Abstract
In our previous report, rosmarinic acid (RA) was revealed to be an antidote active compound in Argusia argentea (family: Boraginaceae). The plant is locally used in Okinawa in Japan as an antidote for poisoning from snake venom, Trimeresurus flavoviridis (habu). This article presents mechanistic evidence of RA’s neutralization of the hemorrhagic effects of snake venom. Anti-hemorrhagic activity was assayed by using several kinds of snake venom. Inhibition against fibrinogen hydrolytic and collagen hydrolytic activities of T. flavoviridis venom were examined by SDS-PAGE. A histopathological study was done by microscopy after administration of venom in the presence or absence of RA. RA was found to markedly neutralize venom-induced hemorrhage, fibrinogenolysis, cytotoxicity and digestion of type IV collagen activity. Moreover, RA inhibited both hemorrhage and neutrophil infiltrations caused by T. flavoviridis venom in pathology sections. These results demonstrate that RA inhibited most of the hemorrhage effects of venom. These findings indicate that rosmarinic acid can be expected to provide therapeutic benefits in neutralization of snake venom accompanied by heat stability.
Keywords: rosmarinic acid, snake venom, hemorrhage, metalloproteinase, Argusia argentea
1. Introduction
Envenomation resulting from snakebites is an important public health hazard in many regions, particularly in tropical and subtropical regions [1,2,3]. Snake envenomation causes various pathophysiological changes such as inflammation, increased body temperature, hemorrhage, necrosis, nephrotoxicity, cardiotoxicity, haemostatic changes and ultimately death [4]. Hemorrhage is a common symptom associated with local tissue damage in snake poisoning by Viperidae. In severe poisoning, hemorrhage can be observed in many internal organs [5,6]. Hemorrhage can occur as a result of cytotoxicity of the venom on endothelial cells, and a degradation of the basement membrane of the vein due to the venom acting as a metalloproteinase. In many countries, plant extracts have been traditionally used in the treatment of snakebite envenomations [7,8,9], although only a few cases have been scientifically validated. The present study aims to examine the venom neutralization potential of rosmarinic acid (RA) isolated from a methanolic extract of Argusia argentea [10] and its mode of action.
2. Materials and Methods
2.1. Materials
Trimeresurus flavoviridis (habu) venom (Okinawa), Gloydius blomhoffii venom and Bitis arietans venom were purchased from Japan Snake Institute, Gunma. Crotalus atrox venom was purchased from Sigma-Aldrich. Hemorrhagic toxin b (HTb) from C. atrox venom was prepared by the method reported previously [11]. Bilitoxin-2 and Ac1-proteinase were isolated using our methods reported previously [12,13] for Agkistrodon bilineatus venom and Deinagkistrodon acutus venom, respectively. Hemorrhagic toxin-1 (HT-1) was obtained from B. arietans venom [14]. Human and bovine fibrinogens were supplied by Sigma-Aldrich, Tokyo, Japan. Type IV collagen was purchased from Nitta Gelatin Inc. Cryo-preserved human umbilical vein endothelial cells (HUVEC), its respective cell culture media (HuMedia EB-2), other cell culture supplements, and reagents were obtained from Kurabo (Osaka, Japan). The cell counting kit was purchased from Dojindo (Kumamoto, Japan). Other chemicals were of analytical grade from commercial sources. All experiments involving the use of animals were carried out in compliance with the guidelines for animal experiments of Faculty of Pharmacy, Meijo University.
2.2. Rosmarinic Acid
Rosmarinic acid (RA) was isolated and purified from methanolic extract of Argusia argentea as reported previously [10]. A voucher sample has been deposited in the Herbarium of the Faculty of Pharmacy, Meijo University, Japan.
2.3. Anti-hemorrhagic Activity Assay
Anti-hemorrhagic activity was assayed by the method of Bjarnason and Tu [15] using ddY mice of 20 g average weight. Two groups of four mice were used for the experiment. All crude venom solutions of T. flavoviridis venom, C. atrox venom, G. blomhoffii venom and B. arietans venom, were prepared at a concentration of 0.14 mg/mL in saline. Concentrations of purified hemorrhagic toxin solutions were as follows: HTb (0.41 mg/mL), bilitoxin-2 (2.75 μg/mL), HT-1 (0.29 mg/mL), and Ac1‑proteinase (1.04 mg/mL). A test solution was prepared by mixing the venom solution or the toxin solution (50 µL) and RA (0.5 mg/mL in 10% DMSO-saline, 50 µL) followed by 10 min incubation at 37 °C. These test solutions (100 µL) were injected subcutaneously (s.c.) in the abdomen of mice. Similarly, a group of mice which were injected with a venom solution without RA was used as a control group, and also a group which was only injected with 10% DMSO-saline (50 μL) served as a blank group. Prior to this study, effects of DMSO at several concentrations were investigated, and DMSO at less than 10% was found to cause no significant inactivation of venom. After 24 h, mice were euthanized by inhalation of chloroform, the skin covering the abdomen was removed and hemorrhagic lesions were analyzed as follows. The area of the lesion was estimated by major and minor axes measurements, since the shape of the lesions are always amorphous, like an ellipse.
2.4. Fibrinogen Hydrolytic Activity Assay
Fibrinogen hydrolytic activity was assayed by the method of Ouyang and Teng [16]. A solution of 0.1% human fibrinogen in 50 mM Tris-HCl buffer (pH 7.5) (1 mL) and a venom solution (50 µL of 0.21 mg/mL of T. flavoviridis venom or 5.5 µg/mL of bilitoxin-2) were incubated in the presence or absence of RA (0.5 mg/mL) at 37 °C. At various time intervals, aliquots of 100 µL of denaturing solution (10 mM phosphate buffer, pH 7.2, containing 10 M urea, 4% sodium dodecyl sulfate (SDS), and 4% β-mercaptoethanol) were added. This solution was incubated at 37 °C for 6 h and then run on 10% polyacrylamide slab gel electrophoresis. Electrophoresis was carried out for 2 h with a current of 25 mA per slab gel. Bromophenol blue (BPB) solution was used as an indicator.
2.5. Collagen Hydrolytic Activity Assay
Collagen hydrolytic activity was assayed as follows. Sodium hydrogen carbonate (60 µL, pH 12) was added to 0.3% type IV collagen (0.9 mL) and adjusted to pH 8. Aliquots of type IV collagen were incubated with T. flavoviridis venom (0.21 μg/mL) in the presence or absence of RA (0.5 mg/mL). At various time intervals, aliquots of 100 µL of denaturing solution (10 mM phosphate buffer, pH 7.2, containing 10 M urea, 4% SDS, and 4% β-mercaptoethanol) were added. This solution was boiled for 3 min and run on SDS-PAGE using a 7.5% polyacrylamide slab gel electrophoresis.
2.6. Cytotoxic Action on HUVEC
The effects of RA and T. flavoviridis venom on cultured human umbilical vein endothelial cells (HUVEC) were investigated [17,18,19]. Frozen HUVEC were cultured and maintained in commercially available media, HuMedia-EB2, supplemented with fetal calf serum (2% v/v), hEGF (10 ng/mL), hFGF-B (5 ng/mL), hydrocortisone (1 µg/mL), heparin (10 µg/mL), gentamicin (50 µg/mL), and amphotericin B (50 ng/mL). At confluency, cells were trypsinized, washed with the same medium and then resuspended in growth media. These cells were seeded in 96-multiwell plates (5 × 103 cells per well in 100 µL medium) and were allowed to attach and reach log phase of growth. Aliquots of venom and RA to be assayed were diluted in saline and were sterilized by filtration with cellulose acetate 0.22 µm membrane filters. Various concentrations of RA (0.5, 0.25, 0.125, 0.06, 0.03 mg/mL in 10% DMSO-saline) in the presence or absence of T. flavoviridis venom (0.14 mg/mL) were added to each well in 100 µL medium. The plate was incubated at 37 °C under 5% CO2 atmosphere for 17 h. Ten microliters of cell counting kit-8 was added to each well, and the microplate was incubated for 1 h, after which cell densities were measured at 450 nm using Bio-RAD Model 550 MicroplateR eader.
2.7. Histopathological Study
Histopathological study for RA was performed by intramuscular (i.m.) injection of T. flavoviridis venom solution into the medial aspect of the thigh muscle of ddY strain white mice. Histopathological study of muscle was conducted in three groups. Group A was injected with the venom (0.21 mg/mL, 100 µL), while group B was injected with RA (0.5 mg/mL, 100 µL). Group C was injected with a mixture of the venom (0.41 mg/mL, 50 µL) and RA (0.25 mg/mL, 50 µL). Test solutions were preincubated at 37 °C for 10 min before injection. The mice were killed by chloroform inhalation 24 h after injection. Tissue samples were immediately fixed in buffered formate fixative for 24 h at room temperature. The tissue was then washed for 4 h in running water, dehydrated in an autotechnicon, and stained with hematoxylin and eosin for observation under light microscope.
2.8. Heat Stability
RA (0.5 mg/mL) in 10% DMSO-saline was heated at 37 °C, 50 °C and 100 °C for 10 min, respectively. Fifty microliters of each heat-treated RA solution was mixed with T. flavoviridis venom (0.21 mg/mL, 50 µL) and incubated at 37 °C for 10 min. An aliquot of 0.1% human fibrinogen in 50 mM Tris-HCl buffer, pH 7.5 was added to each test tube. The reactions were stopped by adding 100 µL of denaturing solution (10 mM phosphate buffer, pH 7.2, containing 10 M urea, 4% SDS, and 4% β-mercaptoethanol). These solutions were incubated at 37 °C for 6 h and then run on 10% polyacrylamide slab gel electrophoresis. The venom solution without a sample was also subjected to SDS-PAGE for comparison. Moreover, by using RA (0.5 mg/mL), which was treated at 100 °C, antihemorrhage activity was also examined as mentioned above.
2.9. Assay for Edema Activity
Hind-paw edema activity was assayed by the method of Ho et al. [20]. Four ddY strain white mice (20–23 g) were individually injected in the right foot pad with Trimeresurus elegans venom (12.5 µg in 50 µL of 10% DMSO-saline). An equal volume of 10% DMSO saline was injected into the left paws as control. Inhibition assays were performed by preincubated rosmarinic acid (0.5 mg/mL in 10% DMSO saline) with toxin for 10 min at 37 °C. The volume of each paw was measured with a slide caliper. The degree of paw swelling was expressed as % increase of the initial paw volume.
3. Results
3.1. Inhibitory Activity of RA on Crude Snake Venoms and Purified Hemorrhagic Toxins
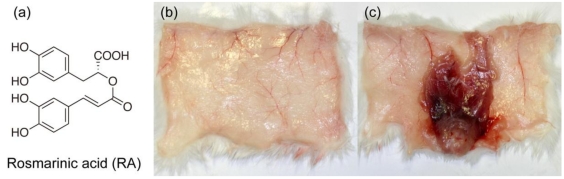
When crude venom (T. flavoviridis (habu) venom, C. atrox venom, G. blomhoffii venom, and B. arietans venom) or purified toxin, (HTb, bilitoxin-2, HT-1 and Ac1-proteinase) was injected s.c. in the abdomen of mice, a distinct hemorrhagic lesion was observed (Figure 1c) [10]. No hemorrhagic spots were produced after s.c. injection of crude venom or purified toxin with RA (Figure 1b). RA effectively inhibited the hemorrhagic activities of crude venoms as well as purified hemorrhagic toxins.
Figure 1.
Inhibitory activity of RA on T. flavoviridis venom (a) Structure of rosmarinic acid, (b) T. flavoviridis venom with RA, (c) T. flavoviridis venom without RA.
3.2. Inhibition of Fibrinogen Hydrolytic Activity
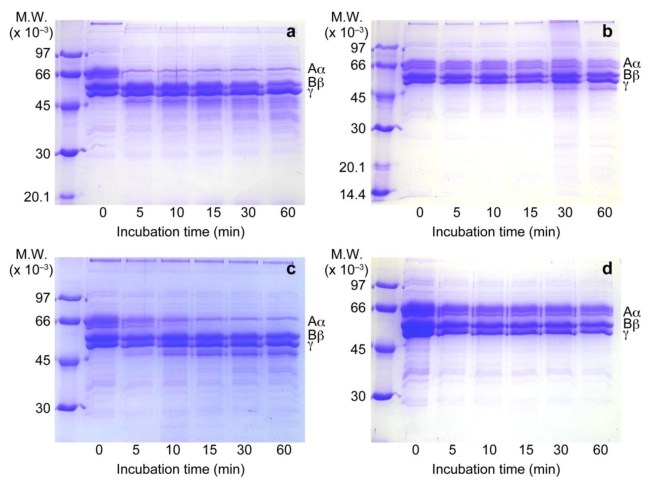
When human fibrinogen was incubated with T. flavoviridis venom, the Aα band of the fibrinogen disappeared on SDS-PAGE, whereas the Bβ chain and γ chain were essentially unaffected (Figure 2a). The venom with RA did not reveal any apparent degradation of human fibrinogen (Figure 2b). RA also inhibited Aα hydrolysis by bilitoxin-2 (Figure 2c and 2d).
Figure 2.
Effect of RA on human fibrinogen hydrolytic activities by T. flavoviridis venom and bilitoxin-2. 10% SDS-PAGE of time-dependent digestion of human fibrinogen by T. flavoviridis venom and bilitoxin-2 in the presence or absence of RA. (a) T. flavoviridis venom without RA; (b) T. flavoviridis venom with RA; (c) bilitoxin-2 without RA; (d) bilitoxin-2 with RA. Molecular weight makers of 97, 66, 43, 30, 20.1, and 14.4 kDa were used.
3.3. Inhibition of Venom Cytotoxic Action on HUVEC
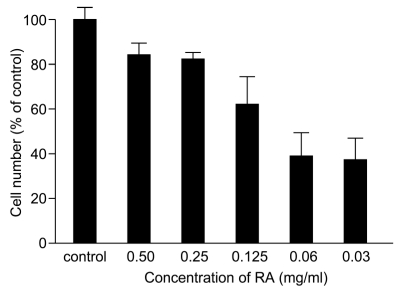
RA alone had no effect on the viability of HUVEC, but it markedly protected HUVEC from the toxic effects of T. flavoviridis venom (0.14 mg/mL) at all concentrations of RA tested (0.50, 0.25, 0.125, 0.06, and 0.03 mg/mL) (Figure 3). The maximum (84.2%) protective effect of RA was exhibited with RA at 0.5 mg/mL.
3.4. Inhibition of Type IV Collagen Hydrolytic Activity
Type IV collagen was incubated with T. flavoviridis venom for different periods of time. The venom completely degraded type IV collagen (104 kDa), especially over 1 h, and degradates with smaller molecular weights (43 and 35 kDa) appeared, as shown in Figure 4a. In the presence of RA (0.5 mg/mL), type IV collagen was not digested by incubation with the venom (Figure 4b).
Figure 3.
Effects of RA against the cytotoxic actions of T. flavoviridis venom on HUVEC.
Figure 4.
Effect of RA on type IV collagen hydrolytic activity by T. flavoviridis venom. 7.5% SDS-PAGE of time-dependent digestion of type IV collagen by T. flavoviridis venom in the presence or absence of RA. (a) venom without RA, (b) venom with RA. Molecular weight makers of 97, 66, 43, and 30 kDa were used.
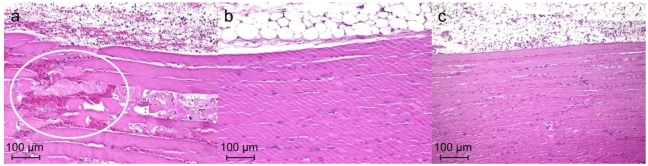
3.5. Histological Study of T. flavoviridis Venom and the Effect of RA
Both hemorrhage and neutrophil infiltrations were observed in a wide area (of the circle) after injection of T. flavoviridis venom (0.21 mg/mL) (Figure 5a). The result showed normal musculature devoid of hemorrhage and neutrophils in the muscle fibers after injection of RA (0.5 mg/mL) (Figure 5b). There was no hemorrhage or neutrolphil infiltration in the muscle fibers after injection of a mixture of the venom (0.41 mg/mL) and RA (0.5 mg/mL, or 0.25 mg/mL; Figure 5c).
Figure 5.
Histological results of thigh muscle after (a) injection of T. flavoviridis venom (0.21 mg/mL) alone (b) injection of RA (0.5 mg/mL) alone (normal muscle), (c) injection of a mixture of RA (0.25 mg/mL) and the venom (0.41 mg/mL).
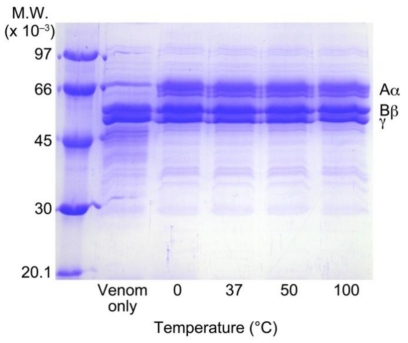
3.6. Heat Stability of RA
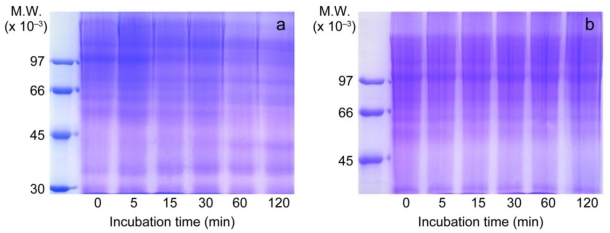
RA, which was heated at various temperatures, was incubated with human fibrinogen and T. flavoviridis venom. RA inhibited the digestion of Aα chain of human fibrinogen after treatment at each temperature (Figure 6). RA (0.5 mg/mL), which was heated at 100 °C for 10 min, also showed complete inhibition against T. flavoviridis venom.
Figure 6.
Heat stability study of RA by human fibrinogen digestion by T. flavoviridis venom. RA was heated at 37 ºC, 50 ºC, or 100 ºC, then RA (0.5 mg/mL) was mixed with human fibrinogen and venom (0.21 mg/mL), and the solution was incubated at 37 °C for 10 min. Aliquots were subjected to SDS-PAGE (7.5% gel).
3.7. Inhibition of Venom-Induced Edema
The edema-forming activity was assayed using four mice. T. elegans venom induced an edema of 30% in the mouse footpad, at a dose of 12.5 µg. When T. elegans venom was preincubated with RA (0.5 mg/mL), the edema-forming was significantly reduced.
4. Discussion
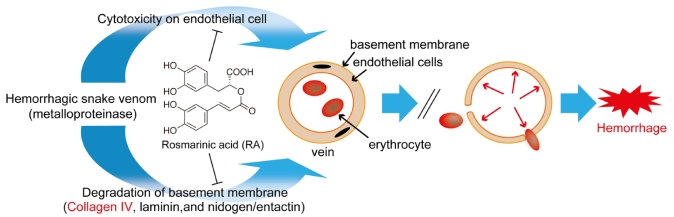
RA effectively inhibited snake venom induced hemorrhage by crude venoms of T. flavoviridis, Crotalus atrox, Gloydius blomhoffii, and B. arietans or purified toxins (HTb, bilitoxin-2, HT-1 and Ac1-proteinase) [10]. As shown in Figure 7, envenomation by snakebites often produces persistent hemorrhage due to considerable degradation of fibrinogen and other coagulation factors, thus preventing clot formation [21]. The pathogenesis of venom-induced hemorrhage involves direct damage to endothelial cells in microvessels by hemorrhagic toxins [22,23]. Snake venom metalloproteinases (especially snake venom metalloproteinase from T. flavoviridis venom) degrade the most important components of the basement membrane, such as laminin, type IV collagen and nidogen/entactin [24,25,26]. In this study, an attempt was made to determine the protective effects of RA on digestion of human fibrinogen, digestion of type IV collagen and cytotoxic action on HUVEC induced by T. flavoviridis venom. The pure compound showed antifibrinogenolytic activity by inhibiting the digestion of the Aα chain of human fibrinogen. RA also effectively inhibited HUVEC against the toxic action of T. flavoviridis venom at various concentrations and digestion of type IV collagen. Moreover, the pathological study of thigh muscles showed that RA inhibited hemorrhage and neutrophil infiltrations. T. flavoviridis venom-induced lethality was significantly antagonized by RA (960 µg), whereas the venom is highly lethal to mice with 200 µg. The compound inhibited the edema‑forming effect of T. elegans venom and lethal action induced by T. flavoviridis venom. The aforementioned evidence demonstrates that RA inhibited most of the hemorrhage effects of venom (Figure 7). It has been reported that RA isolated from Cordia verbenacea (Boraginaceae) inhibits the edema and myotoxic activity induced by crude venom and isolated PLA2s [27]. However, the mechanisms of action of PLA2s are quite different from those of metalloproteinases. This is the first report of RA that demonstrates the inhibitory mechanism for snake venom induced hemorrhage and protection from snakebite envenomation.
Figure 7.
Mechanisms of hemorrhage induction by snake venom, and points of action of RA for inhibition of hemorrhage.
In this study, the inhibitory activities of rosmarinic acid from Argusia argentea against the action of snake venom were investigated. The plant is found on tropical shores of the Pacific and Indian oceans and the extract of the leaves are locally used as an antitoxin for snakebites and fish poisoning [28,29]. In the Okinawa islands, the leaves of Argusia argentea are the first medical treatment against snake venom as well as against jellyfish venom [30]. Hemorrhage is a conspicuous sequela of envenomation by Viperidae. Hemorrhage from damage to the vascular endothelium of vital organs causes death [31]. These observations confirmed that RA possesses potent snake venom neutralizing properties. Furthermore, the heat stability of RA would satisfy a requirement for a first-aid treatment for snakebite. Because snakebites often occur outdoors far from medical institutions, it is necessary to distribute a drug to distant locations without refrigeration, and the drug must be storable at room temperature. Further studies are needed to investigate post-administration of RA, and the effects of different methods of administration. After these issues are resolved, RA could become a potent alternative antidote compound for snake envenomation.
Acknowledgements
This work was supported by the bounty for academic research from the Research Institute of Meijo University. One of the authors (H.T.A.) is supported by the Japanese Government (MEXT) Scholarship 2007 to study in Japan.
References
- 1.Warrell D.A. The global problem of snakebite: Its prevention and treatment. In: Gopalakrishnakone P., Tu C.K., editors. Recent Advances in Toxinology Research. National University of Singapore; Singapore: 1992. pp. 121–153. [Google Scholar]
- 2.Chippaux J.P. Snake-bites: Appraisal of the global situation. Bull. World Health Organ. 1998;76:515–524. [PMC free article] [PubMed] [Google Scholar]
- 3.Swaroop S., Grab B. Snakebite mortality in the world. Bull. World Health Organ. 1954;10:35–76. [PMC free article] [PubMed] [Google Scholar]
- 4.Theakston R.D.G., Reid H.A. Development of simple standard assay procedures for the characterization of snake venom. Bull. World Health Organ. 1983;61:949–956. [PMC free article] [PubMed] [Google Scholar]
- 5.Tu A.T. Venoms: Chemistry and Molecular Biology. Wiley; New York, NY, USA: 1977. [Google Scholar]
- 6.Tu A.T. Rattlesnake Venoms: Their actions and Treatment. Dekker; New York, NY, USA: 1982. [Google Scholar]
- 7.Martz W. Plants with a reputation against snakebite. Toxicon. 1992;30:1131–1142. doi: 10.1016/0041-0101(92)90429-9. [DOI] [PubMed] [Google Scholar]
- 8.Mors W.B., Nascimento M.C., Pereira B.M., Pereira N.A. Plant natural products active against snake-bite —the molecular approach. Phytochemistry. 2000;55:627–642. doi: 10.1016/s0031-9422(00)00229-6. [DOI] [PubMed] [Google Scholar]
- 9.Soares A.M., Januario A.H., Lourenco M.V., Pereira A.M., Pereira P.S. Neutralizing effects of Brazilian plants against snake venoms. Drugs Future. 2004;29:1105–1117. [Google Scholar]
- 10.Aung H.T., Nikai T., Niwa M., Takaya Y. Rosmarinic acid in Argusia argentea inhibits snake venom-induced hemorrhage. J. Nat. Med. 2010;64:482–486. doi: 10.1007/s11418-010-0428-3. [DOI] [PubMed] [Google Scholar]
- 11.Komori Y., Hagihara S., Tu A.T. Specificity of hemorrhagic proteinase from Crotalus atrox (western diamondback rattlesnake) venom. Biochim. Biophys. Acta. 1985;829:127–130. doi: 10.1016/0167-4838(85)90076-7. [DOI] [PubMed] [Google Scholar]
- 12.Nikai T., Taniguchi K., Komori Y., Sugihara H. Hemorrhagic toxin, bilitoxin-2, from Agkistrodon bilineatus venom. J. Nat. Toxins. 1996;5:95–106. [Google Scholar]
- 13.Nikai T., Sugihara H., Tanaka T. Enzymochemical studies on snake venoms II. Purification of lethal protein Ac1-proteinase in the venom of Agkistrodon acutus. Yakugaku Zasshi. 1977;97:507–514. doi: 10.1248/yakushi1947.97.5_507. [DOI] [PubMed] [Google Scholar]
- 14.Nikai T., Suzuki J., Komori Y., Sugihara H. Biochemical and pathological studies on hemorrhagic toxin. J. Nat. Toxins. 1995;4:83–96. [Google Scholar]
- 15.Bjarnason J.B., Tu A.T. Hemorrhagic toxins from western diamondback rattlesnake (Crotalus atrox) venom: Isolation and characterization of five toxins and the role of zinc in hemorrhagic toxin e. Biochemistry. 1978;17:3395–3404. doi: 10.1021/bi00609a033. [DOI] [PubMed] [Google Scholar]
- 16.Ouyang C., Teng C.M. Fibrinogenolytic enzymes of Trimeresurus mucrosquamatus venom. Biochim. Biophys. Acta. 1976;420:298–308. doi: 10.1016/0005-2795(76)90321-4. [DOI] [PubMed] [Google Scholar]
- 17.Ishiyama M., Miyazono Y., Sasamoto K., Ohkura Y., Ueno K. A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta. 1997;44:1299–1305. doi: 10.1016/s0039-9140(97)00017-9. [DOI] [PubMed] [Google Scholar]
- 18.Tominaga H., Ishiyama M., Ohseto F., Sasamoto K., Hamamoto T., Suzuki K., Watanabe M. A water-soluble tetrazolium salt useful for colorimetric cell viability assay. Anal. Commun. 1999;36:47–50. [Google Scholar]
- 19.Komori Y., Nikai T., Taniguchi K., Masuda K., Sugihara H. Vascular endothelial growth factor VEGF-like heparin-binding protein from the venom of Vipera aspis aspis (Aspic Viper) Int. J. Biochem. 1999;38:11796–11803. doi: 10.1021/bi990562z. [DOI] [PubMed] [Google Scholar]
- 20.Ho C.L., Hwang L.L., Chen C.T. Edema-inducing activity of a lethal protein with phospholipase A1 activity isolated from the black-bellied hornet (Vespa basalis) venom. Toxicon. 1993;31:605–613. doi: 10.1016/0041-0101(93)90115-y. [DOI] [PubMed] [Google Scholar]
- 21.Laing G.D., Moura-da-Silva A.M. Jararhagin and its multiple effects on hemostasis. Toxicon. 2005;45:987–996. doi: 10.1016/j.toxicon.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Bjarnason J.B., Fox J.W. Hemorrhagic metalloproteinases from snake venoms. Pharmacol. Ther. 1994;62:325–372. doi: 10.1016/0163-7258(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 23.Markland F.S. Snake venoms and the hemostatic system. Toxicon. 1998;36:1749–1800. doi: 10.1016/s0041-0101(98)00126-3. [DOI] [PubMed] [Google Scholar]
- 24.Baramova E.N., Shannon J.D., Bjarnason J.B., Fox J.W. Degradation of extracellular matrix proteins by hemorrhagic metalloproteinases. Arch. Biochem. Biophys. 1989;275:63–71. doi: 10.1016/0003-9861(89)90350-0. [DOI] [PubMed] [Google Scholar]
- 25.Baramova E.N., Shannon J.D., Bjarnason J.B., Fox J.W. Identification of the cleavage sites by a hemorrhagic metalloproteinase in type IV collagen. Matrix. 1990;10:91–97. doi: 10.1016/s0934-8832(11)80175-7. [DOI] [PubMed] [Google Scholar]
- 26.Mashiko H., Takahashi H. Haemorrhagic factors from snake venoms. II. Structures of haemorrhagic factors and types and mechanisms of haemorrhage. J. Toxicol. Toxin Rev. 1998;17:493–512. [Google Scholar]
- 27.Ticli F.K., Hage L.I.S., Cambraia R.S., Pereira P.S., Magro A.J., Fontes M.R.M., Stábeli R.G., Giglio J.R., França S.C., Soares A.M., Sampaio S.V. Rosmarinic acid, a new snake venom phospholipase A2 inhibitor from Cordia verbenacea (Boraginaceae): Antiserum action potentiation and molecular interaction. Toxicon. 2005;46:318–327. doi: 10.1016/j.toxicon.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 28.Ogihara K., Miyagi Y., Higa M., Yogi S. Pyrrolizidine alkaloids from Messerschmidia argentea. Phytochemistry. 1997;44:545–547. [Google Scholar]
- 29.Noro T., Nishihara G.N., Terada R., Yoropiy A. Ciguatera Fish Poisoning in Ulithi Atoll, Yap State, Micronesia. Kagoshima Univ. Res. Ctr. Pac. Isl. Occas. Pap. 2003;39:83–86. [Google Scholar]
- 30.Ogihara K., Nakazato R., Nishi Y., Higa M., Yogi S. DPPH-radical scavenging constituents from the twigs of Messerschmidia argentea. Bull. Fac. Sci. Univ. Ryukyus. 2002;74:73–80. [Google Scholar]
- 31.Warrell D.A. Russell’s viper: biology, venom and treatment of bites. Trans. R. Soc. Trop. Med. Hyg. 1989;83:732–740. doi: 10.1016/0035-9203(89)90311-8. [DOI] [PubMed] [Google Scholar]