Abstract
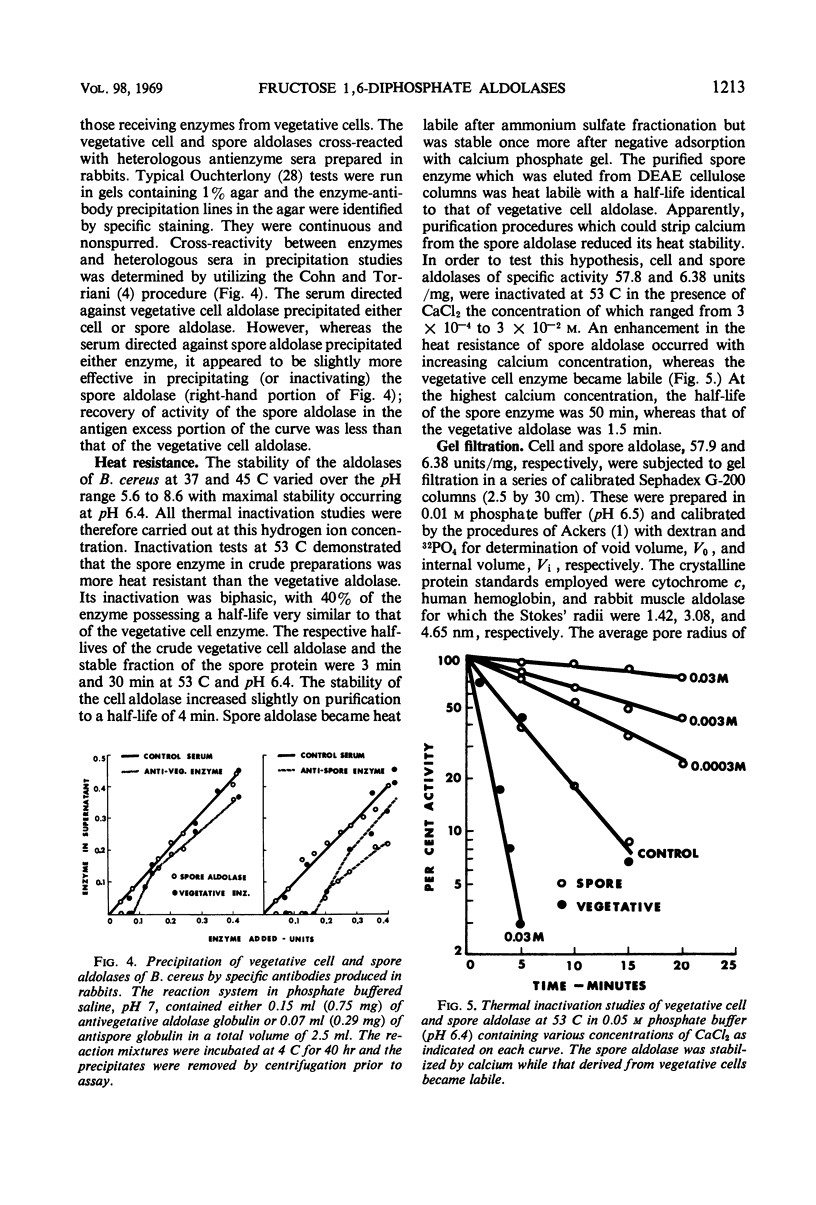
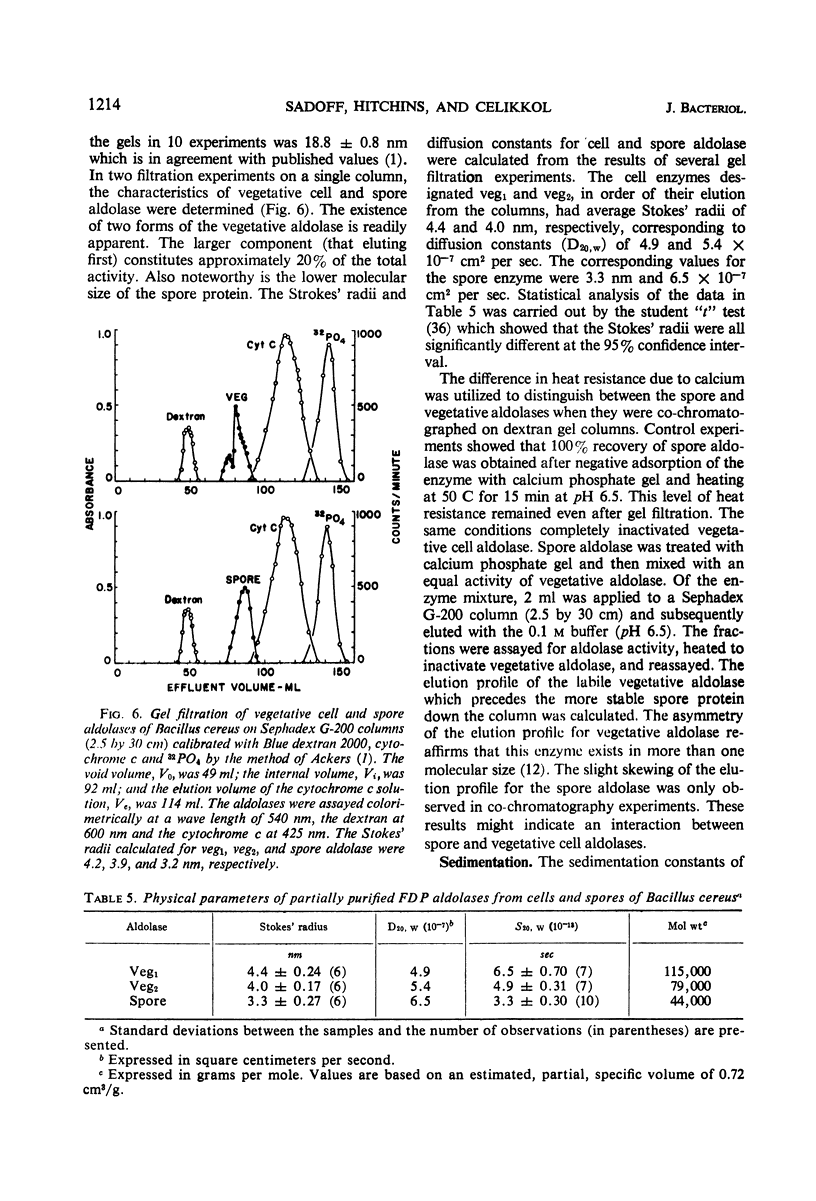
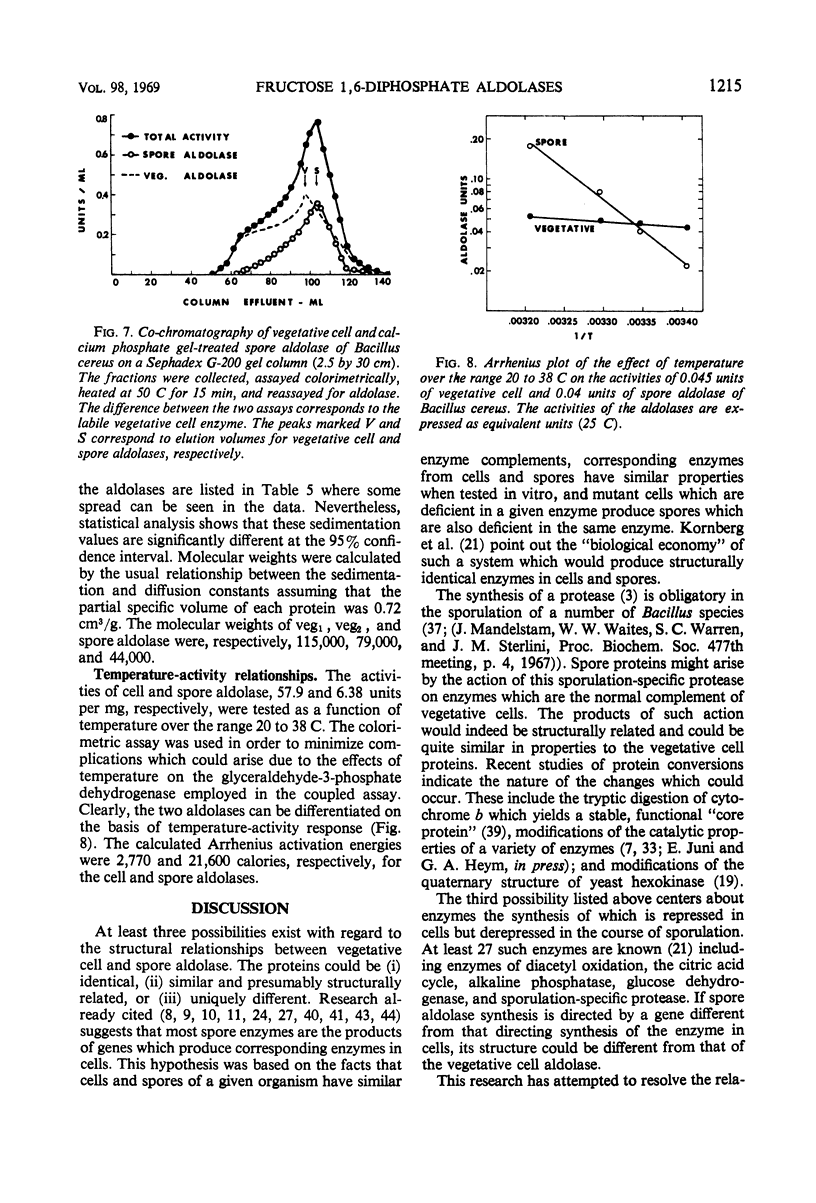
Fructose 1,6-diphosphate aldolase from cells of Bacillus cereus appears to be typical Class II aldolase as judged by its functional and physical properties. Spore and vegetative cell aldolase had similar enzymatic, immunochemical, and heat resistance properties in the absence of calcium, but they differed in their thermal stabilities in the presence of calcium, their Stokes' radii, their mobility in acrylamide gel electrophoresis, and their molecular weights. The pH optimum for both enzymes was 8.5, and their Km with respect to substrate was 2 × 10−3m. Highly purified spore and vegetative cell aldolases were both heat labile with half-lives of 4 min at 53 C and pH 6.4. In the presence of 3 × 10−2m solution of calcium ions, the stability of the spore protein increased 12-fold but the vegetative form became more heat labile. The enhanced stability of the spore aldolase was not diminished by dialysis or gel filtration but was lost after chromatography on diethylaminoethyl cellulose at pH 7.4. Aldolase from vegetative cells exists in an equilibrium mixture of two molecular weights, 115,000 and 79,000 in the approximate ratio of 1:4, respectively. The molecular weight of spore aldolase is 44,000. Spore aldolase was more mobile during electrophoresis than its vegetative cell counterpart because of its smaller size.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERS G. K. MOLECULAR EXCLUSION AND RESTRICTED DIFFUSION PROCESSES IN MOLECULAR-SIEVE CHROMATOGRAPHY. Biochemistry. 1964 May;3:723–730. doi: 10.1021/bi00893a021. [DOI] [PubMed] [Google Scholar]
- BARD R. C., GUNSALUS I. C. Glucose metabolism of Clostridium perfringens: existence of metallo-aldolase. J Bacteriol. 1950 Mar;59(3):387–400. doi: 10.1128/jb.59.3.387-400.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNLOHR R. W. POSTLOGARITHMIC PHASE METABOLISM OF SPORULATING MICROORGANISMS. I. PROTEASE OF BACILLUS LICHENIFORMIS. J Biol Chem. 1964 Feb;239:538–543. [PubMed] [Google Scholar]
- COHN M., TORRIANI A. M. Immunochemical studies with the beta-galactosidase and structurally related proteins of Escherichia coli. J Immunol. 1952 Nov;69(5):471–491. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DRECHSLER E. R., BOYER P. D., KOWALSKY A. G. The catalytic activity of carboxypeptidase-degraded aldolase. J Biol Chem. 1959 Oct;234:2627–2634. [PubMed] [Google Scholar]
- FREESE E., PARK S. W., CASHEL M. THE DEVELOPMENTAL SIGNIFICANCE OF ALANINE DEHYDROGENASE IN BACILLUS SUBTILIS. Proc Natl Acad Sci U S A. 1964 Jun;51:1164–1172. doi: 10.1073/pnas.51.6.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falaschi A., Kornberg A. Biochemical studies of bacterial sporulation. II. Deoxy- ribonucleic acid polymerase in spores of Bacillus subtilis. J Biol Chem. 1966 Apr 10;241(7):1478–1482. [PubMed] [Google Scholar]
- GOLDMAN M., BLUMENTHAL H. U. Pathways of glucose catabolism in intact heat-activated spores of Bacillus cereus. Biochem Biophys Res Commun. 1960 Aug;3:164–168. doi: 10.1016/0006-291x(60)90215-1. [DOI] [PubMed] [Google Scholar]
- GRAZI E., CHENG T., HORECKER B. L. The formation of a stable aldolase-dihydroxyacetone phosphate complex. Biochem Biophys Res Commun. 1962 Apr 20;7:250–253. doi: 10.1016/0006-291x(62)90184-5. [DOI] [PubMed] [Google Scholar]
- GRAZI E., ROWLEY P. T., CHENG T., TCHOLA O., HORECKER B. L. The mechanism of action of aldolases. III. Schiff base formation with lysine. Biochem Biophys Res Commun. 1962 Sep 25;9:38–43. doi: 10.1016/0006-291x(62)90083-9. [DOI] [PubMed] [Google Scholar]
- Gardner R., Kornberg A. Biochemical studies of bacterial sporulation and germination. V. Purine nucleoside phosphorylase of vegetative cells and spores of Bacillus cereus. J Biol Chem. 1967 May 25;242(10):2383–2388. [PubMed] [Google Scholar]
- HASHIMOTO T., BLACK S. H., GERHARDT P. Development of fine structure, thermostability, and dipicolinate during sporogenesis in a bacillus. Can J Microbiol. 1960 Apr;6:203–212. doi: 10.1139/m60-022. [DOI] [PubMed] [Google Scholar]
- JAGANNATHAN V., SINGH K., DAMODARAN M. Carbohydrate metabolism in citric acid fermentation. 4. Purification and properties of aldolase from Aspergillus niger. Biochem J. 1956 May;63(1):94–105. doi: 10.1042/bj0630094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji A. Partial proteolytic digestion of yeast hexokinase and its relation to multiple forms of the enzyme. Arch Biochem Biophys. 1965 Oct;112(1):54–64. doi: 10.1016/0003-9861(65)90009-3. [DOI] [PubMed] [Google Scholar]
- Knox W. E., Stumpf P. K., Green D. E., Auerbach V. H. The Inhibition of Sulfhydryl Enzymes as the Basis of the Bactericidal Action of Chlorine. J Bacteriol. 1948 Apr;55(4):451–458. [PMC free article] [PubMed] [Google Scholar]
- Kornberg A., Spudich J. A., Nelson D. L., Deutscher M. P. Origin of proteins in sporulation. Annu Rev Biochem. 1968;37:51–78. doi: 10.1146/annurev.bi.37.070168.000411. [DOI] [PubMed] [Google Scholar]
- LAI C. Y., TCHOLA O., CHENG T., HORECKER B. L. THE MECHANISM OF ACTION OF ALDOLASES. 8. THE NUMBER OF COMBINING SITES IN FRUCTOSE DIPHOSPHATE ALDOLASE. J Biol Chem. 1965 Mar;240:1347–1350. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MCCORMICK N. G., HALVORSON H. O. PURIFICATION AND PROPERTIES OF L-ALANINE DEHYDROGENASE FROM VEGETATIVE CELLS OF BACILLUS CEREUS. J Bacteriol. 1964 Jan;87:68–74. doi: 10.1128/jb.87.1.68-74.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'CONNOR R. J., HALVORSON H. O. Intermediate metabolism of aerobic spores. V. The purification and properties of L-alanine dehydrogenase. Arch Biochem Biophys. 1960 Dec;91:290–299. doi: 10.1016/0003-9861(60)90503-8. [DOI] [PubMed] [Google Scholar]
- RICHARDS O. C., RUTTER W. J. Preparation and properties of yeast aldolase. J Biol Chem. 1961 Dec;236:3177–3184. [PubMed] [Google Scholar]
- RUTTER W. J. EVOLUTION OF ALDOLASE. Fed Proc. 1964 Nov-Dec;23:1248–1257. [PubMed] [Google Scholar]
- SIEGEL L. M., MONTY K. J. DETERMINATION OF MOLECULAR WEIGHTS AND FRICTIONAL RATIOS OF MACROMOLECULES IN IMPURE SYSTEMS: AGGREGATION OF UREASE. Biochem Biophys Res Commun. 1965 May 3;19:494–499. doi: 10.1016/0006-291x(65)90152-x. [DOI] [PubMed] [Google Scholar]
- STEWART B. T., HALVORSON H. O. Studies on the spores of aerobic bacteria. I. The occurrence of alanine racemase. J Bacteriol. 1953 Feb;65(2):160–166. doi: 10.1128/jb.65.2.160-166.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki R., Sugimoto R., Chiba H. Yeast phosphoglyceric acid mutase-modifying enzyme. Arch Biochem Biophys. 1966 Jul;115(1):53–61. doi: 10.1016/s0003-9861(66)81037-8. [DOI] [PubMed] [Google Scholar]
- Strittmatter P., Ozols J. The restricted tryptic cleavage of cytochrome b5. J Biol Chem. 1966 Oct 25;241(20):4787–4792. [PubMed] [Google Scholar]
- Tono H., Kornberg A. Biochemical studies of bacterial sporulation. 3. Inorganic pyrophosphatase of vegetative cells and spores of Bacillus subtilis. J Biol Chem. 1967 May 25;242(10):2375–2382. [PubMed] [Google Scholar]
- Tono H., Kornberg A. Biochemical studies of bacterial sporulation. IV. Inorganic pyrophosphatase of vegetative cells and spores of Bacillus megaterium. J Bacteriol. 1967 Jun;93(6):1819–1824. doi: 10.1128/jb.93.6.1819-1824.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIDA A., FREESE E. ENZYMATIC PROPERTIES OF ALANINE DEHYDROGENASE OF BACILLUS SUBTILIS. Biochim Biophys Acta. 1965 Feb 22;96:248–262. [PubMed] [Google Scholar]
- YOSHIDA A., FREESE E. PURIFICATION AND CHEMICAL CHARACTERIZATION OF ALANINE DEHYDROGENASE OF BACILLUS SUBTILIS. Biochim Biophys Acta. 1964 Oct 23;92:33–43. doi: 10.1016/0926-6569(64)90266-4. [DOI] [PubMed] [Google Scholar]




