Abstract
Salmonellosis outbreaks involving typhoid fever and human gastroenteritis are important diseases in tropical countries where hygienic conditions are often not maintained. A rapid and sensitive method to detect Salmonella spp., Salmonella Typhi and Salmonella Typhimurium is needed to improve control and surveillance of typhoid fever and Salmonella gastroenteritis. Our objective was the concurrent detection and differentiation of these food-borne pathogens using a multiplex PCR. We therefore designed and optimized a multiplex PCR using three specific PCR primer pairs for the simultaneous detection of these pathogens. The concentration of each of the primer pairs, magnesium chloride concentration, and primer annealing temperature were optimized before verification of the specificity of the primer pairs. The target genes produced amplicons at 429 bp, 300 bp and 620 bp which were shown to be 100% specific to each target bacterium, Salmonella spp., Salmonella Typhi and Salmonella Typhimurium, respectively.
Keywords: optimization, multiplex PCR, Salmonella spp., Salmonella Typhi, Salmonella Typhimurium
Introduction
Salmonellosis is recognized as a global zoonosis and food-borne disease posing a public health risk. It is the most widespread disease in both industrialized and developing countries, although the incidence varies [1]. This group of microorganisms adapts to a wide range of foods, becomes endemic and causes high morbidity with a range of clinical manifestations such as diarrhea, nausea, abdominal cramp, vomiting and fever.
Salmonella Typhi is responsible for enteric fever in humans that remains endemic in locations with untreated water supplies and poor sanitary conditions. Salmonella Typhimurium is a major cause of gastroenteritis and is found in both animals and humans. It causes systemic disease in mice closely resembling the enteric fever in humans [2, 3, 4]. Salmonella Typhi and Salmonella Typhimurium are serovars of Salmonella enterica which cause most of the infections in warm-blooded animals [5]. According to Ngan et al. [6], Salmonella Typhi accounts for more than 25 million infections worldwide, resulting in approximately 200,000 deaths annually. Cardona-Castro and co-workers [2] reported that Salmonella Typhimurium is one of the most prevalent serovars among Salmonella spp. causing gastroenteritis in 49 countries and accounting for an estimated 15% of all food-borne infections in the US [7].
The conventional methods to detect Salmonella spp., Salmonella Typhi and Salmonella Typhimurium are time-consuming, expensive and poor in specificity and sensitivity, resulting in poor identification results [8].
Therefore, the development of a quick and sensitive method to detect these food-borne pathogens is a subject of considerable attention [5]. The polymerase chain reaction (PCR)-based detection method for Salmonella in food samples has advantages over conventional methods such as high sensitivity and specificity as well as shorter time for analysis [9, 10]. PCR has been successfully used to detect bacterial pathogens in clinical samples, aquatic environments and food products [11]. However, PCR cannot distinguish viable cells from non-viable cells. Therefore, standard microbiology tests need to be conducted in parallel with PCR [3]. A multiplex PCR would be practically useful to distinguish between the pathogens, allowing the concurrent detection of two or more pathogens performed in a single tube. Labour and time saving, this would reduce the laboratory test costs for the food industry [8].
Therefore, our objective was to develop a reliable and rapid multiplex PCR for the detection of Salmonella spp., Salmonella Typhi and Salmonella Typhimurium. We optimized the concentration of primer pairs, magnesium ion and the annealing temperature to improve the efficiency of the multiplex PCR. The specificity and reproducibility of the optimized multiplex PCR was determined.
Materials and Methods
Bacterial strains and culture conditions
The Salmonella Typhi, Salmonella Typhimurium, Salmonella Enteritidis and Salmonella Paratyphi strains used in this study were obtained from the Institute for Medical Research, Malaysia. Vibrio parahaemolyticus, V. cholerae, Listeria monocytogenes and Escherichia coli strains were purchased from the American Type Culture Collection (ATCC; Rockville, MD). The strains were grown in Tryptic Soy Broth (TSB; Merck, Darmstadt, Germany) with shaking at 200 rpm at 37°C for 24 h. The NaCl concentration in the TSB was adjusted to 1% and 3% for the optimum growth of V. cholerae and V. parahaemolyticus, respectively.
DNA template preparation
DNA was extracted from the test strains grown as described above using a modified boiled cell method [4, 12]. One millilitre of a broth culture was centrifuged at 15,000 x g for 3 min. The cell pellet was suspended in 500-µL sterile distilled water and vortexed vigorously. The cell suspension was boiled for 10 min; cooled at −20°C for 10 min; and then centrifuged at 15,000 x g for 3 min. The supernatant was collected and used as the DNA template solution for the optimization of the multiplex PCR.
Oligonucleotide primers
Table 1 summarizes the primer pairs used for the optimization of the multiplex PCR. The primer pair, ST11 and ST15, was specific to Salmonella spp. and targeted a randomly selected sequence of unknown function of 429 bp [13]. The primer pair, sty-1 and sty-2, specific to Salmonella Typhi targeted the 23S rRNA gene. [14] The primer pair, Fli15 and Typ04, are specific to the C gene of Salmonella Typhimurium [13]. The primer pairs were first optimized for the Salmonella spp. and Salmonella Typhi. After that, the optimization of primer pairs was performed for Salmonella spp. and Salmonella Typhimurium. Finally, the three primer pairs were assessed in the final multiplex PCR using three repetitions to ensure reproducibility of the assay.
PCR amplification
The multiplex PCR amplification was performed using a VeritiTM 96-Well Thermal Cycler (Applied Biosystems, Foster City, CA). The multiplex PCR reaction mixture for the reference multiplex PCR protocol included: 5 x PCR buffer, 0.2 mM each deoxynucleoside triphosphate mix, 1.5 U Taq DNA polymerase and 4 µL DNA template solution, and the three sets of primer pairs, and magnesium chloride (MgCl2) (respective concentrations were optimized later). The final volume of the reaction mixture was adjusted to 50 µL using sterile distilled water. All the materials used in the PCR except for the DNA templates were purchased from Vivantis Technologies (Selangor, Malaysia). A negative control containing sterile distilled water instead of the template DNA solution was included in each PCR assay. The thermocycling conditions were as follows: initial denaturation at 94°C for 2 min; followed by 35 cycles: denaturation at 94°C for 1 min, primer annealing (temperature was optimized later) for 1 min, and extension at 72°C for 1 min; a final extension at 72°C for 7 min; and maintenance at 4°C before electrophoresis.
Agarose gel electrophoresis
A 5 µL portion of the PCR product was applied to a 1.2% agarose gel in 0.5 x TBE buffer (pH 8.0) and electrophoresed at 90 V for 40 min. The gel was stained with ethidium bromide, and PCR products were visualized as DNA bands under ultraviolet (UV) light. DNA-fragments in a 100-bp ladder (Vivantis Technologies, Selangor, Malaysia) were included as molecular weight markers.
Optimization of the multiplex PCR
Parameters to be optimized included MgCl2 concentration (1.5 to 3.5 mM), primer concentration (0.2 µM to 1.2 µM) and annealing temperature (50 to 60°C). Each component was optimized while others were kept constant. Then concurrent optimized parameters were used in subsequent experiments.
Specificity of the multiplex PCR
A panel of Salmonella and non-Salmonella strains was tested for specificity using the optimized multiplex PCR conditions. The procedure was repeated twice to ensure reproducibility. The Salmonella strains tested in this study were Salmonella Typhi, Salmonella Typhimurium, Salmonella Enteritidis and Salmonella Paratyphi; while non-Salmonella strains included V. parahaemolyticus, V. cholerae, Listeria monocytogenes and Escherichia coli.
Results
The MgCl2 concentration and the annealing temperature for the detection of Salmonella spp. and Salmonella Typhi as well as Salmonella spp. and Salmonella Typhimurium were optimized. Examples of the amplicon analysis using gel electrophoresis are shown in Fig. 1 and 2. Figure 3 shows the optimization of the MgCl2 concentration and the annealing temperature for the detection of Salmonella spp., Salmonella Typhi and Salmonella Typhimurium.
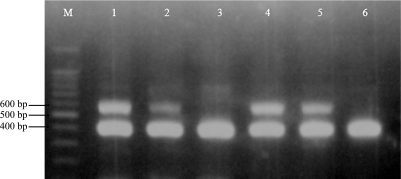
Figure 1.
Optimization of the MgCl2 concentration and the annealing temperature for the detection of Salmonella spp. and Salmonella Typhi. Lane M shows the 100-bp DNA ladder while lane B has no primers. Lanes 1 to 6 show the PCR products obtained with 2.0 mM MgCl2, lanes 7 to 12 with 2.5 mM MgCl2, and lanes 13 to 18 with 3.0 mM MgCl2. The annealing temperature was 50°C for lanes 1, 7 and 13; 53°C for lanes 2, 8 and 14; 55°C for lanes 3, 9 and 15; 57°C for lanes 4, 10 and 16; 59°C for lanes 5, 11 and 17; and 60°C for lanes 6, 12 and 18.
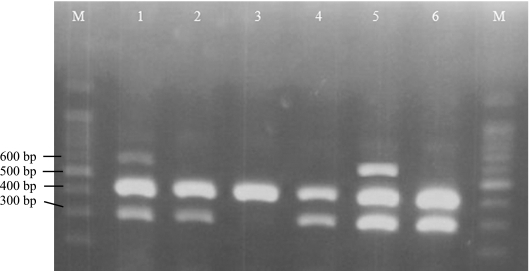
Figure 2.
Optimization of MgCl2 concentration and annealing temperature for the detection of Salmonella spp. and Salmonella Typhimurium. Lane M shows a 100-bp DNA ladder. Lanes 1 to 3 show the PCR products obtained using 2.0 mM MgCl2 and lanes 4 to 6 with 2.5 mM MgCl2. The annealing temperature was 50°C for lanes 1 and 4; 53°C for lanes 2 and 5; and 55°C for lane 3 and 6.
Figure 3.
Optimization of MgCl2 concentration and the annealing temperature to detect Salmonella spp., Salmonella Typhi and Salmonella Typhimurium. Lane M shows a 100-bp DNA ladder. Lanes 1 to 3 show the PCR products with 2.0 mM MgCl2 and lanes 4 to 6 with 2.5 mM MgCl2. The annealing temperature was 50°C for lanes 1 and 4; 53°C for lanes 2 and 5; and 56°C for lanes 3 and 6.
We therefore concluded that the optimum MgCl2 was from 2.0 to 3.0 mM for Salmonella Typhi (Fig. 1) and 2.0 to 2.5 mM for Salmonella Typhimurium (Fig. 2). When Salmonella spp., Salmonella Typhi and Salmonella Typhimurium were examined in a single tube, a 2.5 mM MgCl2 concentration was shown to be optimum (Fig. 3). No amplification was obtained at 1.5 mM MgCl2 while smears were produced at 3.0 mM MgCl2 (data not shown).
The primer concentration was adjusted by increasing the concentration to a point where a faint amplicon band was obtained or by decreasing the concentration a point where a strong band was obtained (data not shown). The adjustments were made to obtain the best combination for optimum primer concentrations of all target DNAs in the multiplex PCR. We found the optimum primer pair concentrations to be: 0.2 µM for ST11/ST15, 1.2 µM for Fli15/Typ 04 and 1.2 µM for sty-1/sty-2.
The thermal cycling conditions were tested at different concentrations of MgCl2. The annealing temperature that yielded the greatest amount of amplicons was 50°C or 53°C for both Salmonella Typhi and Salmonella Typhimurium (Fig. 1 and 2). However, the annealing temperature best for all three Salmonella groups (Salmonella for Salmonella spp., Salmonella Typhi and Salmonella Typhimurium) was 53°C (Fig. 3). Therefore a 53°C annealing temperature was judged to be suitable for the multiplex PCR for these species.
Overall, the optimized multiplex PCR reaction contained the following: 10 µL 5 x PCR buffer, 0.2 mM deoxynucleoside triphosphate mix, 0.2 µM ST11/ST15 and 1.2 µM Fli15/Typ04 and sty-1/sty-2 primers, 2.5 mM MgCl2, 1.5 U Taq DNA polymerase and 4 µL DNA template. The optimized thermocycling conditions were: initial denaturation at 94°C for 2 min; followed by 35 cycles: denaturation at 94°C for 1 min, primer annealing at 53°C for 1 min and extension at 72°C for 1 min; an additional cycle at 72°C for 7 min; and maintenance at 4°C until analysis.
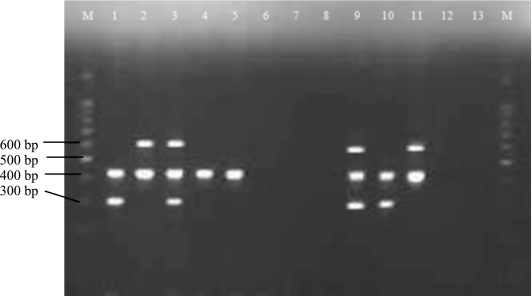
The ST11/ST15 primer pair amplified 429-bp DNA fragments when the DNA templates from Salmonella Typhi, Salmonella Typhimurium, Salmonella Enteritidis and Salmonella Paratyphi were tested. The DNA template of Salmonella Typhimurium specifically amplified 620-bp fragments. Likewise, the DNA template for Salmonella Typhi amplified 300-bp fragments. No amplicons were produced from non-Salmonella strains. Figure 4 shows that the three primer pairs are very specific to the target microorganisms and their respective serotypes.
Figure 4.
Specificity of the primer sets for the optimized multiplex PCR. Lane M shows a 100-bp DNA ladder and lane 13 is the negative control. Lanes 1 to 12 show the PCR products of different microorganisms. Lanes 1 and 10, Salmonella Typhi; Lanes 2 and 11, Salmonella Typhimurium; Lanes 3 and 9, Salmonella Typhi and Salmonella Typhimurium; Lane 4, Salmonella Enteritidis; Lane 5, Salmonella Paratyphi; Lane 6, Vibrio parahaemolyticus; Lane 7, Vibrio cholerae; Lane 8, Listeria monocytogenes; and Lane 12, Escherichia coli.
Discussion
The DNA extraction method can influence the outcome of a multiplex PCR detection of food-borne pathogens. DNA extraction should be fast and simple in order to reduce the possibility of contamination [15] when many different DNA extraction methods are available [9]. The boiling method is an effective way to obtain the genomic DNA of pathogenic bacteria [5]; and DNA can easily be extracted from Gram-negative bacteria such as Salmonella spp. [16]. DNA extraction from Salmonella enterica serovar Brandenburg [17] and Salmonella Enteritidis [11] by the boiling method has been used. We employed the boiling cell DNA extraction method because of its simplicity and speed. No problem was encountered with this method because the expected amplicons were observed using the DNA templates (Figs. 1, 2, 3, 4).
The PCR components in the multiplex PCR are important and include the cycling parameters and the relative concentration of primer pairs, the concentration balance of magnesium chloride and deoxynucleosides, the concentration of the PCR buffer, cycling temperatures, amount of template DNA and Taq DNA polymerase that affect the fidelity of the Taq DNA polymerase and PCR yield [8].
Taq DNA polymerase requires divalent ions such as Mg2+ for activity. Too much Mg2+ will give a false negative reaction because enzyme fidelity will be decreased and non-specific amplification will be increased (smear on gel or a distinct band of an inappropriate size). In general, the concentration of MgCl2 is higher for multiplex PCR than used in monoplex PCR [16].
The nucleoside concentration is also important because Mg2+ will be chelated if too much dNTP mix is used. This is the reason that an increase in Mg2+ concentration often produce positive effects while increases in the dNTP concentration can rapidly inhibit the PCR [18].
Kapley et al. [19] stated that, as the number of target templates increases, non-specific annealing will reduce the effective primer concentration for specific amplicon extension. Hence, the optimization of the multiplex PCR was first performed for Salmonella spp. and Salmonella Typhi and followed by Salmonella spp. and Salmonella Typhimurium before combining all of them in a single tube, because the optimization relies on the sequential investigation of each reaction variable. In the multiplex PCR, the preferential amplification of one target sequence over another can be overcome by decreasing the primer concentration for the stronger amplification while increasing the amount of primers for the weaker amplification [20]. High primer concentration and low annealing temperature can cause mis-priming because the amplicons will compete with the target sequence for primers [11]. The optimum annealing temperature can prevent nonspecific reactions, as shown by the fact that we saw none after optimization [3].
Wang et al. [10] reported that some PCR primers have a very high free energy for overlapping pentamers at the 3’ end that cause non-specific reactions, while others have stable 3’ terminal dimers or a stable hairpin loop. Therefore, careful choice of the PCR primers is one of the important prerequisites. The ST11/ST15 primer pair was found to be specific for Salmonella spp. and to amplify a 429 bp fragment for all strains of the 13 serotypes of Salmonella most frequently found from the analysis of environmental swabs [13]. Zhu et al. [14] found that a 23S rRNA gene cloned sequence was very efficient in the detection of Salmonella Typhi because the variable portions of this rRNA gene provide unique signatures for Salmonella Typhi. Historically, the variation in the flagellar (H), polysaccharide (O) and capsular (Vi) antigens form the basis for serotyping of Salmonella. Therefore, detection of the flagellin gene, fliC, that encodes the phage 1 flagellin of flagellar antigens is recognized as a major component of the flagellum in Salmonella enterica serovar Typhimurium [21].
Specificity is determined if the primer sequences are unique for the target microorganisms where the annealing temperature has to be optimized to avoid non-specific priming [22]. Primer-dimer formation through cross-hybridization was prevented because self-primer annealing reduces the availability of primers for the correct amplification reaction. Consequently, there is a need to design primers longer than those used in a monoplex PCR and characterized by a higher melting temperature as shown here (Table 1). Some primers are species specific, such as Fli05/Typ04 for Salmonella Typhimurium and sty-1/sty-2 for Salmonella Typhi, while others are only genus specific, like ST11/ST15 for Salmonella spp. Our data demonstrated the fact that the primer sets we used are specifically designed to target DNA from Salmonella spp., Salmonella Typhi and Salmonella Typhimurium because no cross-reaction with other non-Salmonella strains occurred. This assay demonstrated specificity fidelity of 100% since all the targeted amplicons were produced while no amplicon was produced for any of the non-Salmonella strains tested.
Table 1.
Primer pairs used for the optimization of the multiplex PCR.
| Primer | Primer sequence 5’ to 3’ | Tm (°C) | Target | Amplicon size (bp) | Reference |
|---|---|---|---|---|---|
| ST11 | GCC AAC CAT TGC TAA ATT GGC GCA | 64.6 | Salmonella spp. | 429 | [13] |
| ST15 | GGT AGA AAT TCC CAG CGG GTA CTG G | 67.9 | 341 | ||
| sty-1 | TGC CGG AAA CGA ATC T | 54.2 | Salmonella Typhi | 300 | [14] |
| sty-2 | GGT TGT CAT GCC AAT GCA CT | 60.4 | 342 | ||
| Fli15 | CGG TGT TGC CCA GGT TGG TAA T | 64.5 | Salmonella Typhimurium | 620 | [13] |
| Typ04 | ACT GGT AAA GAT GGC T | 51.6 | 343 |
During the course of our study, Kumar et al. [23] reported a multiplex PCR system for detection and differentiation of S. tyhi and S. typhimurium. Their multiplex PCR was designed to examine the serum samples of the suspected typhoid patients. Our multiplex PCR system can probably be used for the same purpose although the PCRs of our multiplex system target the nucleotide sequences different from those of Kumar et al. [23]. In addition, our multiplex PCR can also detect Salmonella spp. and differentiate it from S. tyhi and S. typhimurium. It is thus useful for examination of food and other environmental samples for potential pathogens responsible for diarrheal disease.
In conclusion, Salmonella spp. is pathogenic when present in humans and domestic animals and is important for public health and disease prevention in the food industry. Hence, we developed a multiplex PCR to concurrently amplify more than one locus in the same reaction. The application to detect Salmonella spp., Salmonella Typhi and Salmonella Typhimurium using the multiplex PCR system we developed provides a rapid and reliable typing approach that enables effective monitoring of emerging pathogenic Salmonella strains for Salmonella surveillance studies.
Acknowledgements
This study was supported by the Science Fund (project no. 545260) from the Ministry of Science, Technology and Innovation, Malaysia; and in-part by grant-in-aid of Ministry of Health, Labor and Welfare, Japan; by a Grant-in-Aid for Science Research (KAKENHI 19101010) from the Japan Society for the Promotion of the Sciences.
References
- 1.Pieskus J, Kazeniauskas E, Butrimaite-Ambrozeviciene C, et al. Salmonella incidence in broiler and laying hens with the different housing systems. The Journal of Poultry Science 2008; 45: 227-231 [Google Scholar]
- 2.Cardona-Castro N, Sanchez-Jimenez M, Lavalett L, et al. Development and evaluation of a multiplex polymerase chain reaction assay to identify Salmonella serogroups and serotypes. Diagn Microbiol Infect Dis 2009; 65: 327-330 [DOI] [PubMed] [Google Scholar]
- 3.Freitas CGd., Santana AP, Silva PHCd, et al. PCR multiplex for detection of Salmonella Enteritidis, Typhi and Typhimurium and occurrence in poultry meat. Int J Food Microbiol 2010; 139: 15-22 [DOI] [PubMed] [Google Scholar]
- 4.Pui CF, Wong WC, Chai LC, et al. Simultaneous detection of Salmonella spp., Salmonella Typhi and Salmonella Typhimurium in sliced fruits using multiplex PCR. Food Control 2010; 22: 337-342 [Google Scholar]
- 5.Park S-H, Kim H-J, Cho WH, Kim JH, Oh MH, Kim SH, Lee BK, Ricke SC, Kim HY. Identification of Salmonella enterica subspecies I, Salmonella enterica serovars Typhimurium, Enteritidis and Typhi using multiplex PCR. FEMS Microbiol Lett 2009; 301: 137-146 [DOI] [PubMed] [Google Scholar]
- 6.Ngan GJY, Ng LM, Lin RTP, et al. Development of a novel multiplex PCR for the detection and differentiation of Salmonella enterica serovars Typhi and Paratyphi A. Res Microbiol 2010; 161: 243-248 [DOI] [PubMed] [Google Scholar]
- 7.Gray JT, Fedorka-Cray PJ. Salmonella, pp. 55-68 In Cliver DO and Riemann HP (Eds.). Foodborne diseases. Academic Press, San Diego. 2002
- 8.Elizaquivel P, Aznar R. A multiplex RTi-PCR reaction for simultaneous detection of Escherichia coli O157: H7, Salmonella spp. and Staphylococcus aureus on fresh, minimally processed vegetables. Food Microbiol 2008; 25: 705-713 [DOI] [PubMed] [Google Scholar]
- 9.Germini A, Masola A, Carnevali P, et al. Simultaneous detection of Escherichia coli O157: H7, Salmonella spp., and Listeria monocytogenes by multiplex PCR. Food Control 2009; 20: 733-738 [Google Scholar]
- 10.Wang R-F, Cao WW, Cerniglia CE. A universal protocol for PCR detection of 13 species of foodborne pathogens in foods. J Appl Microbiol 1997; 83: 727-736 [DOI] [PubMed] [Google Scholar]
- 11.Moganedi K, Goyvaerts E, Venter S, et al. Optimization of the PCR-invA primers for the detection of Salmonella in drinking and surface waters following a precultivation step.Water SA 2007; 33 (Suppl 2): 195-202 [Google Scholar]
- 12.Tunung R, Margaret SP, Jeyaletchumi P, et al. Prevalence and quantification of Vibrio parahaemolyticus in raw salad vegetables at retail level. J Microbiol Biotechnol 2010; doi: 10.4014/jmb.0908.08009. [PubMed]
- 13.Soumet C, Ermel G, Rose N, et al. Evaluation of a multiplex PCR assay for simultaneous identification of Salmonella sp., Salmonella Enteritidis and Salmonella Typhimurium from environmental swabs of poultry houses. Lett Appl Microbiol 1999; 28: 113-117 [DOI] [PubMed] [Google Scholar]
- 14.Zhu Q, Lim CK, Chan YN. Detection of Salmonella Typhi by polymerase chain reaction. J Appl Bacteriol 1996; 80: 244-251 [DOI] [PubMed] [Google Scholar]
- 15.Sepp R, Uda ISH, Sakamoto H. Rapid techniques for DNA extraction from routinely processed archival tissue for use in PCR. J Clin Pathol 1994; 47: 318-323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mauer J. PCR methods in foods. Springer Science + Business Media, Inc, United States of America. 2006.
- 17.Perera K, Murray A. Development of a PCR assay for the identification of Salmonella enterica serovar Brandenburg. J Med Microbiol 2008; 57: doi: 10.1099/jmm.0.2008/002337-0. [DOI] [PubMed] [Google Scholar]
- 18.Henegariu O, Heerema NA, Dlouhy SA, et al. Multiplex PCR: Critical parameters and step-by-step protocol. Biotechniques 1997; 23: 504-511 [DOI] [PubMed] [Google Scholar]
- 19.Kapley A, Lampel K, Purohit HJ. Thermocycling steps and optimization of multiplex PCR. Biotechnol Lett 2000; 22: 1913-1918 [Google Scholar]
- 20.Khoo C-H, Cheah Y-K, Lee L-H, et al. Virulotyping of Salmonella enterica subsp. enterica isolated from indigenous vegetables and poultry meat in Malaysia using multiplex-PCR. Antonie van Leeuwenhoek 2009; 96: 441-457 doi:10.1007/s10482-009-9358-z. [DOI] [PubMed] [Google Scholar]
- 21.Aldridge P, Gnerer J, Karlinsey JE, et al. Transcriptional and translational control of the Salmonella fliC gene. J Bacteriol 2006; 188 (Suppl 12): 4487-4496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheu PM, Berghof K, Stahl U. Detection of pathogenic and spoilage micro-organisms in food with the polymerase chain reaction. Food Microbiol 1998; 15: 13-31 [Google Scholar]
- 23.Kumar A, Balachandran Y, Gupta S, Khare S, Suman Quick PCR based diagnosis of typhoid using specific genetic markers. Biotechnol Lett 2010; 32: 707–712 [DOI] [PubMed] [Google Scholar]