Abstract
Background:
Atrial fibrillation (AF) is a frequent and serious complication of coronary artery bypass graft (CABG) surgery.
Methods:
We undertook a retrospective review of the records of patients undergoing CABG at Imam Ali Hospital between February 1, 2003 and February 1, 2006. The patients were divided in two groups, ie, Group A (AF) and Group B (no AF). The association between the occurrence of AF following CABG and other variables was compared with respect to continuous or categorical variables by t-test and χ2-test.
Results:
Multivariate logistic regression analysis of potentially predictive factors in univariate analysis showed that opium use, type of operation, and crossclamp time were predictors of AF following CABG.
Conclusion:
This study identifies some new predictors of postoperative AF, control of which could lead to a lower incidence of AF and reduced morbidity, mortality, and resource utilization for patients undergoing cardiac surgery.
Keywords: atrial fibrillation, coronary artery bypass grafting, cardiac surgery, predictors
Introduction
Atrial fibrillation (AF) occurs in approximately 25%–40% of patients following coronary artery bypass grafting (CABG), and increases the risk of an adverse outcome, as well as increasing hospital costs and health care resource consumption.1 AF is a common and serious cardiac complication post surgery. This arrhythmia originates in the atria, and instead of the impulse traveling in an orderly fashion, both the atria quiver at the same time.2 AF was once thought to be harmless, but is now recognized as a dangerous postoperative condition.2 AF is common in patients who require heart surgery for mitral valve, aortic valve, and/or coronary artery disease. AF contributes significantly to mortality and morbidity, particularly in the elderly and in intensive care patients.3 AF is a diverse arrhythmia which is clinically divided into three subtypes, ie, paroxysmal, persistent, and permanent. 4 Patients in whom sinus rhythm cannot be sustained after cardioversion are deemed to have permanent AF.5 Surgeons require strategies to reduce the incidence of AF following CABG, reduce the associated hospital costs, and optimize the outcome of surgery.
Wolfe et al demonstrated that postoperative AF increases mortality and morbidity following CABG.5 Creswell et al6 investigated the relationship between postoperative outcome and postoperative atrial arrhythmia,7 and found that postoperative AF affects postoperative morbidity and mortality. Maisel et al investigated occurrence of AF following CABG and showed that AF leads to longer intensive care and ward stays and more use of hospital resources.8 Other retrospective and prospective studies have also confirmed a relationship between postoperative AF and adverse outcomes.9,10 This study details the results of our investigation of postoperative AF using novel variables to identify predictors of postoperative AF in CABG patients at Imam Ali Hospital.
Methods and Materials
Between February 1, 2003 and February 1, 2006, 700 patients were entered in this retrospective study and data on approximately 31 variables were collected by two dedicated intensive care unit nurses. Thirty patients were excluded for having a history of AF before their operation, incomplete or missing data, CABG with valvular surgery, death in the operating room or during the early hours of intensive care stay, or offpump CABG.
The variables analyzed included preoperative, intraoperative, and postoperative parameters, outcomes, and complications. Risk factors and relevant clinical data were investigated for their relationship with the onset of new postoperative AF. The preoperative variables included in the study were age, gender, body mass index, body weight, ejection fraction, tobacco use, opium use, systemic hypertension, diabetes mellitus, peripheral vascular disease, cerebrovascular disease, preoperative hematocrit, chronic obstructive pulmonary disease, preoperative renal insufficiency, congestive heart failure, preoperative digoxin use, Aspirin using, pO2 on air, pCO2 on air, preoperative beta-blocker use, preoperative nipride use, left main stem disease, preoperative heparin use, and hyperlipidemia. Intraoperative variables were crossclamp time, pump time, left internal mammary artery use, type of operation, need for inotropic support, and number of grafts. Postoperative variables were congestive heart failure, postoperative myocardial infarction, infection, pulmonary complications, renal insufficiency, postoperative infection, cerebrovascular accident, gastrointestinal complications, emergency operation, postoperative bleeding, intra-aortic balloon pump use, reintubation, elective surgery, inotropic drug use, duration of hospital stay, and death.
Definition of Terms
Hypertensive patients were defined as those receiving antihypertensive treatment. Ejection fraction was assessed by catheterization or echocardiography, and cerebrovascular disease included those with a history of stroke, transient ischemic attack, or both. Peripheral vascular disease was defined as a history of intermittent claudication or vascular surgery. Preoperative renal insufficiency was determined as serum creatinine >2 mg/dL. Surgery was considered to be elective if the patient was admitted to the Department of Cardiac Surgery electively on the day preceding the operation. Urgent operations were defined as surgery performed on the day of referral or on the following day.
The operative technique was similar in all patients. After anesthesia, a median sternotomy was performed, followed by routine aortic and atrial cannulation. Cardiopulmonary bypass was carried out using membrane oxygenation, nonpulsatile perfusion, and moderate systemic hypothermia. Myocardial protection was achieved by cold hyperkalemic crystalloid cardioplegia and topical cooling with iced slush and cold saline solution. Cardioplegia was administered in a retrograde and antegrade fashion in all patients and reinfusion was not used routinely during surgery. Coronary artery bypass grafting was performed using the left internal mammary artery and reversed saphenous vein in most instances.
Hospital death was defined as death occurring within 30 days of surgery. Infection included any postoperative complication requiring antibiotic therapy. Pulmonary complications comprised all those leading to prolonged mechanical ventilation. Intra-abdominal complications included only those requiring operative intervention. Low cardiac output was defined as a need for inotropic support or an intra-aortic balloon pump.
Statistical Analysis
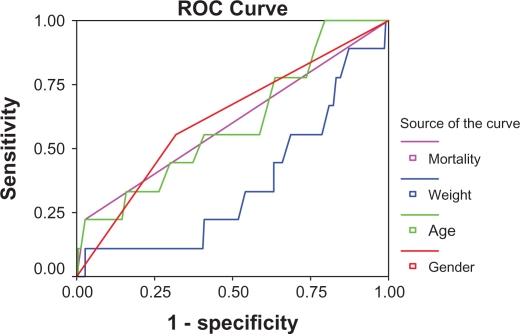
Continuous and categorical variables between patients with postoperative AF (Group A) and those without AF (Group B) were compared by t-test and χ2 test, respectively. Statistically significant variables in the univariate analysis (P ≤ 0.1) were selected and entered into a multiple logistic regression model. The sensitivity and specificity of variables predicting AF were obtained using a receiver-operating characteristic curve (Fig. 1). Patients with a hospital stay ≥12 days and those with a stay <12 days were compared by t-test with respect to postoperative AF. A P value < 0.05 were considered to be statistically significant, and the odds ratio (OR) and 95% confidence interval (CI) for each significant variable in this model were calculated.
Figure 1.
Receiver-operating characteristic curve.
Note: Diagonal segments are produced by ties.
Results
In total 670 patients were included in this study. Of these, 69.5% were men and 30.5% were women, aged 33–87 years, in whom 21.1% had diabetes, 38.9% had hypercholesterolemia, 35% had hypertriglyceridemia, 6% had had a preoperative myocardial infarction, and 4.7% had had a postoperative myocardial infarction. In total, 49.3% were current smokers, 14% were opium addicts, 7% had chronic obstructive pulmonary disease, and 5.9% had congestive heart failure. Mean left ventricular ejection fraction was 53% ± 6%. Some 7.4% had undergone emergency surgery, and 92.6% underwent surgery as elective patients; 13.9% had left main stem disease, 14.4% patients had one graft, 27% had two grafts, 19.8% had three grafts, 37.2% had four grafts, and 1.6% had five grafts. The left internal mammary was used in 96.1% of patients. Eighty-one (11%) patients needed an intra-aortic balloon pump for 24 hours and 19.6% needed inotropic support. Other major immediate postoperative complications included respiratory failure (4%), renal failure (2.3%), cerebrovascular accident (2.08%), infection (2%), bleeding (6.9%), and reintubation (4.1%). Twenty patients (2.9%) died as a result of infection, multiorgan failure, or cerebrovascular accident. Hospital stay was ≤12 days in 60.6% of patients and >12 days in 39.4%.
Preoperative variables and patient demographic factors are shown in Table 1. There was no significant difference in univariate analysis for weight, age, left main stem disease, preoperative hematocrit, pO2 on air, diabetes, chronic obstructive pulmonary disease, smoking, congestive heart failure, preoperative nipride use, and preoperative creatinine >2 mg/dL between Group A and Group B. The majority of the patients were male (68.77%), with a mean age of 55.07 ± 9.1 years. The age range of the whole group was 33–87 years, and 4.1% of male and 7.6% of female patients had AF. Two hundred and fifty patients had a hospital stay ≤12 days and 6.5% of them had AF, while 420 patients had a longer hospital stay, of whom 30 (7.1%) had AF. χ2 showed significant differences with respect to occurrence of AF between the two groups. The two groups of patients (stay ≥12 or <12) were compared with respect to number of grafts and there was a significant difference, with more grafts in patients with a hospital stay ≥12 days (P = 0.006). In univariate analysis, preoperative hypertension and intravenous nitroglycerin use were not significant markers of AF, but preoperative nipride use did predict AF (Table 1).
Table 1.
Preoperative predictors of postoperative atrial fibrillation.
| Variable | AF | No AF | P value |
|---|---|---|---|
| Ejection fraction | 43 ± 0.5 | 54 ± 0.4 | 0.100 |
| Weight | 72.31 ± 11.4 | 68.84 ± 13.7 | 0.006 |
| Preoperative digoxin use | 5% | 13% | 0.040 |
| Preoperative beta-blocker use | 33% | 36% | NS |
| Age | 58.52 ± 9.4 | 54.81 ± 9.07 | 0.011 |
| Preoperative hematocrit | 39.04 ± 5.2 | 41.4 ± 5.6 | 0.004 |
| pO2 on air | 77.01 ± 18.3 | 90.03 ± 24.3 | 0.000 |
| pCO2 on air | 33.47 ± 6.2 | 34 ± 1.1 | NS |
| Left main disease | 8.8% | 3.1% | 0.010 |
| Preoperative serum creatinine | 8% | 2.8% | 0.000 |
| Diabetes | 51.8% | 25.4% | 0.026 |
| Hypercholesterolemia | 46.3% | 38.4% | 0.160 |
| Hypertriglyceridemia | 41.4% | 35.5% | 0.077 |
| Smoking | 50% | 30% | 0.027 |
| Opium use | 12.1% | 14.4% | 0.900 |
| Chronic obstructive pulmonary disease | 8.3% | 4.5% | 0.000 |
| Congestive heart failure | 7.9% | 2.8% | 0.000 |
| Aspirin use | 91% | 93% | 0.450 |
| Preoperative nipride use | 26.8% | 31% | 0.040 |
| Preoperative heparin use | 12.1% | 4.4% | 0.060 |
Notes: Results are expressed as mean ± standard deviation. Differences were considered significant at P < 0.05.
Abbreviations: AF, atrial fibrillation; NS, not statistically significant.
The age and gender distribution of the patients is shown in Table 1. There was no significant difference with respect to gender (P = 0.54). Univariate analysis identified intraoperative variables which had a significant association with AF following CABG. The most significant risk factors were crossclamp time and inotropic drug use (P < 0.05, Table 2). Some postoperative variables showed a significant association with AF following CABG (Table 3). These factors included death, reintubation, and postoperative bleeding. Other important factors in univariate analysis were postoperative myocardial infarction and use of an intra-aortic balloon pump. Eight variables were predictors of AF in a logistic regression model (Table 4). The most important variables among the preoperative patient characteristics were opium use, age, chronic obstructive pulmonary disease, congestive heart failure, and pO2 on air, which predicted AF with ORs of 0.37, 1.05, 67, 3.95, and 15.8, respectively.
Table 2.
Intraoperative predictors of postoperative atrial fibrillation.
| Variable | AF | No AF | P value |
|---|---|---|---|
| No LIMA use | 4.9% | 0% | 0.06 |
| Clamp time | 87 ± 12 | 67 ± 15 | 0.04 |
| Pump time | 136 ± 12 | 111 ± 12 | 0.05 |
| Inotropic support | 12.5% | 4.5% | 0.04 |
Notes: Results are expressed as means ± SD. Differences were considered significant at P < 0.05.
Abbreviations: AF, atrial fibrillation; LIMA, left internal mammary artery.
Table 3.
Postoperative predictors of postoperative atrial fibrillation.
| Variable | AF | No AF | P value |
|---|---|---|---|
| IABP use | 8% | 4% | 0.00 |
| Respiratory complication | 7.3% | 3.7% | 0.00 |
| Renal complication | 5.1% | 2.1% | 0.10 |
| Postoperative infection | 2.4% | 1.9% | 0.70 |
| Gastrointestinal complication | 4.8% | 1.3% | 0.80 |
| Postoperative bleeding | 9.1% | 5.9% | 0.00 |
| Cerebrovascular accident | 2.4% | 1.6% | 0.50 |
| Emergency operation | 10% | 6.5% | 0.06 |
| Duration of hospital stay | 4–12 days | 3–9 days | |
| Postoperative MI | 7.8% | 4.1% | 0.04 |
| Elective operation | 86% | 93.5% | 0.50 |
| Reintubation | 6.7% | 2.4% | 0.00 |
| Death | 5.6% | 1.6% | 0.00 |
Notes: Results are expressed as mean ± standard deviation. Differences were considered significant at P < 0.05.
Abbreviations: AF, atrial fibrillation; IABP, intra-aortic balloon pump; MI, myocardial infarction
Table 4.
Multivariate regression model for atrial fibrillation.
| Variable | B | SE | Wald | DF | Sig | Exp (B) |
95% CI |
|
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Opium use | 0.99 | 0.430 | 5.30 | 1 | 0.020 | 0.37 | 0.937 | 1.003 |
| Age | 0.05 | 0.023 | 6.63 | 1 | 0.010 | 1.05 | 1.01 | 1.1 |
| COPD | 1.37 | 0.455 | 9.10 | 1 | 0.002 | 67 | 29.8 | 154.1 |
| Clamp time | 1.60 | 0.400 | 15.30 | 1 | 0.020 | 5.40 | 2.29 | 12.9 |
| CHF | 4.21 | 0.420 | 100 | 1 | 0.000 | 3.95 | 0.783 | 8.21 |
| pO2 on air | 2.76 | 0.900 | 9.40 | 1 | 0.002 | 15.80 | 2.72 | 92.1 |
| IABP use | 3.50 | 0.510 | 49.40 | 1 | 0.000 | 1 | 36.1 | 13.27 |
| Inotropic support | 1.66 | 0.620 | 6.90 | 1 | 0.008 | 5.20 | 1.53 | 13.04 |
| Constant | 4.73 | 2.070 | 5.10 | 1 | 0.023 | 0.009 | 3.1 | 219.3 |
Abbreviations: CI, confidence interval; SE, standard error of the mean; DF, degrees of freedom; COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure; IABP, intra-aortic balloon pump.
Intraoperative variables associated with AF included a longer crossclamp time (OR 5.4) and intra-aortic balloon pump use (OR 1.0). The need for inotropic agents in the postoperative period and in the intensive care unit was another predictor of AF (OR 5.2). Stroke, defined as regional neurological deficit, such as hemiplegia or paraplegia, or diffuse deficit-like coma, within 30 days after surgery, was not an independent predictor of AF (P > 0.05). However, postoperative AF was related to increased morbidity and other serious events, and the rate of these events was twice as high as in those in whom AF had not developed.
AF occurred in 100 patients (15%) and there were six cases of cerebrovascular accident (6% and 1.4%) between the AF (n = 100) and non-AF (n = 570) patients, respectively (P < 0.05). However, in logistic regression, stroke did not predict postoperative AF. Requirements for inotropic support and reintubation were different between patients with postoperative AF versus those with no AF (P < 0.05). Median duration of stay in intensive care was 96 ± 4 hours for patients with AF, compared with 48 ± 8 hours for patients with no AF. Postoperative mortality at 30 days was significantly higher in patients with postoperative AF (5.6%) compared with those without AF (1.6%, P < 0.05) Fifty percent of the patients were in New York Heart Association functional class I, 33% were in functional class II, 11% were in functional class III, and 6% were in functional class IV (Table 5). Patients in functional class III showed differences with respect to AF; 50% of those with AF were in functional class III, while 26% of those without AF were in functional class III (Table 5). The number of grafts had no effect on the incidence of AF (Table 6). The incidence of AF in patients with crossclamp time ≤60 minutes and >60 minutes was 5% and 30%, respectively. Patients with a pump time ≥100 minutes showed a higher incidence of AF (P < 0.05). Sensitivity and specificity of gender for predicting AF was 78% and 70%, respectively, in a receiver-operating characteristic curve.
Table 5.
Relationship between New York Heart Association functional class and postoperative atrial fibrillation.
| Functional class | AF | No AF | P value |
|---|---|---|---|
| I | 50% | 63% | 0.09 |
| II | 33% | 26% | 0.8 |
| III | 11% | 7.6% | 0.05 |
| IV | 6% | 3.4% | 0.9 |
Note: Differences were considered significant at P < 0.05.
Abbreviation: AF, atrial fibrillation.
Table 6.
Relationship between number of grafts and postoperative atrial fibrillation.
| Grafts (n) | AF | No AF | P value |
|---|---|---|---|
| One | 6.8% (4/58) | 93.1% (54/58) | >0.05 |
| Two | 2.5% (4/160) | 99.5% (156/160) | >0.05 |
| Three | 0.5% (16/320) | 95% (3.4/32) | >0.05 |
| Four | 7.9% (17/151) | 92.1% (139/151) | >0.05 |
Note: Differences were considered significant at P < 0.05.
Abbreviation: AF, atrial fibrillation.
AF is a frequent complication of cardiac surgery. In this setting, AF is usually a transient arrhythmia, but cerebral embolism is a known complication of sustained AF in the postoperative setting. Thus, AF could play a role in stoke following cardiac surgery. In low-risk patients, AF can extend hospital stay by leading to early readmission, and often requires use of antiarrhythmic drugs with significant side effects.9 In high-risk patients, a 10% decrease in cardiac output after onset of rapid AF can lead rapidly to increased morbidity due to the need for inotropic support and an intra-aortic balloon pump, and often requires immediate cardioversion in an ill postoperative patient.10
AF also brings up the issue of anticoagulation in newly postoperative patients, because there is a reported three-fold increased incidence of peripheral emboli and stroke after the onset of AF.11 AF has a low incidence in the general population, which increases with advancing age, as noted by Aranki et al12 who reported an incidence of 0.4% for individuals younger than 70 years which increased to 2%–4% in those older than 70 years.12 The incidence of AF in hospitalized patients was reported to be 24% by Goldman.13 Smith et al14 and Stephenson et al15 reported an AF incidence rate of 10% after cardiac valve surgery and open heart surgery. The highest incidence reported was 65% by Mathew et al16 but the incidence of AF after coronary artery bypass graft surgery is much lower, ranging from 3.3% to 30%.16,17 The risk of AF in some patients is high. For example, Cox et al18 found that the most important risk factor for postoperative AF was chronic obstructive pulmonary disease and hypertension.18 Our study found an incidence of 15% for postoperative AF, which was similar to the findings of some studies, but different to others, which could be explained by differences in the type of operative procedures included. We excluded valvular surgery and combined CABG and valvular surgery because of the likelihood of a lower incidence of AF, and valvular surgery cases constitute 20% of our patients.
Chen et al and Fuller et al in two separate studies suggested that postoperative AF was a benign rhythm disturbance and self-limiting, and does not increase the incidence of stroke or embolism. However, with the accumulation of more and more data from multiple studies, it has become evident that postoperative AF is an important risk factor for a serious postoperative outcome.19,20 The etiology of AF following cardiac surgery may be multifactorial, and includes intraoperative handling of the right atrium,21 suture of the cannulation site,21 suture at the site of a retrograde cannula,21 a large hematoma in the atrial wall,21 venting of the right superior pulmonary vein,22 traumatic laceration of atrial tissue,22 inadequate atrial tissue preservation by cardioplegia,23 inadequate use of iced slush/cooling of the atria,19,24 beta-blocker withdrawal,25 hypothyroidism, withdrawal of thyroid hormone replacement therapy in the preoperative period,26 pericarditis, and many other factors.27,28 Suture line, hematoma, and other traumatic causes of AF lead to a lack of uniformity in the atrial refractory period, and dispersion of refractoriness is a mechanism underlying the vulnerability of atrial tissue to development of AF. Hypothyroidism reduces cardiac contractility and lead to increase peripheral resistance, capillary permeability, and AF. In other hand, thyroid replacement therapy improves cardiac diastolic function and systemic vascular resistance, and may induce myocardial ischemia and cardiac arrhythmia. Therefore, experts have not reached a consensus about the need to replace thyroid hormone in patients with coronary artery disease.26
One of the most important risk factors for postoperative AF in our study was chronic obstructive pulmonary disease (OR 67), as reported by Holford et al.29 Patients with chronic obstructive pulmonary disease, asthma, acute respiratory distress syndrome, and other risk factors for respiratory failure have a high incidence of AF. Patients with these lung diseases may have an intrapulmonary shunt, ventilation-perfusion mismatch associated by atelectasis, reduced vital capacity, and other abnormal ventilatory mechanics following cardiac surgery which aggravate arterial hypoxia, leading to hypoxia of the atrial tissue and AF. Atrial tissue is more vulnerable to hypoxia than myocardium. The OR of chronic obstructive pulmonary disease in our study in logistic regression was 67. It is probable that hypoxia leads to AF, which leads to low cardiac output, and this vicious cycle leads to further deterioration of hypoxia and fatal events. Creswell et al6 showed that these patients have more premature atrial contractions, which can predispose to AF. Our study did not investigate premature atrial contractions as a risk factor for AF, and this is a limitation of our study. In another retrospective study, Leitch et al30 confirmed the relationship between chronic obstructive pulmonary disease and AF. In the past, the preoperative existence of congestive heart failure was thought to increase the incidence of AF. Multiple studies, including the present one, show the effectiveness of using digoxin in this scenario,25 and Roffman has suggested that the combination of beta-blockers and digoxin is effective in reducing the incidence of AF postoperatively in these patients.36 Univariate analysis showed that preoperative digoxin use, but not propranolol, reduced the incidence of AF, but the reduction was not significant in multivariate analysis.
Our study showed that the existence of preoperative congestive heart failure is a risk factor for genesis of postoperative AF (OR 2.5). We believe that digoxin sensitizes the atrial tissue, and that congestive heart failure necessitates its use in the preoperative period. On the other hand, the presence of congestive heart failure, combined with high-dose inotropic support (OR 5.2) and intra-aortic balloon pump use (OR 1.0) lead to tissue hypoxia, acidosis, and arrhythmia, necessitating antiarrhythmic use and also sensitizing atrial tissue to AF (Table 3). This is in contrast with the findings of a study by Hashimoto et al.32 We believe that use of digoxin and propranolol can reduce the incidence of AF, but if these drugs are using to treat congestive heart failure or a combination of congestive heart failure and AF, they actually increase the incidence of AF by the above mechanism. In univariate analysis, preoperative hypertension was not as significant a marker of AF as intravenous nitroglycerin use, but preoperative nipride use was a predictor of AF. We conclude that severe hypertension controlled only by a combination of drugs predicts postoperative AF.
Rubin showed that the use of propranolol in the preoperative period decreased the rate of postoperative AF.33 However, preoperative use of a beta-blocker in our study was not associated with a decreased incidence of AF. Leitch et al found a similar relationship between preoperative beta-blocker use and AF.30 Mathew et al21 found an association between intraoperative variables, such as venting of the left ventricle via the right superior pulmonary vein, or mitral valve repair, and AF.21 This issue might be related to local trauma (suture line) at the site of vent insertion or a combination of venting and high left ventricular diastolic pressure or left ventricular dysfunction and use of digoxin or antiarrhythmic drugs, acidosis, and other factors predisposing to development of AF. Another intraoperative risk factor for positive AF is dissection, and local trauma to the interatrial groove, and has been shown for mitral valve repair or replacement.21–24
Two intraoperative risk factors for development of postoperative AF are pump time and aortic cross clamp time, as shown by Mathew.21 Some types of surgery, such as combined CABG and valve surgery require longer cardiopulmonary bypass and crossclamp times, and these may be confounding factors in the genesis of AF. We excluded combined surgery from our study, which may have led to the short cross clamp and pump times and the low incidence of AF in our study. We found that a long cross clamp time significantly increases the likelihood of developing AF after CABG. Local hypothermia, as reported by Almassi et al22 was found to reduce the risk of AF. Chen et al11 showed that lack of local hypothermia combined with continuous electrical activity during arrest increased the risk of AF.19 After infusion of the first dose of cardioplegia, atrial tissue cooled only to 20 °C (in contrast with the myocardium which rapidly cooled down to 6 °C) with continuous electrical activity, and anaerobic metabolism of atrial tissue in the arrested heart led to AF. We routinely use multiple antegrade and retrograde doses of cardioplegia and iced slush, which may have led to our low rate of AF.
In our study, multivariate regression analysis implied that use of high-dose inotropic agents increased the incidence of postoperative AF (OR 5.2). The need for postoperative inotropic support is associated with several risk factors, including low ejection fraction, high left ventricular diastolic pressure,34 inadequate preservation of myocardium by cardioplegia, lack of retrograde cardioplegia,35 and incomplete revascularization following beating heart surgery in patients with multivessel disease.36,37 Some surgeons use inotropic agents routinely during weaning from cardiopulmonary bypass, which makes it difficult to identify factors contributing to development of AF. We routinely avoid using inotropic support during weaning from cardiopulmonary bypass, even in patients with low ejection fraction, so this problem could not have confounded our findings regarding an association between inotropic agents and AF, and our findings are similar to those reported elsewhere.38
Our study showed that some postoperative events, including congestive heart failure, chronic obstructive pulmonary disease, opium use, and low pO2 levels on air were associated with AF (ORs 3.95, 67, 0.37, and 15.8, respectively). We believe that respiratory failure, ie, congestive heart failure and chronic obstructive pulmonary disease, contributed to development of AF via a hypoxic mechanism. Patients with AF had had other morbid events in the intensive care unit, such as hemodynamic instability, atelectasis, hypoxia, longer ventilation time, and longer duration of stay. All of the these events can be risk factors for AF, and it is difficult to determine whether the presence of respiratory failure contributed to AF, or whether AF caused respiratory failure as a result of congestive heart failure.
Opium use was not statistically significant in univariate analysis, but was significant in multivariate analysis, with a small OR of 0.37. Opium is available in most countries, and is distributed and consumed illegally. Unlike pure opiates, such as morphine and heroin, opium is a mixture of different substances with different effects. The most important extracts of opium are morphine and codeine, which are potent analgesics. Other alkaloids, such as papaverine and noscapine, as well as inert substances and various adulterants, are among the complex constituents of opium consumed by drug abusers. Different substances added to opiates may produce a variety of effects.39 Moreover, patients using opium are invariably cigarette smokers as well and have chronic obstructive pulmonary disease, which could have introduced some bias into the interpretation of our findings.
Stroke is a well recognized complication of cardiac surgery.39 Some strokes occur as a result of intraoperative events, and become evident during recovery from anesthesia. These strokes may result from air or atheromatous emboli.40 Other strokes occur later in the postoperative period after the patient has recovered neurologically intact from anesthesia,41 and despite improvement in surgical and anesthesia techniques, very little progress has been made in decreasing the incidence of postoperative stroke.
Emboli originating from the fibrillating left atrium in AF are a known cause of strokes in the surgical setting; the 15% incidence in our study is consistent with that reported by others.39–42 We found an important relationship between stroke and occurrence of AF in postoperative period. Three percent of our study patients developed both AF and post CABG stroke, with AF preceding the stroke. Postoperative stroke was over 4.2 times more likely to occur if the patient had AF.
Sage and Van Uitert found that AF increased the risk of stroke, and that the risk was independent of any associated heart failure or coronary artery disease.40 Taylor et al41 in a study of 500 patients, found a 6% rate of stroke in AF patients. In the Creswell et al study, this incidence was 3.3%.6 None of these studies reported the temporal relationship between AF and stroke nor the duration of AF, and this is also a limitation of our study. As a result of our experience, we use a protocol to decrease the incidence of postoperative AF that includes routine intraoperative use of amiodarone, with consideration of anticoagulants if AF does not abate within 12 hours. These strategies lead to a lower incidence of AF and reduced morbidity, mortality, and resource utilization in patients undergoing cardiac surgery.
Acknowledgments
This study was funded by the Imam Ali Heart Center, Kermanshah University of Medical Sciences. We very much appreciate the commitment of staff in the division of Cardiac Surgery at Imam Ali Heart Center.
Footnotes
Disclosure
Author(s) have provided signed confirmations to the publisher of their compliance with all applicable legal and ethical obligations in respect to declaration of conflicts of interest, funding, authorship and contributorship, and compliance with ethical requirements in respect to treatment of human and animal test subjects. If this article contains identifiable human subject(s) author(s) were required to supply signed patient consent prior to publication. Author(s) have confirmed that the published article is unique and not under consideration nor published by any other publication and that they have consent to reproduce any copyrighted material. The peer reviewers declared no conflicts of interest.
References
- 1.Ommen SR, Odell JA, Stanton MS. Atrial arrhythmia after cardiothoracic surgery. N Engl J Med. 1997;336:1429–34. doi: 10.1056/NEJM199705153362006. [DOI] [PubMed] [Google Scholar]
- 2.Hogue CW, Hyder ML. Atrial fibrillation after cardiac operation: risks, mechanisms, and treatment. Ann Thorac Surg. 2000;69:300–6. doi: 10.1016/s0003-4975(99)01267-9. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiology features of chronic atrial fibrillation. Framingham study. N Engl J Med. 1982;306:1018–22. doi: 10.1056/NEJM198204293061703. [DOI] [PubMed] [Google Scholar]
- 4.Rose MR, Glassman E, Spencer FC. Arrhythmia following cardiac surgery: relation to serum digoxin levels. Am Heart J. 1995;89:288–94. doi: 10.1016/0002-8703(75)90077-0. [DOI] [PubMed] [Google Scholar]
- 5.Wolfe PA, Kannel WB, Macgee DL. Duration of atrial fibrillation and imminence of stroke. Framingham study. Stroke. 1983;14:537–40. doi: 10.1161/01.str.14.5.664. [DOI] [PubMed] [Google Scholar]
- 6.Creswell LL, Schussler RB, Rozenbloom M, Cox JL. Hazards of postoperative atrial arrhythmia. Ann Thorac Surg. 1993;56:539–49. doi: 10.1016/0003-4975(93)90894-n. [DOI] [PubMed] [Google Scholar]
- 7.Maisel WH, Rawn JD, Stevenson WG. Atrial fibrillation after cardiac surgery. Ann Intern Med. 2001;135:1061–73. doi: 10.7326/0003-4819-135-12-200112180-00010. [DOI] [PubMed] [Google Scholar]
- 8.Humphries JO. Unexpected instant death following successful coronary artery bypass graft surgery (and other clinical settings): atrial fibrillation, quinidine, procainamide, et cetera, and instant death. Clin Cardiol. 1998;21:711–8. doi: 10.1002/clc.4960211004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ACC/AHA/ESC guidelines for the managements of patients with atrial fibrillation. Circulation. 2006 Aug;114:700–52. [Google Scholar]
- 10.Soucier RJ, Mizra S, Abordo MG, et al. Predictors of conversion of atrial fibrillation after cardiac operation in the absence of class I or III antiarrhythmic medications. Ann Thorac Surg. 2001;72:694–8. doi: 10.1016/s0003-4975(01)02817-x. [DOI] [PubMed] [Google Scholar]
- 11.Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythemia:inflammatory mechanism and persistence of atrial fibrillation. Circulation. 2001;104:2886–91. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 12.Aranki SF, Shaw DP, Adams DH, et al. Predictors of atrial fibrillation after coronary artery surgery. Circulation. 1996;94:390–7. doi: 10.1161/01.cir.94.3.390. [DOI] [PubMed] [Google Scholar]
- 13.Goldman L. Supraventricular tachyarrhythmias in hospitalized adults after surgery. Clinical correlates in patients over 40 years of age after major non cardiac surgery. Chest. 1978;73:450–4. doi: 10.1378/chest.73.4.450. [DOI] [PubMed] [Google Scholar]
- 14.Smith R, Grossman W, Johnson L, Segal H, Collins J, Dalen J. Arrhythmias following cardiac valve replacement. Circulation. 1972;45:1018–23. doi: 10.1161/01.cir.45.5.1018. [DOI] [PubMed] [Google Scholar]
- 15.Stephenson LW, MacVaugh H, 3rd, Tomasello DN, Josephson ME. Propranolol for prevention of postoperative cardiac arrhythmias: a randomized study. Ann Thorac Surg. 1980;29:113–6. doi: 10.1016/s0003-4975(10)61647-5. [DOI] [PubMed] [Google Scholar]
- 16.Mathew JP, Parks R, Savino JS, et al. Atrial fibrillation following coronary artery bypass graft surgery: predictors, outcomes, and resource utilization. Multi Center Study of Perioperative Ischemia Research Group. JAMA. 1996;276:300–6. [PubMed] [Google Scholar]
- 17.Mendes LA, Connelly GP, McKenney PA, et al. Right coronary artery stenosis: an independent predictor of atrial fibrillation after coronary artery bypass surgery. JAMA. 1995;25:198–202. doi: 10.1016/0735-1097(94)00329-o. [DOI] [PubMed] [Google Scholar]
- 18.Cox JL. A perspective of post operative atrial fibrillation in cardiac operations. Ann Thorac Surg. 1993;56:405–9. doi: 10.1016/0003-4975(93)90871-e. [DOI] [PubMed] [Google Scholar]
- 19.Chen XZ, Newman M, Rosenfeldt FL. Internal cardiac cooling improves atrial preservation: electrophysiological and biochemical assessment. Ann Thorac Surg. 1988;46:406–11. doi: 10.1016/s0003-4975(10)64653-x. [DOI] [PubMed] [Google Scholar]
- 20.Fuller JA, Adams GG, Buxton B. Atrial fibrillation after coronary artery bypass grafting. Is it a disorder of the elderly? J Thorac Cardiovasc Surg. 1989;97:821–5. [PubMed] [Google Scholar]
- 21.Mathew JP, Fontes ML, Tudor IC, Ramsay, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720–1. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- 22.Almassi GH, Schwalter T, Nicolasi AC, et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg. 1997;226:501–13. doi: 10.1097/00000658-199710000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tchervenkov CI, Wynands JE, Symes JF, Malcolm ID, Dobell AR, Morin JE. Persistent atrial activity during cardioplegic arrest: a possible factor in the etiology of postoperative supraventricular tachyarrhythmias. Ann Thorac Surg. 1983;36:437–43. doi: 10.1016/s0003-4975(10)60484-5. [DOI] [PubMed] [Google Scholar]
- 24.Angelini P, Feldman MI, Lufschanowski R, Leachman RD. Cardiac arrhythmias during and after heart surgery: diagnosis and management. Prog Cardiovasc Dis. 1974;16:469–95. doi: 10.1016/0033-0620(74)90007-3. [DOI] [PubMed] [Google Scholar]
- 25.Roffman JA, Fieldman A. Digoxin and propranolol in the prophylaxis of supraventricular tachydysrhythmias after coronary artery bypass surgery. Ann Thorac Surg. 1981;31:496–501. doi: 10.1016/s0003-4975(10)61337-9. [DOI] [PubMed] [Google Scholar]
- 26.Klemperer JD, Klein IL, Ojamaa K, et al. Triiodothyronine therapy lowers the incidence of atrial fibrillation after cardiac operations. Ann Thorac Surg. 1996;61:1323–9. doi: 10.1016/0003-4975(96)00102-6. [DOI] [PubMed] [Google Scholar]
- 27.Lauer MS, Eagle KA, Buckley MJ, DeSanctis RW. Atrial fibrillation following coronary artery bypass surgery. Prog Cardiovasc Dis. 1989;31:367–78. doi: 10.1016/0033-0620(89)90031-5. [DOI] [PubMed] [Google Scholar]
- 28.Gavaghan TP, Feneley MP, Campbell TJ, Morgan JJ. Atrial tachyarrhythmias after cardiac surgery: results of disopyramide therapy. Aust N Z J Med. 1985;15:27–32. doi: 10.1111/j.1445-5994.1985.tb02726.x. [DOI] [PubMed] [Google Scholar]
- 29.Holford FD, Mithoefer JC. Cardiac arrhythmias in hospitalized patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1973;108:879–85. doi: 10.1164/arrd.1973.108.4.879. [DOI] [PubMed] [Google Scholar]
- 30.Leitch JW, Thomson D, Baird DK, Harris PJ. The importance of age as a predictor of atrial fibrillation and flutter after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 1990;100:338–42. [PubMed] [Google Scholar]
- 31.Weiner B, Rheinlander HF, Decker EL, Cleveland RG. Digoxin prophylaxis following coronary artery bypass surgery. Clin Pharm. 1986;5:55–8. [PubMed] [Google Scholar]
- 32.Hashimoto K, Ilstrup DM, Schaff HV. Influence of clinical and hemodynamic variables on risk of supraventricular tachycardia after coronary artery bypass. J Thorac Cardiovasc Surg. 1991;101:56–65. [PubMed] [Google Scholar]
- 33.Rubin DA, Nieminski KE, Reed GE, Herman MV. Predictors, prevention, and long-term prognosis of atrial fibrillation after coronary artery bypass graft operations. J Thorac Cardiovasc Surg. 1987;94:331–5. [PubMed] [Google Scholar]
- 34.McLenachan JM, Dargie HJ. Ventricular arrhythmias in hypertensive left ventricular hypertrophy. Relationship to coronary artery disease, left ventricular dysfunction, and myocardial fibrosis. Am J Hypertens. 1990;3:735–40. doi: 10.1093/ajh/3.10.735. [DOI] [PubMed] [Google Scholar]
- 35.Smith PK, Buhertman WC, Leveet JM. Supraventricular conduction abnormality following cardiac operation. A complication of inadequate atrial preservation. J Thorac Cardiovasc Surg. 1983;85:105–15. [PubMed] [Google Scholar]
- 36.Hakala T, Pitkanen O, Hippelainen M. Feasibility of predicting the risk of atrial fibrillation after coronary artery bypass surgery with logistic regression model. Scand J Surg. 2002;91:339–44. doi: 10.1177/145749690209100406. [DOI] [PubMed] [Google Scholar]
- 37.Siebert J, Rogowski J, Jagielak D, Anisimowich L, Lango R, Narkiewich M. Atrial fibrillation after coronary artery bypass grafting without cardiopulmonary bypass. Eur J Cardiothorac Surg. 2000;17:520–3. doi: 10.1016/s1010-7940(00)00368-7. [DOI] [PubMed] [Google Scholar]
- 38.Abrey JE, Reilly J, Salzano RP, Khachane VB, Jekel JF, Clyne CA. Comparison of frequencies of atrial fibrillation after coronary artery bypass grafting with and without the use of cardiopulmonary bypass. Am J Cardiol. 1999;83:775–6. doi: 10.1016/s0002-9149(98)00988-6. [DOI] [PubMed] [Google Scholar]
- 39.Kalant H. Opium revisited: a brief review of its nature, composition, non-medical use and relative risks. Addiction. 1997;92:267–77. [PubMed] [Google Scholar]
- 40.Sage JI, Van Uitert RL. Risk of recurrent stroke in patients with atrial fibrillation and non-valvular heart disease. Stroke. 1983;14:537–40. doi: 10.1161/01.str.14.4.537. [DOI] [PubMed] [Google Scholar]
- 41.Taylor GJ, Malik SA, Colliver JA, et al. Usefulness of atrial fibrillation as a predictor of stroke after isolated coronary artery bypass grafting. Am J Cardiol. 1987;60:905–7. doi: 10.1016/0002-9149(87)91045-9. [DOI] [PubMed] [Google Scholar]
- 42.Sabzi F, Moloudi A. Effect of gender on outcome of coronary artery bypass graft surgery. Iran Heart J. 2003;4:17–20. [Google Scholar]