Abstract
Constipation, one of the major side effects of opiates used in palliative care, can impair patients’ quality of life to a point where it prevents sufficient pain control. Methylnaltrexone is a novel μ-receptor antagonist, which does not pass the blood brain barrier. It is licensed to treat opiate induced constipation for patients with advanced diseases. This review article presents an overview of pharmacology and safety of its application, evidence of its efficacy and economic aspects of its use in clinical practice. Available data are limited but strongly suggest that methylnaltrexone causes laxation in less than 24 hours for at least half of those patients over the first two weeks of usage without impairing pain control or causing serious adverse effects. To avoid danger of gastrointestinal perforation it is contraindicated for patients at risk for that complication. More research is needed to evaluate its long-term efficacy and economic impact.
Keywords: opioid-induced constipation, advanced illness, palliative care, methylnaltrexone, opioid-antagonist, μ-receptor antagonist
Introduction
Opiates are among the most commonly prescribed drugs for patients with advanced illness to treat pain and dyspnea. Unlike for nausea and sedation, patients develop no tolerance for opioid-induced bowel dysfunction and constipation. They are the clinical symptoms of different effects of opioids and opiates mainly on μ-receptors in the gut, occurring in up to 90% of those patients.1–3 Gastrointestinal reflux, incomplete evacuation, hard stool, abdominal distension, bloating and discomfort by accumulation of gas and secretions can lead to vomiting, nausea, anorexia and interference with the administration or absorption of medication.4 These debilitating symptoms can seriously impair patients’ quality of life comparable even to pain, up to a point where some prefer inadequate pain control to avoid these side effects.3,5
Commonly, opiate induced constipation (OIC) is treated nonspecifically with stool softeners, osmotic agents and stimulant laxatives. These don’t always succeed and they are sometimes not very comfortable to take, especially for very sick patients.2 The evidence of their efficacy is sparse, mostly because of the lack of adequate clinical trials in that field.6 Recommendations rely on expert opinions and common experience.6,7 There is an urgent need for more research and development of additional, ideally more specific and effective treatments for this burdensome problem.
The neuronal net of the gut comprises the largest and most complicated conglomeration of nerves in the body after the brain and almost every cerebral transmitter or neurohormone with corresponding receptors can be detected there. According to neuroanatomical and neurophysiological studies in animals, OIC is largely mediated by μ-receptors located in the myenteric plexus of the gastrointestinal tract.8,9 Enkephalins, endorphins and dynorphins are their physiological agonists and their activation by exogenous opiates causes a decrease in propulsive motor activity and an increase of netto intestinal fluid absorption.9–11 Blocking μ-receptors by antagonists like naloxone and naltrexone reverses OIC but also antagonizes the opioid’s analgetic effect, which is largely caused by centrally located opioid receptors.
Lately, two opiate receptor antagonists have been developed with restriction of effect to the periphery by their pharmacokinetic behavior.12,13 Alvimopan has been approved in the US by the FDA for the inpatient treatment of postoperative ileus in 2008 but the approval is restricted to eight days as it has been associated with myocardial events in studies for long-term use for opiate-induced constipation.
Methylnaltrexone is a quaternary ammonium derivate of naltrexone with higher polarity, lower lipid solubility and therefore less ability to pass the blood brain barrier.14 This restriction of its clinical effect to the periphery renders methylnaltrexone the perfect drug against mainly peripherally mediated OIC.10,15 For twenty years its efficacy in humans has been researched and lately several phase III studies on patients with opiate induced constipation have been performed with promising results.
Progenics owns the exclusive worldwide rights for methylnaltrexone. In collaboration with Wyeth since 2005, the drug has been licensed for OIC and approved by the FDA (April 2008) and the EMEA (July 2008) for patients with advanced diseases whose treatment of OIC with conventional laxatives has failed.
This article aims to give a short overview of the pharmacological characteristics of methylnaltrexone. It reviews and critically appraises the evidence of its use concerning clinical efficacy, safety and economic value for the treatment of patients with advanced illness. The literature search strategy is described in Table 1.
Table 1.
Search strategy for literature retrieval.
| We performed a literature search using OVID’s interface of the following databases: OVID MEDLINE, including Medline in Process and other non indexed citations (1950–2011), Cochrane Database of Systematic Reviews (4. Quarter 2010), Cochrane Central Register of Controlled Trials (4. Quarter 2010), BIOSIS Previews (1969–2011). The databases were searched for articles identified by the terms ‘methylnaltrexone’, for articles with the terms ‘constipation’, ‘intestinal obstruction’ and for articles retrieved by the terms ‘opiate’, ‘side effect’ and by ‘palliative care’. The results were combined and limited to clinical trials, humans only. In addition, searches were performed in NLM’s PubMed (1966–2011) and on the internet using science-specific search engines Scirus and Google Scholar with the search terms ‘Methylnaltrexone’, ‘constipation’, ‘intestinal obstruction’, and ‘opiate’, ‘side effects’, ‘palliative care’ and related terms. For identifying further trials we went to several trial registers, including Current Controlled Trials Ltd., (http://www.controlled-trials.com), World Health Organisation International Clinical Trials Registry Platform ICTRP (http://www.who.int/trialsearch), National Institutes of Health Randomized Trial Records (http://clinicaltrials.gov) and Pharmaceutical Industry Clinical Trials Database, initiated by the Association of the British Pharmaceutical Industry (http://www.abpi.org.uk). Last date of search was in March 2011. In addition, reference lists of articles retrieved were screened for relevant publications. |
Modified from Deibert 2010.29
Pharmacology
Pharmacokinetics
Methylnaltrexone, a quaternary methylated ammonium derivate from naltrexone (Fig. 1), has shown effectiveness in preclinical studies on humans as oral, subcutaneous and intravenous application.16 Its polarity leads to a low intestinal absorption rate and prevents the passage of the blood brain barrier in therapeutic dosages. As subcutaneous application, the plasma concentration peaks after about half an hour. The drug has a half-life of about eight hours. About half of it is eliminated unchanged through the kidneys and another 40% fecally.17,18 High clearance and therefore low biological half-life lead to a low accumulation rate. This has been clinically confirmed in a study where intravenous methylnaltrexone has been given to healthy subjects repeatedly every six hours twelve times in a row with no significant change in area under curve between the first and last application19 (Table 2).
Figure 1.
Biochemical structure of Methylnaltrexone.
Table 2.
Pharmacology of methylnaltrexone (parameters after repeated intravenous application, healthy volunteers).
| Chemistry | Derivative of naltrexone; molecular weight 436.3 Da; positively charged |
| Receptor antagonism | Peripheral μ-opioid receptor antagonist; 8-fold lower potency at κ-opioid receptor; 120-fold lower potency at δ-opioid receptor |
| Route | Intravenous, subcutaneous, and oral (including enteric-coated) |
| Cmax (ng/mL) | 675 (SD 495–855) (0.3 mg/kg) |
| tmax (h) | 0.10 (SD 0.05–1.15) (0.3 mg/kg) |
| AUC (ng/h*mL) | 353 (SD 262–444) (0.3 mg/kg) |
| t1/2 (h) | 2.9 (SD 2.0–3.8) (0.3 mg/kg) |
| Metabolism | Small percentage undergoes hepatic metabolism (possibly glucuronidation) |
| Active metabolite | None |
| Elimination | 40%–50% renal and unchanged, another 50% fecal elimination |
Only about ten percent of the substance is metabolized by glucuronidation in the liver and no significant interference with the cytochrome system has been noticed. According to the prescribing information there is no data available on patients with severe hepatic impairment, nor on patients with end stage renal disease requiring dialysis. Below a creatinine clearance of 30 ml/min, a dose-reduction of half is recommended by the distributor.
Pharmacodynamics
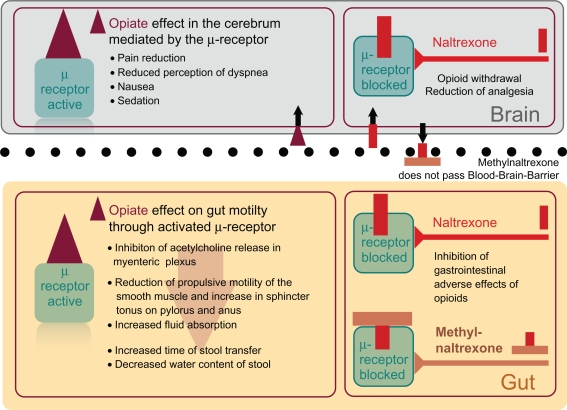
The restriction of the clinical effect to the periphery is only due to its pharmacokinetics (Fig. 2). Intracerebrally applied methylnaltrexone causes antagonism of the mainly centrally mediated analgetic effect of morphium showing its affinity to central μ-receptors as well.20 Methylnaltrexone antagonizes morphium with a medium inhibitory concentration of 75 nm (IC50 75 nm) at the μ-receptor and an exceedingly higher one at the κ-receptor (IC50 575 nm). It has negligible affinity to δ-receptors. Opiates and opioids cause a decrease in acetylcholine release in myenteric neurons leading to a reduction of propulsive motility of the smooth muscles in the gut, an increase in sphincter tonus on pylorus and anus and an increase in fluid absorption. Antagonizing their effect shortens the time of transfer and increases the water content of stool.21
Figure 2.
Opiate effects in brain and gut inhibited by Naltrexone and Methylnaltrexone which cannot pass the blood-brain-barrier because of its chemical structure.
Studies
Preclinical Trials
Phase I and II studies on a small number of healthy volunteers showed efficacy of different applications of methylnaltrexone antagonizing opiate induced effects on the gut, such as the deceleration of gastric emptying or of prolongation of oral-cecal transit time induced by intravenous morphine16,22 (Table 3). Similar results were obtained in phase II studies on chronic methadone users. All 22 patients on methadone experienced fast laxation without any signs of opioid withdrawal by the treatment.23 The effective dosages used did not evoke clinically apparent adverse effects in these homogeneous groups of subjects in controlled settings. Even if the validity of these results for the more complex group of patients in a palliative care situation is low, they opened the path for clinical trials on these patients by repeatedly showing a high rate of efficacy and a low toxicity profile in this small group of healthy humans.
Table 3.
Trials on humans.
| Author | Design | n | Intervention | Results |
|---|---|---|---|---|
| Yuan 1996 | Phase I/II, randomised, double-blind, crossover placebo-controlled | 12 healthy volunteers |
|
Morphine significantly increased oral-cecal transit time from 104.6 +/− 31.1 minutes (mean +/− SD) to 163.3 +/− 39.8 minutes (P < 0.01). Methylnaltrexone prevented 97% of morphine-induced increase in oral-cecal transit time |
| Murphy 1997 | Phase I/II, randomised, double-blind, crossover placebo-controlled | 11 healthy volunteers |
|
Methylnaltrexone given concomitantly with morphine reverses almost completely the morphine-induced delay in gastric emptying |
| Yuan 1997 | Phase II, non-randomised, single-blind dose-escalation | 14 healthy volunteers | Descending doses of oral Methylnaltrexone (from 19.2 mg/kg) with | 6.4 mg/kg oral methylnaltrexone significantly attenuated the morphine-induced delay in oral-cecal transit time dose-dependent response was obtained. |
| Yuan 1997 | Phase II, non-randomised, single-blind dose-escalation | 14 healthy Volunteers | Ascending doses of oral methylnaltrexone (0.64, 6.4, 19.2 mg/kg/KG) | Safe and well tolerated up to maximum dose |
| Yuan 1997 | Phase II, randomized, double-blind, placebo-controlled | 14 healthy volunteers |
|
Oral methylnaltrexone (19.2 mg/kg) completely prevented morphine-induced increase in oral-cecal transit time |
| Yuan 2000 | Phase II, randomised, double-blind, placebo-controlled | 22 methadone users |
|
Decrease from baseline in oral-cecal transit time (min, mean [SD]): Group 1: 1.4 (12); Group 2: 77.7 (37.2) (P < 0.01 vs. group 1); no laxation with group 1 vs. laxation for all of group 2 (P < 0.01); no opioid withdrawal symptoms |
| Yuan 2000 | Phase II, single-blind, dose ranging | 12 methadone users | Placebo followed next day with: Group 1: 0.3 mg/kg Mntx Group 2: 1.0 mg/kg Mntx Group 3: 3.0 mg/kg Mntx all orally |
Time to bowel movement (h, mean [SD]): Group 1 (results for 3 of 4 patients): 18.0 (8.7); Group 2 (4 of 4 patients): 12.3 (8.7); Group 3 (4 of 4 patients): 5.2 (4.5); Dose-response effect with drug, P = 0.04; no adverse effects; no opioid withdrawal symptoms |
| Portenoy 2008 | Phase III, double-blind, randomized, parallel-group, repeated dose, dose-ranging | 33 patients in palliative care |
|
Laxation response in first four hours:
|
| Thomas 2008 | Phase III randomized, double-blind placebo-controlled, clinical trial | 133 patients in palliative care |
|
Laxation response in first four hours after first injection:
Laxation response in first four hours for each of the following injection:
More patients in MNTX group than with placebo had: improvement of stool consistency and bowel status Reduction of difficulty of laxation and distress associated with constipation |
| Slatkin 2009 | Phase III randomized, double-blind placebo-controlled, clinical trial | 154 patients in palliative care |
|
Laxation response in first four hours:
|
Phase III, Clinical Trials
Since 2008, three randomized controlled trials (RCTs) about the use of methylnaltrexone in the target population of palliative care patients with suspected OIC in clinically realistic settings were fully published. These led to the approval of the drug in 2008 by FDA and EMEA (Table 3). Later on, a meta-analysis of data on 288 patients from those trials was performed for a Cochrane review.6
To assess the potentially most effective and safe dosage of methylnaltrexone in this patient population, Portenoy et al performed a one week long double-blinded study in which they randomly assigned 33 patients on stable opiate medication with defined criteria of constipation to receive either 1 mg, 5 mg, 12.5 mg or 20 mg of methylnaltrexone subcutaneously three times in a row every second day.24 This was followed by the option of an open label treatment with an initial dose of 5 mg subcutaneously, which could be adjusted in a range of 2.5 mg to 20 mg by the investigator based on patient response. The primary outcome had been defined as having at least one rescue free bowel movement within four hours after the initial dose. While only 10% of the patients with 1 mg reported a laxation through this period, 48% of all patients receiving any higher dosage achieved that goal without any significant difference between those three groups. This indicates, that there is no additional effect above a certain dosage of the drug.
In the following elegantly designed open label phase, where the dose could be adjusted to the patient response, 49%–63% of all patients reached the primary outcome, while most injections (95 of 100) were between 5 and 12.5 mg. Only four required 15 mg and one got decreased to 1 mg to achieve laxation during the following four hours. Concurring with this, a dosage of 5 mg of Methylnaltrexone seems to be the minimum effective dose. Further increase of dosage would not add any benefit to the effect, again suggesting a ceiling effect concerning the efficacy of the drug. Considering that the average weight of the patients under study was about 64 kg, the dose for optimal treatment would range between 0.08 and 0.20 mg/kg.
Based on these results, Thomas et al performed a multi-center trial where 133 patients with similar inclusion criteria as in the last trial were randomized to receive either placebo or 0.15 mg/kg methylnaltrexone subcutaneously every other day for two weeks. They allowed dose escalation (0.3 mg/kg or twice as much volume of placebo) on the second week according to patient response.25 Again 48% in the verum group versus 15% in the placebo group had a rescue free laxation during the first four hours after initial treatment. Significantly more patients in the verum group had a rescue-free laxation during the first four hours after two or more of the first four dosages than patients receiving placebo. The median time to the first bowel movement of the verum patients, who achieved a laxation within the first four hours, was about 30 minutes. This correlates with Tmax of subcutaneously applied methylnaltrexone in pharmacokinetic trials on humans.17 The median time to laxation after first dose for all patients was 6.3 hours in the verum and more than 48 hours in the placebo group. No difference in pain or opiate withdrawal scores at baseline and each evaluation was found between verum and placebo group. Of adverse effects occurring in more than 5% of all patients, abdominal pain, flatulence, nausea, increased body temperature and dizziness had a 3% higher incidence in the verum group. This was not significant though. Unlike in the placebo group, the majority of patients in the verum group perceived their bowel status as improved. In both groups patients who achieved laxation within the first four hours after the injection reported softer stool consistency. More patients in the verum group sensed a decrease of distress associated with constipation and difficulty of laxation.
Similar results showed the study of Slatkin et al, who randomly assigned a clinically comparable group of 154 patients to the double-blinded application of a single dose of either placebo, 0.15 mg/kg or 0.3 mg/kg methylnaltrexone.26 The percentage of patients defecating within the first four hours after injection was 13.5% (95% CI 4.2%–22.7%) for placebo, 61.7% (95% CI 47.8%–75.6%) for 0.15 mg/kg and 58.2% (95% CI 45.1%–71.2%) for 0.3 mg/kg methylnaltrexone. The median time for all patients to rescue free laxation was 1.1 h, 0.8 h and over 24 h for the 0.15 mg/kg, the 0.3 mg/kg and the placebo group respectively. 147 patients took part in a subsequent three-month open-label phase and again more than 50% of all of those patients achieved a rescue-free laxation within four hours after the first open-label dose. No difference in pain or withdrawal scores between the three groups and compared to the baseline data was reported. The incidence of adverse effects was higher in the verum than in the placebo group for mild or moderate abdominal pain, flatulence, nausea and dizziness. Severe adverse effects possibly related to methylnaltrexone were only reported in mostly one to three cases per symptom and comprised increased sweating (3) and pain (2), burning at injection site, vomiting, diarrhea, asthenia, increased blood pressure, severe dehydration, muscular cramp, loss of consciousness, tremor, delirium, hallucination, dyspnea and flushing.
In a meta-analysis of the data from patients of Thomas’ and Slatkin’s trials, the Odds Ratio concerning the outcome of rescue free laxation within four hours after injection of methylnaltrexone versus placebo was 6.95 (95% CI: 3.83 to 12.61), and within 24 hours it was 5.42 (95% CI: 3.12 to 9.42). The quality of evidence for this outcome in the attending Cochrane review was rated as moderate (three points out of four). Only two studies with positive outcome published for this intervention cannot rule out publication bias. Allocation concealment, selective reporting and other biases could also not be excluded by the presentations of all three trials. In Portenoy’s trial incomplete outcome data weren’t addressed.6
All three multi-centered studies mentioned above, recruited patients from and were conducted in several different palliative care settings (Hospices, palliative care centers and nursing homes). They all had similar and primarily well-defined inclusion and exclusion criteria considering age, type of disease (including ca. 15%–40% non cancer), life expectancy (at least one month to six months), definition of constipation and stability of treatment with opiates and laxatives during trial precedent days.
How patients had been approached has not been described in any publication, which leaves some danger of selection bias, but baseline characteristics of the studied patients were presented to allow for a judgment about the validity of the trial for a palliative care setting concerning the study population. Typical for trials in palliative care, there was a relatively high attrition rate, which was addressed by intention to treat analysis and reasons for attrition were reported. None of the studies provided full information about the reasons why some participants withdrew “by own request”.
The use of additional laxatives before and during the trials was not precisely reported, neither in quantity nor in quality, and can therefore not be rated as optimal. No study has been done yet to compare methylnaltrexone to intensifying or optimising conservative treatment.
As abstract only, and therefore only rudimentarily evaluable in a review, Karver et al reported 124 cancer patients with a life expectancy of 1–6 months receiving either placebo, methylnaltrexone 0.15 mg/kg or methylnaltrexone 0.3 mg/kg subcutaneously. Rescue-free laxation within 4 hours after a single dose of the study drug achieved 59.5% of the patients receiving methylnaltrexone 0.15 mg/kg and 55.6% of the ones on 0.3 mg/kg versus only 16.7% of the placebo group. The same abstract also reported 78 other patients receiving 0.15 mg/kg or placebo over two weeks every second day with similar results. There also was no reporting of reduction of pain control or symptoms of withdrawal.27
Safety
The main side effects of methylnaltrexone in the studies mentioned above were mostly abdominal cramps and flatulence, which appear to be linked to the intentional propulsive effect on the gut. During the first eighteen months since methylnaltrexone had been licensed, this drug has been prescribed to about 6900 patients in the USA. Seven patients have been reported to the FDA, who experienced bowel perforation during two days after the application, most of them with an underlying disease of the gut.28 Therefore extreme caution is recommended in the usage of the drug for patients at risk for gastrointestinal perforation or mechanical ileus by tumor infiltration, inflammation of the bowel wall or treatment with steroids, non-steroidal anti-inflammatory drugs or bevacizumab.
Ascending doses of methylnaltrexone were intravenously applied to healthy male volunteers. The dose-limiting adverse effect of methylnaltrexone was orthostatic hypotension at 0.64 mg/kg or 1.25 mg/kg, which was transient and self-limiting. No such effects were observed at a dosage of 0.32 mg/kg. Plasma levels of methylnaltrexone in excess of 1.400 ng/mL were associated with orthostatic hypotension, a rare side effect in clinical studies later on. No significant subjective changes, release of histamine or changes in physical examination or laboratory studies during the course of the study were observed indicating that methylnaltrexone is well tolerated at doses of 0.32 mg/kg and less in healthy humans.18
Repeated administration of intravenous methylnaltrexone every six hours twelve times in a row was well tolerated in humans with no significant adverse events or clinically noteworthy alterations in pharmacokinetics.19
Methylnaltrexone can block nicotinic ganglionic and cardiac muscarinic receptors by inhibiting the acetylcholine release which might be the mechanism of orthostatic hypotension on moderate to higher dosages and could have a potentially fatal effect.20 Acute animal toxicity studies showed a high LD50 of 100 mg/kgKG intraperitoneally in rats. In primates up to 50 mg/kgKG (iv, sc, im) have been tolerated which is far beyond the recommended dose for treatment of 0.15 mg/kgKG. Unlike rats and mice, humans and dogs cannot demethylate methylnaltrexone to naltrexone in a clinically relevant quantity and even at doses of 10–50 mg/kgKG in dogs and monkeys, methylnaltrexone did not penetrate into the brain. Central effects like the depression of the breathing reflex are therefore not to be expected when peripherally applied.14,15
Economic Aspects
In palliative care, where overall survival is not a valid outcome to judge treatment, quality of life parameters are used to evaluate interventions. This has not been done rigorously enough in the trials mentioned above. They were designed to prove the effect of methylnatrexone on laxation as the primary endpoint. Data on quality of life scores from those trials suggests that once methylnaltrexone induces laxation it decreases patients’ distress related to constipation. This data did not all reach statistical significance.25,26 Therefore future trials have to focus more on the systematical assessment of quality of life aspects for convincing results to support the introduction of this relatively expensive intervention.
Earnshaw has published a model-based cost-effectiveness analysis based on data from the randomized controlled trials mentioned above.31 Resource use, costs (adjusted for the Netherlands), utilities and mortality were obtained from published literature and supplemented with data from clinical experts. The analysis assigned an incremental cost per quality adjusted life year (QALY) of €40,865 and costs per constipation-free day of €25.92 to the additional treatment of opiate induced constipation with methylnaltrexone on top of standard care at an hourly nurse cost of about €65. Even if this kind of analysis is extremely sensitive to bias as it is based on assumptions, which can only marginally be assessed by sensitivity analysis, those numbers can give an idea about the potential economic impact of a treatment. Further research is needed to explore this subject in different health care settings to allow for satisfying judgement.
Discussion
Constipation as one of the major side effects of opiates used in palliative care can impair patients’ quality of life to a point where it prevents sufficient pain control. Conventional laxative therapies are sometimes not satisfactory and there is a lack of evidence on their efficacy.
Despite the limitation to only three fully published double-blinded randomized phase III trials, where potentially biasing flaws cannot entirely be ruled out, due to the way they are presented, the evidence through data available strongly suggests an efficacy of methylnaltrexone in causing laxation within four and up to 24 hours after its subcutaneous application for at least half of these patients compared to placebo during at least the first two weeks of use.
The reason why only about half of the patients react remains unclear and has to be subject to further research. Constipation in those patients might not primarily be caused by opiates at the gut but by other concomitant factors or by centrally mediated opiate effects.
The drug is safe to apply with only minor side effects in therapeutic dosage with a high therapeutic ratio in animal studies, no tendency to accumulate, low metabolism and therefore in theory little danger of pharmacokinetic drug interaction. This can only be concluded from small phase I- and II-studies. Only severe impairment of renal function affects its elimination. A dose reduction of half is required if the creatinine clearance is less than 30 ml/min. The drug requires a minimum dose for effect of about 0.15 mg/kg sc. and the dose-impact relation seems to have a ceiling effect with no further increase in potency after 0.3 mg/kg sc.
Patients receiving melthylnaltrexone experienced mild flatulence and dizziness insignificantly more often than patients on placebo. There were reports of gut perforation, which might have been related to this medication; because methylnaltrexone’s mechanism of action involves an increase of propulsive smooth muscle activity in the gut, patients at risk for ileus or perforation, as well as patients with signs of peritoneal carcinosis or catheters inserted into the peritoneum had been excluded of the trials. Methylnaltrexone is strictly contraindicated for patients under that risk. Few cases where methylnaltrexone was associated with gut perforation have been reported to the adverse effects registry of the FDA.
Concerning efficacy and side effects, studies have been limited to three month follow ups with statistically insufficiently evaluable data for those non-blinded phases. More research has to be done to provide data on medium and long-term effects even in this population with a relatively short life expectancy.
Quality and quantity of conventional laxatives used as baseline medication and for rescue have not been well reported in any of these studies. No study has been done so far to compare methylnaltrexone to an optimisation of conservative treatment.
Conclusion
Subcutaneously applied methylnaltrexone causes laxation in about 50% of patients with advanced illnesses and opiate-induced constipation within the first four hours after its application. The lack of efficacy on the other patients cannot be explained by data available. Considering its pharmacokinetic profile and according to the studies retrieved, it is safe to apply but strictly contraindicated for patients at risk of intestinal perforation. It has only been tested against placebo and not been compared against an optimization of other laxative treatments. Further research has to be focused on long-term usage and on patients’ preference before one can define its place and economic value for patients in a palliative care situation (Table 4).
Table 4.
Executive summary.
| Clinical problem |
|
| Mechanism of action |
|
| Pharmacokinetics |
|
| Clinical efficacy for palliative care |
|
| Safety and tolerability |
|
| Dosage and administration |
|
| License | FDA and EMEA approval since 2008 for patients with advanced diseases if OIC not successfully treated with conventional laxatives |
| Patients preference | Limited data with no statistical significance on quality of life scores suggests that more patients on methylnaltrexone experience an improvement in difficulty of laxation and bowel status and a decrease in distress associated with constipation compared to patients on placebo |
| Economic aspects | Further research needed |
Footnotes
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Droney J, Ross J, Gretton S, Welsh K, Sato H, Riley J. Constipation in cancer patients on morphine. Support Care Cancer. 2008 May;16(5):453–9. doi: 10.1007/s00520-007-0373-1. [DOI] [PubMed] [Google Scholar]
- 2.Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg. 2001 Nov;182(Suppl 5A):11S–8. doi: 10.1016/s0002-9610(01)00782-6. [DOI] [PubMed] [Google Scholar]
- 3.Kurz A, Sessler DI. Opioid-induced bowel dysfunction: pathophysiology and potential new therapies. Drugs. 2003;63(7):649–71. doi: 10.2165/00003495-200363070-00003. [DOI] [PubMed] [Google Scholar]
- 4.Holzer P. Opioid receptors in the gastrointestinal tract. Regul Pept. 2009 Jun 5;155(1–3):11–7. doi: 10.1016/j.regpep.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holmes S. Use of a modified symptom distress scale in assessment of the cancer patient. Int J Nurs Stud. 1989;26(1):69–79. doi: 10.1016/0020-7489(89)90047-3. [DOI] [PubMed] [Google Scholar]
- 6.Candy B, Jones L, Goodman ML, Drake R, Tookman A. Laxatives or methylnaltrexone for the management of constipation in palliative care patients. Cochrane Database Syst Rev. 2011:1CD003448. doi: 10.1002/14651858.CD003448.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Larkin PJ, Sykes NP, Centeno C, Ellershaw JE, Elsner F, Eugene B, et al. The management of constipation in palliative care: clinical practice recommendations. Palliat Med. 2008 Oct;22(7):796–807. doi: 10.1177/0269216308096908. [DOI] [PubMed] [Google Scholar]
- 8.Tavani A, Bianchi G, Ferretti P, Manara L. Morphine is most effective on gastrointestinal propulsion in rats by intraperitoneal route: evidence for local action. Life Sci. 1980 Dec 8;27(23):2211–7. doi: 10.1016/0024-3205(80)90386-0. [DOI] [PubMed] [Google Scholar]
- 9.Manara L, Bianchi G, Ferretti P, Tavani A. Inhibition of gastrointestinal transit by morphine in rats results primarily from direct drug action on gut opioid sites. J Pharmacol Exp Ther. 1986 Jun;237(3):945–9. [PubMed] [Google Scholar]
- 10.Manara L, Bianchi G, Fiocchi R, Notarnicola A, Peracchia F, Tavani A. Inhibition of gastrointestinal transit by morphine and FK 33–824 in the rat and comparative narcotic antagonist properties of naloxone and its N-methyl quaternary analog. Life Sci. 1982;31(12–13):1271–4. doi: 10.1016/0024-3205(82)90359-9. [DOI] [PubMed] [Google Scholar]
- 11.Manara L, Bianchetti A. The central and peripheral influences of opioids on gastrointestinal propulsion. Annu Rev Pharmacol Toxicol. 1985:25249–73. doi: 10.1146/annurev.pa.25.040185.001341. [DOI] [PubMed] [Google Scholar]
- 12.Becker G, Galandi D, Blum HE. Peripherally acting opioid antagonists in the treatment of opiate-related constipation: a systematic review. J Pain Symptom Manage. 2007 Nov;34(5):547–65. doi: 10.1016/j.jpainsymman.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Becker G, Blum HE. Novel opioid antagonists for opioid-induced bowel dysfunction and postoperative ileus. Lancet. 2009 Apr 4;373(9670):1198–206. doi: 10.1016/S0140-6736(09)60139-2. [DOI] [PubMed] [Google Scholar]
- 14.Russell J, Bass P, Goldberg LI, Schuster CR, Merz H. Antagonism of gut, but not central effects of morphine with quaternary narcotic antagonists. Eur J Pharmacol. 1982 Mar 12;78(3):255–61. doi: 10.1016/0014-2999(82)90026-7. [DOI] [PubMed] [Google Scholar]
- 15.Kotake AN, Kuwahara SK, Burton E, McCoy CE, Goldberg LI. Variations in demethylation of N-methylnaltrexone in mice, rats, dogs, and humans. Xenobiotica. 1989 Nov;19(11):1247–54. doi: 10.3109/00498258909043176. [DOI] [PubMed] [Google Scholar]
- 16.Yuan CS, Foss JF, Osinski J, Toledano A, Roizen MF, Moss J. The safety and efficacy of oral methylnaltrexone in preventing morphine-induced delay in oral-cecal transit time. Clin Pharmacol Ther. 1997 Apr;61(4):467–75. doi: 10.1016/S0009-9236(97)90197-1. [DOI] [PubMed] [Google Scholar]
- 17.Yuan CS, Foss JF, O’Connor M, Toledano A, Roizen MF, Moss J. Methylnaltrexone prevents morphine-induced delay in oral-cecal transit time without affecting analgesia: a double-blind randomized placebo-controlled trial. Clin Pharmacol Ther. 1996 Apr;59(4):469–75. doi: 10.1016/S0009-9236(96)90117-4. [DOI] [PubMed] [Google Scholar]
- 18.Foss JF, O’Connor MF, Yuan CS, Murphy M, Moss J, Roizen MF. Safety and tolerance of methylnaltrexone in healthy humans: a randomized, placebo-controlled, intravenous, ascending-dose, pharmacokinetic study. J Clin Pharmacol. 1997 Jan;37(1):25–30. doi: 10.1177/009127009703700105. [DOI] [PubMed] [Google Scholar]
- 19.Yuan CS, Doshan H, Charney MR, O’connor M, Karrison T, Maleckar SA, et al. Tolerability, gut effects, and pharmacokinetics of methylnaltrexone following repeated intravenous administration in humans. J Clin Pharmacol. 2005 May;45(5):538–46. doi: 10.1177/0091270004273491. [DOI] [PubMed] [Google Scholar]
- 20.Brown DR, Goldberg LI. The use of quaternary narcotic antagonists in opiate research. Neuropharmacology. 1985 Mar;24(3):181–91. doi: 10.1016/0028-3908(85)90072-3. [DOI] [PubMed] [Google Scholar]
- 21.Gonenne J, Camilleri M, Ferber I, Burton D, Baxter K, Keyashian K, et al. Effect of alvimopan and codeine on gastrointestinal transit: a randomized controlled study. Clin Gastroenterol Hepatol. 2005 Aug;3(8):784–91. doi: 10.1016/s1542-3565(05)00434-9. [DOI] [PubMed] [Google Scholar]
- 22.Murphy DB, Sutton JA, Prescott LF, Murphy MB. Opioid-induced delay in gastric emptying: a peripheral mechanism in humans. Anesthesiology. 1997 Oct;87(4):765–70. doi: 10.1097/00000542-199710000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Yuan CS, Foss JF, O’Connor M, et al. Methylnaltrexone for reversal of constipation due to chronic methadone use: a randomized controlled trial. JAMA. 2000 Jan 19;283(3):367–72. doi: 10.1001/jama.283.3.367. [DOI] [PubMed] [Google Scholar]
- 24.Portenoy RK, Thomas J, Moehl Boatwright ML, Tran D, Galasso FL, Stambler N, et al. Subcutaneous methylnaltrexone for the treatment of opioid-induced constipation in patients with advanced illness: a double-blind, randomized, parallel group, dose-ranging study. J Pain Symptom Manage. 2008 May;35(5):458–68. doi: 10.1016/j.jpainsymman.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Thomas J, Karver S, Cooney GA, Chamberlain BH, Watt CK, Slatkin NE, et al. Methylnaltrexone for opioid-induced constipation in advanced illness. N Engl J Med. 2008 May 29;358(22):2332–43. doi: 10.1056/NEJMoa0707377. [DOI] [PubMed] [Google Scholar]
- 26.Slatkin N, Thomas J, Lipman AG, Wilson G, Boatwright ML, Wellman C, et al. Methylnaltrexone for treatment of opioid-induced constipation in advanced illness patients. J Support Oncol. 2009;7(1):39–46. [PubMed] [Google Scholar]
- 27.Karver Methylnaltrexone treatment of opioid-induced constipation in cancer patients. ASCO meeting; 2001. Abstracts. [Google Scholar]
- 28.Mackey AC, Green L, Greene P, Avigan M. Methylnaltrexone and gastrointestinal perforation. J Pain Symptom Manage. 2010 Jul;40(1):e1–3. doi: 10.1016/j.jpainsymman.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Deibert P, Xander C, Blum HE, Becker G. Methylnaltrexone: the evidence for its use in the management of opioid-induced constipation. Core Evid. 2010:4247–58. doi: 10.2147/ce.s8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeHaven-Hudkins DL, DeHaven RN, Little PJ, Techner LM. The involvement of the mu-opioid receptor in gastrointestinal pathophysiology: therapeutic opportunities for antagonism at this receptor. Pharmacol Ther. 2008 Jan;117(1):162–87. doi: 10.1016/j.pharmthera.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Earnshaw SR, Klok RM, Iyer S, McDade C. Methylnaltrexone bromide for the treatment of opioid-induced constipation in patients with advanced illness—a cost-effectiveness analysis. Aliment Pharmacol Ther. 2010 Apr;31(8):911–21. doi: 10.1111/j.1365-2036.2010.04244.x. [DOI] [PubMed] [Google Scholar]