Abstract
Ochratoxin A (OTA) is one of the most important mycotoxins that is found in food and feed products. It has proven toxic properties, being primarily known for its nephrotoxicity and carcinogenicity to certain animal species. OTA is produced by several species of Aspergillus and Penicillium that can be found in a wide variety of agricultural products, which makes the presence of OTA in these products common. Many countries have statutory limits for OTA, and concentrations need to be reduced to as low as technologically possible in food and feed. The most important measures to be taken to control OTA are preventive in order to avoid fungal growth and OTA production. However, these measures are difficult to implement in all cases with the consequence of OTA remaining in agricultural commodities. Remediation processes are often used to eliminate, reduce or avoid the toxic effects of OTA. Biological methods have been considered increasingly as an alternative to physical and chemical treatments. However, examples of practical applications are infrequent. This review will focus on the (i) known microorganisms and enzymes that are able to biodegrade OTA; (ii) mode of action of biodegradation and (iii) current applications. A critical discussion about the technical applicability of these strategies is presented.
Keywords: ochratoxin A, biodegradation, detoxification, decontamination
1. Introduction
1.1. Overview
The discovery of aflatoxins in the 1960s [1], with approximately 100,000 turkey poult deaths in England, was the seminal event that made the scientific community realize that mold secondary metabolites could be responsible for food and feed safety problems. Several other mycotoxins were identified when fungi in food were more fully investigated. Ochratoxin A (OTA) was purified and characterized from Aspergillus ochraceus Wilh. strain K‑804 [2,3] isolated from sorghum grain, and proved to be acutely toxic to Pekin ducklings, mice and rats [4]. Nowadays, OTA is one of the most relevant mycotoxins, with its presence in food and feed products being regulated in many countries.
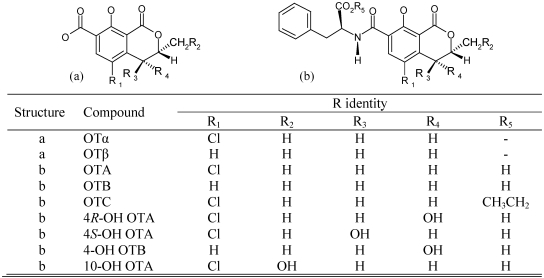
OTA is composed of a 7-carboxy-5-chloro-8-hydroxy-3,4-dihydro-3-R-methylisocoumarin (ochratoxin α) moiety and a L-β-phenylalanine molecule, which are linked through the 7‑carboxy group by an amide bond. The compound belongs to a group of fungal secondary metabolites, commonly known as ochratoxins (Figure 1), which share a similar chemical structure. Ochratoxin B (OTB), ochratoxin C (OTC), ochratoxin α (OTα), ochratoxin β (OTβ), (4‑R)- and (4‑S)-hydroxy-ochratoxin A (4R‑OH OTA and 4S‑OH OTA), 4-hydroxy-ochratoxin B (4‑OH OTB) and 10-hydroxy-ochratoxin A (10‑OH OTA) are naturally produced by certain fungi.
Figure 1.
Molecular structure of ochratoxins naturally produced by filamentous fungi [2,5,6].
Of these, OTA is the predominant compound detected in agriculture commodities and the most relevant to food and feed safety. The IUPAC formula is L‑phenylalanine-N-[(5-chloro-3,4-dihydro-8-hydroxy-3-methyl-1-oxo-1H-2-benzopyran-7-yl)carbonyl]-(R)-isocoumarin and its chemical abstract specification (CAS) is 303-47-9 [7]. OTA is a colorless crystal of empirical formula C20H18O6NCl, which has a molecular weight of 403.822 Da [8]. The melting points are 94-96 °C and 169 °C when crystallized from benzene or xylene, respectively. Maximum absorbances in (a) ![]() are 216, and 330 nm (ε 37,060 and 6050, respectively); and (b)
are 216, and 330 nm (ε 37,060 and 6050, respectively); and (b) ![]() are 214 and 332 nm (ε 37,200 and 6330, respectively) [9]. The infrared spectrum in chloroform presents major peaks at 3380, 1723, 1678 and 1655 cm−1. OTA has weak acid properties with pKa1 4.2-4.4 and pKa2 7.0-7.3, from the carboxyl group of phenylalanine and from the phenolic hydroxyl group of the isocoumarin, respectively [7]. OTA produces a green-bluish fluorescence when excited by UV light (366 nm) using TLC, which changes to a dark blue fluorescence when exposed to ammonia vapors, aqueous NaHCO3 or NaOH [10]. The fluorescence properties are commonly used for detection and identification purposes in TLC and HPLC. LC-MS can be employed to detect OTA. The identity of OTA can be confirmed by converting it to the methyl ester [11], and/or to OTα using carboxypeptidase A. OTA is highly soluble in polar organic solvents, soluble in aqueous sodium hydrogen carbonate and slightly soluble in water.
are 214 and 332 nm (ε 37,200 and 6330, respectively) [9]. The infrared spectrum in chloroform presents major peaks at 3380, 1723, 1678 and 1655 cm−1. OTA has weak acid properties with pKa1 4.2-4.4 and pKa2 7.0-7.3, from the carboxyl group of phenylalanine and from the phenolic hydroxyl group of the isocoumarin, respectively [7]. OTA produces a green-bluish fluorescence when excited by UV light (366 nm) using TLC, which changes to a dark blue fluorescence when exposed to ammonia vapors, aqueous NaHCO3 or NaOH [10]. The fluorescence properties are commonly used for detection and identification purposes in TLC and HPLC. LC-MS can be employed to detect OTA. The identity of OTA can be confirmed by converting it to the methyl ester [11], and/or to OTα using carboxypeptidase A. OTA is highly soluble in polar organic solvents, soluble in aqueous sodium hydrogen carbonate and slightly soluble in water.
1.2. Fungi
OTA is produced by several Aspergillus species and Penicillium verrucosum and P. nordicum. These penicillia are the species most authoritatively listed as OTA-producing. The P. viridicatum strains that were reported as OTA producers were reclassified as P. verrucosum by Pitt [12]. Other species such as P. chrysogenum, P. brevicompactum, P. crustosum, P. olsonii and P. oxalicum have been claimed as OTA producers [13,14]. Nevertheless, a careful confirmation of these findings is required since no other authors report the capacity of these species to produce OTA. Strains of a larger number of species are known to produce OTA in Aspergillus. Species accepted by Frisvad and co-authors [15] are listed in Table 1.
Table 1.
OTA producing fungi.
| Fungi species | References |
|---|---|
| Aspergillus section Circumdati | |
| A. cretensis | [16] |
| A. flocculosus | [16] |
| A. melleus | [17] |
| A. ochraceus | [3,16] |
| A. ostianus | [17] |
| A. persii | [18] |
| A. petrakii | [17] |
| A. pseudoelegans | [16] |
| A. roseoglobulosus | [16] |
| A. sclerotiorum | [16,17,19] |
| A. steynii | [16] |
| A. sulphureus | [16,17,19] |
| A. westerdijkiae | [16] |
| Aspergillus section Flavi | |
| A. alliaceus (Petromycesalliaceus) | [19,20] |
| Petromyces albertensis | [15] |
| Aspergillus section Nigri | |
| A. carbonarius | [21,22,23] |
| A. lacticoffeatus | [23] |
| A. niger | [23,24] |
| A. sclerotioniger | [23] |
| Penicillium | |
| P. nordicum | [25] |
| P. verrucosum | [12,26,27] |
Some other species were reported incorrectly as OTA producers, due to either misidentification of the isolates in question or to the use of contaminated cultures. Others still were reclassified, having now a different scientific name. For example, the original A. ochraceus strain from which OTA was first isolated, was reclassified as A. westerdijkiae, and many other A.ochraceus strains as A. steynii [16].
The major OTA producers in food and feed products are considered to be A. alliaceus, A. carbonarius, A. ochraceus, A. steynii, A. westerdijkiae, P. nordicum and P. verrucosum [15]. These are mainly associated with agricultural crops pre-harvest, or in post harvest storage situations. P. nordicum is a high OTA producer and is isolated predominantly from certain cheeses and fermented meats. Aspergillus niger, which is a very common species, is less relevant since most of the isolates are non-ochratoxigenic [28,29] and the others usually produce small amounts of OTA [30]. However, since it is a widely used species in several biotechnological processes it can pose some safety issues in the manufacture of food grade organic acids and enzymes. The other OTA producers are rare and do not pose a great concern for food safety.
1.3. Biosynthetic pathway
The OTA biosynthesis pathway is not completely established. However, it is known that the isocoumarin is a pentaketide which derives from the polyketide pathway and that L-β-phenylalanine derives from the shikimic acid pathway. More precisely, experimental studies with radioactive labeled precursors demonstrated that the isocoumarin is synthesized by head-to-tail condensation of five acetate units with the subsequent addition in C7 of a unit of methionine, which is subsequently oxidized to carboxyl [31,32,33]. Apparently, L-β-phenylalanine is coupled unaltered to the isocoumarin moiety since an isolated A. ochraceus enzyme fraction was able to link the two portions of the molecule [31]. Finally, the chlorine is incorporated in C5 from NaCl (although it is not known at what exact point of the biosynthesis) and probably through the action of chloroperoxidases [34]. There is little information about the genes responsible for these biosynthetic steps. Only DNA fragments that encode for some OTA polyketide synthases in P. nordicum [35], A. ochraceus [36] and A. carbonarius [37] have been identified.
1.4. Physiology of OTA production
OTA production depends on factors such as temperature, substrate water activity (aw), and micronutrients [9]. The optimal conditions for OTA production by A. carbonarius, A. ochraceus and P. verrucosum are 15-20 °C and 0.95-0.98 aw [38]; 25-30 °C and 0.98 aw [39]; and 24 °C and 0.95-0.99 aw [40], respectively. The culture media most used for its biosynthesis are Yeast Extract Sucrose (YES) and Czapek Yeast Autolysate (CYA) [41].
1.5. Presence in commodities
OTA has been detected in a wide variety of agricultural commodities, livestock products and processed food (Table 2). Concentrations found in the final food products are lower than those found in raw materials since some processing steps can contribute actively to its elimination. For example, (i) malting; (ii) malt fermentation; (iii) white bread and (iv) whole-bread production can contribute to reduce OTA by 56% [42]; 21% [43]; 80% [44] and 40% [44], respectively. Additionally, reductions of 35%, 71% and 83% for mild, medium and strong coffee roasting, respectively, were reported [45]. Finally, the wine-making process contributes to almost 90% reduction of OTA [46].
Table 2.
OTA levels found in some agricultural commodities, livestock products, processed food products.
| Food products | Contamination levels | References |
|---|---|---|
| Beans | 0.25-0.92 µg/Kg | [47] |
| Cocoa beans | 0.35-14.8 µg/Kg | [48] |
| Corn | 0.11-0.15 µg/Kg | [49] |
| Dried figs | <0.1-35.1 µg/Kg | [50] |
| Dried fruits | 0.1-30 µg/Kg | [51] |
| Grapes | 0.008-1.6 µg/Kg | [52] |
| Green coffee beans | 0-48 µg/Kg | [53] |
| Milk | 0.011-0.058 µg/L | [54] |
| Pork kidneys | 0-15 µg/Kg | [55] |
| Pork meat | 0-2.9 µg/Kg | [55] |
| Raisins | 0.2-53.6 μg/Kg | [56] |
| Rice | 1.0-27.3 µg/Kg | [57] |
| Spices | 4.2-103.2 µg/Kg | [58] |
| Wheat, Barley, oats | 0.1-17.8 µg/Kg | [59] |
| Wheat, oats and rye | 0.03-27 µg/Kg | [60] |
| Baby food | 0.06-2.4 µg/Kg | [61] |
| Beer | <0.01-0.135 µg/L | [62] |
| Breakfast cereals | 0.4-8.8 µg/Kg | [63] |
| Cocoa products | 0.22-0.77 µg/Kg | [64] |
| Grape juice | <0.003-0.311 µg/L | [65] |
| Pork products | <0.03-10.0 µg/Kg | [66] |
| Roasted coffee | 3.2-17.0 µg/Kg | [67] |
| Salami | <0.006-0.40 µg/Kg | [68] |
| Wine | <0.003-0.388 µg/L | [65] |
OTA is also detected in feed (Table 3) with the concentrations usually being higher than those in food. Processing steps such as extrusion may contribute to reduce concentrations [69]. Nevertheless, other practices can increase OTA concentrations. For example, some by-products derived from cereal processing, such as cracked grains, cereal cleanings, wheat and corn bran, are often the fractions most contaminated with OTA, and are usually directed for feed proposes [70]. Moreover, it is common practice to direct the lower contaminated commodities for human consumption while the most contaminated are used for feed.
Table 3.
OTA levels found in some feed products and raw materials.
1.6. Toxicity
OTA is the most toxic and relevant of the known ochratoxins. However, some consider that OTC is equally toxic to OTA [74,75] since, when ingested, it is converted rapidly into OTA, which becomes available in the bloodstream [76,77]. OTA is known primarily for its nephrotoxicity. It was nephrotoxic for all tested animals with the exception of adult ruminants [78] and it appears to be the cause of porcine nephropathy, human Balkan endemic nephropathy (BEN) and chronic interstitial nephropathy (CIN) in North Africa [79,80,81]. OTA is also classified as possibly carcinogenic to humans (group 2B) since there is evidence for experimental animals but not for humans [82]. In addition, OTA has mutagenic, teratogenic, neurotoxic, hepatotoxic and immunotoxic properties [80]. Oral LD50 values are 1.0-6.0 mg/kg for pigs, 20-30 mg/kg for rats and 48-58 mg/kg for mouse [83]. In these studies, OTA also caused haemorrhages in almost all vital organs, nephrosis, and necrosis in the liver and lymphoid tissues. OTA is considered to be a cumulative toxic compound since it is easily absorb through the stomach and the small intestine but hardly eliminated through the biliary and urinary routes. Oral OTA half-lives are 35.5 days for humans, 21 days for monkeys, 72-120 hours for pigs, 55-120 hours for rats and 40 hours for mice [83]. The high elimination half-lives observed in some species are due to the strong OTA affinity to serum proteins, which limit its transfer from the blood to the hepatic and renal cells. OTA affinity to bovine serum albumin [84] and to human serum albumin [85] was observed in vitro, and its relationship with OTA excretion confirmed by Kumagai [86] who verified that albumin-deficient rats were able to eliminate this mycotoxin more quickly through urine.
OTα toxicity has not been so extensively studied. Nevertheless, some studies indicated it is essentially non-toxic. For example, OTα was ineffective as an immunosuppressor when tested in mice [87] and was considered 1,000-times less toxic than OTA in brain cells cultures [88]. Furthermore, OTα has an elimination half-live of 9.6 h in rats, which is well below that of OTA (103 h) [77]. Therefore, processes that lead to the conversion of OTA into OTα contributes substantially to reduce OTA toxic effects and, hence, are considered to be routes for OTA detoxification.
A tolerable daily intake (TDI value) of 5 ng OTA/kg bw/day is recommended by the World Health Organization since it has toxic effects and is found in human blood [80,89,90,91] and in breast milk [80,92,93,94], thus proving human exposure. Furthermore, it is recommended that OTA levels in food and feed should be reduced as much as technologically possible.
1.7. Elimination strategies
Several strategies can be employed to reduce OTA levels. The most important are preventive since they avoid the contamination of commodities in the first place. The use of (i) good agricultural practices, (ii) fungal resistant crop varieties, (iii) the correct application of fungicides and (iv) the proper storage of commodities are common measures that could, ideally, be part of a Hazard Analysis Critical Control Point (HACCP) scheme to minimize OTA at critical control points of the food supply chain.
However, fully implemented HACCP schemes are rare, and when the individual measures fail or are not in place, OTA remains in food and feed products. Decontamination or detoxification procedures can be used to remove or to reduce OTA levels. These measures, which are technologically diverse, are usually classified into physical, chemical or biological [95]. Physical methods consist of segregation, sorting, cleaning, peeling and shelling processes that aim to remove the most contaminated fractions of the commodities. They also may involve the utilization of sorbents as nutritional additives that absorb OTA hence reducing bioavailability. Chemical methods consist of the utilization of compounds to destroy OTA: some processes use ammonium (ammoniation), alkaline hydrolysis (nixtamalization), bisulphites and ozone (ozonation). These are reported generally as effective in the elimination of OTA and other mycotoxins [96]. However, the toxicological safety of the final product is not always guaranteed since some chemical residues may remain in products and the toxicity of the reaction products formed is not usually studied. Furthermore, there is a significant reduction in palatability and nutritive quality of treated products.
Biological methods use microorganisms, which can decompose, transform or adsorb OTA to detoxify contaminated products or to avoid the toxic effects when mycotoxins are ingested. These are the technologies of choice for decontamination proposes because they present several advantages from being mediated by enzymatic reactions. For example, they are very specific, efficient, environmentally friendly, and they preserve nutritive quality. However, the non-pathogenicity of the microorganism and the non-toxicity of the reaction products formed are essential [97]. More research is needed to render these methods practical, effective and economically feasible.
2. Biodegradation of Ochratoxin A
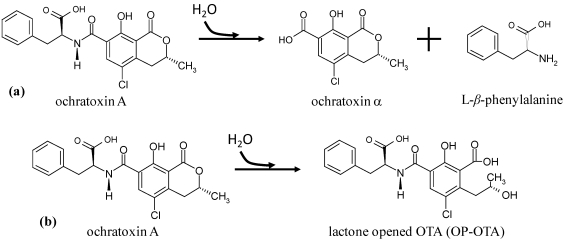
Numerous microorganisms capable of degrading, adsorbing and detoxifying OTA are reported in the literature and some practical processes have been developed. Reports about the capacity of proteolytic enzymes to hydrolyze OTA can also be found. Two pathways may be involved in OTA microbiological degradation. First, OTA can be biodegraded through the hydrolysis of the amide bond that links the L-β-phenylalanine molecule to the OTα moiety (Figure 2a). Since OTα and L-β-phenylalanine are virtually non-toxic, this mechanism can be considered to be a detoxification pathway [97]. Second, a more hypothetical process involves OTA being degraded via the hydrolysis of the lactone ring [97]. In this case, the final degradation product is an opened lactone form of OTA (Figure 2b), which is of similar toxicity to OTA when administered to rats [74,77]. However, it is less toxic to mice and Bacillus brevis [74]. Although this is hypothetical, it is likely to occur since microbiological lactonohydrolases, which undertake a similar transformation, are common [98].
Figure 2.
Ochratoxin A biodegradation pathways. (a) Amide bond hydrolysis of OTA; (b) Lactone ring hydrolysis of OTA.
2.1. Microorganisms which degrade ochratoxin A
Several protozoal, bacterial, yeast and filamentous fungal species are able to biodegrade OTA.
2.1.1. Protozoa
Ruminants are able to biodegrade OTA following the pathway that yields phenylalanine and OTα [99] with rumen protozoa being primarily responsible [100]. Indeed, ruminants are resistant to OTA toxic effects because of this detoxification capability [101]. More evidence is provided when high concentrate-rich diets are fed to animals, which reduce the natural protozoan population of the rumen, leading to increased OTA absorption and accumulation in the blood of animals and excretion into the milk of cows, with resulting carryover to humans [102].
2.1.2. Bacteria
Several bacteria were reported to degrade OTA. Phenylobacterium immobile degraded OTA present in a culture medium containing 0.1 mg OTA/L after three to five hours of incubation at 25 °C [103]. Four degradation products were identified, including OTα, which was not further metabolized. The proposed degradation pathway involved (i) a dioxygenase attack on the phenylalanine moiety; (ii) subsequent dehydrogenation to a catechol derivative that undergoes a meta-ring cleavage and (iii) a hydroxylation step that releases OTα. The authors verified that there was no growth with 200 mg OTA/L as the sole carbon and energy source.
Acinetobacter calcoaceticus removed 0.1005 and 0.0636 mg OTA/L/h when grown at 25 °C and 30 °C, respectively, with 10 mg OTA/L [104]. OTα was detected, and it was assumed that the biodegradation pathway involved the hydrolysis of the peptide bond. Total elimination of OTA was achieved after five days at 30 °C. Bacillus licheniformis degraded 92.5% of 5 mg OTA/L after 48 h at 37 °C and OTα was detected [105]. Also, Streptococcus salivarius subsp. thermophilus, Lactobacillus delbrueckii subsp. bulgaricus and Bifidobacterium bifidum eliminated OTA present in milk samples with 0.05 and 0.1 mg OTA/L [106]. However, the detection of OTα was not reported in this study, which implies that adsorption rather than biodegradation may have occurred. A similar situation was reported in other studies: some Bacilli and Lactobacillus strains were demonstrated to eliminate 0.05 mg OTA/L added to culture medium-in particular, L. bulgaricus, L. helveticus, L. acidophillus, B. lichniformis and B. subtilis eliminated up to 94%, 72%, 46%, 68% and 39%, respectively, of OTA [107]; L. plantarum, L. brevis and L. sanfrancisco were reported to eliminate 54%, 50% and 37%, respectively, of 0.3 mg OTA/L after 24 h of incubation [108].
It is now generally accepted that OTA adsorption to the cells walls is the predominant mechanism involved in this OTA detoxification phenomenon by lactic acid bacteria (LAB). For example, adsorption effects were claimed by Turbic et al. [109], who found that heat and acid treated cells from two Lactobacillusrhamnosus strains were more effective at removing OTA from phosphate buffer solutions than viable cells. The strains removed 36% to 76% in the buffer solution (pH 7.4) after 2 h at 37 °C. Similarly, Piotrowska and Zakowska [110] verified that L. acidophilus and L. rhamnosus caused OTA reductions of 70% and 87% of 1 mg OTA/L after five days at 37 °C, and that significant levels of the OTA were present in the centrifuged bacteria cells. Other LAB (L. brevis, L. plantarum and L. sanfranciscencis) also produced smaller decreases on OTA (approximately 50%). Finally, Del Prete et al. [111] tested 15 strains of oenological LAB in order to determine the in vitro capacity to remove OTA, and reported Oenococcus oeni as the most effective, with OTA reductions of 28%. The involvement of cell-binding mechanisms was confirmed as (i) up to 57% of the OTA absorbed by the cells was recovered through methanol extraction from the bacteria pellets; (ii) crude cell-free extracts were not able to degrade OTA; and (iii) degradation products were not detected.
Nevertheless, some authors consider that metabolism may also be involved. For example, Fuchs et al. confirmed that viable cells of L. acidophilus removed OTA more efficiently then unviable [112]. A L. acidophilus strain was able to decrease ≥95% the OTA in buffer solutions (pH 5.0) containing 0.5 and 1 mg OTA/L when incubated at 37 °C for 4 h. In addition, a detoxification effect was also demonstrated since pre-incubation of OTA with this strain reduced OTA toxicity to human derived liver cells (HepG2) [112]. Other L. acidophilus strains demonstrated only a moderate reduction in OTA contents suggesting that the effect was strain specific.
In summary, some LAB adsorb OTA by a strain specific cell-wall binding mechanism, although some undetected catabolism can also be involved. The detection of this OTA catabolism may only be possible with radiolabeled OTA. The potential of LAB as mycotoxin decontaminating agents has been reviewed [113] and which also considers Saccharomycescerevisiae.
2.1.3. Yeasts
S. cerevisiae and other yeast are described erroneously in some of the literature as OTA biodegradation agents, since most of the effects detected and reported are from wall adsorption mechanisms. Several studies clearly report the adsorption effects, but others do not. S. cerevisiae was claimed to biodegrade 41% of 0.3 mg OTA/L after 24 h at 30 °C, but details were not provided about the mechanism involved [108]. Similarly, Böhm and co‑authors claimed that some strains degraded up to 38% of 0.05 mg OTA/L without describing any resulting degradation metabolites [107].
On the other hand, the adsorption of OTA by oenological Saccharomyces strains was demonstrated by Bejaoui and co-authors, since they verified that heat and acid treated cells could bind significantly more OTA than viable ones [114]. Viable yeast bound up to 35% and 45% of the OTA, depending on the medium and strain, while heat and acid treated cells bound a maximum of 75%. Additionally, yeast are reported to reduce OTA in alcoholic fermentation processes such as brewing or vinification. During wort fermentation, yeasts adsorbed a maximum of 21% of the added OTA [43]. Also, almost 30% of the added OTA was removed after extended contact with yeast biomass [115]. Cecchini and co-authors verified during vinification trials that up to 70% of OTA could be removed from wine and that a significant percentage of the removed OTA was found in yeast lees [116]. Adsorption assays that used several yeasts products or fractions were also carried out in order to understand and explain the mechanisms involved. Moruno and co‑authors tested the capacity of active dried yeasts and yeast lees to remove OTA from wines and reported a reduction of approximately 70% when yeast lees were used [117]. The in vitro biosorption of OTA by vinasse containing yeast cell walls, purified yeast β-glucan and dried yeast cell wall fractions was studied [118]. Dried yeast cell wall fractions were reported to be the most efficient at adsorbing OTA. Several reports explained this phenomena by relating it to yeast β-D-glucans [119], glucomannans [120] and mannanoligosaccharide [121]. The paper of Shetty and Jespersen reviewed the main adsorption mechanisms involved [113].
On the other hand, some studies emphasized the involvement of biodegradation mechanisms. For example, Trichosporon, Rhodotorula and Cryptococcus demonstrated an ability to biodegrade OTA through the cleavage of the amide bond and releasing OTα [122]. In this study, the most effective strain degraded up to 100% of 0.2 mg OTA/L after five hours of incubation at 35 °C. This yeast was classified subsequently as the novel species Trichosporon mycotoxinivorans due to its excellent ability to detoxify OTA and zearalenone [123]. The strain could counteract the OTA toxic effects to some domestic livestock. For example, when (i) introduced to the diet of broiler chickens the yeast completely blocked OTA effects to the immune system [124]; and (ii) fed to pigs with contaminated feed, higher pig body weights were observed [125]. However, a recent study recognized T. mycotoxinivorans as a novel human pathogen associated with cystic fibrosis and the death of a patient with histologically documented Trichosporon pneumonia: this obviously raises safety issues on its practical use [126].
A Phaffia rhodozyma strain was also able to degrade 90% of 7.5 mg OTA/L after 15 days at20 °C [127]. In this study, the authors were able to verify the conversion of OTA into OTα and the adsorption of OTA into viable and heat-treated cells. The involvement of a metalloproteinase similar to carboxypeptidase A in OTA biodegradation was suggested, since it was verified that the chelating agents EDTA and 1,10-phenanthroline inhibited the degradation of the mycotoxin. More recently, Aureobasidium pullulans was reported to degrade OTA through the hydrolysis of the amide bond since OTα was detected [128]. The use as a biocontrol agent was also assessed as a reduction of OTA in grapes and wine was reported. However, the fungus appears to be involved in human disease and this issue needs to be resolved before more general use can be recommended [129].
2.1.4. Filamentous fungi
Some filamentous fungi can biodegrade OTA. Xiao and co-authors reported that A. niger hydrolyzed OTA and OTB [6]. Aspergillus fumigatus, A. japonicus and A. niger degraded 2 mg OTA/L after 10 days of incubation at 30 °C [130]. OTα was detected and further degradation into an unknown compound was observed. Aspergillus niger and other filamentous fungi have also shown to biodegrade OTA completely or partially, after growth in 1 mg OTA/L for six days at 25 °C [131]. OTα was detected, particularly in the assays performed with A. niger and other black aspergilli. An unidentified biodegradation metabolite was observed in the assays carried out with A. ochraceus which did not produce OTA, and some A. wentii strains. Additionally, Rhizopus homothallicus, R. oryzae, R. stolonifer and other Rhizopus species degraded more than 95% of 7.5 mg OTA/L after 16 days of incubation at 25 °C [132]. OTα was also detected in this study. Later, the excellent capacity of some black aspergilli to degrade OTA was confirmed: some A. carbonarius, A. japonicus, and A. niger strains degraded more than 80% of 2 mg OTA/L [133]. More recently and in agreement with Abrunhosa et al. [131], the capacity of Botrytis cinerea to degrade OTA was confirmed with reductions of 24.2% to 26.7% [134]. This provided an explanation for the low OTA contamination of noble rot and late-harvest wines. The white rot fungus Pleurotus ostreatus could degrade OTA (77%) and OTB (97%) when growth on contaminated barley by solid state fermentation, with OTα being detected from OTA biodegradation [135]. Rhizopus japonicus and Phanerochaete chrysosporium were also shown to biodegrade OTA to the lesser extents of 38% and 36%, respectively.
2.1.5. Plant cell cultures
Finally, it was shown that cell cultures of wheat, maize, tomato, soybean, sweet potato and other plants completely transform OTA [136].
2.2. Enzymes which degrade ochratoxin A
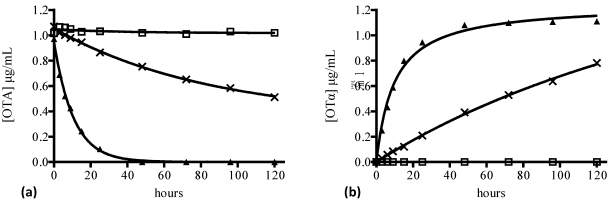
Several enzymes may be involved in the microbiological degradation of OTA. However, little information is available and very few have been purified and characterized. The first reported protease able to hydrolyze OTA was carboxypeptidase A (CPA) (EC 3.4.17.1) from bovine pancreas [137]. Subsequently, a screening study which included several commercial hydrolases, verified that a crude lipase product from A. niger was able to hydrolyze OTA via the amide bond [138]. The enzyme was purified by anion exchange chromatography and was demonstrated to cleave OTA and p‑nitrophenyl palmitate, a specific lipase substrate. Several proteolytic preparations were also studied, which were involved in the hydrolysis of OTA to OTα. These included protease A from A. niger, pancreatin from porcine pancreas and to a lesser extent, Prolyve PAC from A. niger [139]. Additionally, the production and purification of an A. niger cell-free crude enzyme preparation that demonstrated a significant capacity to cleave the amide bond of OTA was reported. The OTA-degrading enzyme involved was purified by anion exchange chromatography and characterized [140]. This enzyme showed higher OTA-degrading activity then CPA at pH 7.5 and 37 °C, and was inhibited by EDTA, which is a specific inhibitor of metalloproteases. In subsequent studies [141], it was found that carboxypeptidase Y (CPY) (EC 3.4.16.1) from S. cerevisiae is also able to hydrolyze OTA with optimal activity at pH 5.6 and 37 °C (Figure 3). However, the specific activity of CPY is very low as indicated by the OTA hydrolyzation reaction being very slow. Nevertheless, after five days of incubation, CPY converted 52% of the OTA present in the reaction assay into OTα. This activity is sufficient to reduce significantly levels of OTA during wine or beer fermentation, since these processes take several days to complete. Hence, a biodegradation pathway is possible for S. cerevisiae in addition to the OTA adsorption phenomenon. It is necessary to consider that CPY is a vacuolar exopeptidase where OTA enters the yeast cells before it is catabolized. However, the S. cerevisiae wall-binding properties can make difficult OTA uptake. Further information concerning the enzymes which are able to hydrolyze OTA is presented in Table 4.
Figure 3.
Ochratoxin A biodegradation by carboxypeptidase Y (CPY) from S. cerevisiae. (a) OTA detected in assays versus time. (b) OTα detected in the same assays versus time.-×- assay with 0.1 mg/mL of CPY at pH 5.6, 37 °C; -▲- assay with 0.5 mg/mL of CPA (control) at pH 7.5, 37 °C, -□- assay with no enzyme at pH 5.6, 37 °C (blank). Material, methods were as reported in [140].
Table 4.
Reported pure enzymes and enzyme formulations that hydrolyze OTA.
| Commercial name | Origin | Main activity | Supplier | Reference |
|---|---|---|---|---|
| Carboxypeptidase A | Bovinus bovis | exopeptidase | Boehringer | [142] |
| Carboxypeptidase Y | Saccharomyces cerevisiae | exopeptidase | Sigma | not reported |
| Lipase | Aspergillus niger | Lipase | Amano Inc. | [138] |
| Enzyme preparations | - | Proteolysis | - | [143] |
| Protease A | Aspergillus niger | Acid protease | Amano Inc. | [139] |
| Prolyve PAC | Aspergillus niger | Acid protease | Lyven | [139] |
| Pancreatin 4XNF-P211P | Porcine pancreas | Amylase, lipase and protease | Biocatalysts | [139] |
| Crude extract | Aspergillus niger | OTA-hydrolase | - | [140] |
- data not available.
3. Conclusions
A significant proportion of the world food crops is contaminated with mycotoxins, and safer methods for decontamination and prevention are required. Microorganisms and enzymes could be a practical way to reduce the concentrations and to avoid the toxic effects via bioremediation. OTA biodegradation occurs with various microorganisms. However, in some cases it is not clear if biodegradation occurred or if adsorption mechanisms were involved. OTA biodegradation is difficult to demonstrate, especially if an extensive catabolization occurs during the assays, i.e., if OTA is broken down into such small units that they are metabolized by the microbiological agent. In these situations, only the use of radiolabeled OTA will clarify the situation, although this approach has not been undertaken.
In addition, the identification of the enzymes involved is complex since it is necessary to employ the appropriate pH, temperature, ionic-strength and time in order to allow the catalytic reaction to occur, and to measure the reaction. Amide bond hydrolysis releases OTα that is detectable by the methods used to detect OTA and so this is an advantage. However, alternative biodegradation pathways can lead to other compounds that are more difficult to detect. Finally, it is important that more OTα and OP‑OTA toxicology studies are undertaken to ensure the safety of the degradation products.
The use of microorganisms such as LAB and S. cerevisiae present great advantages since they have an historical and extensive use in the food industry. In addition, they have well-known probiotic properties. On the other hand, the high purification costs of enzymes can render their practical application inviable and crude enzyme extracts may be a viable alternative. In conclusion, the bioremediation of OTA contaminated foods with microorganisms and enzymes will lead to safer food by using these environmentally-sound processes.
Acknowledgements
Luís Abrunhosa was supported by the grant SFRH/BPD/43922/2008 from Fundação para a Ciência e Tecnologia-FCT, Portugal. Russell Paterson is grateful for the research position in the FCT framework, Commitment to Science ref. C2008-UMINHO-CEB-2.
References
- 1.Sargeant K., Sheridan A., Okelly J. Toxicity associated with certain samples of groundnuts. Nature. 1961;192:1096–1097. [Google Scholar]
- 2.van der Merwe K.J., Steyn P.S., Fourie L. Mycotoxins. Part II. The constitution of ochratoxins A, B, and C, metabolites of Aspergillus ochraceus Wilh. J. Chem. Soc. 1965:7083–7088. doi: 10.1039/jr9650007083. [DOI] [PubMed] [Google Scholar]
- 3.van der Merwe K.J., Steyn P.S., Fourie L., Scott D.B., Theron J.J. Ochratoxin A, a toxic metabolite produced by Aspergillus ochraceus Wilh. Nature. 1965;205:1112–1113. doi: 10.1038/2051112a0. [DOI] [PubMed] [Google Scholar]
- 4.Scott D.B. Toxigenic fungi isolated from cereal and legume products. Mycopathol. Mycol. Appl. 1965;25:213. doi: 10.1007/BF02049914. [DOI] [PubMed] [Google Scholar]
- 5.Hutchison R.D., Steyn P.S., Thompson D.L. The isolation and structure of 4-hydroxyochratoxin A and 7-carboxy-3,4-dihydro-8-hydroxy-3-methylisocoumarin from Penicillium viridicatum . Tetrahedron Lett. 1971;12:4033–4036. [Google Scholar]
- 6.Xiao H., Marquardt R.R., Abramson D., Frohlich A.A. Metabolites of ochratoxins in rat urine and in a culture of Aspergillus ochraceus . Appl. Environ. Microbiol. 1996;62:648–655. doi: 10.1128/aem.62.2.648-655.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ringot D., Chango A., Schneider Y.J., Larondelle Y. Toxicokinetics and toxicodynamics of ochratoxin A, an update. Chem. Biol. Interact. 2006;159:18–46. doi: 10.1016/j.cbi.2005.10.106. [DOI] [PubMed] [Google Scholar]
- 8.Cole R.J., Jarvis B.B., Schweikert M.A. Handbook of Secondary Fungal Metabolites. Vol. 3. Academic; San Diego, CA, USA: 2003. Ochratoxins and related metabolites; pp. 615–624. [Google Scholar]
- 9.Betina V. Mycotoxins: Chemical, Biological and Environmental Aspects. Vol. 9. Elsevier; Amsterdam, The Netherland: 1989. Ochratoxins and related dihydroisocoumarins; pp. 151–173. [Google Scholar]
- 10.Betina V. Chromatography of Mycotoxins: Techniques andApplications. Elsevier; Amsterdam, The Netherlands: 1993. Thin-layer chromatography of mycotoxins. [Google Scholar]
- 11.Li S.Z., Marquardt R.R., Frohlich A.A. Confirmation of ochratoxins in biological samples by conversion into methyl esters in acidified methanol. J. Agric. Food Chem. 1998;46:4307–4312. [Google Scholar]
- 12.Pitt J.I. Penicillium viridicatum, Penicillium verrucosum, and production of ochratoxin. Appl. Environ. Microbiol. 1987;53:266–269. doi: 10.1128/aem.53.2.266-269.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paterson R.R., Venâncio A., Lima N. Solutions to Penicillium taxonomy crucial to mycotoxin research and health. Res. Microbiol. 2004;155:507–513. doi: 10.1016/j.resmic.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Vega F.E., Posada F., Peterson S.W., Gianfagna T.J., Chaves F. Penicillium species endophytic in coffee plants and ochratoxin A production. Mycologia. 2006;98:31–42. doi: 10.3852/mycologia.98.1.31. [DOI] [PubMed] [Google Scholar]
- 15.Frisvad J.C., Thrane U., Samson R.A., Pitt J.I. Important mycotoxins and the fungi which produce them. Adv. Food Mycol. 2006;571:3–31. doi: 10.1007/0-387-28391-9_1. [DOI] [PubMed] [Google Scholar]
- 16.Frisvad J.C., Frank J.M., Houbraken J.A.M.P., Kuijpers A.F.A., Samson R.A. New ochratoxin A producing species of Aspergillus section Circumdati. Stud. Mycol. 2004;50:23–43. [Google Scholar]
- 17.Hesseltine C.W., Vandegraft E.E., Fennell D.I., Smith M.L., Shotwell O.L. Aspergilli as ochratoxin producers. Mycologia. 1972;64:539–550. [PubMed] [Google Scholar]
- 18.Ciegler A. Bioproduction of ochratoxin A and penicillic acid by members of the Aspergillus ochraceus group. Can. J. Microbiol. 1972;18:631–636. doi: 10.1139/m72-100. [DOI] [PubMed] [Google Scholar]
- 19.Varga J., Kevei E., Rinyu E., Téren J., Kozakiewicz Z. Ochratoxin production by Aspergillus species. Appl. Environ. Microbiol. 1996;62:4461–4464. doi: 10.1128/aem.62.12.4461-4464.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bayman P., Baker J.L., Doster M.A., Michailides T.J., Mahoney N.E. Ochratoxin production by the Aspergillus ochraceus group and Aspergillus alliaceus. Appl. Environ. Microbiol. 2002;68:2326–2329. doi: 10.1128/AEM.68.5.2326-2329.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horie Y. Productivity of ochratoxin A of Aspergillus carbonarius in Aspergillus section Nigri. Nippon Kingakkai Kaiho. 1995;36:73–76. [Google Scholar]
- 22.Téren J., Varga J., Hamari Z., Rinyu E., Kevei F. Immunochemical detection of ochratoxin A in black Aspergillus strains. Mycopathologia. 1996;134:171–176. doi: 10.1007/BF00436726. [DOI] [PubMed] [Google Scholar]
- 23.Samson R.A., Houbraken J.A.M.P., Kuijpers A.F.A., Frank J.M., Frisvad J.C. New ochratoxin A or sclerotium producing species in Aspergillus section Nigri. Stud. Mycol. 2004;50:45–61. [Google Scholar]
- 24.Abarca M.L., Bragulat M.R., Castellá G., Cabañes F.J. Ochratoxin A production by strains of Aspergillus niger var. niger. Appl. Environ. Microbiol. 1994;60:2650–2652. doi: 10.1128/aem.60.7.2650-2652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen T.O., Svendsen A., Smedsgaard J. Biochemical characterization of ochratoxin A-producing strains of the genus Penicillium. Appl. Environ. Microbiol. 2001;67:3630–3635. doi: 10.1128/AEM.67.8.3630-3635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Walbeek W., Scott P.M., Harwig J., Lawrence J.W. Penicillium viridicatum Westling: A new source of ochratoxin A. Can. J. Microbiol. 1969;15:1281–1285. doi: 10.1139/m69-232. [DOI] [PubMed] [Google Scholar]
- 27.Ciegler A., Fennell D.I., Sansing G.A., Detroy R.W., Bennett G.A. Mycotoxin-producing strains of Penicillium viridicatum: Classification into subgroups. Appl. Microbiol. 1973;26:271–278. doi: 10.1128/am.26.3.271-278.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serra R., Mendonca C., Venâncio A. Fungi and ochratoxin A detected in healthy grapes for wine production. Lett. Appl. Microbiol. 2006;42:42–47. doi: 10.1111/j.1472-765X.2005.01805.x. [DOI] [PubMed] [Google Scholar]
- 29.Pitt J.I., Taniwaki M.H., Teixeira A.A., Iamanaka B.T. Distribution of Aspergillus ochraceus, A. niger and A. carbonarius in coffee in four regions of Brazil; Proceedings of the 19th Colloquium: Moisture Management for Mould Prevention in Coffee; Trieste, Italy. 2001. [Google Scholar]
- 30.Esteban A., Abarca M.L., Bragulat M.R., Cabañes F.J. Effects of temperature and incubation time on production of ochratoxin A by black aspergilli. Res. Microbiol. 2004;155:861–866. doi: 10.1016/j.resmic.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira N.P., Pitout M.J. Biogenesis of Ochratoxin. J. S. Afr. Chem. I. 1969;22:S1. [Google Scholar]
- 32.Searcy J.W., Davis N.D., Diener U.L. Biosynthesis of Ochratoxin A. Appl. Microbiol. 1969;18:622–627. doi: 10.1128/am.18.4.622-627.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steyn P.S., Holzapfel C.W., Ferreira N.P. The biosynthesis of the ochratoxins, metabolites of Aspergillus ochraceus . Phytochemistry. 1970;9:1977–1983. [Google Scholar]
- 34.Wei R.D., Strong F.M., Smalley E.B. Incorporation of chlorine-36 into ochratoxin A. Appl. Microbiol. 1971;22:276–277. doi: 10.1128/am.22.3.276-277.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karolewiez A., Geisen R. Cloning a part of the ochratoxin A biosynthetic gene cluster of Penicillium nordicum and characterization of the ochratoxin polyketide synthase gene. Syst. Appl. Microbiol. 2005;28:588–595. doi: 10.1016/j.syapm.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 36.O'Callaghan J., Caddick M.X., Dobson A.D.W. A polyketide synthase gene required for ochratoxin A biosynthesis in Aspergillus ochraceus. Microbiology. 2003;149:3485–3491. doi: 10.1099/mic.0.26619-0. [DOI] [PubMed] [Google Scholar]
- 37.Atoui A., Dao H.P., Mathieu F., Lebrihi A. Amplification and diversity analysis of ketosynthase domains of putative polyketide synthase genes in Aspergillus ochraceus and Aspergillus carbonarius producers of ochratoxin. Mol. Nutr. Food Res. 2006;50:488–493. doi: 10.1002/mnfr.200500165. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell D., Parra R., Aldred D., Magan N. Water and temperature relations of growth and ochratoxin A production by Aspergillus carbonarius strains from grapes in Europe and Isra. J. Appl. Microbiol. 2004;97:439–445. doi: 10.1111/j.1365-2672.2004.02321.x. [DOI] [PubMed] [Google Scholar]
- 39.Ramos A.J., Labernia N., Marin S., Sanchís V., Magan N. Effect of water activity and temperature on growth and ochratoxin production by three strains of Aspergillus ochraceus on a barley extract medium and on barley grains. Int. J. Food Microbiol. 1998;44:133–140. doi: 10.1016/s0168-1605(98)00131-7. [DOI] [PubMed] [Google Scholar]
- 40.Northolt M.D., Vanegmond H.P., Paulsch W.E. Ochratoxin A production by some fungal species in relation to water activity and temperature. J. Food Prot. 1979;42:485–490. doi: 10.4315/0362-028X-42.6.485. [DOI] [PubMed] [Google Scholar]
- 41.Pitt J.I., Hocking A.D. Fungi and Food Spoilage. Blackie Academic & Professional; London, UK: 1997. [Google Scholar]
- 42.Baxter E.D., Slaiding I.R., Kelly B. Behavior of ochratoxin A in brewing. J. Am. Soc. Brew. Chem. 2001;59:98–100. [Google Scholar]
- 43.Scott P.M., Kanhere S.R., Lawrence G.A., Daley E.F., Farber J.M. Fermentation of wort containing added ochratoxin A and fumonisins B1 and B2. Food Addit. Contam. 1995;12:31–40. doi: 10.1080/02652039509374276. [DOI] [PubMed] [Google Scholar]
- 44.Scudamore K.A., Banks J., MacDonald S.J. Fate of ochratoxin A in the processing of whole wheat grains during milling and bread production. Food Addit. Contam. 2003;20:1153–1163. doi: 10.1080/02652030310001605979. [DOI] [PubMed] [Google Scholar]
- 45.Suarez-Quiroz M., De Louise B., Gonzalez-Rios O., Barel M., Guyot B., Schorr-Galindo S., Guiraud J.P. The impact of roasting on the ochratoxin A content of coffee. Int. J. Food Sci. Tech. 2005;40:605–611. [Google Scholar]
- 46.Fernandes A., Ratola N., Cerdeira A., Alves A., Venancio A. Changes in ochratoxin A concentration during winemaking. Am. J. Enol. Vitic. 2007;58:92–96. [Google Scholar]
- 47.Domijan A.-M., Peraica M., Zlender V., Cvjetkovic B., Jurjevic Z., Topolovec-Pintaric S., Ivic D. Seed-borne fungi and ochratoxin A contamination of dry beans (Phaseolus vulgaris L.) in the Republic of Croatia. Food Chem. Toxicol. 2005;43:427–432. doi: 10.1016/j.fct.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Amezqueta S., González-Peñas E., Murillo M., De Cerain A.L. Validation of a high-performance liquid chromatography analytical method for ochratoxin A quantification in cocoa beans. Food Addit. Contam. 2004;21:1096–1106. doi: 10.1080/02652030400019422. [DOI] [PubMed] [Google Scholar]
- 49.Shotwell O.L., Hesseltine C.W., Goulden M.L. Ochratoxin A: Occurrence as natural contaminant of a corn sample. Appl. Microbiol. 1969;17:765–766. doi: 10.1128/am.17.5.765-766.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Senyuva H.Z., Gilbert J., Ozcan S., Ulken U. Survey for co-occurrence of ochratoxin A and aflatoxin B1, in dried figs in Turkey by using a single laboratory-validated alkaline extraction method for ochratoxin. J. Food Prot. 2005;68:1512–1515. doi: 10.4315/0362-028x-68.7.1512. [DOI] [PubMed] [Google Scholar]
- 51.Iamanaka B.T., Taniwaki M.H., Menezes H.C., Vicente E., Fungaro M.H.P. Incidence of toxigenic fungi and ochratoxin A in dried fruits sold in Brazil. Food Addit. Contam. 2005;22:1258–1263. doi: 10.1080/02652030500260447. [DOI] [PubMed] [Google Scholar]
- 52.Serra R., Mendonca C., Venancio A. Ochratoxin A occurrence and formation in Portuguese wine grapes at various stages of maturation. Int. J. Food Microbiol. 2006;111:S35–S39. doi: 10.1016/j.ijfoodmicro.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 53.Romani S., Sacchetti G., Chaves Lopez C., Pinnavaia G.G., Dalla Rosa M. Screening on the occurrence of ochratoxin A in green coffee beans of different origins and types. J. Agric. Food Chem. 2000;48:3616–3619. doi: 10.1021/jf990783b. [DOI] [PubMed] [Google Scholar]
- 54.Skaug M.A. Analysis of Norwegian milk and infant formulas for ochratoxin A. Food Addit. Contam. 1999;16:75–78. doi: 10.1080/026520399284235. [DOI] [PubMed] [Google Scholar]
- 55.Jørgensen K., Petersen A. Content of ochratoxin A in paired kidney and meat samples from healthy Danish slaughter pigs. Food Addit. Contam. 2002;19:562–567. doi: 10.1080/02652030110113807. [DOI] [PubMed] [Google Scholar]
- 56.MacDonald S., Wilson P., Barnes K., Damant A., Massey R., Mortby E., Shepherd M.J. Ochratoxin A in dried vine fruit: Method development and survey. Food Addit. Contam. 1999;16:253–260. doi: 10.1080/026520399284019. [DOI] [PubMed] [Google Scholar]
- 57.Gonzalez L., Juan C., Soriano J.M., Molto J.C., Manes J. Occurrence and daily intake of ochratoxin A of organic and non-organic rice and rice products. Int. J. Food Microbiol. 2006;107:223–227. doi: 10.1016/j.ijfoodmicro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 58.Thirumala-Devi K., Mayo M.A., Reddy G., Emmanuel K.E., Larondelle Y., Reddy D.V. Occurrence of ochratoxin A in black pepper, coriander, ginger and turmeric in India. Food Addit. Contam. 2001;18:830–835. doi: 10.1080/02652030117589. [DOI] [PubMed] [Google Scholar]
- 59.Scudamore K.A., Patel S., Breeze V. Surveillance of stored grain from the 1997 harvest in the United Kingdom for ochratoxin A. Food Addit. Contam. 1999;16:281–290. doi: 10.1080/026520399283948. [DOI] [PubMed] [Google Scholar]
- 60.Scott P.M., van Walbeek W., Kennedy B., Anyeti D. Mycotoxins (ochratoxin A, citrinin, and sterigmatocystin) and toxigenic fungi in grains and other agricultural products. J. Agric. Food Chem. 1972;20:1103–1109. doi: 10.1021/jf60184a010. [DOI] [PubMed] [Google Scholar]
- 61.Lombaert G.A., Pellaers P., Roscoe V., Mankotia M., Neil R., Scott P.M. Mycotoxins in infant cereal foods from the Canadian retail market. Food Addit. Contam. 2003;20:494–504. doi: 10.1080/0265203031000094645. [DOI] [PubMed] [Google Scholar]
- 62.Visconti A., Pascale M., Centonze G. Determination of ochratoxin A in domestic and imported beers in Italy by immunoaffinity clean-up and liquid chromatograph. J. Chromatogr. A. 2000;888:321–326. doi: 10.1016/s0021-9673(00)00549-5. [DOI] [PubMed] [Google Scholar]
- 63.Molinié A., Faucet V., Castegnaro M., Pfohl-Leszkowicz A. Analysis of some breakfast cereals on the French market for their contents of ochratoxin A, citrinin and fumonisin B1: development of a method for simultaneous extraction of ochratoxin A and citrinin. Food Chem. 2005;92:391–400. [Google Scholar]
- 64.Tafuri A., Ferracane R., Ritieni A. Ochratoxin A in Italian marketed cocoa products. Food Chem. 2004;88:487–494. [Google Scholar]
- 65.Zimmerli B., Dick R. Ochratoxin A in table wine and grape-juice: Occurrence and risk assessment. Food Addit. Contam. 1996;13:655–668. doi: 10.1080/02652039609374451. [DOI] [PubMed] [Google Scholar]
- 66.Pietri A., Bertuzzi T., Gualla A., Piva G. Occurrence of ochratoxin A in raw ham muscles and in pork products from northern Italy. Ital. J. Food Sci. 2006;18:99–106. [Google Scholar]
- 67.Tsubouchi H., Terada H., Yamamoto K., Hisada K., Sakabe Y. Ochratoxin A found in commercial roast coffee. J. Agric. Food Chem. 1988;36:540–542. [Google Scholar]
- 68.Monaci L., Palmisano F., Matrella R., Tantillo G. Determination of ochratoxin A at part-per-trillion level in Italian salami by immunoaffinity clean-up and high-performance liquid chromatography with fluorescence detection. J. Chromatogr. A. 1090:184–187. doi: 10.1016/j.chroma.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 69.Scudamore K.A., Banks J.N., Guy R.C.E. Fate of ochratoxin A in the processing of whole wheat grain during extrusion. Food Addit. Contam. 2004;21:488–497. doi: 10.1080/02652030410001670166. [DOI] [PubMed] [Google Scholar]
- 70.Scudamore K.A. Prevention of ochratoxin A in commodities and likely effects of processing fractionation and animal feed. Food Addit. Contam. 2005;22:17–25. doi: 10.1080/02652030500309392. [DOI] [PubMed] [Google Scholar]
- 71.Jaimez J., Fente C.A., Franco C.M., Cepeda A., Vazquez B.I. A survey of the fungal contamination and presence of ochratoxin A and zearalenone on Spanish feed and raw material. J. Sci. Food Agric. 2004;84:832–840. [Google Scholar]
- 72.Dalcero A., Magnoli C., Hallak C., Chiacchiera S.M., Palacio G., Rosa C.A.d.R. Detection of ochratoxin A in animal feeds and capacity to produce this mycotoxin by Aspergillus section Nigri in Argentina. Food Addit. Contam. 2002;19:1065–1072. doi: 10.1080/02652030210151895. [DOI] [PubMed] [Google Scholar]
- 73.Thirumala-Devi K., Mayo M.A., Reddy G., Reddy D.V.R. Occurrence of aflatoxins and ochratoxin A in Indian poultry feeds. J. Food Prot. 2002;65:1338–1340. doi: 10.4315/0362-028x-65.8.1338. [DOI] [PubMed] [Google Scholar]
- 74.Xiao H., Madhyastha S., Marquardt R.R., Li S., Vodela J.K., Frohlich A.A., Kemppainen B.W. Toxicity of ochratoxin A, Its opened lactone form and several of its analogs: Structure-activity relationship. Toxicol. Appl. Pharmacol. 1996;137:182–192. doi: 10.1006/taap.1996.0071. [DOI] [PubMed] [Google Scholar]
- 75.Muller G., Rosner H., Rohrmann B., Erler W., Geschwend G., Grafe U., Burkert B., Moller U., Diller R., Sachse K., Kohler H. Effects of the mycotoxin ochratoxin A and some of its metabolites on the human cell line THP-1. Toxicology. 2003;184:69–82. doi: 10.1016/s0300-483x(02)00593-0. [DOI] [PubMed] [Google Scholar]
- 76.Fuchs R., Hult K., Peraica M., Radic B., Plestina R. Conversion of ochratoxin C into ochratoxin A in vivo. Appl. Environ. Microbiol. 1984;48:41–42. doi: 10.1128/aem.48.1.41-42.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li S., Marquardt R.R., Frohlich A.A., Vitti T.G., Crow G. Pharmacokinetics of ochratoxin A and its metabolites in rats. Toxicol. Appl. Pharmacol. 1997;145:82–90. doi: 10.1006/taap.1997.8155. [DOI] [PubMed] [Google Scholar]
- 78.Krogh P. Role of ochratoxin in disease causation. Food Chem. Toxicol. 1992;30:213–224. doi: 10.1016/0278-6915(92)90036-k. [DOI] [PubMed] [Google Scholar]
- 79.Pfohl-Leszkowicz A., Petkova-Bocharova T., Chernozemsky I.N., Castegnaro M. Balkan endemic nephropathy and associated urinary tract tumours: a review on aetiological causes and the potential role of mycotoxin. Food Addit. Contam. 2002;19:282–302. doi: 10.1080/02652030110079815. [DOI] [PubMed] [Google Scholar]
- 80.Pfohl-Leszkowicz A., Manderville R.A. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and human. Mol. Nutr. Food Res. 2007;51:61–99. doi: 10.1002/mnfr.200600137. [DOI] [PubMed] [Google Scholar]
- 81.Pfohl-Leszkowicz A. Ochratoxin A and Aristolochic Acid Involvement in Nephropathies and Associated Urothelial Tract Tumour. Arhiv za Higijenu Rada i Toksikologiju. 2009;60:465–483. doi: 10.2478/10004-1254-60-2009-2000. [DOI] [PubMed] [Google Scholar]
- 82.IARC, authors. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 56. World Health Organization; Lyon, France: 1993. Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins; pp. 489–521. [Google Scholar]
- 83.O'Brien E., Dietrich D.R. Ochratoxin A: The continuing enigma. Crit. Rev. Microbiol. 2005;35:33–60. doi: 10.1080/10408440590905948. [DOI] [PubMed] [Google Scholar]
- 84.Chu F.S. A comparative study of the interaction of ochratoxins with bovine serum albumin. Biochem. Pharmacol. 1974;23:1105–1113. doi: 10.1016/0006-2952(74)90011-2. [DOI] [PubMed] [Google Scholar]
- 85.Perry J.L., Christensen T., Goldsmith M.R., Toone E.J., Beratan D.N., Simon J.D. Binding of ochratoxin A to human serum albumin stabilized by a protein-ligand ion pair. J. Phys. Chem. B. 2003;107:7884–7888. [Google Scholar]
- 86.Kumagai S. Ochratoxin A: Plasma concentration and excretion into bile and urine in albumin-deficient rats. Food Chem. Toxicol. 1985;23:941–943. doi: 10.1016/0278-6915(85)90112-7. [DOI] [PubMed] [Google Scholar]
- 87.Creppy E.E., Stormer F.C., Roschenthaler R., Dirheimer G. Effects of two metabolites of ochratoxin A, (4R)-4-hydroxyochratoxin A and ochratoxin α, on immune response in mice. Infect. Immun. 1983;39:1015–1018. doi: 10.1128/iai.39.3.1015-1018.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bruinink A., Rasonyi T., Sidler C. Differences in neurotoxic effects of ochratoxin A, ochracin and ochratoxin-α in vitro. Nat. Toxins. 1998;6:173–177. doi: 10.1002/(sici)1522-7189(199809/10)6:5<173::aid-nt10>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 89.Zimmerli B., Dick R. Determination of ochratoxin A at the ppt level in human blood, serum, milk and some foodstuffs by high-performance liquid chromatography with enhanced fluorescence detection and immunoaffinity column cleanup: methodology and Swiss data. J. Chromatogr. B. 1995;666:85–99. doi: 10.1016/0378-4347(94)00569-q. [DOI] [PubMed] [Google Scholar]
- 90.Ueno Y., Maki S., Lin J., Furuya M., Sugiura Y., Kawamura O. A 4-year study of plasma ochratoxin A in a selected population in Tokyo by immunoassay and immunoaffinity column-linked HPLC. Food Chem. Toxicol. 1998;36:445–449. doi: 10.1016/s0278-6915(98)00004-0. [DOI] [PubMed] [Google Scholar]
- 91.Sangare-Tigori B., Moukha S., Kouadio J.H., Dano-Djedje S., Betbeder A.-M., Achour A., Creppy E.E. Ochratoxin A in human blood in Abidjan, Cote d'Ivoire. Toxicon. 2006;47:894–900. doi: 10.1016/j.toxicon.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 92.Skaug M.A., Helland I., Solvoll K., Saugstad O.D. Presence of ochratoxin A in human milk in relation to dietary intake. Food Addit. Contam. 2001;18:321–327. doi: 10.1080/02652030117740. [DOI] [PubMed] [Google Scholar]
- 93.Turconi G., Guarcello M., Livieri C., Comizzoli S., Maccarini L., Castellazzi A., Pietri A., Piva G., Roggi C. Evaluation of xenobiotics in human milk and ingestion by the newborn: An epidemiological survey in Lombardy (Northern Italy) Eur. J. Nutr. 2004;43:191–197. doi: 10.1007/s00394-004-0458-2. [DOI] [PubMed] [Google Scholar]
- 94.Micco C., Miraglia M., Brera C., Corneli S., Ambruzzi A. Evaluation of ochratoxin A level in human milk in Italy. Food Addit. Contam. 1995;12:351–354. doi: 10.1080/02652039509374314. [DOI] [PubMed] [Google Scholar]
- 95.Amezqueta S., Gonzalez-Penas E., Murillo-Arbizu M., De Cerain A.L. Ochratoxin A decontamination: A review. Food Control. 2009;20:326–333. [Google Scholar]
- 96.Riley R.T., Norred W.P. Mycotoxin prevention and decontamination-A case study on maize. Food Nutr. Agric. 1999;23:25–32. [Google Scholar]
- 97.Karlovsky P. Biological detoxification of fungal toxins and its use in plant breeding, feed and food production. Nat. Toxins. 1999;7:1–23. doi: 10.1002/(sici)1522-7189(199902)7:1<1::aid-nt37>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 98.Shimizu S., Kataoka M., Honda K., Sakamoto K. Lactone-ring-cleaving enzymes of microorganisms: their diversity and applications. J. Biotechnol. 2001;92:187–194. doi: 10.1016/s0168-1656(01)00359-5. [DOI] [PubMed] [Google Scholar]
- 99.Hult K., Teiling A., Gatenbeck S. Degradation of ochratoxin A by a ruminant. Appl. Environ. Microbiol. 1976;32:443–444. doi: 10.1128/aem.32.3.443-444.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kiessling K.H., Pettersson H., Sandholm K., Olsen M. Metabolism of aflatoxin, ochratoxin, zearalenone, and three trichothecenes by intact rumen fluid, rumen protozoa, and rumen bacteria. Appl. Environ. Microbiol. 1984;47:1070–1073. doi: 10.1128/aem.47.5.1070-1073.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.zpinar H., Bilal T., Abas I., Kutay C. Degradation of ochratoxin A in rumen fluid in vitro. Med. Biol. 2002;9:66–69. [Google Scholar]
- 102.Hohler D., Sudekum K.H., Wolffram S., Frohlich A.A., Marquardt R.R. Metabolism and excretion of ochratoxin A fed to sheep. J. Anim. Sci. 1999;77:1217–1223. doi: 10.2527/1999.7751217x. [DOI] [PubMed] [Google Scholar]
- 103.Wegst W., Lingens F. Bacterial degradation of ochratoxin A. FEMS Microbiol. Lett. 1983;17:341–344. [Google Scholar]
- 104.Hwang C.A., Draughon F.A. Degradation of ochratoxin A by Acinetobacter calcoaceticus. J. Food Prot. 1994;57:410–414. doi: 10.4315/0362-028X-57.5.410. [DOI] [PubMed] [Google Scholar]
- 105.Petchkongkaew A., Taillandier P., Gasaluck P., Lebrihi A. Isolation of Bacillus spp. from Thai fermented soybean (Thua-nao): Screening for aflatoxin B1 and ochratoxin A detoxification. J. Appl. Microbiol. 2008;104:1495–1502. doi: 10.1111/j.1365-2672.2007.03700.x. [DOI] [PubMed] [Google Scholar]
- 106.Skrinjar M., Rasic J.L., Stojicic V. Lowering of ochratoxin A level in milk by yoghurt bacteria and bifidobacteria. Folia Microbiol. (Praha) 1996;41:26–28. doi: 10.1007/BF02816335. [DOI] [PubMed] [Google Scholar]
- 107.Böhm J., Grajewski J., Asperger H., Rabus B., Razzazi E. Study on biodegradation of some trichothecenes (NIV DON, DAS, T-2) and ochratoxin A by use of probiotic microorganisms. Mycot. Res. 2000;16:70–74. doi: 10.1007/BF02942985. [DOI] [PubMed] [Google Scholar]
- 108.Piotrowska M., Zakowska Z. The biodegradation of ochratoxin A in food products by lactic acid bacteria and baker's yeast. In: Bielecki S., Tramper J., Polak J., editors. Progress in Biotechnology (Food Biotechnology) Vol. 17. Elsevier; Amsterdam, The Netherlands: 2000. pp. 307–310. [Google Scholar]
- 109.Turbic A., Ahokas J.T., Haskard C.A. Selective in vitro binding of dietary mutagens, individually or in combination, by lactic acid bacteria. Food Addit. Contam. 2002;19:144–152. doi: 10.1080/02652030110070067. [DOI] [PubMed] [Google Scholar]
- 110.Piotrowska M., Zakowska Z. The elimination of ochratoxin A by lactic acid bacteria strains. Pol. J. Microbiol. 2005;54:279–286. [PubMed] [Google Scholar]
- 111.Del Prete V., Rodriguez H., Carrascosa A.V., Rivas B.D.L., Garcia-Moruno E., Munoz R. In vitro removal of ochratoxin A by wine lactic acid bacteria. J. Food Prot. 2007;70:2155–2160. doi: 10.4315/0362-028x-70.9.2155. [DOI] [PubMed] [Google Scholar]
- 112.Fuchs S., Sontag G., Stidl R., Ehrlich V., Kundi M., Knasmuller S. Detoxification of patulin and ochratoxin A, two abundant mycotoxins, by lactic acid bacter. Food Chem. Toxicol. 2008;46:1398–1407. doi: 10.1016/j.fct.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 113.Shetty P.H., Jespersen L. Saccharomyces cerevisiae and lactic acid bacteria as potential mycotoxin decontaminating agents. Trends Food Sci. Tech. 2006;17:48–55. [Google Scholar]
- 114.Bejaoui H., Mathieu F., Lebrihi A., Taillandier P. Ochratoxin A removal in synthetic and natural grape juices by selected oenological Saccharomyces strains. J. Appl. Microbiol. 2004;97:1038–1044. doi: 10.1111/j.1365-2672.2004.02385.x. [DOI] [PubMed] [Google Scholar]
- 115.Bizaj E., Mavri J., Cus F., Raspor A. Removal of ochratoxin A in Saccharomyces cerevisiae liquid cultures. S. Afr. J. Enol. Vitic. 2009;30:151–155. [Google Scholar]
- 116.Cecchini F., Morassut M., Garcia Moruno E., Di Stefano R. Influence of yeast strain on ochratoxin A content during fermentation of white and red must. Food Microbiol. 2006;23:411–417. doi: 10.1016/j.fm.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 117.Moruno E.G., Sanlorenzo C., Boccaccino B., Di Stefano R. Treatment with yeast to reduce the concentration of ochratoxin A in red wine. Am. J. Enol. Vitic. 2005;56:73–76. [Google Scholar]
- 118.Ringot D., Lerzy B., Bonhoure J.P., Auclair E., Oriol E., Larondelle Y. Effect of temperature on in vitro ochratoxin A biosorption onto yeast cell wall derivatives. Process Biochem. 2005;40:3008–3016. [Google Scholar]
- 119.Yiannikouris A., andre G., Poughon L., Francois J., Dussap C.G., Jeminet G., Bertin G., Jouany J.P. Chemical and conformational study of the interactions involved in mycotoxin complexation with β-D-glucans. Biomacromolecules. 2006;7:1147–1155. doi: 10.1021/bm050968t. [DOI] [PubMed] [Google Scholar]
- 120.Raju M.V.L.N., Devegowda G. Esterified-glucomannan in broiler chicken diets-contaminated with aflatoxin, ochratoxin and T-2 toxin: Evaluation of its binding ability (in vitro) and efficacy as immunomodulator. Asian Australas. J. Anim. Sci. 2002;15:1051–1056. [Google Scholar]
- 121.Oguz H., Parlat S.S. Effects of dietary mannanoligosaccharide on performance of Japanese quail affected by aflatoxicosis. S. Afr. J. Anim. Sci. 2004;34:144–148. [Google Scholar]
- 122.Schatzmayr G., Heidler D., Fuchs E., Mohnl M., Täubel M., Loibner A.P., Braun R., Binder E.M. Investigation of different yeast strains for the detoxification of ochratoxin A. Mycot. Res. 2003;19:124–128. doi: 10.1007/BF02942950. [DOI] [PubMed] [Google Scholar]
- 123.Molnar O., Schatzmayr G., Fuchs E., Prillinger H. Trichosporon mycotoxinivorans sp nov., a new yeast species useful in biological detoxification of various mycotoxins. Syst. Appl. Microbiol. 2004;27:661–671. doi: 10.1078/0723202042369947. [DOI] [PubMed] [Google Scholar]
- 124.Politis I., Fegeros K., Nitsch S., Schatzmayr G., Kantas D. Use of Trichosporon mycotoxinivorans to suppress the effects of ochratoxicosis on the immune system of broiler chicks. Br. Poult. Sci. 2005;46:58–65. doi: 10.1080/00071660400023904. [DOI] [PubMed] [Google Scholar]
- 125.Hofstetter U., Schatzmayr D., Schatzmayr G., Binder E.M. Successful detoxification of ochratoxin A in weaning piglets. J. Anim. Sci. 2006;84:308. [Google Scholar]
- 126.Hickey P.W., Sutton D.A., Fothergill A.W., Rinaldi M.G., Wickes B.L., Schmidt H.J., Walsh T.J. Trichosporon mycotoxinivorans: A novel respiratory pathogen in patients with cystic fibrosis. J. Clin. Microbiol. 2009;47:3091–3097. doi: 10.1128/JCM.00460-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Peteri Z., Teren J., Vagvolgyi C., Varga J. Ochratoxin degradation and adsorption caused by astaxanthin-producing yeasts. Food Microbiol. 2007;24:205–210. doi: 10.1016/j.fm.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 128.de Felice D.V., Solfrizzo M., De Curtis F., Lima G., Visconti A., Castoria R. Strains of Aureobasidium pullulans can lower ochratoxin A contamination in wine grapes. Phytopathology. 2008;98:1261–1270. doi: 10.1094/PHYTO-98-12-1261. [DOI] [PubMed] [Google Scholar]
- 129.Hawkes M., Rennie R., Sand C., Vaudry W. Aureobasidium pullulans infection: Fungemia in an infant and a review of human cases. Diagn. Microbiol. Infect. Dis. 2005;51:209–213. doi: 10.1016/j.diagmicrobio.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 130.Varga J., Rigó K., Téren J. Degradation of ochratoxin A by Aspergillus species. Int. J. Food Microbiol. 2000;59:1–7. doi: 10.1016/s0168-1605(00)00230-0. [DOI] [PubMed] [Google Scholar]
- 131.Abrunhosa L., Serra R., Venâncio A. Biodegradation of ochratoxin A by fungi isolated from grapes. J. Agric. Food Chem. 2002;50:7493–7496. doi: 10.1021/jf025747i. [DOI] [PubMed] [Google Scholar]
- 132.Varga J., Peteri Z., Tabori K., Teren J., Vagvolgyi C. Degradation of ochratoxin A and other mycotoxins by Rhizopus isolates. Int. J. Food Microbiol. 2005;99:321–328. doi: 10.1016/j.ijfoodmicro.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 133.Bejaoui H., Mathieu F., Taillandier P., Lebrihi A. Biodegradation of ochratoxin A by Aspergillus section Nigri species isolated from French grapes: A potential means of ochratoxin A decontamination in grape juices and musts. FEMS Microbiol. Lett. 2006;255:203–208. doi: 10.1111/j.1574-6968.2005.00073.x. [DOI] [PubMed] [Google Scholar]
- 134.Valero A., Sanchis V., Ramos A.J., Marin S. Brief in vitro study on Botrytis cinerea and Aspergillus carbonarius regarding growth and ochratoxin. Lett. Appl. Microbiol. 2008;47:327–332. doi: 10.1111/j.1472-765x.2008.02434.x. [DOI] [PubMed] [Google Scholar]
- 135.Engelhardt G. Degradation of ochratoxin A and B by the white rot fungus Pleurotus ostreatus . Mycot. Res. 2002;18:37–43. doi: 10.1007/BF02946138. [DOI] [PubMed] [Google Scholar]
- 136.Ruhland M., Engelhardt G., Wallnofer P.R. Transformation of the mycotoxin ochratoxin A in plants. 2. Time course and rates of degradation and metabolite production in cell-suspension cultures of different crop plants. Mycopathologia. 1996;134:97–102. doi: 10.1007/BF00436871. [DOI] [PubMed] [Google Scholar]
- 137.Pitout M.J. The hydrolysis of ochratoxin A by some proteolytic enzymes. Biochem. Pharmacol. 1969;18:485–491. doi: 10.1016/0006-2952(69)90224-x. [DOI] [PubMed] [Google Scholar]
- 138.Stander M.A., Bornscheuer U.T., Henke E., Steyn P.S. Screening of commercial hydrolases for the degradation of ochratoxin A. J. Agric. Food Chem. 2000;48:5736–5739. doi: 10.1021/jf000413j. [DOI] [PubMed] [Google Scholar]
- 139.Abrunhosa L., Santos L., Venâncio A. Degradation of ochratoxin A by proteases and by a crude enzyme of Aspergillus niger. Food Biotechnol. 2006;20:231–242. [Google Scholar]
- 140.Abrunhosa L., Venâncio A. Isolation and purification of an enzyme hydrolyzing ochratoxin A from Aspergillus niger. Biotechnol. Lett. 2007;29:1909–1914. doi: 10.1007/s10529-007-9479-2. [DOI] [PubMed] [Google Scholar]
- 141.Abrunhosa L., Venâncio A. 2008 Unpublished work. [Google Scholar]
- 142.Stander M.A., Steyn P.S., van der Westhuizen F.H., Payne B.E. A kinetic study into the hydrolysis of the ochratoxins and analogues by carboxypeptidase A. Chem. Res. Toxicol. 2001;14:302–304. doi: 10.1021/tx000221i. [DOI] [PubMed] [Google Scholar]
- 143.Schatzmayr G., Binder J., Thimm N., Heidler D., Fuchs E., Braun R., Binder E.M. Enzymatic degradation of ochratoxin A; Proceedings of the X. Intern. IUPAC Symposium on Mycotoxins, Phytotoxins; Guaruja, Brazil. 2000. [Google Scholar]