of carbohydrate metabolism in Escherichia coli. III. Glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase in cells grown under different conditions. Biochem J. 1956 Aug;63(4):587–593. doi: 10.1042/bj0630587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCNAIR SCOTT D. B. The oxidative pathway of carbohydrate metabolism in Escherichia coli. IV. Formation of enzymes induced by 2:4-dinitrophenol. Biochem J. 1956 Aug;63(4):593–600. doi: 10.1042/bj0630593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT D. B., CHU E. The oxidative pathway of carbohydrate metabolism in Escherichia coli. 6. Adaptation of glucose 6-phosphate dehydrogenase to growth in complex media. Biochem J. 1959 Jul;72:426–429. doi: 10.1042/bj0720426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman J. A. Pentose phosphate pathway metabolism by normal and glucose-6-phosphate dehydrogenase-deficient human red cell haemolysates. Clin Chim Acta. 1967 Nov;18(2):245–248. doi: 10.1016/0009-8981(67)90164-7. [DOI] [PubMed] [Google Scholar]



