Abstract
Proteases from a variety of sources (viruses, bacteria, fungi, plants, and insects) have toxicity towards insects. Some of these insecticidal proteases evolved as venom components, herbivore resistance factors, or microbial pathogenicity factors, while other proteases play roles in insect development or digestion, but exert an insecticidal effect when over-expressed from genetically engineered plants or microbial pathogens. Many of these proteases are cysteine proteases, although insect-toxic metalloproteases and serine proteases have also been examined. The sites of protease toxic activity range from the insect midgut to the hemocoel (body cavity) to the cuticle. This review discusses these insecticidal proteases along with their evaluation and use as potential pesticides.
Keywords: insecticides, basement membrane, cuticle, peritrophic matrix, plant defense, microbial defense
1. Toxic Proteins as Insecticidal Agents
The use of classical chemical insecticides to control agricultural pests poses hazards to human health, other non-target species including beneficial insects such as pollinators, and the environment. Indiscriminate use of chemical insecticides can also select for pest populations with insecticide resistance. Even the newer insecticidal compounds with less-threatening toxicological profiles can have adverse ecological and environmental impacts [1]. The development of insecticide resistance and the growing awareness of ecological and environmental problems caused by classical chemical insecticides have spurred research into biologically-based, environmentally benign alternatives. Among the compounds identified during the course of this research are proteins from microbes, predators, and plants that have toxicity that is specific to insects. Most prominent among these insecticidal proteins are the Bt crystal (Cry) δ-endotoxin proteins produced by Bacillus thuringiensis that form crystalline inclusions during sporulation [2]. Cry proteins bind to receptors and insert into the membranes of insect midgut epithelial cells, forming pores and causing cellular lysis and fatal damage to the midgut epithelium [3]. Application of Bt toxins towards insect pest management has involved both spray formulations of B. thuringiensis crystals/spores and crops genetically engineered to express Bt toxin genes. The widespread planting of Bt toxin-expressing maize and cotton has significantly reduced pesticide application, with the associated decrease in environmental impact [4]. A number of other insecticidal proteins have been identified which also target the insect midgut, including additional lytic pore-forming proteins from B. thuringiensis such as vegetative insecticidal proteins (vip) and cytolytic proteins (cyt) [3,5,6], cholesterol oxidase [7], biotin-binding proteins [8], toxin complexes produced by nematode symbiotic bacteria from the genus Photorhabdus [9], ribosome-inactivating proteins from plants [10], protease inhibitors [11], and chitinases [12].
There has also been a significant amount of research on insecticidal proteins with targets lying beyond the gut. The most studied among these are insect-selective toxins found in the venoms of predatory invertebrates, particularly scorpions, but also mites, spiders, and sea anemones [13,14,15].
These peptides are neurotoxins that act on ion channels on axonal membranes, disrupting impulse transmission and causing paralysis. Effective delivery of these toxins to their targets has been achieved by genetic engineering of insect viruses (baculoviruses) [16] or entomopathogenic fungi [17] to express the toxins, and by fusion of a toxin with an appropriate delivery system [18,19]. Baculovirus infection of the tracheal system servicing the central nervous system of caterpillars ensures that a supply of synthesized, secreted toxin is available in close proximity to axonal membranes [20]. Other proteins that serve as hormones also exert a toxic effect on insects when expressed from a recombinant baculovirus [16].
Proteases are logical candidates for use as insecticidal agents. One would expect that with insects, as with other animals, proteolytic enzymatic activity can target and destroy essential proteins and tissues to an extent that mortality would result. Indeed, proteases have evolved in plants to defend against herbivorous insects. In microbial pathogens of insects, proteases often play a role in pathogenicity towards the host insect. Proteases have been found as components of the venoms of arthropod predators of insects. Because of what proteases do, even proteases that have not evolved to play a role as a toxin can still have an insecticidal effect when present at the wrong time, the wrong place, and/or the wrong quantity within an insect. This situation has been observed with microbial pathogens that have been engineered to over-express an otherwise innocuous protease during infection and growth of the pathogen within an insect. Hence, this review discusses not only proteases that have evolved as toxins of insects in different contexts, but also proteases that do not normally act as toxins of insects, but have a toxic effect when expressed by a vector (Table 1). A separate review in this volume by Rodou and colleagues provides an in-depth overview of the insecticidal proteases of the bacterium Photorhabdus luminescens. Related to the use of proteolytic enzymes as insecticidal agents is exploitation of the insecticidal impact of the lack of proteases, such as plant expression of inhibitors of herbivore gut proteases [21], and the use of insect-derived, trypsin-modulating oostatic factor that inhibits trypsin biosynthesis in the insect gut [22,23]. The insecticidal effects of protease inhibition will not be considered in the current review.
Table 1.
Proteases with insecticidal activity.
| Target tissue | Class of protease | ||
|---|---|---|---|
| Cysteine protease | Metalloprotease | Serine protease | |
| Midgut (including peritrophic matrix) | Zea mays Mir1-CP | Baculovirus enhancins | Trypsin |
| Carica papaya and Ficus virgata latex proteases | Bacterial (B. thuringiensis) enhancins | ||
| Baculovirus V-CATH | B. thuringiensis InhA2 | ||
| Cuticle | Metarhizium anisopliae PR1A (subtilisin-like) | ||
| Beauveria bassiana CDEP1 | |||
| Hemocoel (including basement membrane and the prophenoloxidase cascade) | Sarcophaga peregrina ScathL (cathepsin L-like) | Eulophus pennicornis reprolysin | |
| Delia coarctata DcCathL | B. thuringiensis InhA | ||
| Helicoverpa armigera cathepsin B-like | |||
| Tuberaphis styraci cathepsin B | |||
2. Proteases with Toxicity towards Insects
2.1. Proteases that target the peritrophic matrix
The peritrophic matrix (PM) is a mesh of chitin fibrils linked to glycoproteins and proteoglycans that lines the midgut epithelium of most insects [24] (Figure 1). The PM is analogous to the protective mucosal layer that lines the digestive tracts of mammals. The PM acts as a mechanical barrier that protects the midgut epithelium from abrasion by the insect diet and blocks access of ingested insect pathogens and toxins to the midgut epithelium. The PM also separates and organizes digestive processes within the midgut. Chemical compounds that disrupt the PM or block its formation or regeneration can lead to a retardation of insect larval growth and even mortality due to the inability of the exposed, damaged midgut epithelium to take up nutrients [25,26]. Thus, the insect PM is a desirable target for insecticidal proteins [27].
Some baculoviruses carry genes that encode zinc metalloproteases known as enhancins [28]. These proteases, previously known as “synergistic factors” [29], promote baculovirus infection of lepidopteran larvae by digesting PM proteins, making the PM more permeable to baculovirus virions [30,31,32]. Enhancins specifically degrade a PM protein known as invertebrate intestinal mucin (IIM) [33]. In addition to promoting viral infection of the midgut epithelium, enhancins can be toxic to insects when expressed outside of the context of a baculovirus infection. The expression of enhancins in transgenic plants results in a retardation in growth and mortality of herbivorous lepidopteran larvae that feed on the plants [34,35]. Enhancin in this context also has been reported to augment the insecticidal effect of Bt Cry toxins, presumably by increasing exposure of the midgut epithelium to the crystal toxins [36].
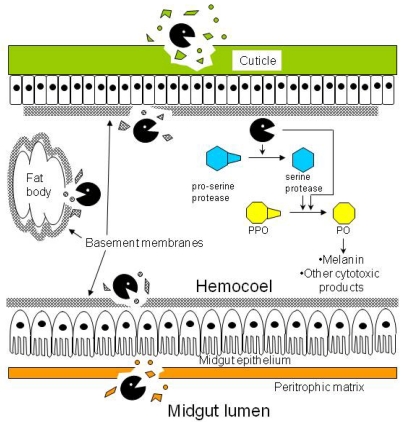
Figure 1.
Diagram of generic insect anatomy, showing targets sites of insecticidal proteases ( ). Besides degrading the cuticle, basement membranes, and peritrophic matrix, proteases in the hemocoel may convert prophenoloxidase (PPO) to phenoloxidase (PO) either directly or indirectly by activating the cascade of serine proteases that lead to the conversion step.
). Besides degrading the cuticle, basement membranes, and peritrophic matrix, proteases in the hemocoel may convert prophenoloxidase (PPO) to phenoloxidase (PO) either directly or indirectly by activating the cascade of serine proteases that lead to the conversion step.
Homologs of baculovirus enhancin genes have been identified in the genomes of the bacteria Yersinia pestis, Bacillus anthracis, Bacillus thuringiensis, and Bacillus cereus [37,38]. Only one of the Bacillus spp. enhancin-like genes, the bel gene from B. thuringiensis, was found to be required for normal levels of Cry protein toxicity against lepidopteran larvae [39]. Purfied bel-encoded protease enhanced the oral toxicity of Cry toxin and was found to digest IIM proteins of T. ni and Helicoverpa armigera larvae [39]. The acrystalliferous B. thuringiensis strain 407 (Cry_) encodes a metalloprotease, InhA2, which is unrelated to Bel. InhA2 was required for the synergistic enhancement of oral toxicity of CryIC crystals observed when waxmoth (Galleria mellonella) larvae were co-inoculated with crystals and B. thuringiensis strain 407 spores [40,41]. It is unknown if InhA2 degrades PM proteins or a different set of proteins such as those in the extracellular matrix.
A plant-encoded enzyme has also been found that targets the PM of insect pests. Inbred maize lines resistant to lepidopteran larval herbivory were found to produce a 33 kDa papain-like cysteine protease, Mir1-CP, in response to feeding by larvae of the fall armyworm, Spodoptera frugiperda [42,43,44]. Larvae feeding on calluses transformed with a Mir1-CP expression construct exhibited growth inhibition as seen for larvae fed on the resistant maize lines [45]. Scanning electron microscopy of the midguts of larvae fed on maize plants or calluses expressing Mir1-CP revealed cracks and perforations in the PM [46]. PM dissected from S. frugiperda and other insect species could also be degraded by recombinant Mir1-CP purified from baculovirus vector-infected S. frugiperda larvae [47]. Purified recombinant Mir1-CP kills lepidopteran larvae and can enhance the toxicity of Bt Cry toxins [48], similar to baculovirus-expressed enhancin [36] and bacterial homologs [39].
Similarly, cysteine proteases in the latex of papaya (Carica papaya) and a wild fig (Ficus virgata) were found to kill or retard the growth of larvae from three different lepidopteran species [49]. In this case, Konno and co-workers suggested that the toxic effect of the proteases was due to the very high concentration of the proteases in the latex of these plants. Other plant cysteine proteases have been identified by microarray and proteomics studies of tobacco (Nicotiana attenuata) and Arabidopsis thaliana as having a potential role in defense against lepidopteran herbivores [50].
Baculoviruses encode a cathepsin L-like protease, V-CATH, which is required for late-infection and postmortem liquefaction of the internal anatomy and weakening of the cuticle of baculovirus-infected lepidopteran larvae [51,52]. Unlike enhancin, V-CATH does not normally target the peritrophic matrix during baculovirus infection. However, expression of V-CATH in transgenic tobacco plants was found to retard the growth of Helicoverpa armigera (Old World bollworm) larvae that fed on the plants, suggesting that this cysteine protease may also damage or disrupt the PM. While enhancin metalloproteases and a variety of cysteine proteases can degrade PM proteins, serine proteases do not appear to be capable of degrading PM proteins in in vitro assays [53,54]. In a study testing the oral toxicity of five different proteases to pea aphids (Acyrthosiphon pisum) when included as part of an artificial diet, trypsin was the second most toxic protease, with an IC50 of 22 μg/mL [55]. As aphids lack peritrophic matrices, the mode of insecticidal action for the ingested trypsin is unclear, but may represent non-specific degradation of gut epithelial proteins.
2.2. Proteases that target the cuticle
The cuticle is the extracellular layer of the integument, the outer covering of insects and other arthropods, and is secreted by the insect epidermis. The cuticle covers the whole of the outside of the insect as well as the foregut, hindgut, and tracheal invaginations. The cuticle occurs in layers, typically with a waxy epicuticle covering an exocuticle and endocuticle consisting of protein, lipid, and chitin cross-linked to a varying degree to provide elasticity and hardness [56].
The entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana infect a wide range of insects and related arthropods and are used in many insecticidal biocontrol products [57]. Fungal infection is initiated when spores or conidia come into contact with the cuticle of a suitable host. Under favorable environmental conditions, germination occurs and specialized structures for penetrating the cuticle develop. These events are accompanied by the synthesis and secretion of a host of cuticle-degrading enzymes, particularly chitinases and proteases [58,59,60]. Several fungal protease genes that are up-regulated in response to cuticle exposure encode proteinase K-like class II subtilisins [61]. Of these, the M. anisopliae protease PR1A digests cuticle proteins and is essential for virulence and cuticle penetration [62,63,64]. Expression of this enzyme is repressed in the presence of readily catabolizable carbon and nitrogen sources, but induced in the presence of insect cuticle [64,65,66].
A clone of M. anisopliae which was genetically modified with additional copies of the pr1a gene under the control of a constitutive promoter killed larvae of the tobacco hornworm, Manduca sexta 25% faster than wild-type M. anisopliae [67]. While expression of PR1A is normally turned off when the invading fungus enters the hemocoel [68], the expression of PR1A in the hemocoel of larvae infected with the recombinant fungus resulted in degradation of hemolymph proteins and melanization of the hemolymph and the internal anatomy of the larvae. Injection of purified PR1A into the hemocoel of M. sexta larvae also killed larvae and caused melanization. Hence, although the normal mode of action of PR1A is to degrade cuticle proteins and facilitate the cuticular penetration of invading fungi, PR1A is also toxic to insects when introduced into the hemocoel.
Melanin formation in insects is a normal process that takes place during post-molt hardening and darkening of the insect cuticle and in response to wounding or the appearance of foreign matter in the insect [69]. The enzyme phenoloxidase catalyzes key steps in a series of reactions leading to the production of melanin from tyrosine. Phenoloxidase is synthesized as an inactive zymogen prophenoloxidase, which is activated in response to developmental and environmental cues by a cascade of trypsin-like serine proteases [70]. The PR1A protease likely triggered melanization of larvae by activating the proteolytic cascade that leads to the cleavage and activation of prophenoloxidase. The activation of prophenoloxidase is under tight control due to the toxic nature of the products and by-products of phenoloxidase, which include quinones and oxygen free radicals [71,72]. Unregulated phenoloxidase activity and/or non-specific proteolysis of proteins within the hemocoel by PR1A may have contributed to its toxicity.
Recombinant B. bassiana clones engineered to constitutively express PR1A exhibited similar toxicity when used to infect larvae of the pine caterpillar, Dendrolimus punctatus, and the wax moth, G. mellonella [73]. Larvae infected with these clones exhibited extensive melanization. These clones had also been engineered to express the scorpion peptide neurotoxin AaIT, but whereas B. bassiana clones that expressed only AaIT caused contractile paralysis in larvae that were infected with them, the fungal clones expressing both AaIT and PR1A did not cause paralysis. Medium from cultures of the AaIT-expressing fungus caused paralysis when injected into larvae, but the paralytic activity vanished if the medium was mixed with PR1A-containing medium, suggesting that PR1A was degrading AaIT. B. bassiana engineered to constitutively express a B. bassiana PR1A-like protease (CDEP1) killed G. mellonella larvae 12.5% faster than wild-type B. bassiana [74]. Additional clones in the same study were produced which constitutively expressed a fusion protein consisting of CDEP1 linked to the N-terminus of a B. bassiana chitinase, CHIT1. This clone killed larvae 67% faster than wild-type and also faster than clones expressing only CHIT1, indicating that a synergistic reduction in survival time was obtained by expressing a fusion protein with both proteolytic and chitinolytic activities. However, melanization was not reported in larvae infected with either the CDEP1-expressing recombinant fungus or the fungus expressing the CDEP1:CHIT1 fusion.
2.3. Proteases that target the basement membrane
Basement membranes (BMs), also referred to as basal laminae, are extracellular sheets of proteins that surround the tissues of all animals, providing structural support, a filtration function, and a surface for cell attachment, migration, and differentiation [75]. The BM has been reported to act as a physical barrier to the movement of viruses [76,77,78,79,80]. Given the size exclusion limit of insect BMs [81] and the size of baculovirus virions [82], it seems likely that the BM would also act as a physical barrier to baculovirus infection and dissemination, and the results of some studies support this idea [83,84,85].
The time taken for baculoviruses to kill insect pests has been cited repeatedly as a disadvantage hindering their use and commercialization as pest management tools [16]. To overcome this disadvantage, baculoviruses have been engineered with insect-selective toxins or development-disrupting enzymes and hormones [86]. Expression of these genes causes a cessation of feeding and the death of the infected host faster than the time taken for the infected host to succumb to wild type baculovirus infection. Given their size exclusion properties, BMs are a logical target for enzymes that enhance baculovirus insecticidal efficacy [87].
To evaluate the potential for BM-degrading enzymes to enhance the insecticidal efficacy of baculoviruses, the nucleopolyhedrovirus Autographa californica multiple nucleopolyhedrovirus (AcMNPV) was engineered to express three different BM protein-degrading proteases, including two vertebrate matrix metalloproteases (rat stromelysin, human gelatinase A) and a cathepsin L, ScathL, from the flesh fly, Sarcophaga peregrina [88]. Expression of the ScathL protease had the most profound effect on baculovirus insecticidal activity, with the median survival time of infected larvae of the tobacco budworm, Heliothis virescens reduced to approximately 50% that of wild-type virus-infected larvae. Viruses expressing this enzyme killed larvae faster than viruses expressing scorpion peptide neurotoxins from the same promoter. ScathL had been reported to specifically digest large (>200 kDa) proteins in the BMs of imaginal discs and larval brain of flesh flies during larval development [89,90,91], but the high level of expression of ScathL from a strong, late-phase viral promoter (p6.9) augmented the virulence of an infecting baculovirus and greatly reduced survival time of infected larvae. In contrast, expression from a weaker, early-phase viral promoter (ie1) had no impact on survival time of infected hosts, suggesting that the expression level of the protease was an important determinant of the insecticidal effect. The viruses expressing vertebrate matrix metalloproteases showed no improvement in virulence relative to the wild type virus.
Fifth instar larvae of H. virescens infected with the ScathL-expressing virus AcMLF9.ScathL exhibited a significant degree of cuticlar melanization (Figure 2). Dissection of these larvae also showed patches of melanization over parts of the internal anatomy, as well as fragmentation of some of the tissues [88] (Figure 3). Further experiments with viruses carrying marker genes suggested that ScathL activity did not promote systemic spread of viral infection or alter tissue trophism of the infecting virus [92]. Microscopic examination of tissues in larvae infected with AcMLF9.ScathL showed damage to basement membranes overlying the midgut, fat body, and muscle fibers [93]. Purified ScathL protease was also toxic to insects (specifically, H. virescens; the tomato moth, Lacanobia oleracera; and the pea aphid, Acyrthosiphon pisum) when injected into the hemocoel, and caused melanization in the lepidopteran larvae similar to the melanization seen when ScathL was expressed during baculovirus infection [94,95]. The purified enzyme was capable of causing basement membrane damage in vivo and degraded basement membrane proteins in vitro [93,94]. Melanization, mortality, and the level of hemolymph cysteine protease activity were closely correlated in lepidopteran larvae infected with AcMLF9.ScathL [95].
ScathL may activate the phenoloxidase cascade indirectly by damaging the BM and underlying tissues, or directly by cleaving prophenoloxidase or an upstream serine protease. The total potential phenoloxidase activity (from prophenoloxidase and phenoloxidase combined) was reduced by approximately 50% in wild-type virus infected H. virescens larvae and by 75% in AcMLF9.ScathL-infected larvae, while the actual activity from activated phenoloxidase in infected larvae and in non-melanized vs. melanized larvae was unaffected by ScathL expression [95]. Purified ScathL did not activate prophenoloxidase in vitro when added to larval hemolymph. Finally, melanization was not observed in aphids or mosquitoes injected with purified ScathL [95]. These results indicate that the toxic effect of ScathL on insects can be explained primarily as a result of the proteolytic damage done to basement membranes and the underlying tissues [93]. The formation of melanin by-products may contribute to its toxicity in lepidopteran larvae.
Figure 2.
Cuticular melanization resulting from baculovirus expression of ScathL in larvae of Heliothis virescens. Control (uninfected) and wild type virus-infected larvae are shown for comparison.
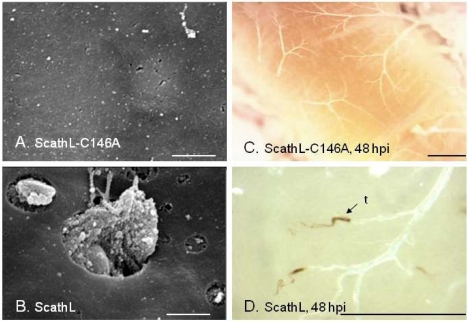
Figure 3.
Impact of ScathL on internal tissues of H. virescens. A, B, Scanning electron micrographs of the basement membrane overlying the fat body of larvae infected with recombinant baculoviruses expressing the catalytic site mutant ScathL-C146A (A) or wild-type ScathL (B) showing ScathL-mediated perforations. Bars, 2.5 µm. C, D, Light microscopy images of trachea of larvae infected with viruses expressing ScathL-C146A (A) or wild-type ScathL (B) showing melanized tracheal tips (t). Scale bar, 0.5 mm. (Reprinted with permission from Elsevier, [95].)
A homolog of ScathL has been identified from the guts of larvae of the wheat bulb fly, Delia coarctata [96]. This protease, DcCathL, is also toxic when injected into the hemocoel of larvae of L. oleracea, and seems to be less specific in protein degradation than ScathL. DcCathL selectively degraded serine protease inhibitors (serpins) from the cabbage moth, Mamestra brassicae, and M. brassicae serpins could block the insecticidal activity of DcCathL when they were co-injected along with DcCathL into the hemocoel of M. brassicae larvae. The melanization normally observed with larvae injected with DcCathL was also blocked when the serpins were co-injected, indicating that the serpins could block the proteolytic cascade, presumably initiated by DcCathL-mediated activation of a serine protease in the cascade, that led to prophenoloxidase activation and melanin formation. This result suggests that melanization in larvae injected with DcCathL may be facilitated by the degradation of endogenous serpins by DcCathL.
A cathepsin B-like protease identified from Helicoverpa armigera reduced the survival time of baculovirus-infected H. armigera larvae by 12 hr when expressed by a recombinant baculovirus during viral infection [97]. Like the S. peregrina cathepsin L-like protease, this cathepsin B played a normal role in the development of H. armigera [98,99,100], but had an insecticidal effect when indiscriminately expressed or expressed at levels far above those found under normal physiological conditions.
The venoms of arthropod predators sometimes contain metalloproteases and gelatinolytic serine proteases that are capable of cleaving basement membrane proteins [101,102,103,104,105,106]. These proteases may also have an insecticidal effect when delivered to the hemocoel of an insect. The reprolysin metalloprotease homolog EpMP3 from the venom of the parasitic wasp Eulophus pennicornis [107] caused mortality in L. oleracea larvae when purified, recombinant enzyme was injected into the hemocoel. The mortality occurred just prior to or during molting to the next instar, and surviving larvae exhibited a slower rate of growth and development after injection. Soldier-caste nymphs of the social aphid species, Tuberaphis styraci, produce a toxic cathepsin B protease in their intestine [108]. When aphid predators threaten the galls produced by reproductive aphids, the soldier nymphs thrust their piercing mouthparts (stylets) into the intruders and orally excrete this cathepsin B, causing paralysis or death of the intruders. Purified recombinant T. styraci cathepsin B killed larvae of the wax moth, G. mellonella, within 2-4 h when injected into the hemocoel.
Pathogenic bacteria often express proteases that are toxic to their hosts [109]. B. thuringiensis encodes a metalloprotease, InhA, with collagenolytic activity [110]. Genetic studies with inhA mutants indicated that it was not required for B. thuringiensis virulence [40], but infections of culture filtrates containing InhA caused mortality in waxmoth (G. mellonella) larvae.
3. Application of Proteases with Biocontrol Potential
Some of the proteases described above, particularly those that target the PM, act as “stomach poisons” upon ingestion by an insect. However, the toxic effect of proteases that hydrolyze proteins on the cuticle or in the hemocoel generally depends upon an appropriate means to deliver the proteases to their targets. As described above, one popular means to deliver proteases into the insect hemocoel is by means of an insect pathogen, such as entomopathogenic fungi or viruses. However, these proteases can be applied as an insecticide by themselves, without the requirement for an insect pathogen to deliver them to their target sites.
The toxicity of PR1A and other fungal cuticle-degrading enzymes described above occurred when those enzymes were expressed in the hemocoel via a fungal vector. However, significant mortality of an arthropod, the hard tick, Haemaphysalis longicornis, could be achieved by topical application of medium from insect cells infected with a recombinant baculovirus that expressed an H. longicornis chitinase [111]. Most of the observed mortality could be achieved with medium from which budded virus had been clarified by ultracentrifugation. As it is not expected that the baculovirus used in this study (AcMNPV) would infect and kill ticks, this result suggests that the chitinase alone was toxic to the ticks. Cuticle-degrading proteases such as PR1A also may have toxicity towards insects when applied topically. There is further evidence for this idea as the chitinolytic and proteolytic activities in a B. bassiana culture supernatant may cause mortality when the supernatant is sprayed on aphids (A. gossypii) [112].
In addition, there is substantial evidence that proteins can be transported intact from the guts of insects and related arthropods to the hemocoel [113]. Translocation of protein from the gut to the hemocoel is inefficient, with less than 0.1% to approximately 2% of the protein of interest detected in the hemolymph after ingestion. One promising means for enhancing the degree of protein translocation across the gut is to fuse the protein of interest to a lectin. Fusion proteins consisting of snowdrop lectin, Galanthus nivalis agglutinin (GNA) linked to neuropeptide hormones or venom peptide neurotoxins retained the activity of the hormone or neurotoxin portion of the fusion and were transported intact across the gut wall into the hemolymph [114,115,116,117,118]. Insects that were fed diets containing these fusion proteins exhibited reduced growth and mortality. However, it remains to be determined whether this strategy can work with insecticidal proteins such as proteases that are larger than the peptide hormones and neurotoxins employed to date.
4. Conclusions
Taken together, proteases represent a group of diverse and relatively unexplored agents for use in insect pest management. A key issue before broad application of such proteases for pest control relates to target specificity. For reduced risk associated with any insect control technology, insect specificity is highly desirable. The use of proteases employed in plant defense against herbivory holds particular promise for future development of insect resistant transgenic plants. Greater understanding of the biology of virulence factors in the genomics and transcriptomics era may facilitate identification of candidate proteases for use in pest management.
Acknowledgements
The authors thank Hailin Tang for electron micrographs of the physiological impact of ScathL. This material is based upon work supported by USDA NRI 2003-35302-13558 as well as Hatch Act and State of Iowa funds. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture.
References
- 1.Devine G.J., Furlong M.J. Insecticide use: Contexts and ecological consequences. Agric. Human Values. 2007;24:281–306. doi: 10.1007/s10460-007-9067-z. [DOI] [Google Scholar]
- 2.Schnepf E., Crickmore N., Van Rie J., Lereclus D., Baum J., Feitelson J., Zeigler D.R., Dean D.H. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 1998;62:775–806. doi: 10.1128/mmbr.62.3.775-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bravo A., Gill S.S., Soberon M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon. 2007;49:423–435. doi: 10.1016/j.toxicon.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brookes G., Barfoot P. Global impact of biotech crops: Socio-economic and environmental effects, 1996-2006. AgBioForum. 2008;11:21–38. [Google Scholar]
- 5.Lee M.K., Walters F.S., Hart H., Palekar N., Chen J.S. The mode of action of the Bacillus thuringiensis vegetative insecticidal protein Vip3A differs from that of Cry1Ab delta-endotoxin. Appl. Environ. Microbiol. 2003;69:4648–4657. doi: 10.1128/AEM.69.8.4648-4657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez-Rodriguez C.S., Boets A., Van Rie J., Ferre J. Screening and identification of vip genes in Bacillus thuringiensis strains. J. Appl. Microbiol. 2009;107:219–225. doi: 10.1111/j.1365-2672.2009.04199.x. [DOI] [PubMed] [Google Scholar]
- 7.Shen Z., Corbin D.R., Greenplate J.T., Grebenok R.J., Galbraith D.W., Purcell J.P. Studies on the mode of action of cholesterol oxidase on insect midgut membranes. Arch. Insect Biochem. Physiol. 1997;34:429–442. doi: 10.1002/(SICI)1520-6327(1997)34:4<429::AID-ARCH3>3.0.CO;2-N. [DOI] [Google Scholar]
- 8.Markwick N.P., Docherty L.C., Phung M.M., Lester M.T., Murray C., Yao J.L., Mitra D.S., Cohen D., Beuning L.L., Kutty-Amma S., Christeller J.T. Transgenic Res. 2003;12:671–681. doi: 10.1023/B:TRAG.0000005103.83019.51. [DOI] [PubMed] [Google Scholar]
- 9.ffrench-Constant R.H., Dowling A., Waterfield N.R. Insecticidal toxins from Photorhabdus bacteria and their potential use in agriculture. Toxicon. 2007;49:436–451. doi: 10.1016/j.toxicon.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Shahidi-Noghabi S., Van Damme E.J., Smagghe G. Carbohydrate-binding activity of the type-2 ribosome-inactivating protein SNA-I from elderberry (Sambucus nigra) is a determining factor for its insecticidal activity. Phytochemistry. 2008;69:2972–2978. doi: 10.1016/j.phytochem.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Haq S.K., Atif S.M., Khan R.H. Protein proteinase inhibitor genes in combat against insects, pests, and pathogens: Natural and engineered phytoprotection. Arch. Biochem. Biophys. 2004;431:145–159. doi: 10.1016/j.abb.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Arakane Y., Muthukrishnan S. Insect chitinase and chitinase-like proteins. Cell. Mol. Life Sci. 2010;67:201–216. doi: 10.1007/s00018-009-0161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon D., Karbat I., Ilan N., Cohen L., Kahn R., Gilles N., Dong K., Stuhmer W., Tytgat J., Gurevitz M. The differential preference of scorpion alpha-toxins for insect or mammalian sodium channels: Implications for improved insect control. Toxicon. 2007;49:452–472. doi: 10.1016/j.toxicon.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Gurevitz M., Karbat I., Cohen L., Ilan N., Kahn R., Turkov M., Stankiewicz M., Stuhmer W., Dong K., Gordon D. The insecticidal potential of scorpion beta-toxins. Toxicon. 2007;49:473–489. doi: 10.1016/j.toxicon.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson G.M. Insect-selective spider toxins targeting voltage-gated sodium channels. Toxicon. 2007;49:490–512. doi: 10.1016/j.toxicon.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 16.Inceoglu A.B., Kamita S.G., Hammock B.D. Genetically modified baculoviruses: A historical overview and future outlook. Adv. Virus Res. 2006;68:323–360. doi: 10.1016/S0065-3527(06)68009-3. [DOI] [PubMed] [Google Scholar]
- 17.St Leger R.J., Wang C. Genetic engineering of fungal biocontrol agents to achieve greater efficacy against insect pests. Appl. Microbiol. Biotechnol. 2010;85:901–907. doi: 10.1007/s00253-009-2306-z. [DOI] [PubMed] [Google Scholar]
- 18.Liu S., Li H., Sivakumar S., Bonning B.C. Virus-derived genes for insect resistant transgenic plants. In: Bonning B.C., editor. Insect Viruses: Biotechnological Applications. Academic Press; San Diego, CA, USA: 2006. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt N.R., Bonning B.C. Intrahemocoelic toxins for lepidopteran pest management. In: Goldsmith M., Marc F., editors. Molecular Biology and Genetics of Lepidoptera. Taylor and Francis; Baton Rouge, FL, USA: 2010. pp. 307–319. [Google Scholar]
- 20.Elazar M., Levi R., Zlotkin E. Targeting of an expressed neurotoxin by its recombinant baculovirus. J. Exp. Biol. 2001;204:2637–2645. doi: 10.1242/jeb.204.15.2637. [DOI] [PubMed] [Google Scholar]
- 21.Haq S.K., Atif S.M., Khan R.H. Protein proteinase inhibitor genes in combat against insects, pests, and pathogens: Natural and engineered phytoprotection. Arch. Biochem. Biophys. 2004;431:145–159. doi: 10.1016/j.abb.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 22.Lemeire E., Borovsky D., Van Camp J., Smagghe G. Effect of ace inhibitors and TMOF on growth, development, and trypsin activity of larval Spodoptera littoralis. Arch. Insect Biochem. Physiol. 2008;69:199–208. doi: 10.1002/arch.20270. [DOI] [PubMed] [Google Scholar]
- 23.Borovsky D., Rabindran S., Dawson W.O., Powell C.A., Iannotti D.A., Morris T.J., Shabanowitz J., Hunt D.F., DeBondt H.L., DeLoof A. Expression of Aedes trypsin-modulating oostatic factor on the virion of TMV: A potential larvicide. Proc. Natl. Acad. Sci. USA. 2006;103:18963–18968. doi: 10.1073/pnas.0606146103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hegedus D., Erlandson M., Gillott C., Toprak U. New insights into peritrophic matrix synthesis, architecture, and function. Annu. Rev. Entomol. 2009;54:285–302. doi: 10.1146/annurev.ento.54.110807.090559. [DOI] [PubMed] [Google Scholar]
- 25.Wang P., Granados R.R. Calcofluor disrupts the midgut defense system in insects. Insect Biochem. Mol. Biol. 2000;30:135–143. doi: 10.1016/S0965-1748(99)00108-3. [DOI] [PubMed] [Google Scholar]
- 26.Sobotnik J., Kudlikova-Krizkova I., Vancova M., Munzbergova Z., Hubert J. Chitin in the peritrophic membrane of Acarus siro (Acari: Acaridae) as a target for novel acaricides. J. Econ. Entomol. 2008;101:1028–1033. doi: 10.1603/0022-0493(2008)101[1028:citpmo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Wang P., Granados R.R. Molecular structure of the peritrophic membrane (PM): identification of potential PM target sites for insect control. Arch. Insect Biochem. Physiol. 2001;47:110–118. doi: 10.1002/arch.1041. [DOI] [PubMed] [Google Scholar]
- 28.Lepore L.S., Roelvink P.R., Granados R.R. Enhancin, the granulosis virus protein that facilitates nucleopolyhedrovirus (NPV) infections, is a metalloproteas. J. Invertebr. Pathol. 1996;68:131–140. doi: 10.1006/jipa.1996.0070. [DOI] [PubMed] [Google Scholar]
- 29.Tanada Y. A synopsis of studies on the synergistic property of an insect baculovirus: A tribute to Edward A. Steinhaus. J. Invertebr. Pathol. 1985;45:125–138. [Google Scholar]
- 30.Derksen A.C., Granados R.R. Alteration of a lepidopteran peritrophic membrane by baculoviruses and enhancement of viral infectivity. Virology. 1988;167:242–250. doi: 10.1016/0042-6822(88)90074-8. [DOI] [PubMed] [Google Scholar]
- 31.Wang P., Hammer D.A., Granados R.R. Interaction of Trichoplusia ni granulosis virus-encoded enhancin with the midgut epithelium and peritrophic membrane of four lepidopteran insects. J. Gen. Virol. 1994;75:1961–1967. doi: 10.1099/0022-1317-75-8-1961. [DOI] [PubMed] [Google Scholar]
- 32.Peng J., Zhong J., Granados R.R. A baculovirus enhancin alters the permeability of a mucosal midgut peritrophic matrix from lepidopteran larvae. J. Insect Physiol. 1999;45:159–166. doi: 10.1016/s0022-1910(98)00110-3. [DOI] [PubMed] [Google Scholar]
- 33.Wang P., Granados R.R. An intestinal mucin is the target substrate for a baculovirus enhancin. Proc. Natl. Acad. Sci. USA. 1997;94:6977–6982. doi: 10.1073/pnas.94.13.6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao J., Ibrahim H., Garcia J.J., Mason H., Granados R.R., Earle E.D. Transgenic tobacco plants carrying a baculovirus enhancin gene slow the development and increase the mortality of Trichoplusia ni larvae. Plant Cell Rep. 2002;21:244–250. [Google Scholar]
- 35.Mori M., Kitamura H., Kondo A., Dohi K., Mori M., Kaido M., Mise K., Shimojyo E., Hashimoto Y. Expression of an enhancin gene from the Trichoplusia ni granulosis virus confers resistance to lepidopterous insect pests to rice. Plant Biotechnol. 2006;23:55–61. doi: 10.5511/plantbiotechnology.23.55. [DOI] [Google Scholar]
- 36.Granados R.R., Fu Y., Corsaro B., Hughes P.R. Enhancement of Bacillus thuringiensis toxicity to lepidopterous species with the enhancin from Trichoplusia ni granulovirus. Biol. Control. 2001;20:153–159. doi: 10.1006/bcon.2000.0891. [DOI] [Google Scholar]
- 37.Galloway C.S., Wang P., Winstanley D., Jones I.M. Comparison of the bacterial Enhancin-like proteins from Yersinia and Bacillus spp. with a baculovirus Enhancin. J. Invertebr. Pathol. 2005;90:134–137. doi: 10.1016/j.jip.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Hajaij-Ellouze M., Fedhila S., Lereclus D., Nielsen-LeRoux C. The enhancin-like metalloprotease from the Bacillus cereus group is regulated by the pleiotropic transcriptional activator PlcR but is not essential for larvicidal activity. FEMS Microbiol. Lett. 2006;260:9–16. doi: 10.1111/j.1574-6968.2006.00289.x. [DOI] [PubMed] [Google Scholar]
- 39.Fang S., Wang L., Guo W., Zhang X., Peng D., Luo C., Yu Z., Sun M. Bacillus thuringiensis bel protein enhances the toxicity of Cry1Ac protein to Helicoverpa armigera larvae by degrading insect intestinal mucin. Appl. Environ. Microbiol. 2009;75:5237–5243. doi: 10.1128/AEM.00532-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fedhila S., Nel P., Lereclus D. The InhA2 metalloprotease of Bacillus thuringiensis strain 407 is required for pathogenicity in insects infected via the oral route. J. Bacteriol. 2002;184:3296–3304. doi: 10.1128/JB.184.12.3296-3304.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fedhila S., Gohar M., Slamti L., Nel P., Lereclus D. The Bacillus thuringiensis PlcR-regulated gene inhA2 is necessary, but not sufficient, for virulence. J. Bacteriol. 2003;185:2820–2825. doi: 10.1128/JB.185.9.2820-2825.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang B., Siregar U., Willeford K.O., Luthe D.S., Williams W.P. Association of a 33-kilodalton cysteine proteinase found in corn callus with the inhibition of fall armyworm larval growth. Plant Physiol. 1995;108:1631–1640. doi: 10.1104/pp.108.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopez L., Camas A., Shivaji R., Ankala A., Williams P., Luthe D. Mir1-CP, a novel defense cysteine protease accumulates in maize vascular tissues in response to herbivory. Planta. 2007;226:517–527. doi: 10.1007/s00425-007-0501-7. [DOI] [PubMed] [Google Scholar]
- 44.Pechan T., Jiang B., Steckler D., Ye L., Lin L., Luthe D.S., Williams W.P. Characterization of three distinct cDNA clones encoding cysteine proteinases from maize (Zea mays L.) callus. Plant Mol. Biol. 1999;40:111–119. doi: 10.1023/A:1026494813936. [DOI] [PubMed] [Google Scholar]
- 45.Pechan T., Ye L., Chang Y., Mitra A., Lin L., Davis F.M., Williams W.P., Luthe D.S. A unique 33-kD cysteine proteinase accumulates in response to larval feeding in maize genotypes resistant to fall armyworm and other Lepidoptera. Plant Cell. 2000;12:1031–1040. doi: 10.1105/tpc.12.7.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pechan T., Cohen A., Williams W.P., Luthe D.S. Insect feeding mobilizes a unique plant defense protease that disrupts the peritrophic matrix of caterpillars. Proc. Natl. Acad. Sci. USA. 2002;99:13319–13323. doi: 10.1073/pnas.202224899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohan S., Ma P.W., Pechan T., Bassford E.R., Williams W.P., Luthe D.S. Degradation of the S. frugiperda peritrophic matrix by an inducible maize cysteine protease. J. Insect Physiol. 2006;52:21–28. doi: 10.1016/j.jinsphys.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 48.Mohan S., Ma P.W., Williams W.P., Luthe D.S. A naturally occurring plant cysteine protease possesses remarkable toxicity against insect pests and synergizes Bacillus thuringiensis toxin. PLoS One. 2008;3:e1786. doi: 10.1371/journal.pone.0001786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Konno K., Hirayama C., Nakamura M., Tateishi K., Tamura Y., Hattori M., Kohno K. Papain protects papaya trees from herbivorous insects: Role of cysteine proteases in latex. Plant J. 2004;37:370–378. doi: 10.1046/j.1365-313x.2003.01968.x. [DOI] [PubMed] [Google Scholar]
- 50.Zhu-Salzman K., Luthe D.S., Felton G.W. Arthropod-inducible proteins: Broad spectrum defenses against multiple herbivores. Plant Physiol. 2008;146:852–858. doi: 10.1104/pp.107.112177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Slack J.M., Kuzio J., Faulkner P. Characterization of v-cath, a cathepsin L-like proteinase expressed by the baculovirus Autographa californica multiple nuclear polyhedrosis virus. J. Genl. Virol. 1995;76:1091–1098. doi: 10.1099/0022-1317-76-5-1091. [DOI] [PubMed] [Google Scholar]
- 52.Hawtin R.E., Zarkowska T., Arnold K., Thomas C.J., Gooday G.W., King L.A., Kuzio J.A., Possee R.D. Liquefaction of Autographa californica nucleopolyhedrovirus-infected insects is dependent on the integrity of virus-encoded chitinase and cathepsin genes. Virology. 1997;238:243–253. doi: 10.1006/viro.1997.8816. [DOI] [PubMed] [Google Scholar]
- 53.Wang P., Li G., Granados R.R. Identification of two new peritrophic membrane proteins from larval Trichoplusia ni: Structural characteristics and their functions in the protease rich insect gut. Insect Biochem. Mol. Biol. 2004;34:215–227. doi: 10.1016/j.ibmb.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 54.Li C., Song X., Li G., Wang P. Midgut cysteine protease-inhibiting activity in Trichoplusia ni protects the peritrophic membrane from degradation by plant cysteine proteases. Insect Biochem. Mol. Biol. 2009;39:726–734. doi: 10.1016/j.ibmb.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Rahbe Y., Febvay G. Protein toxicity to aphids: an in vitro test on Acyrthosiphon pisum. Entomol. Exp. Appl. 1993;67:149–160. doi: 10.1111/j.1570-7458.1993.tb01663.x. [DOI] [Google Scholar]
- 56.Chapman R.F. The Insects: Structure and Function. Cambridge University Press; Cambridge, UK: 1998. Integument; pp. 415–440. [Google Scholar]
- 57.St Leger R.J., Wang C. Genetic engineering of fungal biocontrol agents to achieve greater efficacy against insect pests. Appl. Microbiol. Biotechnol. 2010;85:901–907. doi: 10.1007/s00253-009-2306-z. [DOI] [PubMed] [Google Scholar]
- 58.Freimoser F.M., Screen S., Bagga S., Hu G., St Leger R.J. Expressed sequence tag (EST) analysis of two subspecies of Metarhizium anisopliae reveals a plethora of secreted proteins with potential activity in insect hosts. Microbiology. 2003;149:239–247. doi: 10.1099/mic.0.25761-0. [DOI] [PubMed] [Google Scholar]
- 59.Freimoser F.M., Hu G., St Leger R.J. Variation in gene expression patterns as the insect pathogen Metarhizium anisopliae adapts to different host cuticles or nutrient deprivation in vitro. Microbiology. 2005;151:361–371. doi: 10.1099/mic.0.27560-0. [DOI] [PubMed] [Google Scholar]
- 60.Cho E.M., Boucias D., Keyhani N.O. EST analysis of cDNA libraries from the entomopathogenic fungus Beauveria (Cordyceps) bassiana. II. Fungal cells sporulating on chitin and producing oosporein. Microbiology. 2006;152:2855–2864. doi: 10.1099/mic.0.28845-0. [DOI] [PubMed] [Google Scholar]
- 61.Bagga S., Hu G., Screen S.E., St Leger R.J. Reconstructing the diversification of subtilisins in the pathogenic fungus Metarhizium anisopliae. Gene. 2004;324:159–169. doi: 10.1016/j.gene.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 62.St Leger R.J., Charnley A.K., Cooper R.M. Characterization of cuticle-degrading proteases produced by the entomopathogen Metarhizium anisopliae. Arch. Biochem. Biophys. 1987;253:221–232. doi: 10.1016/0003-9861(87)90655-2. [DOI] [PubMed] [Google Scholar]
- 63.St Leger R.J., Durrands P.K., Charnley A.K., Cooper R.M. Role of extracellular chymoelastase in the virulence of Metarhizium anisopliae for Manduca sexta. J. Invertebr. Pathol. 1988;52:285–293. doi: 10.1016/0022-2011(88)90137-1. [DOI] [Google Scholar]
- 64.St Leger R.J., Frank D.C., Roberts D.W., Staples R.C. Molecular cloning and regulatory analysis of the cuticle-degrading-protease structural gene from the entomopathogenic fungus Metarhizium anisopliae. Eur. J. Biochem. 1992;204:991–1001. doi: 10.1111/j.1432-1033.1992.tb16721.x. [DOI] [PubMed] [Google Scholar]
- 65.St Leger R.J., Durrands P.K., Cooper R.M., Charnley A.K. Regulation of production of proteolytic enzymes by the entomopathogenic fungus Metarhizium anisopliae. Arch. Microbiol. 1988;150:413–416. doi: 10.1007/BF00408316. [DOI] [Google Scholar]
- 66.Paterson I.C., Charnley A.K., Cooper R.M., Clarkson J.M. Partial characterization of specific inducers of a cuticle-degrading protease from the insect pathogenic fungus Metarhizium anisopliae. Microbiology. 1994;140:3153–3159. doi: 10.1099/13500872-140-11-3153. [DOI] [PubMed] [Google Scholar]
- 67.St Leger R., Joshi L., Bidochka M.J., Roberts D.W. Construction of an improved mycoinsecticide overexpressing a toxic protease. Proc. Natl. Acad. Sci. USA. 1996;93:6349–6354. doi: 10.1073/pnas.93.13.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang C., Hu G., St Leger R.J. Differential gene expression by Metarhizium anisopliae growing in root exudate and host (Manduca sexta) cuticle or hemolymph reveals mechanisms of physiological adaptation. Fungal Genet. Biol. 2005;42:704–718. doi: 10.1016/j.fgb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 69.Marmaras V.J., Charalambidis N.D., Zervas C.G. Immune response in insects: The role of phenoloxidase in defense reactions in relation to melanization and sclerotization. Arch. Insect Biochem. Physiol. 1996;31:119–133. doi: 10.1002/(SICI)1520-6327(1996)31:2<119::AID-ARCH1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 70.Cerenius L., Lee B.L., Soderhall K. The proPO-system: Pros and cons for its role in invertebrate immunity. Trends Immunol. 2008;29:263–271. doi: 10.1016/j.it.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 71.Carton Y., Nappi A.J. Drosophila cellular immunity against parasitoids. Parasitol. Today. 1997;13:218–227. doi: 10.1016/S0169-4758(97)01058-2. [DOI] [PubMed] [Google Scholar]
- 72.Ashida M., Brey P.T. Recent advances in research on the insect phenoloxidase cascade. In: Brey P.T., Hultmark D., editors. Molecular Mechanisms of Immune Responses in Insects. Chapman & Hall; London, UK: 1997. pp. 135–172. [Google Scholar]
- 73.Lu D., Pava-Ripoll M., Li Z., Wang C. Insecticidal evaluation of Beauveria bassiana engineered to express a scorpion neurotoxin and a cuticle degrading protease. Appl. Microbiol. Biotechnol. 2008;81:515–522. doi: 10.1007/s00253-008-1695-8. [DOI] [PubMed] [Google Scholar]
- 74.Fang W., Feng J., Fan Y., Zhang Y., Bidochka M.J., Leger R.J., Pei Y. Expressing a fusion protein with protease and chitinase activities increases the virulence of the insect pathogen Beauveria bassiana. J. Invertebr. Pathol. 2009;102:155–159. doi: 10.1016/j.jip.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 75.Timpl R., Brown J.C. Supramolecular assembly of basement membranes. Bioessays. 1996;18:123–132. doi: 10.1002/bies.950180208. [DOI] [PubMed] [Google Scholar]
- 76.Grimstad P.R., Walker E.D. Aedes triseriatus (Diptera: Culicidae) and La Crosse virus. IV. Nutritional deprivation of larvae affects the adult barriers to infection and transmission. J. Med. Entomol. 1991;28:378–386. doi: 10.1093/jmedent/28.3.378. [DOI] [PubMed] [Google Scholar]
- 77.Huard J., Feero W.G., Watkins S.C., Hoffman E.P., Rosenblatt D.J., Glorioso J.C. The basal lamina is a physical barrier to herpes simplex virus-mediated gene delivery to mature muscle fibers. J. Virol. 1996;70:8117–8123. doi: 10.1128/jvi.70.11.8117-8123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peiffer M.L., Gildow F.E., Gray S.M. Two distinct mechanisms regulate luteovirus transmission efficiency and specificity at the aphid salivary gland. J. Gen. Virol. 1997;78:495–503. doi: 10.1099/0022-1317-78-3-495. [DOI] [PubMed] [Google Scholar]
- 79.Weeks B.S., Ramchandran R.S., Hopkins J.J., Friedman H.M. Herpes simplex virus type-1 and -2 pathogenesis is restricted by the epidermal basement membrane. Arch. Virol. 2000;145:385–396. doi: 10.1007/s007050050030. [DOI] [PubMed] [Google Scholar]
- 80.Romoser W.S., Turell M.J., Lerdthusnee K., Neira M., Dohm D., Ludwig G., Wasieloski L. Pathogenesis of Rift Valley fever virus in mosquitoes-tracheal conduits & the basal lamina as an extra-cellular barrier. Arch. Virol. Suppl. 2005:89–100. doi: 10.1007/3-211-29981-5_8. [DOI] [PubMed] [Google Scholar]
- 81.Reddy J.T., Locke M. The size limited penetration of gold particles through insect basal laminae. J. Insect Physiol. 1990;36:397–407. doi: 10.1016/0022-1910(90)90057-M. [DOI] [Google Scholar]
- 82.Jehle J.A., Blissard G.W., Bonning B.C., Cory J.S., Herniou E.A., Rohrmann G.F., Theilmann D.A., Thiem S.M., Vlak J.M. On the classification and nomenclature of baculoviruses: A proposal for revision. Arch. Virol. 2006;151:1257–1266. doi: 10.1007/s00705-006-0763-6. [DOI] [PubMed] [Google Scholar]
- 83.Hess R.T., Falcon L.A. Temporal events in the invasion of the codling moth, Cydia pomonella, by a granulosis virus. J. Invertebr. Pathol. 1987;50:85–105. doi: 10.1016/0022-2011(87)90108-X. [DOI] [Google Scholar]
- 84.Engelhard E.K., Kam-Morgan L.N., Washburn J.O., Volkman L.E. The insect tracheal system: A conduit for the systemic spread of Autographa californica M nuclear polyhedrosis virus. Proc. Nat. Acad. Sci. USA. 1994;91:3224–3227. doi: 10.1073/pnas.91.8.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith-Johannsen H., Witkiewicz H., Iatrou K. Infection of silkmoth follicular cells with Bombyx mori nuclear polyhedrosis virus. J. Invertebr. Pathol. 1986;48:74–84. doi: 10.1016/0022-2011(86)90145-X. [DOI] [Google Scholar]
- 86.van Beek N.A.M., Hughes P.R. The response time of insect larvae infected with recombinant baculoviruses. J. Invertebr. Pathol. 1998;72:338–347. doi: 10.1006/jipa.1998.4814. [DOI] [PubMed] [Google Scholar]
- 87.Keddie B.A., Aponte G.W., Volkman L.E. The pathway of infection of Autographa californica nuclear polyhedrosis virus in an insect host. Science. 1989;243:1728–1730. doi: 10.1126/science.2648574. [DOI] [PubMed] [Google Scholar]
- 88.Harrison R.L., Bonning B.C. Use of proteases to improve the insecticidal activity of baculoviruses. Biol. Control. 2001;20:199–209. doi: 10.1006/bcon.2000.0899. [DOI] [Google Scholar]
- 89.Fujii-Taira I., Tanaka Y., Homma K.J., Natori S. Hydrolysis and synthesis of substrate proteins for cathepsin L in the brain basement membranes of Sarcophaga during metamorphosis. J. Biochem. 2000;128:539–542. doi: 10.1093/oxfordjournals.jbchem.a022784. [DOI] [PubMed] [Google Scholar]
- 90.Homma K., Kurata S., Natori S. Purification, characterization, and cDNA cloning of procathepsin L from the culture medium of NIH-Sape-4, an embryonic cell line of Sarcophaga peregrina (flesh fly), and its involvement in the differentiation of imaginal discs. J. Biol. Chem. 1994;269:15258–15264. [PubMed] [Google Scholar]
- 91.Homma K., Natori S. Identification of substrate proteins for cathepsin L that are selectively hydrolyzed during the differentiation of imaginal discs of Sarcophaga peregrina. Eur. J. Biochem. 1996;240:443–447. doi: 10.1111/j.1432-1033.1996.0443h.x. [DOI] [PubMed] [Google Scholar]
- 92.Li H., Tang H., Harrison R.L., Bonning B.C. Impact of a basement membrane-degrading protease on dissemination and secondary infection of Autographa californica multiple nucleopolyhedrovirus in Heliothis virescens (Fabricus) J. Gen. Virol. 2007;88:1109–1119. doi: 10.1099/vir.0.82691-0. [DOI] [PubMed] [Google Scholar]
- 93.Tang H., Li H., Lei S.M., Harrison R.L., Bonning B.C. Tissue specificity of a baculovirus-expressed, basement membrane-degrading protease in larvae of Heliothis virescens. Tissue Cell. 2007;39:431–443. doi: 10.1016/j.tice.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 94.Philip J.M., Fitches E., Harrison R.L., Bonning B., Gatehouse J.A. Characterisation of functional and insecticidal properties of a recombinant cathepsin L-like proteinase from flesh fly (Sarcophaga peregrina), which plays a role in differentiation of imaginal discs. Insect Biochem. Mol. Biol. 2007;37:589–600. doi: 10.1016/j.ibmb.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 95.Li H., Tang H., Sivakumar S., Philip J., Harrison R.L., Gatehouse J.A., Bonning B.C. Insecticidal activity of a basement membrane-degrading protease against Heliothis virescens (Fabricius) and Acyrthosiphon pisum (Harris). J. Insect Physiol. 2008;54:777–789. doi: 10.1016/j.jinsphys.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 96.Pyati P.S., Bell H.A., Fitches E., Price D.R., Gatehouse A.M., Gatehouse J.A. Cathepsin L-like cysteine proteinase (DcCathL) from Delia coarctata (wheat bulb fly): Basis of insecticidal activity. Insect Biochem. Mol. Biol. 2009;39:535–546. doi: 10.1016/j.ibmb.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 97.Hong-Lian S., Du-Juan D., Jin-Dong H., Jin-Xin W., Xiao-Fan Z. Construction of the recombinant baculovirus AcMNPV with cathepsin B-like proteinase and its insecticidal activity against Helicoverpa armigera. Pestic. Biochem. Physiol. 2008;91:141–146. [Google Scholar]
- 98.Yang X.M., Hou L.J., Dong D.J., Shao H.L., Wang J.X., Zhao X.F. Cathepsin B-like proteinase is involved in the decomposition of the adult fat body of Helicoverpa armigera. Arch. Insect. Biochem. Physiol. 2006;62:1–10. doi: 10.1002/arch.20115. [DOI] [PubMed] [Google Scholar]
- 99.Yang X.M., Hou L.J., Wang J.X., Zhao X.F. Expression and function of cathepsin B-like proteinase in larval hemocytes of Helicoverpa armigera during metamorphosis. Arch. Insect Biochem. Physiol. 2007;64:164–174. doi: 10.1002/arch.20169. [DOI] [PubMed] [Google Scholar]
- 100.Zhao X.F., An X.M., Wang J.X., Dong D.J., Du X.J., Sueda S., Kondo H. Expression of the Helicoverpa cathepsin B-like proteinase during embryonic development. Arch. Insect Biochem. Physiol. 2005;58:39–46. doi: 10.1002/arch.20030. [DOI] [PubMed] [Google Scholar]
- 101.Veiga S.S., Zanetti V.C., Braz A., Mangili O.C., Gremski W. Extracellular matrix molecules as targets for brown spider venom toxins. Braz. J. Med. Biol. Res. 2001;34:843–850. doi: 10.1590/s0100-879x2001000700002. [DOI] [PubMed] [Google Scholar]
- 102.da Silveira R.B., dos Santos Filho J.F., Mangili O.C., Veiga S.S., Gremski W., Nader H.B., von Dietrich C.P. Identification of proteases in the extract of venom glands from brown spiders. Toxicon. 2002;40:815–822. doi: 10.1016/s0041-0101(02)00078-8. [DOI] [PubMed] [Google Scholar]
- 103.Devaraja S., Nagaraju S., Mahadeswaraswamy Y.H., Girish K.S., Kemparaju K.A. low molecular weight serine protease: Purification and characterization from Hippasa agelenoides (funnel web) spider venom gland extract. Toxicon. 2008;52:130–138. doi: 10.1016/j.toxicon.2008.04.168. [DOI] [PubMed] [Google Scholar]
- 104.Malta M.B., Lira M.S., Soares S.L., Rocha G.C., Knysak I., Martins R., Guizze S.P., Santoro M.L., Barbaro K.C. Toxic activities of Brazilian centipede venoms. Toxicon. 2008;52:255–263. doi: 10.1016/j.toxicon.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 105.Trevisan-Silva D., Gremski L.H., Chaim O.M., da Silveira R.B., Meissner G.O., Mangili O.C., Barbaro K.C., Gremski W., Veiga S.S., Senff-Ribeiro A. Astacin-like metalloproteases are a gene family of toxins present in the venom of different species of the brown spider (genus Loxosceles) Biochimie. 2010;92:21–32. doi: 10.1016/j.biochi.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 106.Çalışkan F., Sivas H., Şahin Y. A preliminary study for the detection of gelatinolytic proteases from the scorpion Androctonus crassicauda (Turkish Black Scorpion) venom. Turk. J. Biochem. 2009;34:148–153. [Google Scholar]
- 107.Price D.R., Bell H.A., Hinchliffe G., Fitches E., Weaver R., Gatehouse J.A. A venom metalloproteinase from the parasitic wasp Eulophus pennicornis is toxic towards its host, tomato moth (Lacanobia oleracae) Insect Mol. Biol. 2009;18:195–202. doi: 10.1111/j.1365-2583.2009.00864.x. [DOI] [PubMed] [Google Scholar]
- 108.Kutsukake M., Nikoh N., Shibao H., Rispe C., Simon J.C., Fukatsu T. Evolution of soldier-specific venomous protease in social aphids. Mol. Biol. Evol. 2008;25:2627–2641. doi: 10.1093/molbev/msn203. [DOI] [PubMed] [Google Scholar]
- 109.Miyoshi S., Shinoda S. Microbial metalloproteases and pathogenesis. Microbes Infect. 2000;2:91–98. doi: 10.1016/S1286-4579(00)00280-X. [DOI] [PubMed] [Google Scholar]
- 110.Dalhammar G., Steiner H. Characterization of inhibitor A, a protease from Bacillus thuringiensis which degrades attacins and cecropins, two classes of antibacterial proteins in insect. Eur. J. Biochem. 1984;139:247–252. doi: 10.1111/j.1432-1033.1984.tb08000.x. [DOI] [PubMed] [Google Scholar]
- 111.Assenga S.P., You M., Shy C.H., Yamagishi J., Sakaguchi T., Zhou J., Kibe M.K., Xuan X., Fujisaki K. The use of a recombinant baculovirus expressing a chitinase from the hard tick Haemaphysalis longicornis and its potential application as a bioacaricide for tick control. Parasitol. Res. 2006;98:111–118. doi: 10.1007/s00436-005-0007-9. [DOI] [PubMed] [Google Scholar]
- 112.Kim J.S., Roh J.Y., Choi J.Y., Wang Y., Shim H.J., Je Y.H. Correlation of the aphicidal activity of Beauveria bassiana SFB-205 supernatant with enzymes. Fungal Biol. 2010;114:120–128. doi: 10.1016/j.mycres.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 113.Jeffers L.A., Michael Roe R. The movement of proteins across the insect and tick digestive system. J. Insect Physiol. 2008;54:319–332. doi: 10.1016/j.jinsphys.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 114.Fitches E., Audsley N., Gatehouse J.A., Edwards J.P. Fusion proteins containing neuropeptides as novel insect contol agents: Snowdrop lectin delivers fused allatostatin to insect haemolymph following oral ingestion. Insect Biochem. Mol. Biol. 2002;32:1653–1661. doi: 10.1016/s0965-1748(02)00105-4. [DOI] [PubMed] [Google Scholar]
- 115.Fitches E., Edwards M.G., Mee C., Grishin E., Gatehouse A.M., Edwards J.P., Gatehouse J.A. Fusion proteins containing insect-specific toxins as pest control agents: snowdrop lectin delivers fused insecticidal spider venom toxin to insect haemolymph following oral ingestion. J. Insect Physiol. 2004;50:61–71. doi: 10.1016/j.jinsphys.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 116.Down R.E., Fitches E.C., Wiles D.P., Corti P., Bell H.A., Gatehouse J.A., Edwards J.P. Insecticidal spider venom toxin fused to snowdrop lectin is toxic to the peach-potato aphid, Myzus persicae (Hemiptera: Aphididae) and the rice brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae). Pest Manag. Sci. 2006;62:77–85. doi: 10.1002/ps.1119. [DOI] [PubMed] [Google Scholar]
- 117.Pham Trung N., Fitches E., Gatehouse J.A. A fusion protein containing a lepidopteran-specific toxin from the South Indian red scorpion (Mesobuthus tamulus) and snowdrop lectin shows oral toxicity to target insects. BMC Biotechnol. 2006;6:18. doi: 10.1186/1472-6750-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fitches E.C., Bell H.A., Powell M.E., Back E., Sargiotti C., Weaver R.J., Gatehouse J.A. Insecticidal activity of scorpion toxin (ButaIT) and snowdrop lectin (GNA) containing fusion proteins towards pest species of different orders. Pest Manag. Sci. 2009;66:74–83. doi: 10.1002/ps.1833. [DOI] [PubMed] [Google Scholar]