Abstract
Endoplasmic reticulum calcium homeostasis is involved in several essential cell functions including cell proliferation, protein synthesis, stress responses or secretion. Calcium uptake into the endoplasmic reticulum is performed by Sarco/Endoplasmic Reticulum Calcium ATPases (SERCA enzymes). In order to study endoplasmic reticulum calcium homeostasis in situ in mammary tissue, in this work SERCA3 expression was investigated in normal breast and in its benign and malignant lesions in function of the cell type, degree of malignancy, and histological and molecular parameters of the tumors. Our data indicate, that although normal breast acinar epithelial cells express SERCA3 abundantly, its expression is strongly decreased already in very early non-malignant epithelial lesions such as adenosis, and remains low in lobular carcinomas. Whereas normal duct epithelium expresses significant amounts of SERCA3, its expression is decreased in several benign ductal lesions, as well as in ductal adenocarcinoma. The loss of SERCA3 expression is correlated with Elston-Ellis grade, negative hormone receptor expression or triple negative status in ductal carcinomas. The concordance between decreased SERCA3 expression and several histological, as well as molecular markers of ductal carcinogenesis indicates that endoplasmic reticulum calcium homeostasis is remodeled during tumorigenesis in the breast epithelium.
Keywords: breast cancer, calcium signaling, endoplasmic reticulum, SERCA, calcium pump, ion transport
Introduction
Breast carcinoma, which represents 40% of all cancers in women, is a heterogeneous disease with a wide variety of pathological entities and range of clinical behaviors. For appropriate clinical management a complex set of various clinical, histological and molecular factors of prognostic and predictive value has to be integrated.1 However, although clinicopathological algorithms are largely used for stratification, it is known that breast cancer of patients belonging to the same currently used clinically and pathologically defined disease groups may display different behaviors and response to treatment. It seems therefore, that current prognostic factors, although useful, are not yet fully sufficient to take into account the molecular and clinical heterogeneity of breast cancer, and that further knowledge about molecular differences that distinguish normal breast tissue from benign and malignant lesions is required for the understanding of the disease.
Based on work on colon carcinogenesis,2,3 neoplasic progression is often regarded as a linear multistep process where progressively accumulated mutations drive cells sequentially through normal to dysplastic to in situ and invasive carcinoma phenotypes. The complexities of breast molecular carcinogenesis are, however, reflected by the observations that benign tumoral lesions of the breast are not inevitably preneoplastic, that breast carcinoma can arise without prior preneoplastic stages, and that the carcinomas can arise equally within histologically low or high grade premalignant lesions. Breast carcinogenesis is therefore now considered as a process in which several parallel oncogenic pathways are involved in a somewhat combinatorial manner, giving rise to several tumor phenotypes with distinct gene expression profiles, hormone dependency, histological type and sensitivity to various types of treatment.1,4–9
The functional units of normal breast epithelium, where most breast tumors arise, are the terminal ductal lobular units (TDLU) that are composed of acini and intralobular and extralobular terminal ducts. Acini and ducts are layered by an inner (luminal) epithelial, and an outer (basal) myoepithelial cell layer.10 Luminal ductal, acinar and basal myoepithelial cells are functionally and phenotypically distinct,10 and benign, as well as malignant breast lesions can also display ductal or lobular characteristics. The study of breast epithelial differentiation and its defects in benign, premalignant and malignant lesions is therefore essential for the better understanding of changes that occur during neoplastic transformation.
Calcium-dependent cellular signaling is involved in the regulation of many cellular functions such as secretion, differentiation, motility, the control of cell proliferation, as well as survival or apoptosis.11–16 Calcium-induced cell activation is critically dependent on the release of calcium from the endoplasmic reticulum accumulated by Sarco/Endoplasmic Reticulum Calcium transport ATPases (SERCA enzymes). SERCA enzymes are coded by three genes (ATP2A1, 2 and 3), the expression and alternative splicing of which is tissue dependent and developmentally regulated.17,18 Whereas the SERCA1 and SERCA2a isoforms are expressed in skeletal and cardiac muscle, respectively, the SERCA2b isoform is ubiquitous. SERCA3 expression has been reported in cells of hematopoietic origin and selected epithelial cells such as colonic and gastric epithelium.17–19 Interestingly, the expression of SERCA3 has been previously shown to be negatively regulated by the APC/β-catenin/TCF4 oncogenic pathway in colon cancer.19 In addition, SERCA3 expression is induced during the differentiation of colon and gastric carcinoma cells,20 and SERCA activity is involved in the control of cell differentiation.19,21 These observations when taken together show, that the remodeling of ER calcium homeostasis due to the specific loss of SERCA3 expression is involved in the establishment of the tumoral phenotype in the colon.
Based on these data, in order to explore the role of endoplasmic reticulum calcium homeostasis and signaling in breast pathology, in the present work we investigated the expression of the SERCA3 calcium pump in normal breast epithelium, in benign breast lesions and in invasive ductal and lobular breast carcinoma. Our data show, that SERCA3 expression undergoes significant changes during the neoplastic process in the breast and point at the involvement of endoplasmic reticulum calcium accumulation in the control of breast epithelial differentiation and carcinogenesis.
Materials and Methods
Tissue samples
Formalin fixed and paraffin embedded tissue sections from archival biopsies and surgical specimens were obtained retrospectively from the collection of the Pathology Department of Hôpital Lariboisière, Paris, France. Samples were processed anonymously and no direct patient data were collected. The study did not interfere with the routine management and follow-up of cases. Additional samples were commercially obtained in a tissue microarray format (N °Z7020005) from BioChain Institute (Hayward, CA, USA, Clini-Sciences, Montrouge, France) comprising 70 cases of all major types of breast carcinoma deposited in duplicate. Altogether, a total number of 100 invasive lesions and 20 non-invasive lesions were analyzed.
Histology
Lesions were analyzed after standard hematoxylineosin-saffron (HES) coloration, and were classified according to the WHO classification.22 Carcinomas were graded according to the criteria of the grading system of Elston and Ellis,23,24 based on tubule differentiation, nuclear pleomorphism and mitotic count.
Immunohistochemistry
Immunohistochemistry was performed on deparaffinized formalin-fixed sections using an indirect ABC-peroxidase method revealed with 3,3′diaminobenzidine (DAB) as chromogene. Endogenous peroxidase activity was blocked by incubation in 3% hydrogen peroxide in phosphate-buffered saline for 10 minutes. Immunohistochemistry was performed with an automated immunostainer system (Benchmark®, Ventana Medical Systems, Illkirch, France) using primary antibodies raised against E-cadherin, estrogen receptor, progesterone receptor, Ki-67, and HER-2 (Table 1). HER-2 immunostaining was done in parallel with negative and 3+ positive external controls calibrated by in situ hybridization. For each antigen, omission of the primary antibody and replacement by an isotype-matched irrelevant antibody were used as negative controls and these gave no staining. Staining for SERCA3 was performed using the clone 2H3 (H00000489-MO1) monoclonal mouse anti-SERCA3 antibody (IgG2a kappa) from Abnova (Tebu-bio, Le Perray en Yvelines, France) raised against a GST-tagged peptide sequence encompassing the 501–621 amino acid fragment of SERCA3, at 3 μg/ml final concentration diluted in Dako Real® antibody diluent (Dako France S.A.S., Trappes, France). Briefly, after inhibition of endogenous peroxydase and antigen retrieval of deparaffinized sections by a tris-(hydroxymethyl)-aminomethane-based antigen retrieval reagent (Ventana CC1 cell conditioning solution) at 95–100 °C for 30 minutes, slides were incubated for 30 minutes at 37 °C with the anti-SERCA3 antibody, and staining was revealed using the Ventana I-View Biotin-Ig-streptavidin-biotin-horseradish peroxydase system with copper enhancement, according to the instructions of the manufacturer. Slides were counterstained with hematoxylin and bluing agent (Ventana Medical Systems, Illkirch, France). For estrogen receptor (nuclear labeling), progesterone receptor (nuclear labeling), SERCA3 (cytoplasmic labeling) and HER2 (membrane labeling), the intensity of immunostaining was semi-quantitatively evaluated using a 4 grade scale (0 to 3+) and by determining the percentage of stained cells. For Ki-67, percentage of positive cells (nuclear labeling) was evaluated using a 3 grade scale (S1 < 15%, 15% ≤ S2 < 40%, S3 ≥ 40%). For SERCA3 immunostaining, endothelial cells and lymphocytes present in the sections were used as internal positive control. For estrogen and progesterone receptors, in addition to external positive controls, normal breast structures present in the specimens served as internal positive controls.
Table 1.
Antibodies used in this study for the characterization of breast lesions.
| Antibody | Supplier | Catalog number | Dilution |
|---|---|---|---|
| E-cadherin | DakoCytomation, Glostrup, Denmark | M3612 | 1/50 |
| Estrogen receptor | Novocastra, Newcastle upon Tyne, UK | NCL-ER-6F11 | 1/50 |
| Progesteron receptor | Coulter-Immunotech, Marseille, France | 2147 | 1/50 |
| Ki-67 | DakoCytomation | M-7240 | 1/50 |
| HER-2/C-erbB-2 | DakoCytomation | A-0485 | 1/3500 |
Note: Dilutions and sources of the antibodies used in this study for the immunohistochemical characterization of breast lesions.
Statistical analysis
Non-parametric statistical analysis was performed using Kruskal-Wallis and Mann-Whitney tests for comparison of quantitative variables using the XLSTAT 2010 software (Addinsoft, Paris, France). A p-value less than 0.05 was considered as significant.
Results
Normal breast
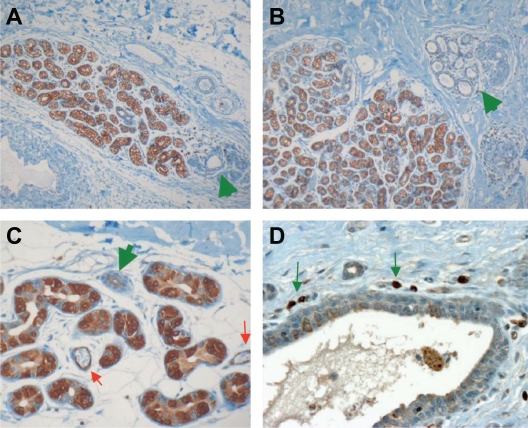
SERCA3 labeling in normal breast epithelium is located in the cytoplasmic area and appears finely granular, corresponding to the endoplasmic reticulum on the light microscopic level. As shown in Figure 1, SERCA3 labeling in normal breast structures is markedly heterogeneous. The strongest labeling (+++) is seen in luminal cells of acini, which express SERCA3 at levels similar to that observed in lymphocytes and endothelial cells present in the samples. In marked contrast with acinar luminal cells, SERCA3 expression in terminal duct luminal epithelium is very weak to weak (0/+ to +; Figure 1, Panels A, B and C green arrows), whereas in larger ducts SERCA3 labeling is somewhat more heterogeneous (Fig. 1, Panel D), and varies from weak to moderate (+ to ++). Myoepithelial cells in acinar, as well as in ductal structures are consistently negative.
Figure 1.
Expression of SERCA3 in normal breast structures. Luminal cells of acini strongly express SERCA3. Intensity of labeling is comparable to that of endothelial cells (Panel C, red arrows) or lymphocytes (Panel D, small green arrows). SERCA3 labeling in terminal duct luminal epithelium is very weak to weak (Panels A, B and C, large green arrows). In larger ducts SERCA3 labeling is somewhat more heterogeneous, and varies from weak to moderate (Panel D). Myoepithelial cells in acinar, as well as ductal structures are negative. Original magnification: ×20 (Panels A and B), ×40 (Panels C and D).
Non-invasive lesions
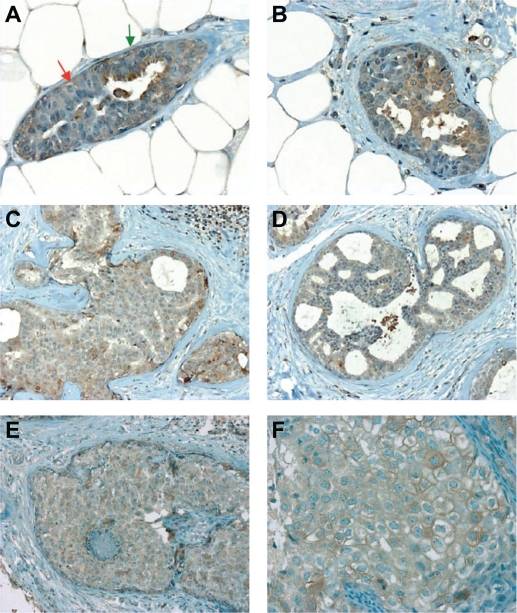
Adenosis is a benign condition without increased relative risk of cancer, where the lobules are enlarged and composed of small acini made of epithelial cells and myoepithelial cells. In sharp contrast with normal acini (Fig. 2, Panel A), epithelial cells in adenosis exhibited a very weak or negative staining for SERCA3 (Fig. 2, Panels B and C).
Figure 2.
SERCA3 expression in adenosis. Expression is very weak or negative in adenosis (Panels B and C), in contrast with normal acini (Panel A). Original magnification: ×20.
Apocrine metaplasia is a common benign condition without increased relative risk of cancer, found in female breast after the age of 30 years. The lesion is composed of cuboidal or flat cells that form a single luminal layer or blunt papillae. Nuclei appear homogeneous and regular without atypia. As shown in Figure 3, SERCA3 is weakly and homogeneously expressed in the cytoplasmic area of the cells, whereas myoepithelial cells are negative.
Figure 3.
SERCA3 expression in apocrine metaplasia. Expression is weak and homogeneous in the cytoplasmic area of the cells. Lymphocytes are strongly positive. Original magnification: ×40.
Columnar cell lesions
Columnar cell changes without atypia are characterized by dilation of terminal ductal lobular units lined by columnar epithelial cells with basally oriented nuclei and luminal cytoplasmic tufts and ranging from one to two cell layers.
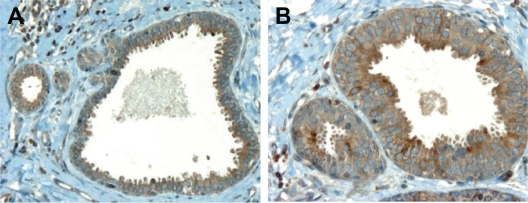
Columnar cell changes with atypia (ductal intraepithelial neoplasia; DIN 1A) are characterized by a stratified epithelium which may form micropapillary or fenestrated structures.22 Although known since a long time, these lesions are being increasingly detected as mammographic calcifications, and have been shown to be associated with invasive carcinomas, usually of the lobular type.25 As shown in Figure 4, SERCA3 expression in columnar changes with (Panel B), or without atypia (Panel A) is moderate and somewhat heterogeneous with apical reinforcement.
Figure 4.
SERCA3 expression in columnar changes with (Panel B) or without atypia (Panel A). Expression is moderate and somewhat heterogeneous with apical reinforcement. Original magnification: ×20 (Panel A), ×40 (Panel B).
Ductal hyperplasia of the breast is a common lesion characterized by an increase in the cellularity of duct epithelium leading to a partial or complete obliteration of the duct lumen.
In usual ductal hyperplasia (low risk DIN) nuclei may seem to overlap, and nucleoli remain inconspicuous even when hyperplasia is florid.22 This entity is considered to carry a very low to low relative risk of cancer (RR: 1.2 to 2).
In comparison, atypical ductal hyperplasia (DIN 1B)22 is considered to carry a moderate relative risk (RR: 4 to 5) of subsequent invasive carcinoma. It exhibits cytological atypia such as enlarged hyperchromatic nuclei, clumped irregular chromatin and enlarged pleomorphic nucleoli.
As shown in Figure 5, SERCA3 expression in ductal hyperplasia is always weaker than in normal acini and its intensity is similar to that of normal ducts, independently from the presence (Fig. 5, Panels C and D) or absence of atypia (Fig. 5, Panels A and B).
Figure 5.
SERCA3 expression in epithelial hyperplasia with (Panels C and D), or without atypia (Panels A and B) and in ductal carcinoma in situ (Panels E and F). Expression is moderate, and always weaker than in normal acini or lymphocytes. In Panel A, epithelial hyperplasia (red arrow) can be distinguished from columnar change (green arrow) by the lack of apical reinforcement of SERCA3 staining in the former. Original magnification: ×40 (Panels A, B and F), ×20 (Panels C, D and E).
In situ ductal carcinomas are classified as DIN 1C, 2 and 3 according to the degree of the proliferation and/or cytological features.22 As shown in Figure 5 (Panels E and F), the expression of SERCA3 is significantly lower than in normal acini or lymphocytes and is globally of moderate intensity.
Lobular in situ neoplasia (LIN) are located in terminal duct-lobular units and are characterized by conserved lobular architecture whereas the acini of one or more lobules are distended by a loosely cohesive monomorphic cell proliferation.22 According to the extent and degree of the proliferation and/or cytological features, LIN is graded into LIN 1, LIN 2 and LIN 3. Cytologically, proliferative cells are usually of small size, with round nuclei (LIN 1 and 2) and homogenous chromatin with inconspicuous nucleoli. In some cases, proliferative cells may appear larger and more pleomorphic (LIN 3). In all grades, SERCA3 expression is always weaker than in normal acini (Fig. 6).
Figure 6.
SERCA3 expression in lobular in situ neoplasia (LIN). Expression is always weaker than in normal acini, and seems to slightly increase with increased atypia. (Panel A: LIN 1, Panels B and C: LIN 2, Panel D: LIN3). Original magnification: ×20 (Panel A), ×40 (Panels B, C and D).
Invasive lesions
Invasive breast carcinoma can be graded based on histological criteria. The current three tiered classification of Elston and Ellis, based on the combined grading of tubule formation, nuclear pleomorphism and mitotic activity is widely used in clinical practice, and is prognostically, as well as therapeutically pertinent.23,24 Invasive ductal and lobular carcinomas always exhibit a decreased labeling for SERCA3 when compared to normal acini.
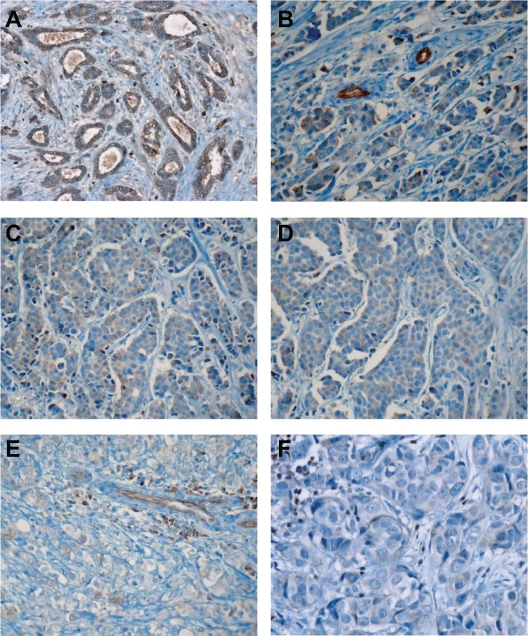
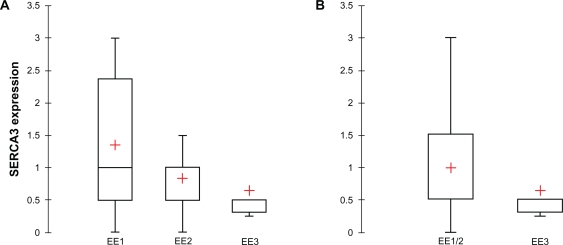
When investigated in invasive ductal carcinomas, an inverse relationship could be observed between SERCA3 expression and Elston-Ellis grade (EE). As shown in Figures 7 and 8, SERCA3 expression decreases significantly among EE groups (Kruskal-Wallis test, p = 0.012). SERCA3 expression is significantly higher in Elston-Ellis groups EE1 (mean = 1.350) and EE2 (mean = 0.833), than in group E3 (mean = 0.644; Fig. 8, Panel A). Although SERCA3 expression does not differ significantly between groups EE1 and EE2 (Mann-Whitney test, p = 0.114), as shown in Figure 8, Panel B, when groups EE1 and EE2 are pooled for analysis, a highly significant difference can be observed when compared to group EE3 (Mann-Whitney test, p = 0.006).
Figure 7.
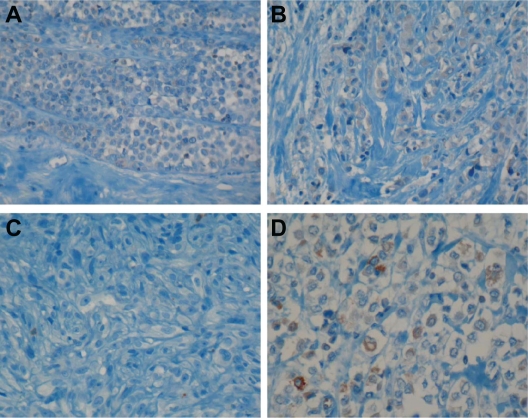
SERCA3 expression in invasive ductal carcinomas. Expression is weaker than in normal acini. The decrease of SERCA3 expression correlates with the increase of Elston-Ellis (EE) grade. Panel A: tubular carcinoma (very well differentiated, EE grade I); Panel B: ductal carcinoma (EE grade I); Panels C and D: EE grade II ductal carcinomas; Panels E and F: EE grade III ductal carcinomas. Original magnification: ×20 (Panels A, C and E), ×40 (Panels B, D and F).
Figure 8.
Statistical analysis of SERCA3 expression according to Elston-Ellis (EE) grade in ductal carcinomas. Panel A: SERCA3 expression significantly decreases from EE I to EE II groups (Kruskal-Wallis test, p = 0.012). Although EE I and EE II groups don’t differ significantly, when EE I and EE II groups are pooled (Panel B), a significant difference is observed when compared to EE III tumors (Mann-Whitney test, p = 0.006). Box plot shows median.
SERCA3 expression of invasive lobular carcinomas was always weaker than that of normal acini, as shown in Figure 9. No significant differences could be observed among various Elston-Ellis groups in these lesions (Kruskal-Wallis test, p = 0.056), although a tendency of increased SERCA3 expression could be observed between EE1 (m = 1.063) and EE2 (m = 1.542) groups, and a decrease from EE2 to EE3 (m = 0.167). SERCA3 expression does not differ between the groups EE1 and EE2 according to the Mann-Whitney test (p = 0.274). However, when groups EE1 and EE2 were pooled, they significantly differed from the group EE3 (Mann-Whitney test, p = 0.033, not shown).
Figure 9.
SERCA3 expression in invasive lobular carcinomas. Panel A: EE grade I, Panel B: EE grade II lobular carcinomas. Panels C and D: pleomorphic EE grade II lobular carcinomas. SERCA3 expression is variable, but always markedly decreased when compared to normal acini or normal lymphocytes. Original magnification: ×40.
Regarding individual parameters of the Elston-Ellis grading system (ie, tubule formation, nuclear pleomorphism and mitotic activity), the loss of SERCA3 expression in ductal carcinomas varies significantly among nuclear grade (NG) groups (Kruskal-Wallis test, p = 0.0003; Fig. 10, Panel A). SERCA3 expression is significantly higher in NG groups 1 and 2 (m = 1.906 and 1.060, respectively), than in NG group 3 (m = 0.552). NG1 and NG2 groups do not differ significantly when analyzed by the Mann-Whitney test (p = 0.062). However, when NG1 and NG2 groups were pooled and compared to NG3, a highly significant difference could be obtained by the Mann-Whiney test (p = 0.0001; Fig. 10, Panel B).
Figure 10.
Statistical analysis of SERCA3 expression in invasive ductal carcinomas according to nuclear grade (NG). Panel A: SERCA3 expression decreases significantly with increasing nuclear pleomorphism (NG1 to NG3; Kruskal-Wallis test, p = 0.0003). Panel B: When NG1 and NG2 groups are pooled and compared to NG3, SERCA3 expression is significantly decreased in NG3 tumors when analyzed with the Mann-Whitney test (p = 0.0001).
On the other hand, SERCA3 expression does not vary significantly among tubule differentiation (TG) grades as measured by the Kruskal-Wallis test (p = 0.069, not shown), although a tendency of decreased expression can be observed with decreased tubule differentiation (ie, increased TG). However, when TG1 and TG2 groups are pooled, a significant decrease of SERCA3 expression can be observed in the TG3 group by the Mann-Whitney test (p = 0.035; Fig. 11, Panel A).
Figure 11.
Statistical analysis of SERCA3 in invasive ductal carcinomas. Panel A: Although SERCA3 doesn’t vary significantly according to tubular differentiation (TG) among TG1, 2 and 3 grades (Kruskal-Wallis test, p = 0.069, not shown), when TG1 and TG2 tumors are pooled, a significant decrease of SERCA3 expression is observed in TG3 tumors (Mann-Whitney test, p = 0.035). Panel B: Statistical analysis of SERCA3 expression in invasive ductal carcinomas stratified simultaneously for nuclear grade (NG) and proliferation index as detected by Ki-76 staining (S). Although SERCA3 expression doesn’t differ significantly according to mitotic activity alone (not shown), a significant decrease of SERCA3 expression is observed when NG1/S1, NG2/S2 and NG3/S3 tumors are compared (Kruskal-Wallis test, p = 0.008). Box plot shows median.
In order to investigate the correlation between SERCA3 expression and mitotic activity, three grades for Ki-67 expression (S1, S2 and S3) were used, corresponding to less than 15%, 15%–39% and 40%–100% Ki-67 positivity, respectively. SERCA3 expression did not differ significantly among S groups (Kruskal-Wallis test, p = 0.668, not shown).
In order to further analyze the correlation between SERCA3 expression and tumor histology, samples were stratified simultaneously for nuclear grade (NG) and Ki-67 expression (S), and groups that display low, intermediate and high grades simultaneously for nuclear grade and for mitotic activity (NG1/S1, NG2/S2 and NG3/S3, respectively) were established. As shown in Figure 11, Panel B, using this combined stratification scheme, a highly significant correlation could be observed between decreased SERCA3 expression and NG/S groups (Kruskal-Wallis test, p = 0.008).
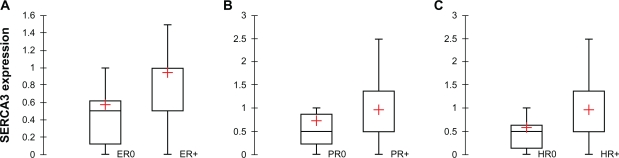
Analysis of estrogen (ER) and progesterone receptor (PR) expression is widely used in the clinical management of breast carcinoma, and has also been shown to be relevant for the molecular classification of tumors.4–6,26,27 As shown in Figure 12, when invasive ductal carcinomas were stratified according to estrogen (Mann-Whitney test, p = 0.007; Panel A), or progesterone receptor expression (Mann-Whitney test, p = 0.041; Panel B), significantly higher SERCA3 expression could be observed in estrogen or progesterone receptor positive tumors. When double hormone receptor negative (HR0) and double positive (HR+) tumors were compared, SERCA3 expression was also significantly higher in double positive tumors (Mann-Whitney test, p = 0.003; Fig. 12, Panel C).
Figure 12.
Statistical analysis of SERCA3 in invasive ductal carcinomas according to hormone receptor expression. Panel A: SERCA3 expression is decreased in estrogen receptor negative, when compared to estrogen receptor positive tumor cells (Mann-Whitney test, p = 0.007). Panel B: SERCA3 expression in decreased in progesterone receptor negative tumor cells when compared to progesterone receptor positive tumor cells (Mann-Whitney test, p = 0.041). Panel C: SERCA3 expression in estrogen and progesterone receptor double negative (HR0), and double positive (HR+) cells. SERCA3 expression is significantly inferior in double negative cells when compared to estrogen and progesterone receptor double positive cells (Mann-Whitney test, p = 0.003).
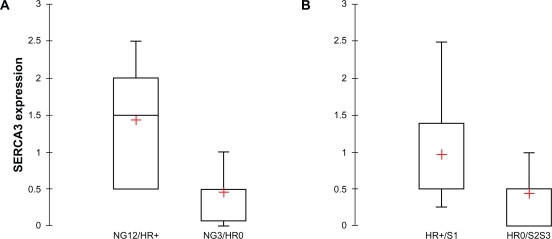
In order to explore the combined effect of hormone receptor status (HR) and nuclear grade (NG), invasive ductal carcinomas were stratified simultaneously according to nuclear grade and hormone receptor expression. When tumors with low/medium nuclear grade (NG1/2) that express hormone receptors (HR+) were compared to high nuclear grade, hormone receptor negative (NG3/HR0) tumors, a highly significantly decreased SERCA3 expression could be observed in high nuclear grade, hormone receptor negative tumors (Mann-Whitney test, p = 0.00035; Figure 13, Panel A). Moreover, when tumors with low proliferation index and simultaneous positive hormone receptor status (HR+/S1) were compared to highly proliferative, hormone receptor negative (HR0/S2,3) tumors, a highly significant decrease of SERCA3 expression could be observed in the latter (Mann-Whitney test, p = 0.00025; Figure 13, Panel B). When HER-2 expression was studied, SERCA3 expression did not differ significantly between HER-2 0/+ and HER-2 +++ tumors (Mann-Whitney test, p = 0.883; not shown). Interestingly, on the other hand, there was a significant decrease of SERCA3 expression (Mann-Whitney test, p = 0.004; not shown) in triple negative tumors (HR0, HER-2 0; m = 0.333) when compared to all other tumor types as a group (m = 0.897).
Figure 13.
Statistical analysis of SERCA3 expression in invasive ductal carcinomas. Panel A: When tumors are stratified simultaneously according to nuclear grade (NG) and hormone receptor expression (HR), SERCA3 expression is significantly decreased in high nuclear grade, hormone receptor negative (NG3/HR0) tumors when compared to low nuclear grade, hormone receptor positive (NG1/HR+) tumors (Mann-Whitney test, p = 0.00035). Panel B: When tumors are stratified simultaneously according to hormone receptor expression (HR) and proliferation index (S), SERCA3 expression is significantly decreased in hormone receptor negative, highly proliferative (HR0/S2,3) tumors, when compared to hormone receptor positive tumors with a low proliferative index (HR+/S1; Mann-Whitney test, p = 0.00025).
Discussion
Data presented in this work show, for the first time, that SERCA3 protein is expressed in normal breast tissue, with the highest expression levels found in normal lobular epithelial cells. SERCA3 expression was also observed, although at a lower level, in luminal ductal cells as well, whereas myoepithelial cells were consistently negative. This indicates that SERCA3 constitutes a new phenotypic marker that displays a cell-type specific expression pattern in the normal mammary gland.
Of particular interest, we show that SERCA3 expression in acini markedly decreases already in very early and common lobular lesions such as adenosis and lobular hyperplasia without atypia which are non-obligatory low risk precancerous lesions. This observation indicates that fully differentiated normal breast acinar epithelium possesses a specialized endoplasmic reticulum calcium uptake system, which, as reflected by the loss of SERCA3 expression, undergoes significant remodeling at a very early step of hyperplasia/dysplasia.
Calcium-dependent signal transduction constitutes a key component of intracellular signaling networks that control cell growth, differentiation and survival. Calcium-dependent cell activation is initiated by the release of calcium from the endoplasmic reticulum by second messengers such as inositol-1,4,5-tris-phosphate.12,14 Calcium accumulation in this organelle depends entirely on the activity of SERCA enzymes. Therefore, the extent, the spatiotemporal characteristics, frequency and amplitude of calcium release from the endoplasmic reticulum are critically dependent upon, and modulated by, SERCA-dependent calcium transport.28–33 SERCA activity therefore constitutes a major negative feedback mechanism on calcium mobilization from the organelle.
It has been shown earlier that SERCA3 expression undergoes major changes during colon and gastric carcinogenesis.19,20 Whereas SERCA3 is abundantly expressed in gastric and colonic epithelium,20 SERCA3 expression is decreased in colon adenomas, and lost in adenocarcinomas.19 In addition, it has been shown, that the induction of cell differentiation by histone deacetylase inhibitors, as well as the inhibition of the APC/β-catenin-TCF-4 oncogenic pathway by dominant negative TCF-4 lead to the induction of the expression of SERCA3 in colon cancer cells in vitro.19,20 SERCA3 expression is correlated also with the state of differentiation of various lymphoid34,35 and myeloid36,37 leukemia cells, and the direct pharmacological modulation of SERCA activity can induce growth factor independence, growth arrest or differentiation in a cell-type dependent manner.19,21,40 These observations, when taken together indicate that SERCA-dependent calcium sequestration into the endoplasmic reticulum can modulate cell growth and differentiation in various cell types.
Epidemiologic, as well as experimental studies indicate that calcium plays an important role in breast cancer prevention and in the control of breast cancer cell growth.38,39 The observation that endoplasmic reticulum calcium pump expression becomes anomalous already in very early non-obligatory precancerous lesions indicates that proper calcium uptake into the organelle is involved in the establishment and maintenance of the fully differentiated breast acinar phenotype, and suggests that a previously unknown defect of calcium accumulation into the endoplasmic reticulum, related to the loss of SERCA3 expression, may be involved in the formation of early/premalignant steps of breast tumorigenesis.
When investigated in invasive ductal or lobular breast carcinoma, SERCA3 expression, although variable, was always decreased when compared to normal acini, and in several cases was below detection limits. While tubular carcinoma, a histologically well differentiated lesion, displayed SERCA3 expression almost similar to normal acini, and stronger than normal ducts, SERCA3 expression in invasive ductal carcinoma was lower than in normal controls (lymphocytes, endothelial cells and normal acini). SERCA3 expression in invasive ductal carcinoma was inversely proportional to Elston-Ellis (EE) grade, as well as to individual components of the EE grading system, with the strongest correlation being observed with nuclear grade. Moreover, when tumors were stratified simultaneously for nuclear grade (NG) and proliferative activity (S), a highly significant correlation could be observed between grade and SERCA3 expression among the NG1/S1, NG2/S2 and NG3/S3 groups. In benign ductal lesions (columnar changes, apocrine metaplasia, ductal hyperplasia with or without atypia), the expression of SERCA3 was decreased when compared to normal controls and remained heterogeneous in the range of weak to moderate expression levels.
When investigated in the context of estrogen and progesterone receptor expression, the loss of SERCA3 expression was found to be correlated with hormone receptor negative status in invasive ductal carcinomas, and an even stronger correlation could be seen when lesions were stratified simultaneously for hormone receptor status and nuclear grade. In addition, SERCA3 expression was also significantly decreased in triple negative (HR0, HER-2 0) carcinomas.
Taken together, data presented in this work show that normal acinar differentiation in breast is associated with strong SERCA3 expression. This is drastically decreased already at the earliest stages of morphologically detectable anomalies of normal acinar architecture, and remains low at further stages of lobular carcinogenesis. Regarding ductal pathology, we show that although normal breast ducts express SERCA3 at significant levels, its expression is comparable or decreased in early benign ductal lesions. In invasive ductal carcinoma SERCA3 expression is inversely correlated with nuclear grade and proliferative activity, as well as with hormone receptor expression. SERCA3 loss is the most marked in high nuclear grade, highly proliferating, hormone receptor negative tumors. The correlation of the degree of SERCA3 expression with several established markers of breast cancer biology connects, for the first time, abnormal endoplasmic reticulum calcium pump expression to breast tumorigenesis.
Cellular calcium homeostasis is maintained by a functionally interconnected network of calcium pumps, calcium channels and calcium binding proteins. The activity of many components of this network is regulated by calcium itself in a concentration-dependent manner, and interconnectedness and partial functional redundancies of the components are typical of the cellular calcium homeostatic “toolkit”.14,33 Decreased SERCA3 expression may thus have complex consequences on global cellular calcium homeostasis, cell survival and responsiveness to external stimuli. SERCA3 has been shown earlier to be associated with the inositol-1,4,5-tris-phosphate-mobilisable sub-compartment of the endoplasmic reticulum in platelets.41 It is tempting to speculate that SERCA3 loss in breast epithelial cells may also lead to decreased calcium accumulation into the inositol-1,4,5-tris-phosphate-mobilisable calcium pool, leading to the acquisition by the cell of a more autonomous signaling configuration42 with a decreased or lost capacity to respond to extracellular stimuli that signal via inositol-1,4,5-tris-phosphate-induced calcium mobilization. These effects may be explored in further investigations using, for example, genetically engineered calcium indicators (GECIs43) expressed in mammary epithelium in model systems that recapitulate the formation and progression of benign and malignant breast tumors.
In conclusion, observations presented in this work indicate that endoplasmic reticulum calcium pump function may be involved in the process of breast tumorigenesis already at a very early stage. Detection of SERCA3 expression may prove useful for the immunophenotypic characterization of benign and malignant lesions. A better understanding of the molecular implications of the endoplasmic reticulum calcium homeostatic defect reflected by the loss of SERCA3 expression may open new avenues in the understanding of early steps of breast tumorigenesis.
Acknowledgments
We are grateful for the support of Pr. Françoise Gray (Service d’Anatomie et Cytologie Pathologiques, Hôpital Lariboisière) during this work, and we thank Patrice Castagnet for his excellent technical help. This work was supported by the Institut National de la Santé et de la Recherche Médicale (Inserm) and the Association pour la Recherche sur le Cancer (ARC), France.
Footnotes
Disclosures
Authors have provided signed confirmations to the publisher of their compliance with all applicable legal and ethical obligations in respect to declaration of conflicts of interest, funding, authorship and contributorship, and compliance with ethical requirements in respect to treatment of human and animal test subjects. If this article contains identifiable human subject(s) author(s) were required to supply signed patient consent prior to publication. Authors have confirmed that the published article is unique and not under consideration nor published by any other publication and that they have consent to reproduce any copyrighted material. The peer reviewers declared no conflicts of interest.
References
- 1.Simpson PT, Reis-Filho JS, Gale T, Lakhani SR. Molecular evolution of breast cancer. J Pathol. 2005 Jan;205(2):248–54. doi: 10.1002/path.1691. [DOI] [PubMed] [Google Scholar]
- 2.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 3.Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–32. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 4.Reis-Filho JS, Simpson PT, Gale T, Lakhani SR. The molecular genetics of breast cancer: the contribution of comparative genomic hybridization. Pathol Res Pract. 2005;201(11):713–25. doi: 10.1016/j.prp.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000 Aug 17;406(6797):747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 6.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001 Sep 11;98(19):10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borresen-Dale AL, Sorlie T, Kristensen VN. On the molecular biology of breast cancer. Mol Oncol. 2010;4(3):171–3. doi: 10.1016/j.molonc.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weigelt B, Geyer FC, Reis-Filho JS. Histological types of breast cancer: how special are they? Mol Oncol. 2010;4(3):192–208. doi: 10.1016/j.molonc.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bombonati A, Sgroi DC. The molecular pathology of breast cancer progression. J Pathol. 2011 Jan;223(2):307–17. doi: 10.1002/path.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mills SE, editor. Histology for pathologists. 3rd ed. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 57–71. [Google Scholar]
- 11.Berridge MJ. Calcium signal transduction and cellular control mechanisms. Biochim Biophys Acta. 2004 Dec 6;1742(1–3):3–7. doi: 10.1016/j.bbamcr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Berridge MJ. Inositol trisphosphate and calcium oscillations. Biochem Soc Symp. 2007;74:1–7. doi: 10.1042/BSS0740001. [DOI] [PubMed] [Google Scholar]
- 13.Berridge MJ. Smooth muscle cell calcium activation mechanisms. J Physiol. 2008 Nov 1;586(Pt 21):5047–61. doi: 10.1113/jphysiol.2008.160440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berridge MJ. Inositol trisphosphate and calcium signalling mechanisms. Biochim Biophys Acta. 2009 Jun;1793(6):933–40. doi: 10.1016/j.bbamcr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008 Oct 27;27(50):6407–18. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Høyer-Hansen M, Jäättelä Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007 Sep;14(9):1576–82. doi: 10.1038/sj.cdd.4402200. [DOI] [PubMed] [Google Scholar]
- 17.Strehler EE, Treiman M. Calcium pumps of plasma membrane and cell interior. Curr Mol Med. 2004 May;4(3):323–35. doi: 10.2174/1566524043360735. [DOI] [PubMed] [Google Scholar]
- 18.Wuytack F, Raeymaekers L, Missiaen L. Molecular physiology of the SERCA and SPCA pumps. Cell Calcium. 2002 Nov-Dec;32(5–6):279–305. doi: 10.1016/s0143416002001847. [DOI] [PubMed] [Google Scholar]
- 19.Brouland JP, Gélébart P, Kovàcs T, Enouf J, Grossmann J, Papp B. The loss of sarco/endoplasmic reticulum calcium transport ATPase 3 expression is an early event during the multistep process of colon carcinogenesis. Am J Pathol. 2005 Jul;167(1):233–42. doi: 10.1016/S0002-9440(10)62968-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gélébart P, Kovàcs T, Brouland JP, et al. Expression of endomembrane calcium pumps in colon and gastric cancercells. Induction of SERCA3 expression during differentiation. J Biol Chem. 2002 Jul 19;277(29):26310–20. doi: 10.1074/jbc.M201747200. [DOI] [PubMed] [Google Scholar]
- 21.Launay S, Giannì M, Diomede L, Machesky LM, Enouf J, Papp B. Enhancement of ATRA-induced cell differentiation by inhibition of calcium accumulation into the endoplasmic reticulum: cross-talk between RAR alpha and calcium-dependent signaling. Blood. 2003 Apr 15;101(8):3220–8. doi: 10.1182/blood-2002-09-2730. [DOI] [PubMed] [Google Scholar]
- 22.Tavassoli FA, Devilee P. Pathology and genetics of tumours of the breast and female genital organs. Vol. 432. IARC Press; Lyon: 2003. International Agency for Research on Cancer, World Health Organization; pp. 9–110. [Google Scholar]
- 23.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991 Nov;19(5):403–10. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee AH, Ellis IO. The Nottingham prognostic index for invasive carcinoma of the breast. Pathol Oncol Res. 2008 Jun;14(2):113–5. doi: 10.1007/s12253-008-9067-3. [DOI] [PubMed] [Google Scholar]
- 25.Feeley L, Quinn CM. Columnar cell lesions of the breast. Histopathology. 2008 Jan;52(1):11–9. doi: 10.1111/j.1365-2559.2007.02890.x. [DOI] [PubMed] [Google Scholar]
- 26.Orlando L, Schiavone P, Fedele P, et al. Molecularly targeted endocrine therapies for breast cancer. Cancer Treat Rev. 2010;36:S67–71. doi: 10.1016/S0305-7372(10)70023-2. [DOI] [PubMed] [Google Scholar]
- 27.Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med. 2009 Feb 19;360(8):790–800. doi: 10.1056/NEJMra0801289. [DOI] [PubMed] [Google Scholar]
- 28.Bertram R, Arceo RC., 2nd A mathematical study of the differential effects of two SERCA isoforms on Ca2+ oscillations in pancreatic islets. Bull Math Biol. 2008 Jul;70(5):1251–71. doi: 10.1007/s11538-008-9298-1. [DOI] [PubMed] [Google Scholar]
- 29.Dellen BK, Barber MJ, Ristig ML, Hescheler J, Sauer H, Wartenberg M. Ca2 oscillations in a model of energy-dependent Ca2+ uptake by the endoplasmic reticulum. J Theor Biol. 2005 Dec 7;237(3):279–90. doi: 10.1016/j.jtbi.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Diederichs F. Ion homeostasis and the functional roles of SERCA reactions in stimulus-secretion coupling of the pancreatic beta-cell: a mathematical simulation. Biophys Chem. 2008 May;134(3):119–43. doi: 10.1016/j.bpc.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Higgins ER, Cannell MB, Sneyd J. A buffering SERCA pump in models of calcium dynamics. Biophys J. 2006 Jul 1;91(1):151–63. doi: 10.1529/biophysj.105.075747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Juska A. Dynamics of calcium fluxes in nonexcitable cells: mathematical modeling. J Membr Biol. 2006;211(2):89–99. doi: 10.1007/s00232-005-7019-3. [DOI] [PubMed] [Google Scholar]
- 33.Yano K, Petersen OH, Tepikin AV. Dual sensitivity of sarcoplasmic/endoplasmic Ca2+-ATPase to cytosolic and endoplasmic reticulum Ca2+ as a mechanism of modulating cytosolic Ca2+ oscillations. Biochem J. 2004 Oct 15;383(Pt 2):353–60. doi: 10.1042/BJ20040629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dellis O, Arbabian A, Brouland JP, et al. Modulation of B-cell endoplasmic reticulum calcium homeostasis by Epstein-Barr virus latent membrane protein-1. Mol Cancer. 2009;8:59. doi: 10.1186/1476-4598-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Launay S, Bobe R, Lacabaratz-Porret C, et al. Modulation of endoplasmic reticulum calcium pump expression during T lymphocyte activation. J Biol Chem. 1997 Apr 18;272(16):10746–50. doi: 10.1074/jbc.272.16.10746. [DOI] [PubMed] [Google Scholar]
- 36.Launay S, Giannì M, Kovàcs T, et al. Lineage-specific modulation of calcium pump expression during myeloid differentiation. Blood. 1999 Jun 15;93(12):4395–405. [PubMed] [Google Scholar]
- 37.Lacabaratz-Porret C, Launay S, Corvazier E, Bredoux R, Papp B, Enouf J. Biogenesis of endoplasmic reticulum proteins involved in Ca2+ signalling during megakaryocytic differentiation: an in vitro study. Biochem J. 2000 Sep 15;350(Pt 3):723–34. [PMC free article] [PubMed] [Google Scholar]
- 38.Lee WJ, Robinson JA, Holman NA, McCall MN, Roberts-Thomsosn SJ, Monteith GR. Antisense-mediated inhibition of the plasma membrane calcium-ATPase suppresses proliferation of MCF-7 cells. J Biol Chem. 2005 Jul 22;280(29):27076–84. doi: 10.1074/jbc.M414142200. [DOI] [PubMed] [Google Scholar]
- 39.Peterlik M, Grant WB, Cross HS. Calcium, vitamin D and cancer. Anticancer Res. 2009 Sep;29(9):3687–98. [PubMed] [Google Scholar]
- 40.Apàti A, Jànossy J, Bròzik A, Gàti R, Schaefer A, Magòcsi M. Calcium induces cell survival and proliferation through the activation of the MAPK pathway in a human hormone-dependent leukemia cell line, TF-1. J Biol Chem. 2003 Mar 14;278(11):9235–43. doi: 10.1074/jbc.m205528200. [DOI] [PubMed] [Google Scholar]
- 41.Papp B, Pàszty K, Kovàcs T, Sarkadi B, Gàrdos G, Enouf J, et al. Characterization of the inositol trisphosphate-sensitive and insensitive calcium stores by selective inhibition of the endoplasmic reticulum-type calcium pump isoforms is isolated platelet membrane vesicles. Cell Calcium. 1993 Jul;14(7):531–8. doi: 10.1016/0143-4160(93)90074-g. [DOI] [PubMed] [Google Scholar]
- 42.Papp B, Brouland J-P, Gélébart P, Kovàcs T, Chomienne C. Endoplasmic reticulum calcium transport ATPase expression during differentiation of colon cancer and leukaemia cells. Biochem Biophys Res Commun. 2004;322:1223–36. doi: 10.1016/j.bbrc.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 43.Gerasimenko O, Tepikin A. How to measure Ca2+ in cellular organelles? Cell Calcium. 2005;38:201–11. doi: 10.1016/j.ceca.2005.06.025. [DOI] [PubMed] [Google Scholar]