Abstract
Photobacterium damselae subsp. piscicida (Phdp) is a Gram-negative pathogen agent of an important fish septicemia. The key virulence factor of Phdp is the plasmid-encoded exotoxin AIP56, which is secreted by exponentially growing pathogenic strains. AIP56 has 520 amino acids including an N-terminal cleavable signal peptide of 23 amino acid residues, two cysteine residues and a zinc-binding region signature HEXXH that is typical of most zinc metallopeptidases. AIP56 induces in vitro and in vivo selective apoptosis of fish macrophages and neutrophils through a caspase-3 dependent mechanism that also involves caspase-8 and -9. In vivo, the AIP56-induced phagocyte apoptosis progresses to secondary necrosis with release of cytotoxic phagocyte molecules including neutrophil elastase. Fish injected with recombinant AIP56 die with a pathology similar to that seen in the natural infection.
Keywords: Photobacterium damselae subsp. piscicida, AIP56, AB toxin, apoptosis, secondary necrosis
1. The Apoptogenic Exotoxin AIP56 Is the Key Virulence Factor of Photobacterium damselae subsp. piscicida
Photobacterium damselae subsp. piscicida (Phdp) is a Gram-negative pathogen that was isolated for the first time from a massive fish kill in Chesapeake Bay [1]. The disease has been named pseudotuberculosis and, after the initial classification of this agent as Pasteurella piscicida [2], as pasteurellosis. The pathogen was later reassigned to the genus Photobacterium as Photobacterium damsela subsp. piscicida [3], the name afterwards corrected to Photobacterium damselae subsp. piscicida [4]. Consequently, the disease is also referred to as photobacteriosis. Phdp is highly pathogenic for wild and cultured marine fish and has been found to affect more than 20 warm waterfish species worldwide, including sea bass, sole, yellowtail, gilt-head sea bream and turbot [5,6,7]. Phdp infection is now recognized as one of the most threatening bacterial diseases in mariculture worldwide due to its wide host range, massive mortality, ubiquitous geographical distribution, widespread antibiotic resistance and lack of efficient vaccines [8]. In its acute form, this disease has a septicemic nature and has a short course and very high mortality [5,7,9].
Early descriptions of the histopathology of Phdp infection and of the toxicity of Phdp extracellular products recognized the occurrence of cytotoxic alterations [6,10,11,12,13,14,15,16], but the cytological characterization of these alterations and the mechanism for their production were not elucidated. The re-evaluation of the interaction of virulent Phdp with fish phagocytes was undertaken in our laboratory using the intraperitoneal (i.p.) inoculation in sea bass. This analysis revealed that the cytopathology previously described in Phdp infection is due to pathogen-induced macrophage and neutrophil apoptotic death [17]. The virulence of Phdp strains from different geographical locations, including Portugal, Spain, Italy, Greece and Japan, was associated to the apoptogenic activity of the pathogen [17].
Different studies have identified several factors/mechanisms that could be involved in the virulence of Phdp [6,11,18]. However, the recognition that (i) systemic macrophage and neutrophil apoptosis was a marked feature of experimental Phdp infections; (ii) apoptosis of macrophages and neutrophils was observed after injection of virulent bacterial culture supernatants but not of UV-killed virulent bacteria; and (iii) the apoptogenic activity of culture supernatants was abolished by heat-treatment, led us to admit that one (or several) protein(s) secreted by virulent Phdp strains would be responsible for the apoptogenic activityof these bacteria [17].
Considering that infecting Phdp in the host would be metabolically active, we searched for the putative apoptogenic factor in supernatants of mid-exponential cultures of Phdp [19]. Surprisingly, this search revealed a major protein band in the electrophoretic profiles of those supernatants of virulent Phdp but not in nonvirulent strains. This finding was in contrast to previous studies suggesting the secretion of a highly complex mixture of different proteins by Phdp [20]. The larger protein complexity of the culture supernatants observed by those researchers could be explained by the fact that stationary-phase cultures were used in their studies.
Concentrated cell-free culture supernatants from a virulent Phdp strain were resolved by native-PAGE, fractionated, and the fraction containing the major protein showed in vivo capacity to induce apoptosis of sea bass macrophages and neutrophils, pointing to the likelihood of it being the virulent apoptogenic factor of pathogenic Phdp. SDS-PAGE analysis of that fraction revealed a single protein band of approximately 56 kDa. The protein was named AIP56 (apoptosis inducing protein of 56 kDa) [19].
Recombinant AIP56 expressed in Escherichia coli BL21 was found to retain the apoptogenic activity of Phdp AIP56 towards macrophages and neutrophils when injected i.p. in sea bass or when the toxin was incubated ex vivo with peritoneal sea bass phagocytes. These results demonstrated that the exotoxin AIP56 is the factor responsible for the apoptogenic activity of virulent Phdp [19].
The primary structure of AIP56 has been characterized [19]. AIP56 was annotated as a 513 amino acid precursor protein, including an N-terminal cleavable signal peptide of 16 amino acid residues (GeneBank Accession number DQ066884). However, an alternative GTG start codon exists 21 nucleotides ahead of the previously annotated ATG start codon, extending the signal peptide to 23 amino acids and the full length protein to 520 amino acids. Moreover, AIP56 displays a hydropathic profile typical of a non-membrane protein.
Table 1.
Sequences producing significant alignments from Blast analysis of the AIP56 protein sequence against the non-redundant protein database at http://blast.ncbi.nlm.nih.gov/Blast.cgi.
| Accession | Description | Max score | Total score | Query coverage | E value |
|---|---|---|---|---|---|
| YP_003422532.1 | Aip56 [Photobacterium damselae subsp. piscicida] | 1064 | 1064 | 98% | 0.0 |
| BAF99004.1 | apoptosis inducing protein [Photobacterium damselae subsp. piscicida] | 1046 | 1046 | 98% | 0.0 |
| ZP_02194626.1 | hypothetical protein 1103602000593_AND4_00648 [Vibrio sp. AND4] | 597 | 597 | 99% | 9.00E-169 |
| CBA72068.1 | non-LEE encoded type III effector C [Arsenophonus nasoniae] | 306 | 306 | 94% | 3.00E-81 |
| CBA72300.1 | apoptosis inducing protein [Arsenophonus nasoniae] | 281 | 281 | 93% | 1.00E-73 |
| CBA76058.1 | non-LEE encoded type III effector C [Arsenophonus nasoniae] | 275 | 275 | 93% | 7.00E-72 |
| YP_002308522.1 | hypothetical protein D [Bacteriophage APSE-2] | 194 | 194 | 36% | 2.00E-47 |
| CBA74519.1 | non-LEE encoded type III effector C [Arsenophonus nasoniae] | 192 | 192 | 94% | 8.00E-47 |
| ZP_04620510.1 | Non-LEE encoded type III effector C [Yersinia aldovae ATCC 35236] | 178 | 178 | 48% | 2.00E-42 |
| NP_286533.1 | hypothetical protein Z0986 [Escherichia coli O157:H7 EDL933] | 178 | 178 | 50% | 2.00E-42 |
| YP_003229213.1 | T3SS secreted effector NleC-like protein [Escherichia coli O26:H11 str. 11368] | 178 | 178 | 50% | 2.00E-42 |
| YP_002328603.1 | T3SS secreted effector NleC homolog [Escherichia coli O127:H6 str. E2348/69] | 178 | 178 | 50% | 2.00E-42 |
| YP_003234807.1 | T3SS secreted effector NleC-like protein [Escherichia coli O111:H- str. 11128] | 177 | 177 | 50% | 4.00E-42 |
| YP_003234967.1 | T3SS secreted effector NleC-like protein [Escherichia coli O111:H- str. 11128] | 174 | 174 | 50% | 2.00E-41 |
| YP_003365223.1 | T3SS effector protein NleC [Citrobacter rodentium ICC168] | 173 | 173 | 48% | 6.00E-41 |
| ZP_03043710.1 | non-LEE encoded type III effector C [Escherichia coli E22] | 172 | 172 | 50% | 7.00E-41 |
| ZP_02576318.1 | non-LEE encoded type III effector C [Salmonella enterica subsp. enterica serovar 4,[5],12:i:- str. CVM23701] | 169 | 169 | 48% | 9.00E-40 |
| YP_001762636.1 | hypothetical protein Swoo_4286 [Shewanella woodyi ATCC 51908] | 83.2 | 83.2 | 28% | 6.00E-14 |
| ZP_03318593.1 | hypothetical protein PROVALCAL_01527 [Providencia alcalifaciens DSM 30120] | 38.9 | 38.9 | 25% | 1.7 |
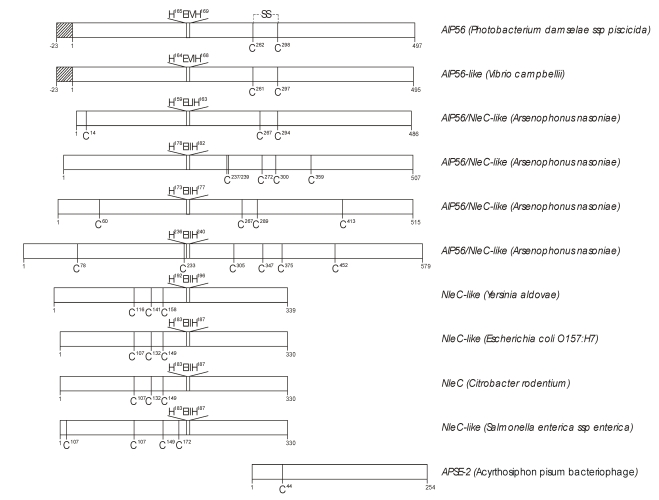
Two other relevant aspects of AIP56 primary structure are the presence of only two cysteine residues (C278 and C314) and of a zinc-binding region signature HEXXH that is typical of most zinc metallopeptidases. Further characterization, using Blast analysis on non-redundant protein sequences database (http://blast.ncbi.nlm.nih.gov/Blast.cgi), revealed as the closest hit a homologous hypothetical protein of Vibrio campbellii, another widely distributed pathogen of cultured marine organisms [21] (Table 1). The next hits with the full length AIP56 were four recently annotated proteins of Arsenophonus nasoniae, a son-killer bacterium of the wasp Nasonia vitripennis, resulting from four open reading frames dispersed throughout the genome [22] (Table 1). These AIP56-like proteins present a primary structure highly similar to that of AIP56 (Figure 1). The Blast analysis also revealed the similarity of the N-terminal of AIP56 (first 324 amino-acids) with type III secreted effectors C from enteric pathogenic bacteria, and of the C-terminal with a hypothetical protein of the temperate lambda-like bacteriophage APSE-2, a phage that infects Hamiltonella defense, the endosymbiont of the pea aphid Acyrthosiphon pisum (reviewed in ref. [23]). Most remarkable is the conservation of the zinc-metalloprotease signature in the referred type III secreted effectors (Figure 1). The above mentioned analysis suggests that AIP56 has two domains, possibly linked by a disulfide bridge. This hypothesis has been supported in our laboratory by limited proteolysis experiments, which showed the existence of two major digestion fragments with sizes consistent with the two putative AIP56 domains predicted by homology analysis (unpublished results). N-terminal sequencing and MS analysis of the chymotrypsin digestion fragments revealed that the cleavage occurs between F285 and F286 lying in the amino-acid stretch between the two unique cysteine residues (C278 and C314), and analysis of the digests under non-reducing conditions confirmed the existence of a disulfide bridge linking the two proteolytic fragments, as a single band of 56 kDa was obtained.
The above data led us to hypothesize that AIP56 is an AB-toxin, similar to tetanus and botulinum neurotoxins. Tetanus and botulinum neurotoxins are AB toxins that possess an A domain displaying metalloprotease activity linked to a B domain by a single disulfide bridge [24,25]. Considering that the AIP56 N-terminal region has a high identity with type III effectors from enteric pathogenic bacteria, and that a zinc-metalloprotease signature is conserved both in the AIP56 N-terminal and in the type III effectors, we hypothesize that the N-terminal region is responsible for the toxin’s apoptogenic activity and the C-terminal region would be responsible for the toxin binding/entry into the cells. Further studies are presently ongoing to assess this hypothesis.
As observed for many bacterial toxin-specific genes, which are often located on mobile genetic elements [28], AIP56 was also found to be encoded in a high-copy plasmid present in all virulent Phdp strains tested and from diverse geographical locations [19]. The location of the AIP56 gene in a high-copy plasmid is probably the reason why Phdp virulent strains are capable of producing large amounts of exotoxin.
Like in other pathogens, the virulence of Phdp certainly is multifactorial. However, the AIP56-dependent pathogenicity mechanism most likely is central in the etiopathogenesis of fish pasteurellosis because: (i) the AIP56 gene is present in all virulent strains and absent in the non-virulent isolates tested [19]; (ii) systemic phagocyte destruction similar to that seen in fish with advanced natural or experimental pasteurellosis was seen in moribund fish following the inoculation of recombinant AIP56 [29]; (iii) passive immunization with anti-AIP56 rabbit serum protects sea bass against Phdp infection [19].
The importance of this novel pathogenicity factor of Phdp is emphasized by the observation that AIP56-induced apoptotic phagocyte destruction observed in experimental infections also occurs in sea bass and sole with natural Phdp infection [29].
Figure 1.
Schematic diagram comparing the main features of the primary structure of AIP56 with proteins retrieved by Blast analysis of the AIP56 protein sequence against the non-redundant protein sequences database. Striped bars represent signal peptides according to SignalP at http://www.cbs.dtu.dk/services/SignalP/ [26,27].The conserved zinc-metalloprotease signature HEXXH and cysteine residues are represented at their relative positions. Numbers represent amino acid residues.
2. AIP56 Induces a Typical Apoptotic Death in Fish Macrophages and Neutrophils
The initial observations in sea bass experimental Phdp infection pointed to the selective targeting of both professional phagocytes by a Phdp cytotoxin [17]. To better dissect the anti-phagocyte apoptogenic activity of AIP56, an ex vivo model was used. The cells used in the ex vivo studies were inflammatory peritoneal leukocytes collected from peritoneal cavities of sea bass injected with Incomplete Freund’s Adjuvant 15 days before collection [19] (Figure 2). The apoptotic nature of the alterations seen in these leukocytes was established by the presence of a set of indicators [30,31,32]: cell shrinkage, chromatin condensation, nuclear fragmentation, production of apoptotic bodies, internucleosomal DNA degradation revealed by nuclear TUNEL positivity and by DNA electrophoresis, and activation of the apoptosis executioner caspase-3. These experiments showed that the only cells in the inflamed peritoneal population affected by the apoptogenic activity of AIP56 were macrophages and neutrophils [19]. The apoptogenic activity of AIP56 against the murine macrophage cell line J774, bone-marrow derived mouse macrophages and Drosophila S2 macrophage-like cell line was also tested. AIP56 did not display apoptogenic activity against those cells (unpublished results).
Recent results [33,34] showed that the AIP56-induced selective apoptosis of fish macrophages and neutrophils also involves activation of caspases-8 and -9. Following the molecular cloning and characterization of sea bass caspases-3, -8, and -9, the expression of these caspases was analyzed in sea bass with experimental Phdp infection or injected with AIP56-containing Phdp culture supernatants. The very low basal expression of those caspases increased in the initial 12-24 h post-infection or post-injection. Additionally, caspase-3, -9, and -8 proteolytic activities were detected in organs of sea bass with terminal infection [29,33,34].
Involvement of mitochondria was also found, as shown by loss of mitochondrial membrane potential, translocation of cytochrome c to the cytosol and over-production of ROS.
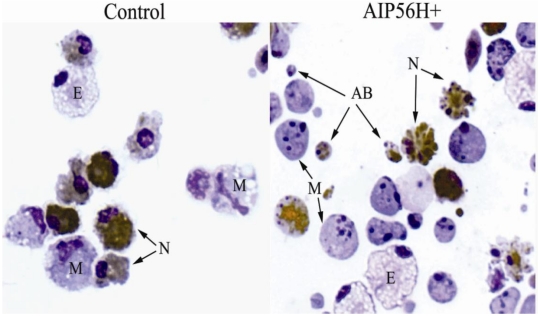
Figure 2.
Wright-stained cytospins of sea bass peritoneal leukocytes 4 h after i.p. injection of 2 μg of recombinant AIP56 (AIP56H+) or the same amount of heat-inactivated recombinant AIP56 (control). Neutrophils were labeled by peroxidase detection (brown granules). In cells exposed to AIP56H+, note the occurrence of extensive nuclear fragmentation and chromatin condensation in macrophages (M) and neutrophils (N), cell blebbing in neutrophils, and the presence of apoptotic bodies (AB). The eosinophilic granular cells (E) look normal in both samples. Objective 100 ×. From reference [19].
3. Pathogenetic Consequences of the in Vivo Cytopathological Effects of AIP56 Toxin
Apoptosis [35] is one mode of active cell death that, in multicellular animals, is a major physiological cell killing process used during embryonic morphogenesis and in adult life in tissue homeostasis and immune responses for the elimination of unnecessary, unwanted or dangerous cells [36,37]. Typically, in physiological situations, the apoptotic mode of cell elimination is a useful process since it is induced when appropriate, comprises the induction of a safe mechanism for cell disposal through the removal of apoptosing cells by scavenger cells, and, although spending energy, is economical in terms of re-utilization of the products of this disposal [35]. However, the perspective of apoptosis as a useful process is restrictive, since this mode of cell elimination can be subverted, leading to harmful pathological situations. In this context, it has to be noted that fully developed apoptosis leads to necrotic cell disintegration. Indeed, soon after the proposal the term apoptosis [35], it was recognized that in vitro apoptosis proceeds to necrosis leading to cell disruption [38,39], a process called secondary necrosis [30]. However, in multicellular organisms, the apoptotic process comprises a mechanism for the removal and degradation of the apoptosing cell through phagocytosis by scavenger cells, mainly macrophages [40,41]. This removal occurs while the apoptosing cell still is enveloped by a membrane that retains potentially harmful components, thus preventing the progression to secondary necrosis. When insufficient removal of apoptosing cells occurs, secondary necrosis ensues leading to cell lysis, which is pathogenetic [42]. Insufficient clearance of apoptosing cells in vivo has been described in situations of massive apoptosis that overwhelms the available scavenging capacity [43,44,45], or when this capacity is directly impaired by deleterious effects on macrophages [46,47,48,49].
Apoptosis also turns pathological when the killed cells are necessary and functional, for example in degenerative disorders and in infectious diseases when pathogens destroy protective immune cells leading to evasion from host anti-microbial mechanisms [50,51,52,53].
In the early phase of infection, Phdp is seen within macrophages in natural [9] and experimental Phdp infection [13,29]. However, as the infection progresses, systemic dissemination of the pathogen occurs as indicated by the isolation of increasing numbers of bacteria from the blood and organs in fish experimentally infected with Phdp [29]. Systemic dissemination is accompanied by extensive extracellular bacterial multiplication as suggested by the observation of dense aggregates of extracellular bacteria (micro-colonies) in infected tissues. Extracellular Phdp are present in the spleen, headkidney, liver, gut lamina propria and blood, indicating that advanced Phdp infection is a septicemic situation. The septicemic spread of Phdp in the infected host is paralleled by the occurrence of AIP56 in the plasma [29].
Extensive infiltration of macrophages and neutrophils occurs in the initial phase of infection when local multiplication of Phdp becomes detectable in infected tissues [29]. This initial accumulation of phagocytes is followed by extensive phagocyte depletion in advanced experimental Phdp infections [29]. This depletion correlates with the appearance of high numbers of macrophages and neutrophils with apoptotic features [17,29]. Apoptosing cells, identified by an apoptotic morphotype, TUNEL positivity and presence of active caspase-3, were seen in foci and scattered in the splenic and head kidney parenchymas, in the peripheral blood, in blood of the spleen, liver and head kidney vasculature and in gut lamina propria. In fish experimentally infected with Phdp or injected with the toxin AIP56, detachment of enterocytes was observed [54]; the detached enterocytes enter detachment-induced epithelial cell apoptosis or anoikis [55]. Supporting the interpretation that AIP56 is the apoptogenic factor of Phdp towards fish macrophages and neutrophils, leukocytes with apoptotic features present in Phdp-infected tissues immunostain for AIP56 [29] (Figure 3).
Figure 3.
Blood in a vessel of the spleen of a sea bass with advanced natural Phdp infection labeled by immunocytochemistry for AIP56 exotoxin. Apoptosing cells with fragmented nuclei and/or condensed chromatin are immunostained for AIP56 (brown). Objective 100 ×. From ref. [29].
In advanced Phdp infection, foci of necrosis with abundant cell debris are prominent [29]. There are data that support the interpretation that this necrosis is the result of the progression of phagocyte apoptosis to secondary necrosis due to the lack of removal of abundant apoptosing phagocytes. Activated caspases are released by cells undergoing apoptotic secondary necrosis when rupture of the cytoplasmic membrane occurs [56] and occurrence of relevant apoptotic secondary necrosis can be detected in vivo by the presence of increased levels of extracellular activated caspases, which can be quantified in the blood [57,58,59]. An indication that the extensive phagocyte lysis in the advanced phase of the disease is due to the progression to secondary necrosis of the phagocytes is the observation in terminal infection of elevated blood levels of activated caspase-3 [29]. This result agrees with the observation by electron microscopy in fish with advanced Phdp infections of phagocytes with condensed chromatin and fragmented nuclei (typical of apoptosing cells) together with ruptured cytoplasmic membrane (typical of necrosis) [17], a hallmark of secondary necrosis [42].
Lysis of phagocytes by secondary necrosis, leading to the release of highly cytotoxic components, is particularly pathogenetic in the case of neutrophils. Neutrophils are extraordinarily rich in highly cytotoxic components which, if released in excess, can damage many types of cells, with the potential to produce tissue injury [60,61,62]. One such molecule is the destructive enzyme neutrophil elastase [62,63]. Active neutrophil elastase was found in the plasma of fish with terminal Phdp infection [29]. In fish with advanced disease the levels of circulating active neutrophil elastase highly correlated with the levels of circulating activated caspase-3, suggesting that in terminal Phdp infection neutrophil lysis was due to secondary necrosis.
Experimental [17,29] and natural [5,7] acute Phdp infections are rapid, severe infections with a very high mortality. This may be related to the characteristics of the AIP56-dependent Phdp pathogenicity mechanism that indicate that it is a highly potent one, as follows. First, using an exotoxin, this mechanism can operate at a distance without requiring contact between the pathogen and the target cells. Second, apoptotic destruction of both macrophages and neutrophils, AIP56 not only impairs the participation of these leukocytes in phagocytosis and killing of Phdp, but also releases intraphagocytic bacteria present in the initial phase of the infection, two effects that promote survival of the pathogen and its unrestricted extracellular multiplication. Concomitantly, the AIP56-induced apoptosis of both professional phagocytes leads to tissue damage with deleterious consequence for the host. In fact, destruction of macrophages, the cells with the crucial role of eliminating apoptotic cells [40,41], results in deficient clearance of AIP56-induced abundant apoptotic neutrophils, which leads to their lysis by secondary necrosis [17], with the severe consequences due to the release of highly cytotoxic neutrophil molecules [60,61,62]. Finally, relying on a potent protein exotoxin, this pathogenicity mechanism requires the production of anti-AIP56 antibodies as a defensive response from the host, an adaptive immune response that cannot be achieved in the short time lapse of this fulminant infectious disease.
In innate and adaptive anti-bacterial immune responses macrophages and neutrophils work in concert as crucial elements of host defense against infection as the effectors of the myeloid phagocyte system [64,65]. The ability of bacterial pathogens to cause timely phagocyte cell death is important for virulence [66], and this anti-phagocytic mechanism may follow phagocytosis of the pathogen, depend on direct contact between the pathogen and the host cell target with transfer of effector molecules by secretion systems [67,68], or be mediated by exotoxins as in the case of Phdp. The simultaneous targeting of macrophages and neutrophils by Phdp through the AIP56 exotoxin thus represents a very effective pathogenicity strategy which contributes to the severity of acute Phdp infections. This ability of pathogens to simultaneously target the two professional phagocytes in vivo is not common, but a few examples have been reported, as is the case of Pseudomonas aeruginosa [69,70], Yersinia pestis [71] and Francisella tularensis [72,73].
In conclusion, the identification, characterization and production of recombinant active AIP56 allowed the clarification of the key virulence factor of an important fish pathogen and revealed a relevant bacterial pathogenicity weapon relying on a largely underestimated anti-phagocyte mechanism based on the lysis by apoptotic secondary necrosis of macrophages and neutrophils by a bacterial cytotoxin. The observation that neutralization of AIP56 activity by transfer of anti-AIP56 antibodies is effective in protecting sea bass against experimental Phdp infection open promising prospects towards the development of an effective anti-Phdp vaccine based on AIP56. Finally, the observed homology between AIP56 and type III secreted effectors from enteric pathogenic bacteria suggests possible commonalities between pathogenicity mechanisms of Phdp and those enteropathogens, with implications for human infections.
Acknowledgements
The work discussed in this review was funded by the FCT projects POCI/MAR/56111/2004 (funded by POCI 2010, co-funded by FEDER), PPCDT/MAR/56111 (funded by Programa 3599 PPCDT) and PTDC/CVT/69086/2006 (funded by PTDC). Ana do Vale was funded by “Ciência 2008”.
References and Notes
- 1.Snieszko S.F., Bullock G.L., Hollis E., Boone J.G. Pasteurella Sp. from an Epizootic of White Perch (Roccus Americanus) in Chesapeake Bay Tidewater Areas. J. Bacteriol. 1964;88:1814–1815. doi: 10.1128/jb.88.6.1814-1815.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janssen W.A., Surgalla M.J. Morphology, physiology, and serology of a Pasteurella species pathogenic for white perch. (Roccus americanus) J. Bacteriol. 1968;96:1606–1610. doi: 10.1128/jb.96.5.1606-1610.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gauthier G., Lafay B., Ruimy R., Breittmayer V., Nicolas J.L., Gauthier M., Christen R. Small-subunit rRNA sequences and whole DNA relatedness concur for the reassignment of Pasteurella piscicida (Snieszko et al.) Janssen and Surgalla to the genus Photobacterium as Photobacterium damsela subsp. piscicida comb. nov. Int. J. Syst. Bacteriol. 1995;45:139–144. doi: 10.1099/00207713-45-1-139. [DOI] [PubMed] [Google Scholar]
- 4.Trüper H.G., de’Clari L. Taxonomic note: necessary correction of specific epithets formed as substantives (nouns) ‘in apposition’. Int. J. Syst. Bacteriol. 1997;47:908–909. [Google Scholar]
- 5.Thune R.L., Stanley L.A., Cooper R.K. Pathogenesis of Gram-negative bacterial infections in warmwater fish. Annu. Rev. Fish Dis. 1993;3:337–368. [Google Scholar]
- 6.Magariños B., Toranzo A.E., Romalde J.L. Phenotypic and pathobiological characteristics of Pasteurella piscicida. Ann. Rev. Fish Dis. 1996;6:41–64. [Google Scholar]
- 7.Romalde J.L. Photobacterium damselae subsp. piscicida: an integrated view of a bacterial fish pathogen. Int. Microbiol. 2002;5:3–9. doi: 10.1007/s10123-002-0051-6. [DOI] [PubMed] [Google Scholar]
- 8.Barnes A.C., dos Santos N.M.S., Ellis A.E. Update on bacterial vaccines: Photobacterium damselae subsp. piscicida. In: Mydtlyng P.J., editor. Progress in Fish Immunology. Vol. 121. Karger; Basel, Switzerland: 2005. pp. 75–84. [PubMed] [Google Scholar]
- 9.Hawke J.P., Plakas S.M., Vernon Minton R., McPhearson R.M., Snider T.G., Guarino A.M. Fish pasteurellosis of cultured striped bass (Morone saxatilis) in coastal Alabama. Aquaculture. 1987;65:193–204. [Google Scholar]
- 10.Toranzo A.E., Barreiro S., Casal J.F., Figueras A., Magarinos B., Barja J.L. Pasteurellosis in cultured gilthead seabream (Sparus aurata): first report in Spain. Aquaculture. 1991;99:1–15. [Google Scholar]
- 11.Magariños B., Santos Y., Romalde J.L., Rivas C., Barja J.L., Toranzo A.E. Pathogenic activities of live cells and extracellular products of the fish pathogen Pasteurella piscicida. J. Gen. Microbiol. 1992;138:2491–2498. doi: 10.1099/00221287-138-12-2491. [DOI] [PubMed] [Google Scholar]
- 12.Kusuda R., Salati F. Major bacterial diseases affecting mariculture in Japan. Annu. Rev. Fish Dis. 1993;3:69–85. [Google Scholar]
- 13.Noya M., Magarinos B., Toranzo A.E., Lamas J. Sequential pathology of experimental pasteurellosis in gilthead seabream Sparus aurata. A light- and electron-microscopic study. Dis. Aquat. Org. 1995;21:177–186. doi: 10.3354/dao021177. [DOI] [Google Scholar]
- 14.Bakopoulos V., Peric Z., Rodger H., Adams A., Richards R. First report of fish pasteurellosis from Malta. J. Aquat. Anim. Health. 1997;9:26–33. [Google Scholar]
- 15.Poulos C., Bakopoulos V., Zolota V., Dimitriadis G.J. Histopathological findings after sea bass (Dicentrarhus labrax L.) exposure to extracellular products of Photobacterium damselae subsp. piscicida produced in vivo. Aquaculture Res. 2004;35:931–936. doi: 10.1111/j.1365-2109.2004.01086.x. [DOI] [Google Scholar]
- 16.Bakopoulos V., Hanif A., Poulos K., Galeotti M., Adams A., Dimitriadis G.J. The effect of in vivo growth on the cellular and extracellular components of the marine bacterial pathogen Photobacterium damsela subsp. piscicida. J. Fish Dis. 2004;27:1–13. doi: 10.1046/j.1365-2761.2003.00513.x. [DOI] [PubMed] [Google Scholar]
- 17.do Vale A., Marques F., Silva M.T. Apoptosis of sea bass (Dicentrarchus labrax L.) neutrophils and macrophages induced by experimental infection with Photobacterium damselae subsp. piscicida. Fish Shellfish Immunol. 2003;15:129–144. doi: 10.1016/S1050-4648(02)00144-4. [DOI] [PubMed] [Google Scholar]
- 18.Romalde J.L., Magariños B. Immunization with bacterial antigens: pasteurellosis. In: Gudding R., Lillehaug A., Midtlyng P.J., Brown F., editors. Fish Vaccinology. Karger; Basel, Switzerland: 1997. pp. 167–177. [PubMed] [Google Scholar]
- 19.do Vale A., Silva M.T., dos Santos N.M., Nascimento D.S., Reis-Rodrigues P., Costa-Ramos C., Ellis A.E., Azevedo J.E. AIP56, a novel plasmid-encoded virulence factor of Photobacterium damselae subsp. piscicida with apoptogenic activity against sea bass macrophages and neutrophils. Mol. Microbiol. 2005;58:1025–1038. doi: 10.1111/j.1365-2958.2005.04893.x. [DOI] [PubMed] [Google Scholar]
- 20.Bakopoulos V., Adams A., Richards R.H. The effect of iron limitation growth conditions on the cell and extracellular components of the fish pathogen Pasteurella piscicida. J. Fish Dis. 1997;20:297–305. [Google Scholar]
- 21.San Luis B.B., Hedreyda C.T. Analysis of a gene (vch) encoding hemolysin isolated and sequenced from Vibrio campbellii. J. Gen. Appl. Microbiol. 2006;52:303–313. doi: 10.2323/jgam.52.303. [DOI] [PubMed] [Google Scholar]
- 22.Darby A.C., Choi J.H., Wilkes T., Hughes M.A., Werren J.H., Hurst G.D., Colbourne J.K. Characteristics of the genome of Arsenophonus nasoniae, son-killer bacterium of the wasp Nasonia. Insect. Mol. Biol. 2010;19 (Suppl 1):75–89. doi: 10.1111/j.1365-2583.2009.00950.x. [DOI] [PubMed] [Google Scholar]
- 23.Degnan P.H., Yu Y., Sisneros N., Wing R.A., Moran N.A. Hamiltonella defensa, genome evolution of protective bacterial endosymbiont from pathogenic ancestors. Proc. Natl. Acad. Sci. USA. 2009;106:9063–9068. doi: 10.1073/pnas.0900194106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiavo G., Benfenati F., Poulain B., Rossetto O., Polverino de Laureto P., DasGupta B.R., Montecucco C. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359:832–835. doi: 10.1038/359832a0. [DOI] [PubMed] [Google Scholar]
- 25.Rossetto O., Seveso M., Caccin P., Schiavo G., Montecucco C. Tetanus and botulinum neurotoxins: turning bad guys into good by research. Toxicon. 2001;39:27–41. doi: 10.1016/s0041-0101(00)00163-x. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen H., Engelbrecht J., Brunak S., von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Bendtsen J.D., Nielsen H., von Heijne G., Brunak S. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 2004;340:783–795. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 28.Hacker J., Blum-Oehler G., Muhldorfer I., Tschape H. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol. Microbiol. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
- 29.do Vale A., Costa-Ramos C., Silva A., Silva D.S., Gartner F., dos Santos N.M., Silva M.T. Systemic macrophage and neutrophil destruction by secondary necrosis induced by a bacterial exotoxin in a Gram-negative septicaemia. Cell. Microbiol. 2007;9:988–1003. doi: 10.1111/j.1462-5822.2006.00846.x. [DOI] [PubMed] [Google Scholar]
- 30.Wyllie A.H., Kerr J.F., Currie A.R. Cell death: the significance of apoptosis. Int. Rev. Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- 31.Pallardy M., Biola A., Lebrec H., Breard J. Assessment of apoptosis in xenobiotic-induced immunotoxicity. Methods. 1999;19:36–47. doi: 10.1006/meth.1999.0825. [DOI] [PubMed] [Google Scholar]
- 32.Vermes I., Haanen C., Reutelingsperger C. Flow cytometry of apoptotic cell death. J. Immunol. Methods. 2000;243:167–190. doi: 10.1016/s0022-1759(00)00233-7. [DOI] [PubMed] [Google Scholar]
- 33.Reis M.I., do Vale A., Pinto C., Nascimento D.S., Costa-Ramos C., Silva D.S., Silva M.T., dos Santos N.M. First molecular cloning and characterisation of caspase-9 gene in fish and its involvement in a gram negative septicaemia. Mol. Immunol. 2007;44:1754–1764. doi: 10.1016/j.molimm.2006.07.293. [DOI] [PubMed] [Google Scholar]
- 34.Reis M.I., Nascimento D.S., do Vale A., Silva M.T., dos Santos N.M. Molecular cloning and characterisation of sea bass (Dicentrarchus labrax L.) caspase-3 gene. Mol. Immunol. 2007;44:774–783. doi: 10.1016/j.molimm.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 35.Kerr J.F., Wyllie A.H., Currie A.R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henson P.M., Hume D.A. Apoptotic cell removal in development and tissue homeostasis. Trends Immunol. 2006;27:244–250. doi: 10.1016/j.it.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Domingos P.M., Steller H. Pathways regulating apoptosis during patterning and development. Curr. Opin. Genet. Dev. 2007;17:294–299. doi: 10.1016/j.gde.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Don M.M., Ablett G., Bishop C.J., Bundesen P.G., Donald K.J., Searle J., Kerr J.F. Death of cells by apoptosis following attachment of specifically allergized lymphocytes in vitro. Aust. J. Exp. Biol. Med. Sci. 1977;55:407–417. doi: 10.1038/icb.1977.38. [DOI] [PubMed] [Google Scholar]
- 39.Robertson A.M., Bird C.C., Waddell A.W., Currie A.R. Morphological aspects of glucocorticoid-induced cell death in human lymphoblastoid cells. J. Pathol. 1978;126:181–187. doi: 10.1002/path.1711260307. [DOI] [PubMed] [Google Scholar]
- 40.Wood W., Turmaine M., Weber R., Camp V., Maki R.A., McKercher S.R., Martin P. Mesenchymal cells engulf and clear apoptotic footplate cells in macrophageless PU.1 null mouse embryos. Development. 2000;127:5245–5252. doi: 10.1242/dev.127.24.5245. [DOI] [PubMed] [Google Scholar]
- 41.Parnaik R., Raff M.C., Scholes J. Differences between the clearance of apoptotic cells by professional and non-professional phagocytes. Curr. Biol. 2000;10:857–860. doi: 10.1016/s0960-9822(00)00598-4. [DOI] [PubMed] [Google Scholar]
- 42.Silva M.T., do Vale A., dos Santos N.M. Secondary necrosis in multicellular animals: an outcome of apoptosis with pathogenic implications. Apoptosis. 2008;13:463–482. doi: 10.1007/s10495-008-0187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogasawara J., Watanabe-Fukunaga R., Adachi M., Matsuzawa A., Kasugai T., Kitamura Y., Itoh N., Suda T., Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- 44.Haslett C. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am. J. Respir. Crit. Care Med. 1999;160:S5–S11. doi: 10.1164/ajrccm.160.supplement_1.4. [DOI] [PubMed] [Google Scholar]
- 45.Devitt A., Parker K.G., Ogden C.A., Oldreive C., Clay M.F., Melville L.A., Bellamy C.O., Lacy-Hulbert A., Gangloff S.C., Goyert S.M., Gregory C.D. Persistence of apoptotic cells without autoimmune disease or inflammation in CD14-/- mice. J. Cell Biol. 2004;167:1161–1170. doi: 10.1083/jcb.200410057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Medan D., Wang L., Yang X., Dokka S., Castranova V., Rojanasakul Y. Induction of neutrophil apoptosis and secondary necrosis during endotoxin-induced pulmonary inflammation in mice. J. Cell Physiol. 2002;191:320–326. doi: 10.1002/jcp.10105. [DOI] [PubMed] [Google Scholar]
- 47.Knapp S., Leemans J.C., Florquin S., Branger J., Maris N.A., Pater J., van Rooijen N., van der Poll T. Alveolar macrophages have a protective antiinflammatory role during murine pneumococcal pneumonia. Am. J. Respir. Crit. Care Med. 2003;167:171–179. doi: 10.1164/rccm.200207-698OC. [DOI] [PubMed] [Google Scholar]
- 48.Li M.O., Sarkisian M.R., Mehal W.Z., Rakic P., Flavell R.A. Phosphatidylserine receptor is required for clearance of apoptotic cells. Science. 2003;302:1560–1563. doi: 10.1126/science.1087621. [DOI] [PubMed] [Google Scholar]
- 49.Bianchi S.M., Prince L.R., McPhillips K., Allen L., Marriott H.M., Taylor G.W., Hellewell P.G., Sabroe I., Dockrell D.H., Henson P.W., Whyte M.K. Impairment of apoptotic cell engulfment by pyocyanin, a toxic metabolite of Pseudomonas aeruginosa. Am. J. Respir. Crit. Care Med. 2008;177:35–43. doi: 10.1164/rccm.200612-1804OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weinrauch Y., Zychlinsky A. The induction of apoptosis by bacterial pathogens. Annu. Rev. Microbiol. 1999;53:155–187. doi: 10.1146/annurev.micro.53.1.155. [DOI] [PubMed] [Google Scholar]
- 51.Narayanan S.K., Nagaraja T.G., Chengappa M.M., Stewart G.C. Leukotoxins of gram-negative bacteria. Vet. Microbiol. 2002;84:337–356. doi: 10.1016/s0378-1135(01)00467-9. [DOI] [PubMed] [Google Scholar]
- 52.DeLeo F.R. Modulation of phagocyte apoptosis by bacterial pathogens. Apoptosis. 2004;9:399–413. doi: 10.1023/B:APPT.0000031448.64969.fa. [DOI] [PubMed] [Google Scholar]
- 53.Kennedy A.D., DeLeo F.R. Neutrophil apoptosis and the resolution of infection. Immunol. Res. 2009;43:25–61. doi: 10.1007/s12026-008-8049-6. [DOI] [PubMed] [Google Scholar]
- 54.do Vale A., Costa-Ramos C., Silva D.S., Macedo P.M., Fernandes R., Sampaio P., dos Santos N.M., Silva M.T. Cytochemical and ultrastructural study of anoikis and secondary necrosis in enterocytes detached in vivo. Apoptosis. 2007;12:1069–1083. doi: 10.1007/s10495-006-0040-x. [DOI] [PubMed] [Google Scholar]
- 55.Frisch S.M., Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell. Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Denecker G., Vercammen D., Steemans M., Vanden Berghe T., Brouckaert G., Van Loo G., Zhivotovsky B., Fiers W., Grooten J., Declercq W., Vandenabeele P. Death receptor-induced apoptotic and necrotic cell death: differential role of caspases and mitochondria. Cell. Death Differ. 2001;8:829–840. doi: 10.1038/sj.cdd.4400883. [DOI] [PubMed] [Google Scholar]
- 57.Sun F., Hamagawa E., Tsutsui C., Ono Y., Ogiri Y., Kojo S. Evaluation of oxidative stress during apoptosis and necrosis caused by carbon tetrachloride in rat liver. Biochim. Biophys. Acta. 2001;1535:186–191. doi: 10.1016/s0925-4439(00)00098-3. [DOI] [PubMed] [Google Scholar]
- 58.Hentze H., Schwoebel F., Lund S., Keel M., Ertel W., Wendel A., Jaattela M., Leist M. In vivo and in vitro evidence for extracellular caspase activity released from apoptotic cells. Biochem. Biophys. Res. Commun. 2001;283:1111–1117. doi: 10.1006/bbrc.2001.4918. [DOI] [PubMed] [Google Scholar]
- 59.Holdenrieder S., Eichhorn P., Beuers U., Samtleben W., Schoenermarck U., Zachoval R., Nagel D., Stieber P. Nucleosomal DNA fragments in autoimmune diseases. Ann. N.Y. Acad. Sci. 2006;1075:318–327. doi: 10.1196/annals.1368.043. [DOI] [PubMed] [Google Scholar]
- 60.Henson P.M., Johnston R.B., Jr. Tissue injury in inflammation. Oxidants, proteinases, and cationic proteins. J. Clin. Invest. 1987;79:669–674. doi: 10.1172/JCI112869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weiss S.J. Tissue destruction by neutrophils. N. Engl. J. Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 62.Kawabata K., Hagio T., Matsuoka S. The role of neutrophil elastase in acute lung injury. Eur. J. Pharmacol. 2002;451:1–10. doi: 10.1016/s0014-2999(02)02182-9. [DOI] [PubMed] [Google Scholar]
- 63.Voynow J.A., Fischer B.M., Zheng S. Proteases and cystic fibrosis. Int. J. Biochem. Cell Biol. 2008;40:1238–1245. doi: 10.1016/j.biocel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Silva M.T. When two is better than one: macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. J. Leukoc. Biol. 2010;87:93–106. doi: 10.1189/jlb.0809549. [DOI] [PubMed] [Google Scholar]
- 65.Silva M.T. Neutrophils and macrophages work in concert as inducers and effectors of adaptive immunity against extracellular and intracellular microbial pathogens. J. Leukoc. Biol. 2010 doi: 10.1189/jlb.1109767. [DOI] [PubMed] [Google Scholar]
- 66.Monack D., Falkow S. Apoptosis as a common bacterial virulence strategy. Int. J. Med. Microbiol. 2000;290:7–13. doi: 10.1016/S1438-4221(00)80096-X. [DOI] [PubMed] [Google Scholar]
- 67.Bhavsar A.P., Guttman J.A., Finlay B.B. Manipulation of host-cell pathways by bacterial pathogens. Nature. 2007;449:827–834. doi: 10.1038/nature06247. [DOI] [PubMed] [Google Scholar]
- 68.Cascales E. The type VI secretion toolkit. EMBO Rep. 2008;9:735–741. doi: 10.1038/embor.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tateda K., Ishii Y., Horikawa M., Matsumoto T., Miyairi S., Pechere J.C., Standiford T.J., Ishiguro M., Yamaguchi K. The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect. Immun. 2003;71:5785–5793. doi: 10.1128/IAI.71.10.5785-5793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Allen L., Dockrell D.H., Pattery T., Lee D.G., Cornelis P., Hellewell P.G., Whyte M.K. Pyocyanin production by Pseudomonas aeruginosa induces neutrophil apoptosis and impairs neutrophil-mediated host defenses in vivo. J. Immunol. 2005;174:3643–3649. doi: 10.4049/jimmunol.174.6.3643. [DOI] [PubMed] [Google Scholar]
- 71.Lukaszewski R.A., Kenny D.J., Taylor R., Rees D.G., Hartley M.G., Oyston P.C. Pathogenesis of Yersinia pestis infection in BALB/c mice: effects on host macrophages and neutrophils. Infect. Immun. 2005;73:7142–7150. doi: 10.1128/IAI.73.11.7142-7150.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wickstrum J.R., Bokhari S.M., Fischer J.L., Pinson D.M., Yeh H.W., Horvat R.T., Parmely M.J. Francisella tularensis induces extensive caspase-3 activation and apoptotic cell death in the tissues of infected mice. Infect. Immun. 2009;77:4827–4836. doi: 10.1128/IAI.00246-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parmely M.J., Fischer J.L., Pinson D.M. Programmed cell death and the pathogenesis of tissue injury induced by type A Francisella tularensis. FEMS Microbiol. Lett. 2009;301:1–11. doi: 10.1111/j.1574-6968.2009.01791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]