Abstract
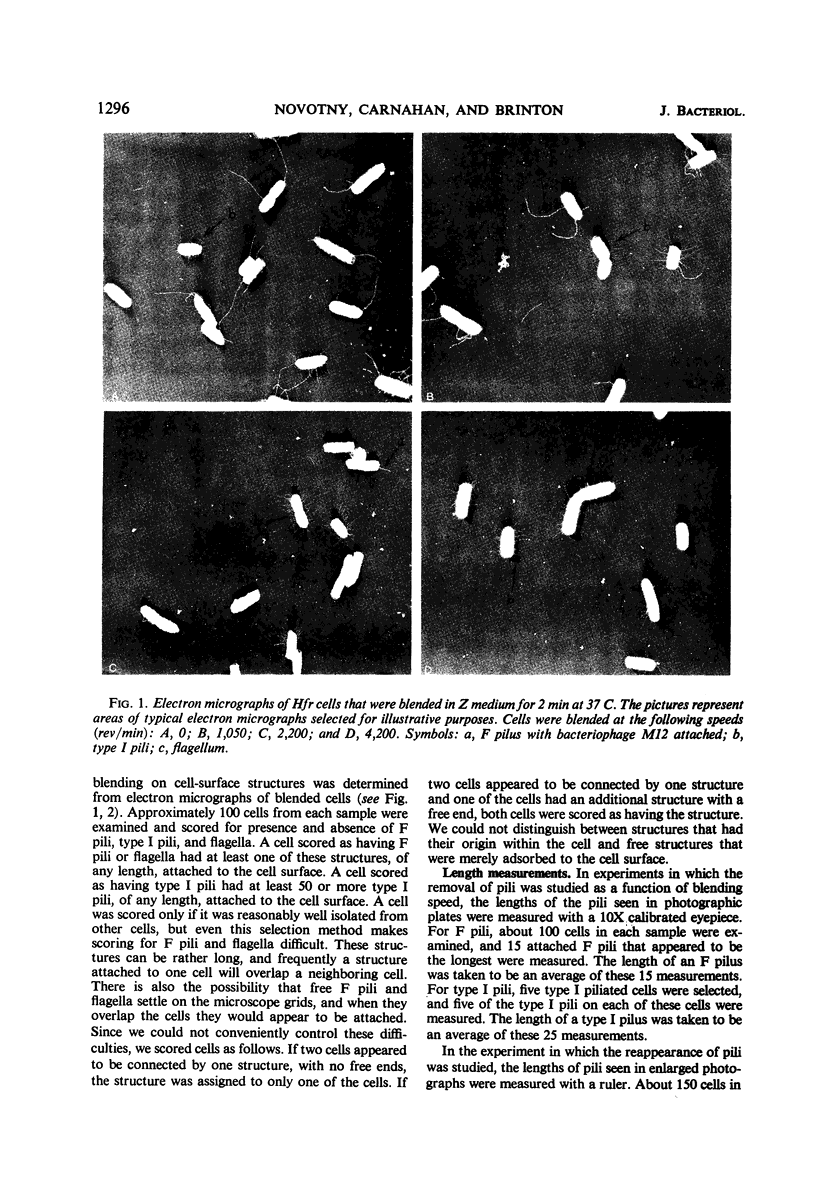
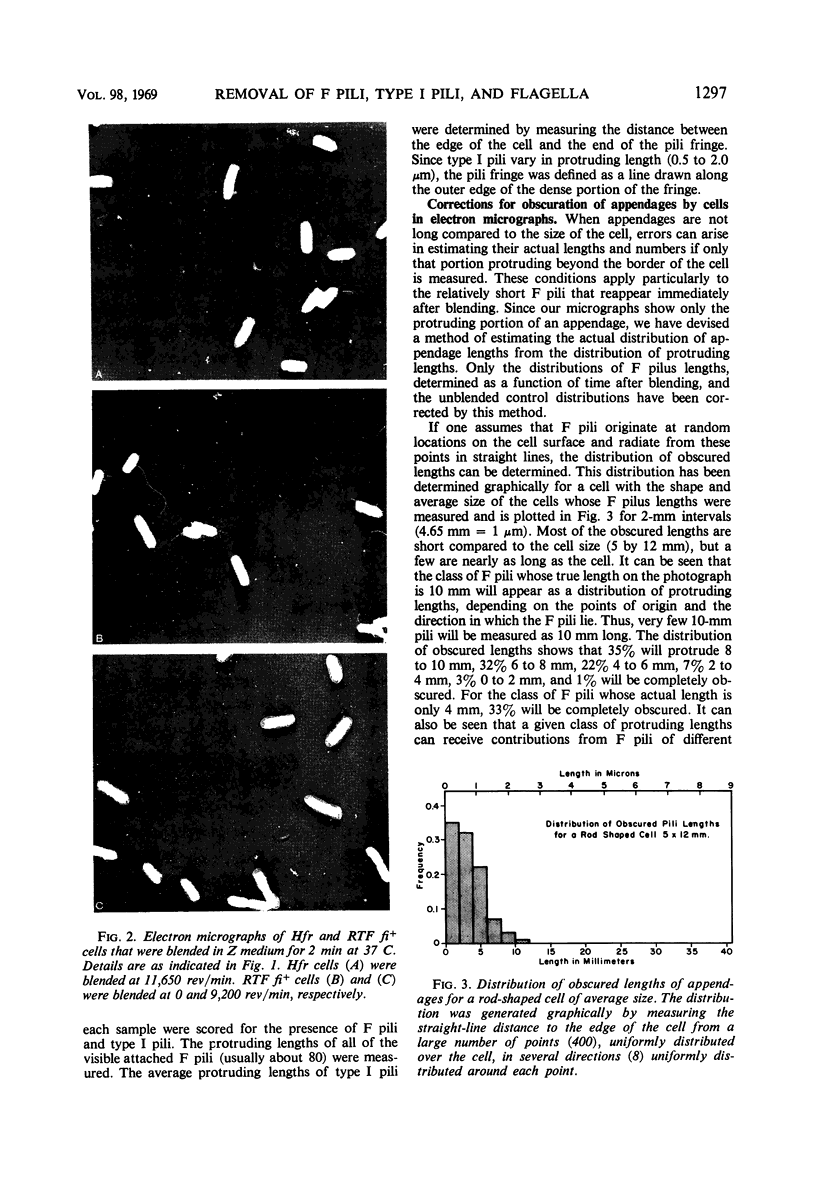
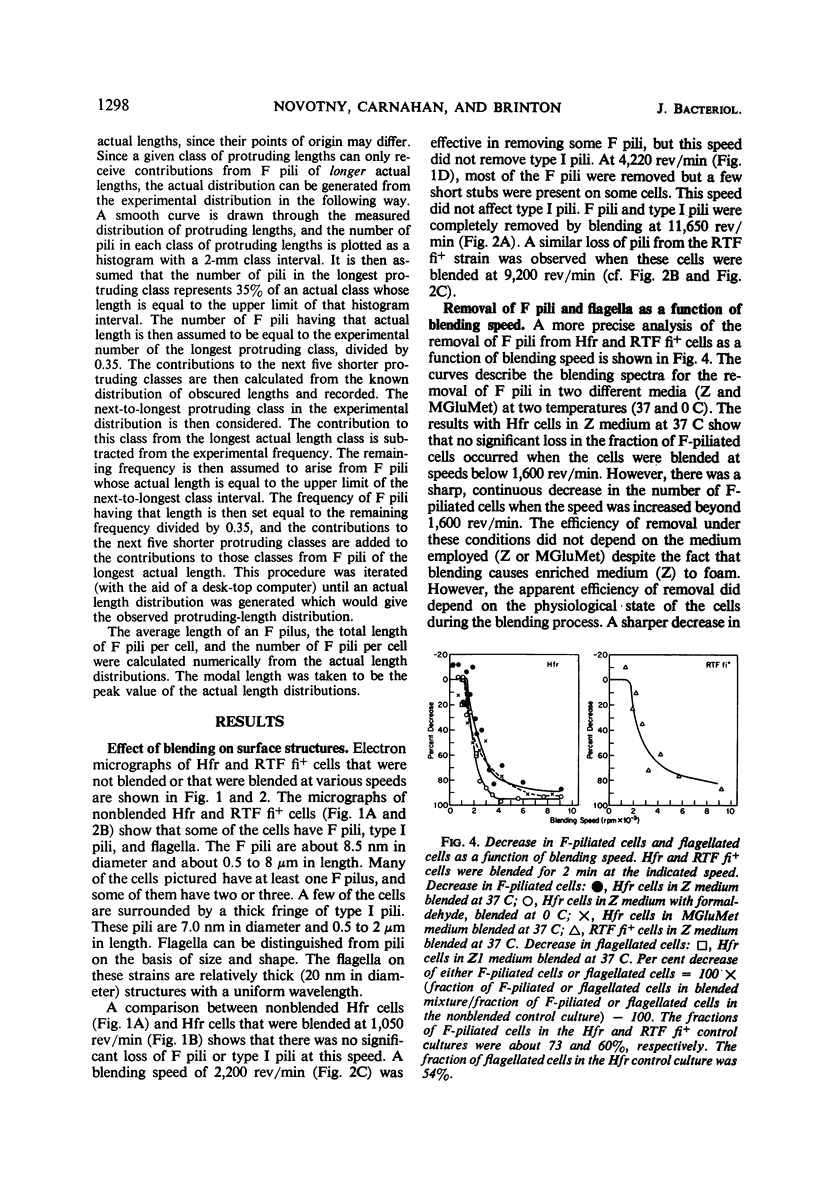
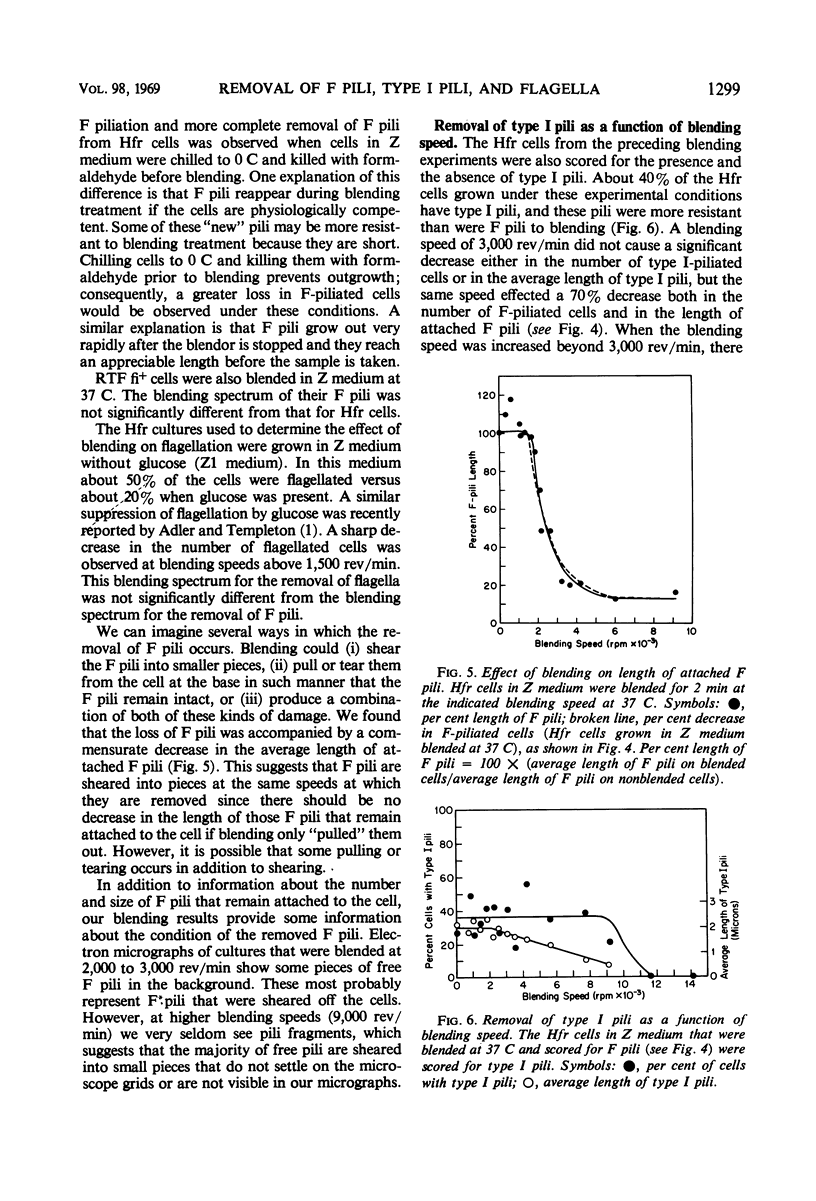
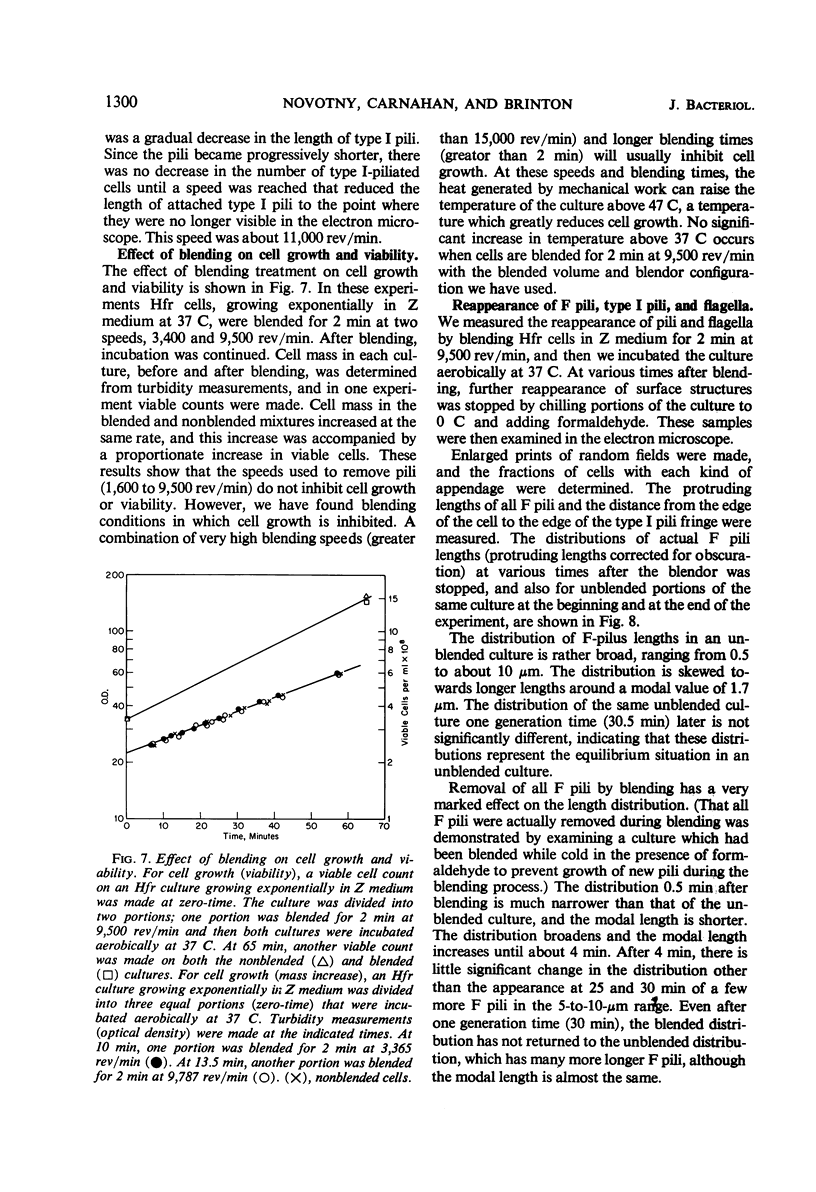
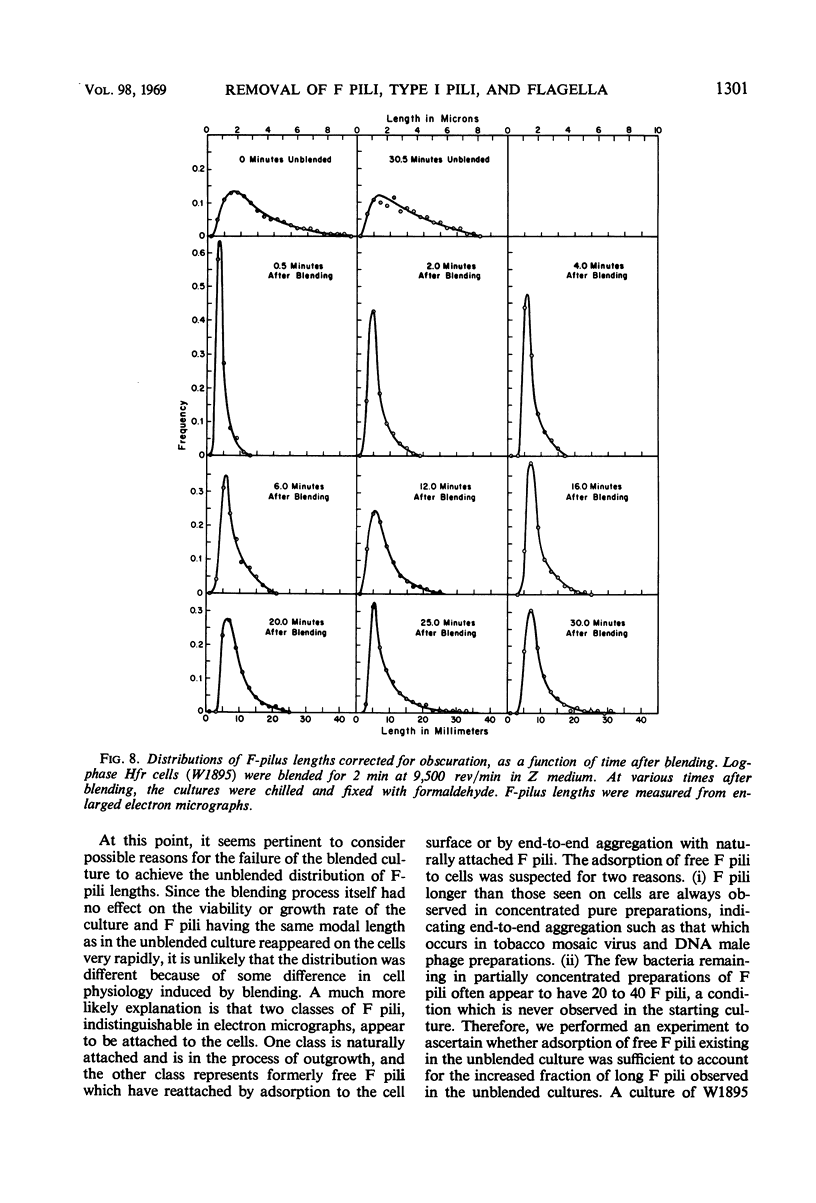
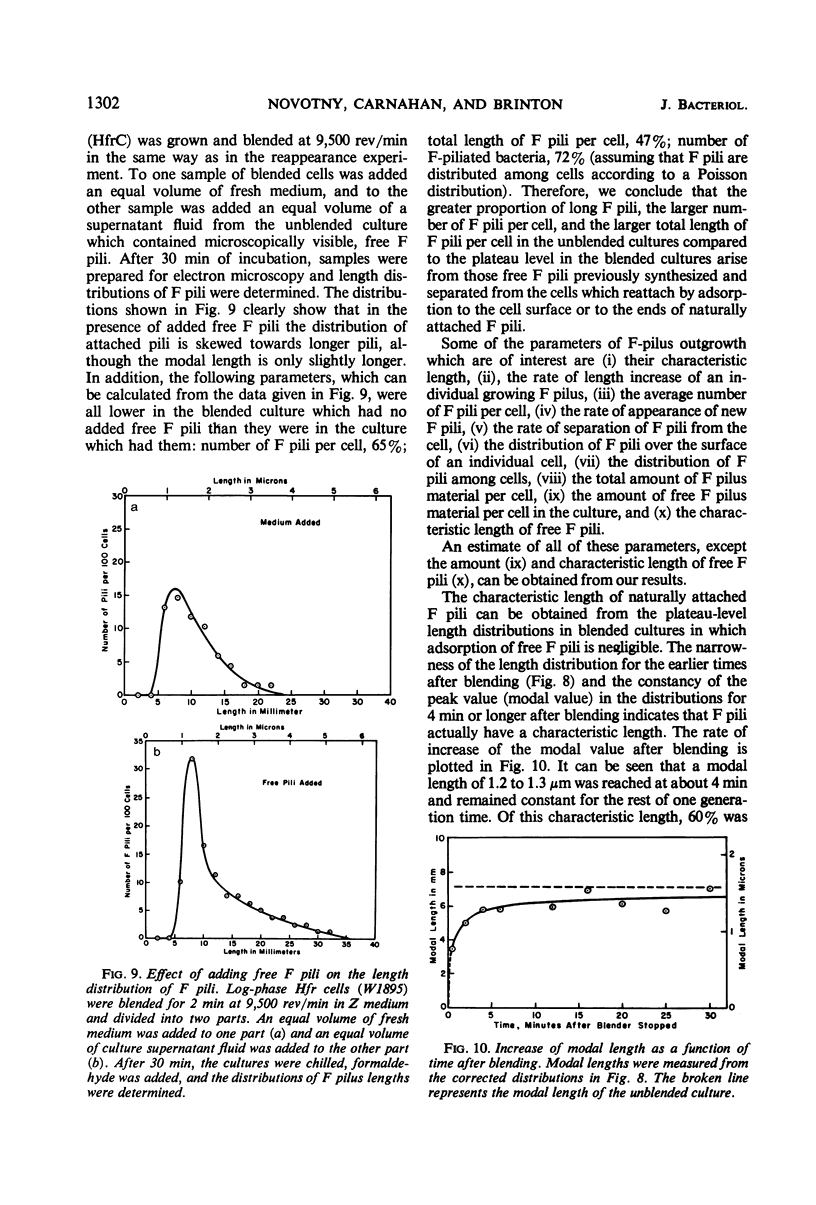
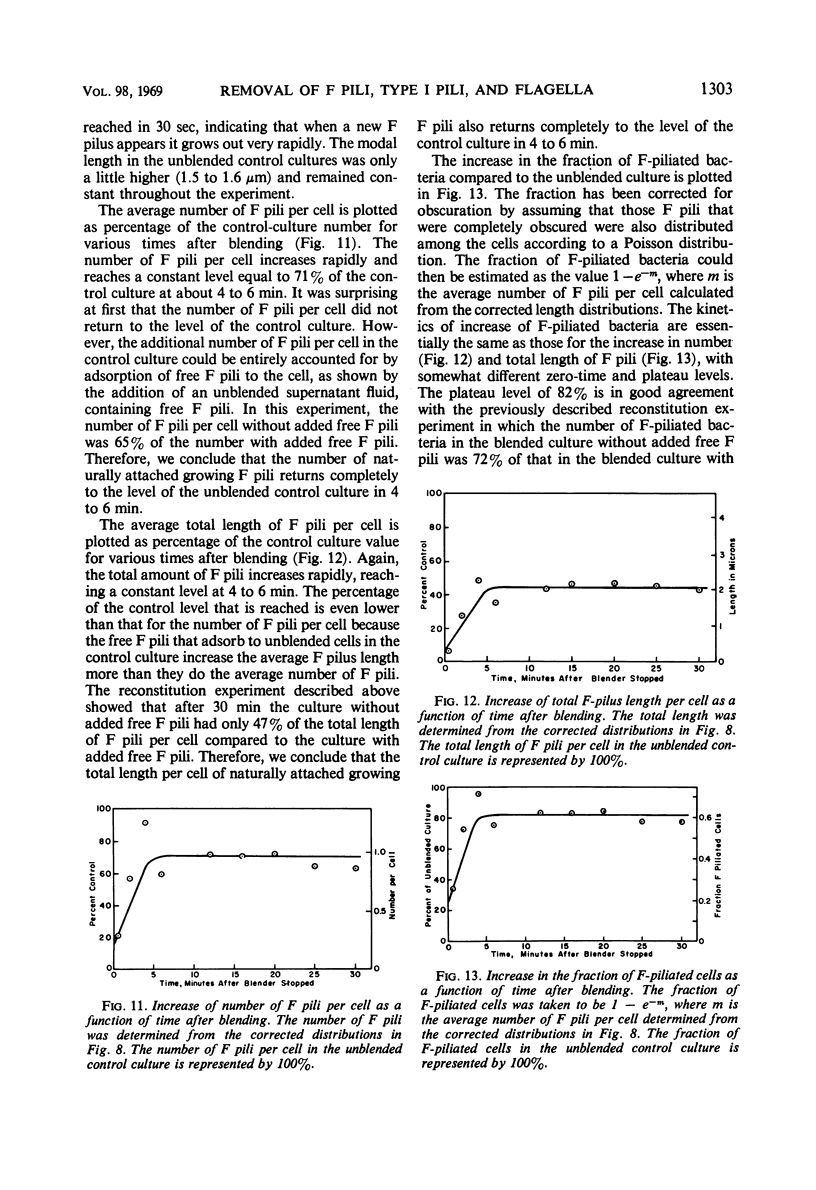
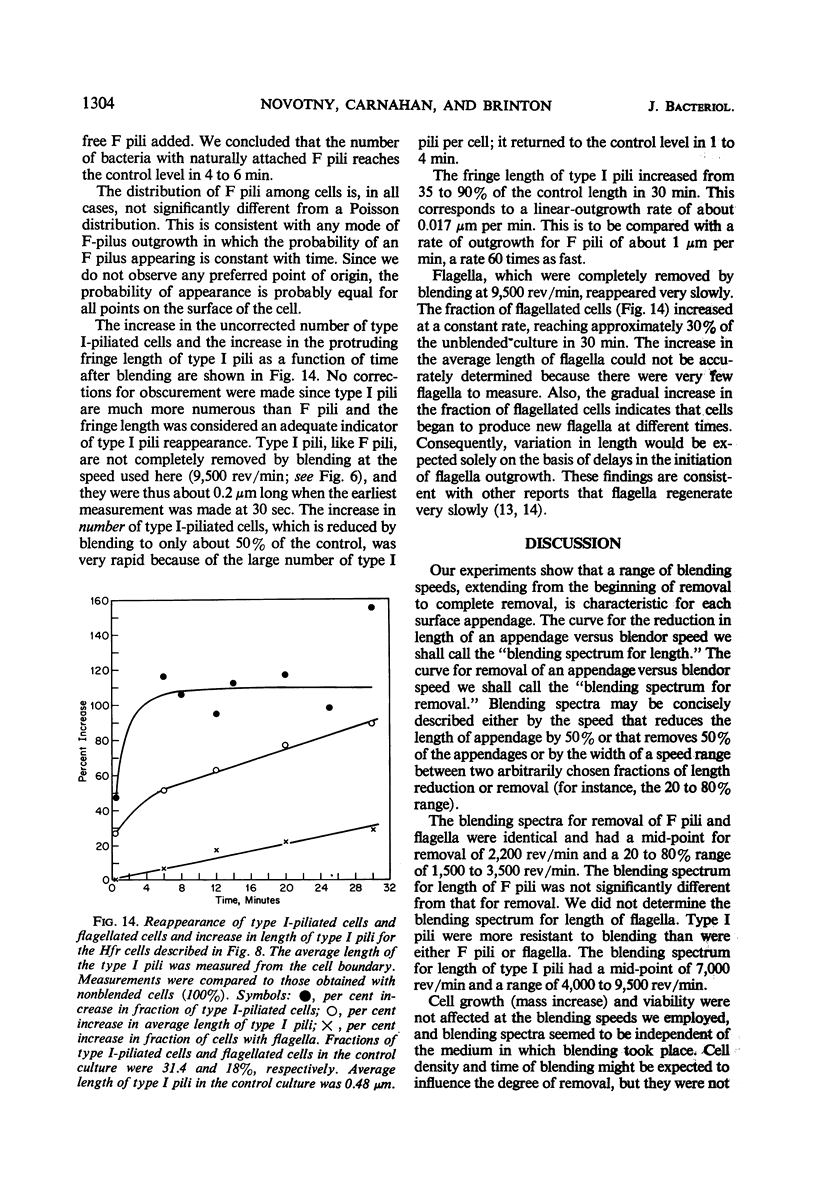
The effect of mechanical agitation (blending) on the removal of F pili, type I pili, and flagella from Hfr (high-frequency recombinant) and resistance transfer factor (RTF) fi+Escherichia coli cells was studied by electron microscopy. The reduction in number and length of appendages was measured as a function of blendor speed under standard conditions of temperature, medium, cell density, and blendor configuration. F pili and flagella were removed within the same narrow range of blendor speeds. Type I pili were removed within a higher and broader range of speeds. The speed which reduced the average length of type I pili to 50% was 3.5 times the speed which reduced the average length of F pili to 50%. None of the speeds employed inhibited cell growth, viability, or the ability to produce cell appendages. The kinetics of reappearance of F pili and type I pili after removal by blending were also different. F pili grew out very rapidly, reaching 50% of their full length in 30 sec and their full length in 4 to 5 min. The number of attached F pili per cell also increased rapidly, reaching a constant value in 4 to 5 min. After 5 min, F pilus lengths were distributed around a modal value of about 1.2 μm, and the numbers of F pili per cell were distributed according to a Poisson distribution, with an average of 1.0 per cell. These reappearance kinetics, length distributions, and number distributions are consistent with a model of F-pilus outgrowth in which new F pili appear at random locations on the cell surface at an average rate of about once every 4 min, grow to their characteristic length in about 4 min, and then separate from the cell. F pili which had separated could absorb to the cells, leading to the presence of two classes of F pili on cells: those in the process of natural out-growth and those attached by absorption. Type I pili increased in length much more slowly than did F pili, although the fraction of cells having visible type I pili increased very rapidly after blending because of the large number of type I pili per cell. The fraction of flagellated cells increased even more slowly, reaching only 30% of the unblended fraction in 30 min. The application of blending spectra and reappearance kinetics to the identification of cell functions with surface structures is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J., Templeton B. The effect of environmental conditions on the motility of Escherichia coli. J Gen Microbiol. 1967 Feb;46(2):175–184. doi: 10.1099/00221287-46-2-175. [DOI] [PubMed] [Google Scholar]
- BRINTON C. C., Jr, BUZZELL A., LAUFFER M. A. Electrophoresis and phage susceptibility studies on a filament-producing variant of the E. coli B bacterium. Biochim Biophys Acta. 1954 Dec;15(4):533–542. doi: 10.1016/0006-3002(54)90011-6. [DOI] [PubMed] [Google Scholar]
- BRINTON C. C., Jr, GEMSKI P., Jr, CARNAHAN J. A NEW TYPE OF BACTERIAL PILUS GENETICALLY CONTROLLED BY THE FERTILITY FACTOR OF E. COLI K 12 AND ITS ROLE IN CHROMOSOME TRANSFER. Proc Natl Acad Sci U S A. 1964 Sep;52:776–783. doi: 10.1073/pnas.52.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- DUGUID J. P., SMITH I. W., DEMPSTER G., EDMUNDS P. N. Non-flagellar filamentous appendages (fimbriae) and haemagglutinating activity in Bacterium coli. J Pathol Bacteriol. 1955 Oct;70(2):335–348. doi: 10.1002/path.1700700210. [DOI] [PubMed] [Google Scholar]
- Jahn T. L., Bovee E. C. Movement and locomotion of microorganisms. Annu Rev Microbiol. 1965;19:21–58. doi: 10.1146/annurev.mi.19.100165.000321. [DOI] [PubMed] [Google Scholar]
- Meynell E., Datta N. Mutant drug resistant factors of high transmissibility. Nature. 1967 May 27;214(5091):885–887. doi: 10.1038/214885a0. [DOI] [PubMed] [Google Scholar]
- Meynell G. G., Lawn A. M. Sex pili and common pili in the conjugational transfer of colicin factor Ib by Salmonella typhimurium. Genet Res. 1967 Jun;9(3):359–367. doi: 10.1017/s0016672300010636. [DOI] [PubMed] [Google Scholar]
- Novotny C., Knight W. S., Brinton C. C., Jr Inhibition of bacterial conjugation by ribonucleic acid and deoxyribonucleic acid male-specific bacteriophages. J Bacteriol. 1968 Feb;95(2):314–326. doi: 10.1128/jb.95.2.314-326.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOCKER B. A., CAMPBELL J. C. The effect of non-lethal deflagellation on bacterial motility and observations on flagellar regeneration. J Gen Microbiol. 1959 Jun;20(3):670–685. doi: 10.1099/00221287-20-3-670. [DOI] [PubMed] [Google Scholar]
- Stirm S., Orskov F., Orskov I., Mansa B. Episome-carried surface antigen K88 of Escherichia coli. II. Isolation and chemical analysis. J Bacteriol. 1967 Feb;93(2):731–739. doi: 10.1128/jb.93.2.731-739.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALENTINE R. C., STRAND M. COMPLEXES OF F-PILI AND RNA BACTERIOPHAGE. Science. 1965 Apr 23;148(3669):511–513. doi: 10.1126/science.148.3669.511. [DOI] [PubMed] [Google Scholar]