Abstract
Ochratoxin A (OTA) producing fungi are members of the genera Aspergillus and Penicillium. Nowadays, there are about 20 species accepted as OTA producers, which are distributed in three phylogenetically related but distinct groups of aspergilli of the subgenus Circumdati and only in two species of the subgenus Penicillium. At the moment, P. verrucosum and P. nordicum are the only OTA producing species accepted in the genus Penicillium. However, during the last century, OTA producers in this genus were classified as P. viridicatum for many years. At present, only some OTA producing species are known to be a potential source of OTA contamination of cereals and certain common foods and beverages such as bread, beer, coffee, dried fruits, grape juice and wine among others. Penicillium verrucosum is the major producer of OTA in cereals such as wheat and barley in temperate and cold climates. Penicillium verrucosum and P. nordicum can be recovered from some dry-cured meat products and some cheeses.
Keywords: foods, ochratoxin A, Penicillium, taxonomy
1. Introduction
Ochratoxin A (OTA) is a potent nephrotoxic mycotoxin that has been linked to kidney problems in both livestock and human populations. It has also carcinogenic, genotoxic and immunotoxic properties. Natural occurrence of OTA has been reported from temperate to tropical climates mainly on cereals and their products. However, it is also found in a variety of common foods and beverages, including bread, beer, chocolate, coffee, dried fruits, grape juice, pork, poultry and wine, among others [1]. In a recent study about the health risk assessment of OTA in a market economy [2], the major food contributors of OTA for children were wheat based foods followed by oats, rice and raisins; beer, coffee and wine also contributed to total OTA exposure in older individuals. The presence of OTA in blood from healthy humans confirms a continuous worldwide exposure [3]. In Europe, OTA maximum levels have been established for most of the above mentioned foodstuffs [4], some spices and liquorice [5] and also some guidance values for this mycotoxin have been also recommended for cereals, cereal products intended for animal feed and complete and complementary feedingstuffs for pigs and poultry [6].
Some species of the genera Penicillium and Aspergillus are known to form OTA, but few of them are known to contaminate foods with this mycotoxin. OTA contamination of food and feeds was until recently believed to be produced only by A. ochraceus and by P. verrucosum, which affect mainly dried stored foods and cereals respectively, in different regions of the world. However, some recent surveys have clearly shown that certain species belonging to the black aspergilli, including the A. niger aggregate and A. carbonarius, are sources of OTA in food commodities such as wine, grapes and dried vine fruits worldwide [7,8,9,10,11,12,13,14,15,16,17,18,19]. Recent studies also indicated that in addition to these species, A. westerdijkiae, A. steynii and A. ochraceus are responsible for the formation of OTA in coffee [20,21].
Species included in some of these taxa are difficult to distinguish from each other and molecular methods are usually necessary to identify them. For some of them, taxonomy is not fully resolved, as the number of accepted species depends on the methodology used. So far there has not been complete agreement between phenotypical and molecular data. In part for these reasons, a larger number of species have been cited incorrectly as OTA producers. This is also due mainly to the use of unsuitable analytical techniques in determining OTA production and misidentification of the fungal isolates tested [22]. A set of recommendations have been recently published to avoid incorrect reporting of fungal species producing particular mycotoxins [23]. The taxonomy of the OTA producing species in the genus Aspergillus and their potential for mycotoxin production in different foods have been reviewed in different papers [24,25,26,27,28,29,30]. A new subgeneric classification based on phylogenetic analysis of multilocus sequence data has been recently proposed [31].
In this paper, an overview of the current taxonomy of OTA producing species in the genus Penicillium arising from the most relevant approaches published in this field is presented. This review pays special attention to the natural occurrence of these species on food commodities where they are potential sources of OTA contamination. Emphasis has been placed on literature published within this decade, but prior noteworthy review papers and seminal works are included.
2. OTA Producing Species in the Genus Penicillium
2.1. Taxonomy
Penicillium taxonomy is not easy for the inexperienced, and compared to Aspergillus it is a more diverse genus, in terms of numbers of species and range of habitats [32]. At present, P. verrucosum and P. nordicum are the only OTA producers known and accepted in this genus, despite some reports on OTA production by other species [33,34]. Penicilliumcasei and P. mediolanense are synonyms for P. verrucosum and P. nordicum, respectively [34]. Nevertheless, different examples of incorrect citations of some Penicillium spp. producing OTA (e.g., P. cyclopium, P. viridicatum, P. chrysogenum) have been recently listed [23,34]. It is worth bearing in mind that in the last century, OTA producers in this genus were classified as P. viridicatum for many years. Main species concepts for P. viridicatum, P. verrucosum and P. nordicum are summarized in Table 1.
Table 1.
Main species concepts of OTA producing species in the genus Penicillium.
| References | Strains | ||
|---|---|---|---|
| OTA - and CIT - | OTA + and CIT + | OTA + and CIT - | |
| Frisvad & Samson., 2004 [33] | P. viridicatum | P. verrucosum | P. nordicum |
| P. nordicum II OTA? | |||
| Larsen et al., 2001 [35] | P. viridicatum | P. verrucosum | P. nordicum |
| Frisvad & Filtenborg, 1989 [36] | P. viridicatum | P. verrucosum chemotype II | P. verrucosum chemotype I |
| Pitt, 1987 [37] | P. viridicatum | P. verrucosum chemotype CIT | P. verrucosum |
| Pitt, 1979 [38] | P. viridicatum | P. viridicatum | P. verrucosum |
| Samson et al., 1976 [39] | P. verrucosum var. verrucosum | P. verrucosum var. verrucosum | P. verrucosum var. verrucosum |
| Ciegler et al., 1973 [40] | P. viridicatum I | P. viridicatum II | P. viridicatum III |
| Raper & Thom, 1949 [41] | P. viridicatum | P. viridicatum | P. viridicatum |
OTA, ochratoxin A; CIT, citrinin; +, producing strains; - non producing strains.
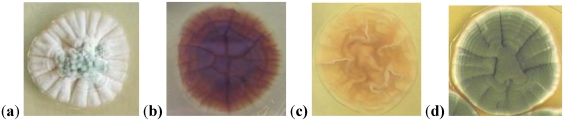
Penicillium verrucosum and P. nordicum have common morphological characteristics such as very similar colony diameters on many culture media or rough stipes (Figure 1). These are slow growing species of the subgenus Penicillium, which is by far the most difficult taxonomically, both because there are numerous species and because apparent differences between species are small. Many species classified in this subgenus are morphologically similar, and identification using traditional morphological techniques remains difficult [32]. Recently, Frisvad and Samson [33] pointed out that a polyphasic approach, including a combination of DNA sequences, extrolite production and other phenotypical characters, is necessary to classify and identify species of Penicillium subgenus Penicillium. These authors keyed a total of 58 species in this subgenus. Many of these species are very common, being associated mainly with stored foods. The OTA producing species are placed in the series Verrucosa of the section Viridicata [33].
Figure 1.
Colonies of some Penicillium spp. on Yeast Extract Sucrose agar after seven days of incubation at 25 °C (a) Colony pattern of P. verrucosum or P. nordicum; (b) P. verrucosum (reverse); (c)P. nordicum (reverse); (d) P. viridicatum.
The series Verrucosa is monophyletic based on the phylogenetic analysis of partial β-tubulin sequences of strains representing all accepted species in this subgenus. This series includes two subclades, one consisting of the non OTA producing species P. thymicola and the other subclade consisting of strains of the two OTA producing species, P. verrucosum and two genetic groups of P. nordicum strains [42]. A similar genetic profile among these species was detected using CO1 (mitochondrial cytochrome c oxidase 1) DNA barcoding [43]. In this study, P. verrucosum and three strains of P. nordicum had identical CO1 barcodes, whereas four P. nordicum strains showed their own unique barcode. Currently, P. viridicatum is placed in the series Viridicata of the section Viridicata [33]. This series also includes other incorrectly cited OTA producing species such as P. aurantiogriseum, P. cyclopium or P. polonicum. Some years before, Larsen et al. [35] had described two distinct groups of OTA producing Penicillium strains based mainly on differences in secondary metabolite profiles. Some strains grouped with the ex-type culture of P. nordicum ATCC 44219 were classified as P. nordicum. Other strains grouped with the type culture of P. verrucosum NRRL 965 were classified as P. verrucosum.
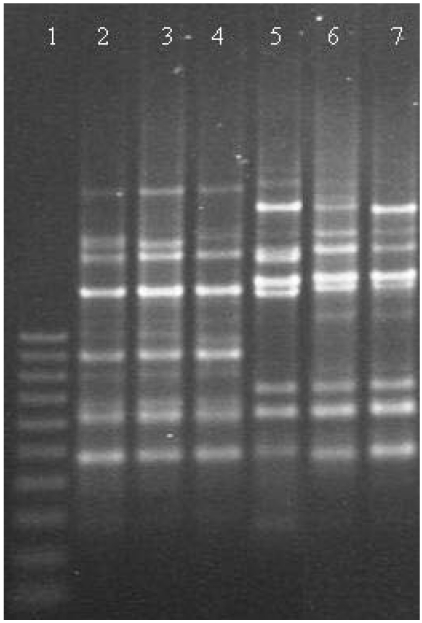
Figure 2.
Agarose gel of representative RAPD banding patterns of P. nordicum and P. verrucosum. Lane 1, 100 bp ladder; lanes 2-4, P. nordicum patterns; lanes 5-7, P.verrucosum patterns.
These species are ecologically different. P. nordicum is generally recovered from meat and cheese products whereas P. verrucosum is recovered mainly from plant-derived material. Besides, most of the isolates of this latter species have a characteristic dark brown reverse color on Yeast Extract Sucrose agar (YES), whereas almost all the P. nordicum strains show a pale, creamy or dull yellow reverse color in this culture medium [33,35]. The colony pattern of P. viridicatum is different on YES (Figure 1). Among other differences, Frisvad and Samson [33] considered P. verrucosum among the species always negative (no reaction) or occasionally producing a yellow reaction for the Ehrlich test and P. nordicum among the species with a yellow reaction. These colored reactions are related to the production of some alkaloids. Castellá et al [44] confirmed these two groups of OTA producing strains in the genus Penicillium by Random Amplified Polymorphic DNA (RAPD) (Figure 2) and Amplified Fragment Length Polymorphism (AFLP) analyses, which are useful to differentiate these two species. However, the analysis of the ITS-5.8S rRNA gene sequences of these strains was not able to discriminate these two groups, indicating a close phylogenetic relationship between P. verrucosum and P. nordicum.
2.2. Occurrence and Significance
Penicillum verrucosum is an important ochratoxigenic species because it is the major producer of OTA in cereals such as wheat, barley, oats and rye, in temperate and cold climates. This species is the main source of OTA contamination in cereals associated to the porcine and avian nephropathy detected in temperate and cold countries such as Denmark, Sweden, Canada or the United States [45,46]. An association of OTA with the human Balkan endemic nephropathy also has been suggested, but to date the etiology of this disease remains unresolved and controversial [47,48]. Contamination of animal feeds with OTA may result in the presence of residues in edible offal and blood products, whereas the OTA contamination in meat, milk and eggs is negligible. However, higher concentrations of OTA may occur in certain local specialties such as blood puddings and sausages prepared with pig blood serum [49]. At present, maximum levels for OTA in meat and meat products are not established in the European Community. However, the consideration of setting a maximum level for OTA for edible offal and blood products is under discussion. In Denmark, since 1978, the contamination of pig meat with OTA has been assessed indirectly by the inspection of pigs’ kidneys for the presence of macroscopic lesions of porcine nephropathy [50]. Nephritis is a common cause of condemnation of pig kidneys in Great Britain, but there are few studies of OTA in cases of porcine nephropathy identified at slaughter in other countries [51]. In France, the first national monitoring program showed that pigs are clearly exposed to OTA and monitoring of pork products and of feed for swine is necessary. Swine, like poultry, are exposed to OTA through their feed, which is composed of cereals such as barley, maize, oats and wheat that are susceptible to contamination by this mycotoxin [52]. In a recent study [53], more than 80% of the combine harvested samples (including rye, barley and wheat, among others) contained P. verrucosum, showing that much grain was contaminated prior to drying and storage. These authors pointed out that the early contamination with this species is a latent risk of OTA production if the grain is not handled properly after harvest.
It is well known that P. verrucosum is much more frequently found on cereals in countries where they occasionally have OTA problems as in the North European countries compared with South Europe where levels of OTA generally seem to be lower or they are not detected [54]. Recently, the occurrence of P. verrucosum on feedstuff and retail wheat flours purchased in the Spanish market was determined [55,56]. This species was the only OTA producing Penicillium species detected in these substrates. Although the occurrence and abundance of P. verrucosum producing species were moderately low in this study, these results confirmed the potential risk of OTA and CIT production in these products and the occurrence of P. verrucosum in South European countries.
Penicillium nordicum is normally present in the air and on the surface of hams in dry-cured ham manufacturing plants in Italy [57]. Recently, both species were also detected on the surface of sausages from northern Italy [58]. In this study, approximately 45% of these samples were positive for the presence of OTA. However, this toxin was not identified inside the dry meat. These authors [58] concluded that the presence of OTA on the surface of sausage constitutes a health risk when moulds are not removed from casings. Penicillium verrucosum has been also isolated from Speck [59] and Istrian dried ham [60].
In contrast, the OTA producing species P. nordicum and P. verrucosum were not isolated during some studies of the mycobiota of the processing areas of North European meat products, such as fermented sausage, liver pâté [61] and smoked dry-cured ham and dry-cured lamb leg [62]. Mould growth is not accepted on most types of North European meat products and is considered as both an economic and aesthetic problem for the producers. At the moment, neither of the two species, P. nordicum or P. verrucosum, have been cited from Spanish dry-cured meat products such as Iberian ham [63] or fermented meat sausages [64].
3. Conclusions
A high number of Penicillium species have been cited incorrectly as OTA producers. Species included in the subgenus Penicillium are difficult to distinguish from each other and molecular methods are usually necessary to identify them. On the other hand, different species concepts are used in the identification of these OTA producing species, causing confusion in the literature. At present, P. verrucosum and P. nordicum are the only OTA producers known and accepted in this genus. These species are phenotypically similar and phylogenetically closely related. However, they can be distinguished from each other mainly because they have different reverse color in YES agar and they produce different secondary metabolite profiles. They are also clearly differentiated by RAPD and AFLP analyses. On the other hand, the species are ecologically different. Penicillium verrucosum is the main source of OTA contamination in cereals and their products in cold and temperate climates. In contrast, P. nordicum is usually recovered from dry-cured meat products and cheese and it may be the cause of OTA contamination in these foods.
Acknowledgements
The authors acknowledge the financial support of the Spanish Government (Ministry of Science and Innovation AGL2007-66416-C05-05).
References
- 1.Clark H.A., Snedeker S.M. Ochratoxin A: Its cancer risk and potential for exposure. J. Toxicol. Environ. Health B. 2006;9:265–296. doi: 10.1080/15287390500195570. [DOI] [PubMed] [Google Scholar]
- 2.Kuiper-Goodman T., Hilts C., Billiard S.M., Kiparissis Y., Richard I.D., Hayward S. Health risk assessment of ochratoxin A for all age-sex strata in a market economy. Food Addit. Contam. A. 2010;27:212–240. doi: 10.1080/02652030903013278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfohl-Leszkowicz A., Manderville R.A. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007;51:61–99. doi: 10.1002/mnfr.200600137. [DOI] [PubMed] [Google Scholar]
- 4.Commission of the European Communities, authors. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Commun. 2006;L364:5–29. [Google Scholar]
- 5.Commission of the European Communities, authors. Commission Regulation (EC) No 105/2010 of 5 February 2010 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards ochratoxin Al. Off. J. Eur. Commun. 2010;L35:7–8. [Google Scholar]
- 6.Commission of the European Communities, authors. Commission Recommendation (EC) No 576/2006 of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Commun. 2006;L229:7–9. [Google Scholar]
- 7.Abarca M.L., Accensi F., Bragulat M.R., Castellá G. Aspergillus carbonarius as the main source of ochratoxin A contamination in dried vine fruits from the Spanish market. J. Food Prot. 2003;66:504–506. doi: 10.4315/0362-028x-66.3.504. [DOI] [PubMed] [Google Scholar]
- 8.Battilani P., Pietri A., Bertuzzi T., Languasco L., Giorni P., Kozakiewicz Z. Occurrence of ochratoxin A-producing fungi in grapes grown in Italy. J. Food Prot. 2003;66:633–636. doi: 10.4315/0362-028x-66.4.633. [DOI] [PubMed] [Google Scholar]
- 9.Bau M., Bragulat M.R., Abarca M.L., Mínguez S., Cabañes F.J. Ochratoxigenic species from Spanish wine grapes. Int. J. Food Microbiol. 2005;98:125–130. doi: 10.1016/j.ijfoodmicro.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Bejaoui H., Mathieu F., Taillandier P., Lebrihi A. Black aspergilli and ochratoxin A production in French vineyards. Int. J. Food Microbiol. 2006;111(Suppl. 1):S46–S52. doi: 10.1016/j.ijfoodmicro.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Cabañes F.J., Accensi F., Bragulat M.R., Abarca M.L., Castellá G., Mínguez S., Pons A. What is the source of ochratoxin A in wine? Int. J. Food Microbiol. 2002;79:213–215. doi: 10.1016/S0168-1605(02)00087-9. [DOI] [PubMed] [Google Scholar]
- 12.Chulze S.N., Magnoli C.E., Dalcero A.M. Occurrence of ochratoxin A in wine and ochratoxigenic mycoflora in grapes and dried vine fruits in South America. Int. J. Food Microbiol. 2006;111:S5–S9. doi: 10.1016/j.ijfoodmicro.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Gómez C., Bragulat M.R., Abarca M.L., Mínguez S., Cabañes F.J. Ochratoxin A-producing fungi from grapes intended for liqueur wine production. Food Microbiol. 2006;23:541–545. doi: 10.1016/j.fm.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Guzev L., Danshin A., Ziv S., Lichter A. Occurrence of ochratoxin A producing fungi in wine and table grapes in Israel. Int. J. Food Microbiol. 2006;111:S67–S71. doi: 10.1016/j.ijfoodmicro.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 15.Lasram S., Bellí N., Chebil S., Nahla Z., Ahmed M., Sanchis V., Ghorbel A. Occurrence of ochratoxigenic fungi and ochratoxin A in grapes from a Tunisian vineyard. Int. J. Food Microbiol. 2007;114:376–379. doi: 10.1016/j.ijfoodmicro.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 16.Leong S.L., Hocking A.D., Scott E.S. Aspergillus species producing ochratoxin A: isolation from vineyard soils and infection of Semillon bunches in Australia. J. Appl. Microbiol. 2007;102:124–133. doi: 10.1111/j.1365-2672.2006.03056.x. [DOI] [PubMed] [Google Scholar]
- 17.Ponsone M.L., Combina M., Dalcero A., Chulze S. Ochratoxin A and ochratoxigenic Aspergillus species in Argentinean wine grapes cultivated under organic and non-organic systems. Int. J. Food Microbiol. 2007;114:131–135. doi: 10.1016/j.ijfoodmicro.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Serra R., Abrunhosa L., Kozakiewicz Z., Venâncio A. Black Aspergillus species as ochratoxin A producers in Portuguese wine grapes. Int. J. Food Microbiol. 2003;88:63–68. doi: 10.1016/s0168-1605(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 19.Tjamos S.E., Antoniou P.P., Tjamos E.C. Aspergillus spp., distribution, population composition and ochratoxin A production in wine producing vineyards in Greece. Int. J. Food Microbiol. 2006;111:S61–S66. doi: 10.1016/j.ijfoodmicro.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 20.Noonim P., Mahakarnchanakul W., Varga J., Samson R.A. Aspergilli and ochratoxin A in coffee. In: Varga J., Samson R.A., editors. Aspergillus in the Genomic Era. Wageningen Academic Publishers; Wageningen, The Netherlands: 2008. pp. 213–231. [Google Scholar]
- 21.Taniwaki M.H. An update on ochratoxigenic fungi and ochratoxin A in coffee. In: Hocking A.D., Pitt J.I., Samson R.A., Thrane U., editors. Advances in Food Mycology. Advances in Experimental Medicine and Biology. Springer Science; New York, NY, USA: 2006. [DOI] [PubMed] [Google Scholar]
- 22.Cabañes F.J., Bragulat M.R. Ochratoxin A in profiling and speciation. In: Varga J., Samson R.A., editors. Aspergillus in the Genomic Era. Wageningen Academic Publishers; Wageningen, The Netherlands: 2008. pp. 57–70. [Google Scholar]
- 23.Frisvad J.C., Nielsen K.F., Samson R.A. Recommendations concerning the chronic problem of misidentification of mycotoxigenic fungi associated with foods and feeds. In: Hocking A.D., Pitt J.I., Samson R.A., Thrane U., editors. Advances in Food Mycology. Advances in Experimental Medicine and Biology. Springer Science; New York, NY, USA: 2006. [DOI] [PubMed] [Google Scholar]
- 24.Abarca M.L., Accensi F., Cano J., Cabañes F.J. Taxonomy and significance of black aspergilli. Antonie van Leeuwenhoek. 2004;86:33–49. doi: 10.1023/B:ANTO.0000024907.85688.05. [DOI] [PubMed] [Google Scholar]
- 25.Frisvad J.C., Frank J.M., Houbraken J.A.M.P., Kuijpers A.F.A., Samson R.A. New ochratoxin A producing species of Aspergillus section. Circumdati. Stud. Mycol. 2004;50:23–43. [Google Scholar]
- 26.Nielsen K.F., Mogensen J.M., Johansen M., Larsen T.O., Frisvad J.C. Review of secondary metabolites and mycotoxins from the Aspergillus niger group. Anal. Bioanal. Chem. 2009;395:1225–1242. doi: 10.1007/s00216-009-3081-5. [DOI] [PubMed] [Google Scholar]
- 27.Palencia E.R., Hinton D.M., Bacon C.W. The black Aspergillus species of maize and peanuts and their potential for mycotoxin production. Toxins. 2010;2:399–416. doi: 10.3390/toxins2040399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perrone G., Gallo A., Susca A., Varga J. Aspergilli in grapes: Ecology, biodiversity and genomics. In: Varga J., Samson R.A., editors. Aspergillus in the Genomic Era. Wageningen Academic; Wageningen, The Netherlands: 2008. pp. 179–212. [Google Scholar]
- 29.Samson R.A., Houbraken J.A.M.P., Kuijpers A.F.A., Frank J.M., Frisvad J.C. New ochratoxin A or sclerotium producing species of Aspergillus section Nigri. Stud. Mycol. 2004;50:45–61. [Google Scholar]
- 30.Samson R.A., Noonim P., Meijer M., Houbraken J., Frisvad J.C., Varga J. Diagnostic tools to identify black aspergilli. Stud. Mycol. 2007;59:129–145. doi: 10.3114/sim.2007.59.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterson S.W., Varga J., Frisvad J.C., Samson R.A. Phylogeny and subgeneric taxonomy of Aspergillus. In: Varga J., Samson R.A., editors. Aspergillus in the Genomic Era. Wageningen Academic Publishers; Wageningen, The Netherlands: 2008. pp. 33–56. [Google Scholar]
- 32.Pitt J.I., Hocking A.D. Fungi and Food Spoilage. 3rd. Springer; Dordrecht, The Netherlands: 2009. [Google Scholar]
- 33.Frisvad J.C., Samson R.A. Polyphasic taxonomy of Penicillium subgenus Penicillium. A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Stud. Mycol. 2004;49:1–173. [Google Scholar]
- 34.Frisvad J.C., Smedsgaard J., Larsen T.O., Samson R.A. ycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud. Mycol. 2004;49:201–241. [Google Scholar]
- 35.Larsen T.O., Svendsen A., Smedsgaard J. Biochemical characterization of ochratoxin A-producing strains of the genus Penicillium. Appl. Environ. Microbiol. 2001;67:3630–3635. doi: 10.1128/AEM.67.8.3630-3635.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frisvad J.C., Filtenborg O. Terverticillate penicillia: Chemotaxonomy and mycotoxin production. Mycologia. 1989;81:837–861. [Google Scholar]
- 37.Pitt J.I. Penicillium viridicatum, Penicillium verrucosum and production of ochratoxin A. Appl. Environ. Microbiol. 1987;53:266–269. doi: 10.1128/aem.53.2.266-269.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pitt J.I. The genus Penicillium and Its Teleomorphic States Eupenicillium and Talaromyces. Academic Press; New York, NY, USA: 1979. [Google Scholar]
- 39.Samson R.A., Stolk A.C., Hadlok R. Revision of the Subsection Fasciculata of Penicillium and some Allied Species. Stud. Mycol. 1976;11:1–47. [Google Scholar]
- 40.Ciegler A., Fenell D.I., Sansing G.A., Detroy R.W., Bennett G.A. Mycotoxin-producing strains of Penicillium viridicatum: Classification into subgroups. Appl. Microbiol. 1973;26:271–278. doi: 10.1128/am.26.3.271-278.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raper K.B., Thom C. A Manual of Penicillia. The Williams & Wilkins Company; Baltimore, MD, USA: 1949. [Google Scholar]
- 42.Samson R.A., Seifert K.A., Kuijpers A.F.A., Frisvad J.C. Phylogenetic analysis of Penicillium subgenus Penicillium using partial β-tubulin sequences. Stud. Mycol. 2004;49:175–200. [Google Scholar]
- 43.Seifert K.A., Samson R.A., Dewaard J.R., Houbraken J., Lévesque C.A., Moncalvo J.M., Louis-Seize G., Hebert P.D. Prospects for fungus identification using CO1 DNA barcodes, with Penicillium as a test case. Proc. Natl. Acad. Sci. USA. 2007;104:3901–3906. doi: 10.1073/pnas.0611691104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castellá G., Larsen T.O., Cabañes F.J., Schmidt H., Alboresi A., Niessen L., Färber P., Geisen R. Molecular characterization of ochratoxin A producing strains of the genus Penicillium. System. Appl. Microbiol. 2002;25:74–83. doi: 10.1078/0723-2020-00094. [DOI] [PubMed] [Google Scholar]
- 45.Krogh P. Mycotoxicoses of animals. Mycopathologia. 1978;65:43–45. doi: 10.1007/BF00447172. [DOI] [PubMed] [Google Scholar]
- 46.Marquardt R.R., Frohlich A.A. A review of recent advances in understanding ochratoxicosis. J. Anim. Sci. 1992;70:3968–3988. doi: 10.2527/1992.70123968x. [DOI] [PubMed] [Google Scholar]
- 47.Pfohl-Leszkowicz A. Ochratoxin A and aristolochic acid involvement in nephropathies and associated urothelial tract tumours. Arh. Hig. Rada. Toksikol. 2009;60:465–483. doi: 10.2478/10004-1254-60-2009-2000. [DOI] [PubMed] [Google Scholar]
- 48.Stefanovic V., Polenakovic M. Fifty years of research in Balkan endemic nephropathy: Where are we now? Nephron Clin. Pract. 2009;112:c51–c56. doi: 10.1159/000213081. [DOI] [PubMed] [Google Scholar]
- 49.EFSA, authors. Opinion of the Scientific Panel on Contaminants in Food Chain on a request from the Commission related to ochratoxin A (OTA) as an undesirable substance in animal feed. EFSA J. 2004;101:1–36. [Google Scholar]
- 50.Jorgensen K., Petersen A. Content of ochratoxin in paired kidney and meat samples from healthy Danish slaughter pigs. Food Addit. Contam. 2002;19:562–567. doi: 10.1080/02652030110113807. [DOI] [PubMed] [Google Scholar]
- 51.Gresham A., Done S., Livesey C., MacDonald S., Chan D., Sayers R., Clark C., Kemp P. Survey of pig’ kidneys with lesions consistent with PMWS and PDNS and ochratoxicosis. Part 1: concentrations and prevalence of ochratoxin A. Vet. Rec. 2006;159:737–742. doi: 10.1136/vr.159.22.737. [DOI] [PubMed] [Google Scholar]
- 52.Dragacci S., Grosso F., Bire R., Fremy J.M., Coulon S. A french monitoring programme for determining ochratoxin A occurrence in pig kidneys. Nat. Toxins. 1999;7:167–173. doi: 10.1002/(sici)1522-7189(199907/08)7:4<167::aid-nt55>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 53.Elmholt S., Rasmussen P.H. Penicillium verrucosum occurrence and ochratoxin A contents in organically cultivated grain with special reference to ancient wheat types and drying practice. Mycopathologia. 2005;159:421–432. doi: 10.1007/s11046-005-1152-5. [DOI] [PubMed] [Google Scholar]
- 54.Lund F., Frisvad J.C. Penicillium verrucosum in wheat and barley indicates presence of ochratoxin A. J. Appl. Microbiol. 2003;95:1117–1123. doi: 10.1046/j.1365-2672.2003.02076.x. [DOI] [PubMed] [Google Scholar]
- 55.Bragulat M.R., Martínez E., Castellá G., Cabañes F.J. Ochratoxin A and citrinin producing species of the genus Penicillium from feedstuffs. Int. J. Food Microbiol. 2008;126:43–48. doi: 10.1016/j.ijfoodmicro.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 56.Cabañas R., Bragulat M.R., Abarca M.L., Castellá G., Cabañes F.J. Occurrence of Penicillium verrucosum in retail wheat flours from the Spanish market. Food Microbiol. 2008;25:642–647. doi: 10.1016/j.fm.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 57.Battilani P., Pietri V.A., Giorni P., Formenti S., Bertuzzi T., Toscani T., Virgili R., Kozakiewicz Z. Penicillium populations in dry-cured ham manufacturing plants. J. Food Prot. 2007;70:975–980. doi: 10.4315/0362-028x-70.4.975. [DOI] [PubMed] [Google Scholar]
- 58.Iacumin L., Chiesa L., Boscolo D., Manzano M., Cantoni C., Orlic S., Comi G. Moulds and ochratoxin A on surfaces of artisanal and industrial dry sausages. Food Microbiol. 2009;26:65–70. doi: 10.1016/j.fm.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 59.Peintner U., Geiger J., Pöder R. The mycobiota of speck, a traditional Tyrolean smoked and cured ham. J. Food Prot. 2000;63:1399–1403. doi: 10.4315/0362-028x-63.10.1399. [DOI] [PubMed] [Google Scholar]
- 60.Comi G., Orlic S., Redzepovic S., Urso R., Iacumin L. Moulds isolated from Istrian dried ham at the pre-ripening and ripening level. Int. J. Food Microbiol. 2004;96:29–34. doi: 10.1016/j.ijfoodmicro.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 61.Sørensen L.M., Jacobsen T., Nielsen P.V., Frisvad J.C., Koch A.G. Mycobiota in the processing areas of two different meat products. Int. J. Food Microbiol. 2008;124:58–64. doi: 10.1016/j.ijfoodmicro.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 62.Asefa D.T., Gjerde R.O., Sidhu M.S., Langsrud S., Kure C.F., Nesbakken T., Skaar I. Moulds contaminants on Norwegian dry-cured meat products. Int. J. Food Microbiol. 2009;128:435–439. doi: 10.1016/j.ijfoodmicro.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 63.Núñez F., Rodríguez M.M., Bermúdez M.E., Córdoba J.J., Asensio M.A. Composition and toxigenic potential of the mould population on dry-cured Iberian ham. Int. J. Food Microbiol. 1996;32:185–197. doi: 10.1016/0168-1605(96)01126-9. [DOI] [PubMed] [Google Scholar]
- 64.López-Díaz T.M., Santos J.A., García-López M.L., Otero A. Surface mycoflora of a Spanish fermented meat sausage and toxigenicity of Penicillium isolates. Int. J. Food Microbiol. 2001;68:69–74. doi: 10.1016/s0168-1605(01)00472-x. [DOI] [PubMed] [Google Scholar]