1 Geographic features relating to the endemicity
Japan is located between latitude 45°33’ 28” north (Etorofu) and lat. 20°25’ 31” N (Okino-Torishima) and consists of 6,852 islands scattered between Taiwan and Saharin. Among them there are 4 main islands, Honshu, Kyushu, Shikoku and Hokkaido. Of all islands, 456 are inhabited. From the viewpoint of filariasis transmission, Kyushu and Okinawa were very important in terms of their high endemicity. The Okinawa archipelago is located south of Kyushu at the southwestern extremity of Japan. The archipelago consists of 60 inhabited islands with subtropical or tropical meteorological features. Many of these small islands are essentially the elevated coral reefs. In order to get potable water in such islands, wells are useless because of salt content, thus pots or tanks are necessary to collect rainwater. However, this type of water pool provides a favorite feeding place for Culex spp. mosquitoes. From the demographic viewpoint, people were not migrating among islands. The conservative habit of the inhabitants kept their traditional way of living in each place, thus limiting filariasis transmission in each restricted island for a long time.
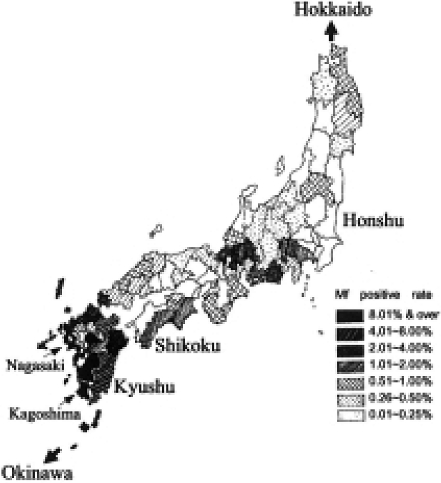
In 1911, the Ministry of Army examined the night blood of recruited soldiers in order to determine the filariasis infection at a state level [1]. As seen in Fig. 1, the survey revealed dense infections in the southwestern part of Japan. Okinawa, Kagoshima, Nagasaki and some areas of Shikoku showed particularly high infection rates. The data are graphically illustrated in Fig. 2. Zone 1 includes 9,359 soldiers, who were examined, from Hokkaido and Tohoku areas; Zone 2, l4,620 from Kanto area; Zone 3, 12,885 from Shin-etsu and Hokuriku areas; Zone 4, 7,977 from Tokai area; Zone 5, 13,438 from Kinki area; Zone 6, 16,003 from Chugoku area; Zone 7, 9,750 from Shikoku area; Zone 8, 26,671 from Kyushu area; and Zone 9, 1,650 from Okinawa area, arranged from north to south. This survey clarified a marked feature of filariasis prevalence in Japan. Namely in Hokkaido, no positives were found and the infected cases were few in Zones 1 to 6. On the contrary, soldiers from southwestern areas of Japan with temperate to subtropical climate showed high prevalence. Zone 9 (Okinawa) had the highest positive rate of 17.6% among the soldiers approximately 20 years of age. In the Zone 8 (Kyushu), Kagoshima and Nagasaki showed very high rates of 8.5% and 13.5% respectively. It will be noted that sporadic endemicity was present even in the northern cool areas of Japan.
Fig. 1.
Prevalence of microfilaremia (%) in recruited soldiers (1911) [Quoted from Sasa [15]].
Fig. 2.
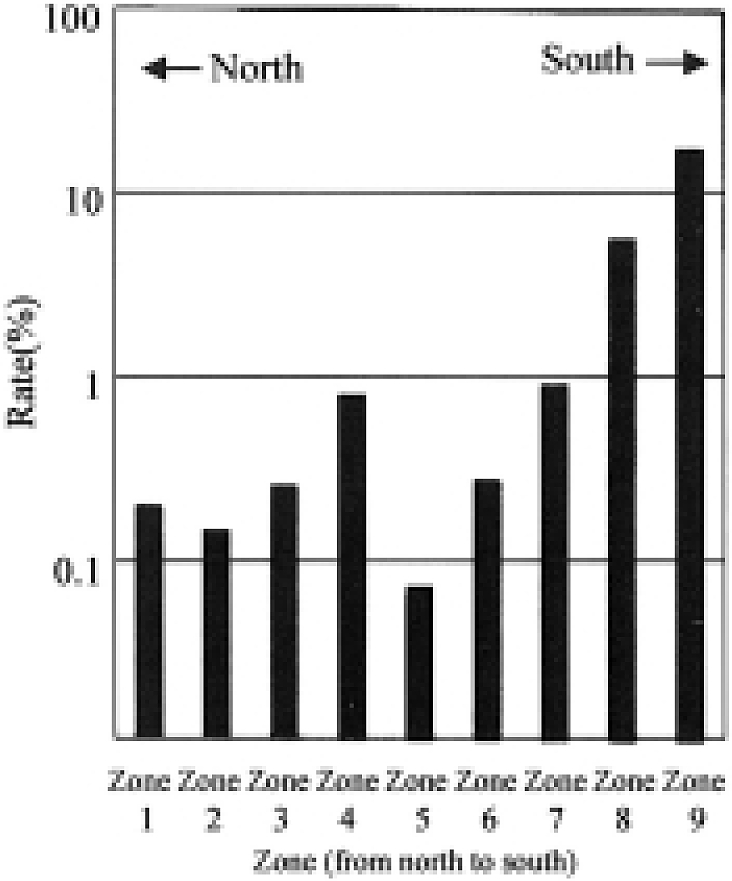
Prevalence of microfilaremia (%) in recruited soldiers according to geographic zone.
The results of this survey indicate the original distribution of transmission of lymphatic filariasis in relation to the geographic/ meteorological characteristic of Japan.
2 Meteorological features of endemic areas
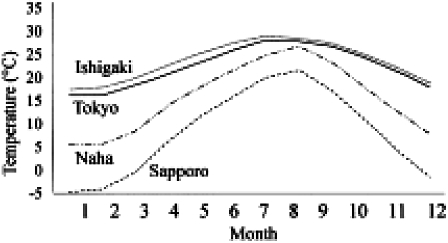
The Fig. 3 shows the monthly mean temperatures of 4 cities: Sapporo, the prefectural capital of Hokkaido, located at the northern extremity; Tokyo, the capital of Japan at the center of Honshu; Naha the prefectural capital of Okinawa at the south-westernmost part of Japan; and Ishigaki, a city on Ishigaki Is. in the western part of Okinawa archipelago. In the summer, the average temperature of Sapporo surpasses 20°C, while the winter-time temperature falls down below zero in Dec., Jan., Feb. and Mar. In Tokyo, the average monthly temperature of each month is approximately 5-7 degrees higher than that of Hokkaido. In July and Aug., the temperature is higher than 25°C, while in winter it is quite low and unsuitablet for the hibernation of adult mosquitoes. On the contrary, Naha and Ishigaki show very similar annual temperature patterns, high and more flat through the whole year. The annual average temperature in Ishigaki is 23.8°C. This level of temperature is quite favorable for blood sucking activity, reproduction and growth of mosquitoes, particularly the vector Culex quinguefasciatus. Further more, under these conditions filarial larvae taken by the vector mosquito are able to grow smoothly through the whole year.
Fig. 3.
Monthly mean temperatures of 4 areas in Japan.
3 Vector mosquitoes
In the endemic areas in Shikoku and Kyushu, the most abundant mosquito species is Culex pipiens pallens and the second is Cx. tritaeniorhynchus. Then Anopheles hyrcanus sinensis and Aedes togoi follow. Since the early 20th century, there have been various studies on the vector mosquitoes of lymphatic filariasis in southwestern Japan (Taniguchi, 1905; Mochizuki, 1911) [2, 3]. Mochizuki clarified the transmission efficiency of Cx. p. pallens in terms of natural and experimental infections. After World War II, extensive studies were made in this field (see the review of Omori, 1962 [4]).
Actually, Cx. p. pallens is an anthropophilic species and breeds in ditches and other places surrounding human dwellings. It sucks human blood actively between May/June and Nov. in Japan. According to Omori (1962) [4] the blood-fed mosquitoes hibernate below 16°C in the natural condition. The microfilariae taken by mosquitoes are not able to mature at or under 16°C and the optimal temperature for the larval growth lies between 24-26°C. Omori (1958) [5] assessed the filarial larval growth in Cx. p. pallens and concluded that there existed a regression Y = 0.703 (X - 14.6) between development velocity (Y) and mosquito rearing temperature (X) at the environmental temperature over 18°C. This formula would suggest that the development does not occur at 14.6°C in the natural environment. The average temperature of the period between Nov. and April in Nagasaki is below 16°C and therefore not favorable for the filarial transmission.
Considering the biological characteristic of Cx. p.pallens, it may be concluded that there is a marked seasonal interruption of filariasis transmission in most endemic areas in Shikoku and Kyushu. Thus it was suggested that treatment of infected people should be accomplished during the autumn/winter season. This is the climatic factor that enabled us to implement selective treatment of infected people successfully in endemic foci, apart from subtropical Okinawa and Amami. As is seen in the monthly temperature of Ishigaki Island Groups in Okinawa (Fig. 3), the average annual temperature exceeds 23°C and only 3 or 4 months in a year show a monthly average of slightly below 20°C. The climatic condition in the Nansei Shoto (including Okinawa and Amami Archipelagos) is of (sub-)tropical nature and favorable for the filarial transmission, where Cx. quinquefasciatus (=Cx. fatigans), a very close species to Cx. p. pallens, was the main vector.
4 Community and people: a main player in the successful program
In the foreyard of Miyako Health Center on Miyako Is., Okinawa, we see a stone monument constructed in Nov. 1988 celebrating the completion of the filariasis control campaign (Fig. 4a, 4b). It reads as follows: In Jan. 1965, filariasis control campaign was started in Miyako Islands based on the dedication of the community inhabitants. This control activity then encouraged campaigns in other islands, Yaeyama and Okinawa (main island). Eventually lymphatic filariasis was eliminated from all the Okinawa Islands in 1978 (Fig. 5). In order to attain the goal of filariasis elimination program, “Araragama spirit” of Miyako communities played a major role, that is, trust in the scientific evidence of filariology and the sincerity of the administrative sector in health control. People wanted to improve health conditions by combating the long-lasting scourge of filariasis by their own means. This achievement, which required intelligence and power of the community, would certainly be a valuable example for any coming health project. Commemorating the 10th annual meeting of filariasis elimination and the 20th annual meeting for the Okinawa Association of Public Health, this monument was constructed as a tribute to the people of Miyako, and all of Okinawa too.
Fig. 4a.
The stone monument erected at Miyako Health Center commemorating the eradication of filariasis.
Fig. 4b.
The inscription on the other side of the monument.
Fig. 5.
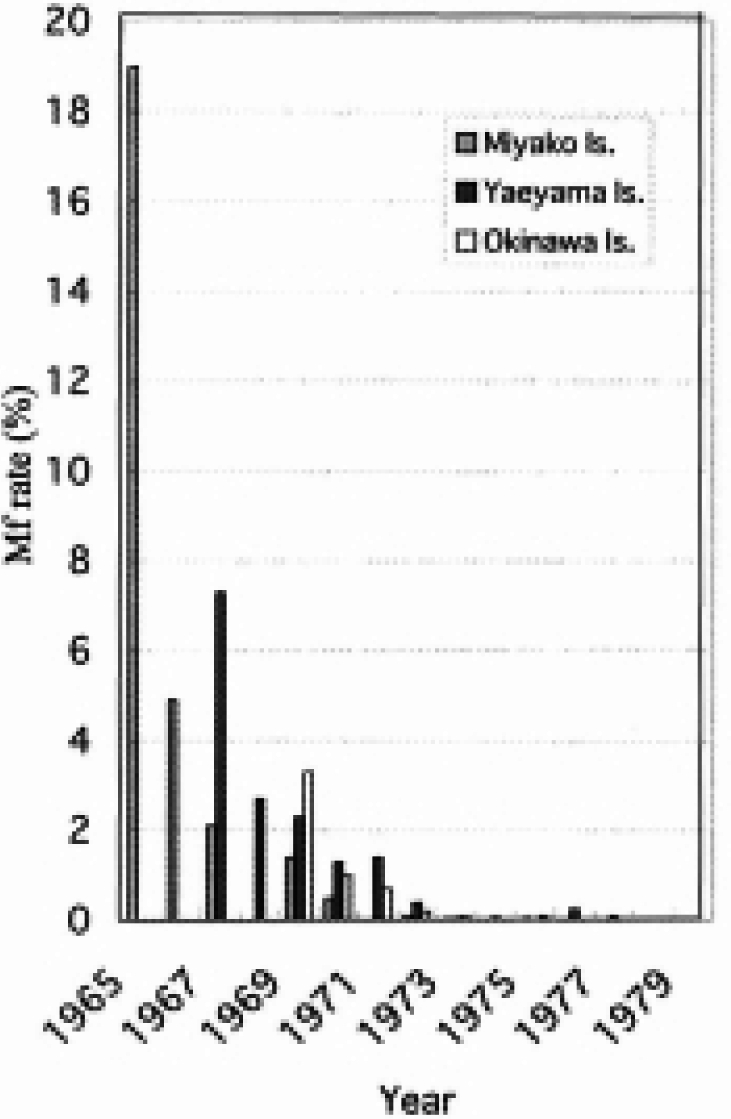
Change in microfilaria rate in Okinawa.
Okinawa had the highest endemicity of filariasis in Japan and for this reason, the control campaign was considered difficult, taking account of meteorological and entomological situations as well. However, the control campaign revealed that the absentee rate was the lowest in blood examination and DEC administration. Aiming at the 100% participation of the inhabitants in the campaign, the staff performed, if necessary, door-to-door examinations at night. Inhabitants were previously educated repeatedly by radio, newspaper and community meetings on the historical meaning of the program. Therefore the whole community made up their mind to achieve elimination sooner than other communities. This community-based competition is traditionally called““Araragama spirit” in Miyako Islands. As seen in Okinawa, the participation of community people with high motivation will be very important to implement any health activity.
Yoshida (1988) [6] noted the important role of the public health nurse system in Okinawa in order to improve participation rate, because nurses have been working among the community and thus involved in various consultations for the inhabitants with regard to pregnancy, delivery, vaccination, infant health examination, etc. Thus they were well known among the community people and could be efficient advocators for filariasis control campaign.
The morale of health staff involved in the campaign was also commendable. One health technologist invented a slide glass holder using a pair of wire springs and a wooden plate (Fig. 6). This device made the process of blood collection, drying and staining of blood smears quite easy in the field. In 1967, Mr. Z. Heishiki, the inventor, was awarded the Kojima Technology Medal for his hand-made but brilliant device.
Fig. 6.
Heishiki’s handmade slide rack made of a pair of wire springs and a wood block.
In this way the accumulation of all the positive actions and factors made it possible to eliminate filariasis in Okinawa and the other parts of Japan, too.
5 Effects of economy and its growth
In 2000, the opening ceremony of the Global Elimination Program of Lymphatic Filariasis, convened by WHO, was held at Santiago de Compostera, Spain. The proposed catchword for this ambitious program was “Combat poverty,” quite an impressive phrase. Disease burden among humans due to filarial morbidity like filarial fever, elephantiasis, hydrocele, etc. had been disturbing the productive activities in many fields. Thus the elimination of this disease would not only mean the extinction of a disease, but also the promotion of economic and agricultural activities in affected countries. In this global program, the support of allied partners including pharmaceutical companies was very important to promote elimination programs in various developing countries with low GNPs.
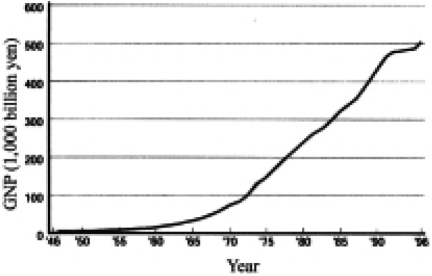
In order to establish a filariasis control program in Japan during the 1960s, we needed to prepare necessary financial and human resources. Fig. 7 depicts the historical change of per capita GNP in Japan between 1885 and 1998. World War II started in 1941 and ended in 1945. After the war, Japan lost everything and took about 10 years to bring its GNP back to the pre-war level. In the mid 1950s, only 51.5% of pupils entered high school after graduation from primary and middle schools that had a compulsory 9-year basic course in total. Further more, only 10.1% of high school graduates entered university/college. In 1956 the white paper on economy, issued by the Japanese government, stated that the country was no longer in the postwar era. Actually this year was the beginning of rapid economic and industrial growth in Japan. This trend is evident in the rising GNP curve after 1960. In the health sector, budgets increased in the field of prevention of diseases. This trend of national finance played an important role in the control of various parasites such as filaria, schistosome and intestinal parasltes.
Fig. 7.
Increase of GNP in Japan.
6 Historical progress in filariology
6.1 Accumulation of research evidence in filariasis and filariology
In Japan, filariasis was distributed in various regions since the Heian Era, about 1200 years ago (Fig. 8). Many investigators and clinicians noted this disease and plenty of studies were reported in the field of clinical medicine, epidemiology and parasitology before 1947, when DEC appeared as a filaricide.
Fig. 8.

Cover picture of a WHO booklet [WHO/FIL/99. 198] issued for the global elimination program of lymphatic filariasis, showing an ancient elephantiasis case in Japan.
Further more, many physicians noted the impact of lymphatic filariasis as an endemic disease in Kyushu and Okinawa. Mine (1913) [7] reported the prevalence of endemic filariasis among soldiers recruited in Okinawa for the first time. A state survey was thus conducted in 1912. Heated discussions on the etiology of elephantiasis unfolded between bacteriologists of Kyoto University and internists of Kyushu University (see Chapter 3, 7.1.1). Forty-eight reports were found to mention studies on filariasis in Okinawa before World War II according to Kuniyoshi (1970) [8]. It is worthy of note that Ohama, a practitioner with his clinic in Ishigaki Is., wrote 10 reports on this endemic disease. His passion for energetic reporting seems to have originated from love for his native archipelago.
After the war, during the period between 1945 and 1960, 25 papers were published; likewise between 1961 and 1970, 84 papers were published in local and international journals. Considering this number of scientific achievements on endemic filariasis, we can recognize the strong advocative effect of academic activities influencing endemic communities. Through these activities, people were well acquainted with the pathogenicity of the disease and its transmission mechanism. They recognized gradually that the symptoms were not a social stigma but a disease that needed proper treatment.
With regard to the treatment of filariasis, various chemical compounds had been tested, particularly for chyluria, and with animal models, before the appearance of diethylcarbamazine (DEC). These include salvarsan, neostibunal (Wada, 1927) [9], stibunal and quinine hydrochloride (Hojo, 1931) [10], potassium picrate (Ihara, 1930) [11], and trypaflavin (Tamashiro, 1931) [12]. The effects, however, were rather obscure. Among them, the remarkable effect of pulverized antimony colloid (AMC) administered to chyluria cases was reported by Miyagawa et al. (1943) [13]. In this way, there were various physicians who tried to treat lymphatic filariasis before DEC, and this situation accelerated control trials using DEC.
6.2 Adverse reactions to DEC and management: a key to compliance
One of the major reasons that infected people dislike DEC administration is febrile reactions of varying severity. Sasa et al. (1963) [14] revealed a direct correlation between microfilarial density in the peripheral blood and the grade of fever. Severe febrile reaction occurred in more than 80% of people whose microfilaria count was 1 or more per 1 µl of blood, while this rate was reduced to less than 10% in those with 0.1 microfilaria per 1 µl (Sasa 1976) [15].
On the other hand, counting of microfilariae in blood samples was undertaken even in the early days. After the standardization of blood sampling with 30 µl, counting became a routine task in the control campaign. Therefore, medical staff in charge of treatment could explain individually not only whether the febrile reaction would occur or not, but even its severity. This prediction of the expected side reactions persuaded people to accept treatment with more ease. This approach of blood examination and following selective DEC treatment was quite smoothly accepted in each endemic area in Japan.
Acknowledgement
This paper is revised from Asian Parasitology Vol. 3 Filariasis in Asia and Western Pacific Islands, 93-99 by The Federation of Asian Parasitologists in 2004.
References
- 1.Bureau of Military Surgeon. Distribution of filariasis in Japan. J Bureau of Military Surgeon 1912; 41: 332-348 (in Japanese). [Google Scholar]
- 2.Taniguchi N. Supplements to the biology of Filaria bancrofi, Cobbold, and clinical survey on filariasis. Chinzei Iho 1905; 90: 129 (in Japanese). [Google Scholar]
- 3.Mochizuki D. Relation between various mosquitoes and Wuchereria bancrofti. Fukuoka Ikadaigaku Zasshi 1911; 4(3), 384-444 (in Japanese). [Google Scholar]
- 4.Omori N. Japanese mosquitoes involved in the transmission of Wuchereria bancrofti: Special reference to the role of Culex pipiens pallens. Progress of Medical Parasitology in Japan. Vol. 2, 1962: 35-65 [Google Scholar]
- 5.Omori N. Experimental studies on the role of the house mosquito, Culex pipiens pallens in the transmission of bancroftian filariasis. 4. Development and longevity in days of filariae in mosquitoes kept at a series of constant temperatures. Nagasaki Igakkai Zasshi 1958; 33 (Suppl.): 61-70 [Google Scholar]
- 6.Yoshida C. A requiem for filariasis. Okinawa Association of Public Health: Naha1988. (in Japanese).
- 7.Mine J. A report on the filariasis in soldiers from Okinawa. Gun’ idan Zasshi 1913; 12: 253 (in Japanese). [Google Scholar]
- 8.Kuniyoshi S. Reference list of papers concerned to the filariasis in Okinawa (1911-1970). Okinawa Association of Public Health: Naha 1970 (in Japanese).
- 9.Wada K. Treatment of dirofilariasis with neo-stibunal. Zikkenn-Igaku Zasshi 1927; 11(7).
- 10.Hojo E. Treatment of human fi1ariasis and a study on microfilaria. Gun’ idan Zasshi l931;(222) 2487 (in Japanese).
- 11.Ihara A. Treatment of filariasis with potassium picrate. Gun’ idan Zasshi 1930; (209) 1764 (in Japanese).
- 12.Tamashiro F. Treatment of microfilaremia by trypaflavin. Chiryo Yakuho 1931; (342) 17 (in Japanese).
- 13.Miyagawa Y, Kitamura K, Morishita T, et al. A new treatment of human filariasis by Wuchereria bancrofti. Nihon Kiseityu-gakkai Kizi 1943; 15: 38 (in Japanese). [Google Scholar]
- 14.Sasa M, et al. Studies on epidemiology and control of filariasis: Observations on the carriers of Wuchereria bancrofti in the Amami Islands with special reference to the effects and side-reactions of diethylcarbamazine. Jpn J Exp Med 1963; 33: 213. [PubMed] [Google Scholar]
- 15.Sasa M. Human filariasis. A global survey of epidemiology and control. Univ Tokyo Press: Tokyo, 1976.