Abstract
The bacteria Edwardsiella ictaluri and Flavobacterium columnare cause enteric septicemia and columnaris disease, respectively, in channel catfish (Ictalurus punctatus). Natural therapeutants may provide an alternative to current management approaches used by producers. In this study, a rapid bioassay identified plant compounds as potential therapeutants. Chelerythrine chloride and ellagic acid were the most toxic toward E. ictaluri, with 24-h IC50 of 7.3 mg/L and 15.1 mg/L, respectively, and MIC of 2.1 mg/L and 6.5 mg/L, respectively. Chelerythrine chloride, ellagic acid, β-glycyrrhetinic acid, sorgoleone, and wogonin were the most toxic towards two genomovars of F. columnare, and wogonin had the strongest antibacterial activity (MIC = 0.3 mg/L).
Keywords: antibacterial, channel catfish, chelerythrine, columnaris, ellagic acid, enteric septicemia of catfish, β-glycyrrhetinic acid, sorgoleone, therapeutant, wogonin
1. Introduction
Enteric septicemia of catfish (ESC) is the leading cause of mortality in pond-raised channel catfish in the United States and is caused by the Gram-negative bacterium Edwardsiella ictaluri [1]. The second leading cause of mortality in pond-raised catfish is columnaris disease which is caused by the Gram-negative bacterium Flavobacterium columnare [2]. The use of antibiotic-laden feed is one management approach that catfish producers use in the treatment of ESC and columnaris disease. However, concerns about the development of antibiotic resistant strains of E. ictaluri and F. columnare from the use of these antibiotics and public concerns about the environmental impact from the use of antibiotic-laden feeds in agriculture make the future use of medicated feed in catfish aquaculture uncertain.
Another management approach in dealing with ESC and columnaris infection in catfish aquaculture is the use of therapeutants. Therapeutic chemicals have been used less commonly to manage ESC than columnaris disease. Potassium permanganate (KMnO4) and copper sulfate have been used to treat columnaris [3]. Recent research demonstrated that KMnO4 has a prophylactic value, but only a marginal therapeutic value once an infection of columnaris is established in channel catfish [4]. However, these chemicals have several drawbacks including the following: (1) their toxicity can be affected by water chemistry which makes efficacious application rates more difficult; (2) they are highly phytotoxic and their use may result in poisoning of the non-target phytoplankton community, with subsequent water quality deterioration (e.g., low dissolved oxygen levels); and (3) their broad-spectrum toxicity requires careful application so that catfish are not killed [5]. Chloramine-T, diquat, and hydrogen peroxide have also been investigated as bath treatments for columnaris disease in channel catfish, and diquat was the most effective in reducing the mortality of acutely infected catfish [6]. A more recent efficacy study conducted in tanks also found that diquat was effective in reducing the mortalities of catfish from an acute columnaris infection [7]. However, diquat is currently labeled for aquatic use as an herbicide only and would require additional testing and evaluation prior to United States Food and Drug Administration (USFDA) approval for use against columnaris. In addition, diquat may be too expensive for use in large catfish production ponds, and a reduction of efficacy could occur due to the binding of diquat to organic matter in the ponds. Currently, the only bath treatment approved by the USFDA for the treatment of external columnaris infection in channel catfish is the commercial product 35% PEROX-AID® which has an application dose as H2O2 of 50–75 mg/L for 60 minutes for channel catfish fingerlings and adults (http://www.fda.gov/AnimalVeterinary/DevelopmentApprovalProcess/Aquaculture/ucm132954.htm). However, this commercial product is not recommended for use in earthen ponds due to the rapid degradation of hydrogen peroxide in the presence of organic matter. Columnaris disease can also occur during episodes of ESC in channel catfish [8]. The efficacy of 35% PEROX-AID® against ESC is still unproven, and the USFDA has not approved 35% PEROX-AID® for the treatment of ESC.
Plants offer a very large and relatively unexplored source of phytochemicals for evaluation as pesticides and antimicrobials. Previous research found that extracts from clove (Caryophyllus aromaticum (Myrtaceae)) and jambolan (Syzygyum joabolanum (Myrtaceae)) contain certain compounds with toxicity against human pathogenic bacteria resistant to antibiotics [9]. In addition, various plant extracts were identified to possess antibacterial activity towards the common human pathogen Staphyl ococcus aureus [10,11]. While these examples demonstrate the potential of plants to provide antibacterial compounds, there has so far been very limited research in the discovery of compounds from plants with antibacterial activity towards fish pathogenic bacteria.
Previously, a rapid 96-well microplate bioassay developed by Schrader and Harries [12] has been utilized to evaluate natural compounds and crude extracts from plants and other organisms for antibacterial activity towards E. ictaluri and F. columnare. Subsequently, promising compounds have been identified; for example, tannic acid which is highly toxic towards E. ictaluri and F. columnare [13]. Additional research is needed to discover and develop other natural compounds (not antibiotics) from plants and other organisms for use as therapeutants to manage ESC and columnaris disease in catfish aquaculture. In this study, a variety of plant compounds were evaluated to determine their potential for use as antibacterial compounds against E. ictaluri and F. columnare.
2. Results and Discussion
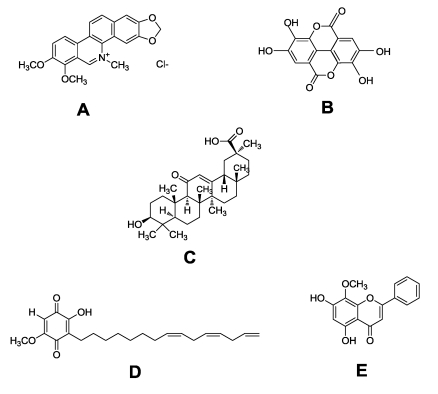
Chelerythrine chloride (Figure 1a) and ellagic acid (Figure 1b) had moderate to strong toxicity against E. ictaluri based upon 24-h IC50 and MIC results (Table 1). Chelerythrine chloride had significant toxicity, with a 24-h IC50 of 7.3 ± 0.8 mg/L and MIC of 2.1 ± 1.7 mg/L. Chelerythrine is a benzophenanthridine alkaloid that has been isolated from the extracts of several plants including Chelidonium majus (Papaveraceae) and Zanthoxylum clava-herculis (Rutaceae). Previous studies have cited the antibacterial activity of this alkaloid against methicillin-resistant S. aureus [14,15].
Figure 1.
Chemical structures of: (a) Chelerythrine chloride; (b) Ellagic acid; (c) β-Glycyrrhetinic acid; (d) Sorgoleone; (e) Wogonin.
Ellagic acid also had significant toxicity toward E. ictaluri, with a 24-h IC50 of 15.1 ± 8.6 mg/L and MIC of 6.5 ± 5.0 mg/L. Ellagic acid is a phenolic compound found in many plants, and this phytochemical can be formed by the lactonization of hexahydroxydiphenic acid, a product of the hydrolysis of ellagitannin. Previous research by Chung et al. [16] found that ellagic acid was not strongly inhibitory towards the growth of 15 different bacterial species, though none of their test species were members of Edwardsiella or Flavobacteriaceae. Cai and Wu [17] also reported that ellagic acid did not contribute to the antimicrobial activity of the methanol crude extract of clove (S. aromaticum) towards the Gram-negative anaerobic bacteria Porphyromonas gingivalis and Prevotella intermedia. However, Thiem and Goślińska [18] attributed the significant antimicrobial activity of leaves of cloudberry (Rubus chamaemorus (Rosaceae)) towards several species of Gram-positive bacteria to the presence of ellagic acid in the leaves.
Table 1.
Bioassay results of the toxicities of various plant compounds toward Edwardsiella ictaluri.
| Compound | 24-h IC50a | MICb | 24-h IC50 | MIC | ||
|---|---|---|---|---|---|---|
| (mg/L) | (mg/L) | RDCFc | RDCOd | RDCFc | RDCOd | |
| Acacetin | >284.3 | >284.3 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| Allyl disulfide | >146.3 | 146.3 | >2040.8 | >3846.2 | 1000.0 | 1111.1 |
| Apigenin | >270.2 | >270.2 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| Apigenin 7-glucoside | >432.4 | >432.4 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| Baicalein | 243.2 | 27.0 | 1839.7 | 3461.5 | 100.0 | 111.1 |
| Baicalin | >446.4 | >446.4 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| Camphor | >152.2 | >154.2 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| Chelerythrine chloride | 7.3 | 2.1 | 38.8 | 73.1 | 10.0 | 11.1 |
| Cichoriin | 54.4 | 178.1 | 622.3 | 1173.1 | 1000.0 | 1111.1 |
| p-Coumaric acid | >164.2 | 164.2 | >2040.8 | >3846.2 | 1000.0 | 1111.1 |
| Cyanidin chloride | >287.2 | >287.2 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| p-Cymene | >134.2 | >134.2 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| Daidzein | >254.2 | >254.2 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| Delphinidin chloride | 101.6 | >338.7 | 612.2 | 1153.9 | >1000.0 | >1111.1 |
| Ellagic acid | 15.1 | 6.5 | 83.3 | 143.6 | 10.0 | 10.6 |
| Embelin | >294.4 | >294.4 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| Emetine | >553.6 | >553.6 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| Eucalyptol | >154.3 | >154.3 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| Flavone | >222.2 | >222.4 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| Gallocatechin | 260.3 | 306.3 | 1734.7 | 3269.2 | 1000.0 | 1111.1 |
| Genistein | >270.2 | >270.2 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| Glycyrrhetinic acid (α) | >470.7 | >470.7 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| Glycyrrhetinic acid (β) | >470.7 | >470.7 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| Hyperforin | >536.8 | >536.8 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| Inulin | >522.5 | >522.5 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| Lavandulol | 50.9 | 154.3 | 673.5 | 1269.2 | 1000.0 | 1111.1 |
| (S)-Limonene | >136.2 | >136.2 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| Myrcene | >136.2 | >136.2 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| Myricetin | >318.2 | 318.2 | >2040.8 | >3846.2 | 1000.0 | 1111.1 |
| Naringenin | >272.3 | 27.2 | >2040.8 | >3846.2 | 100.0 | 111.1 |
| Pelargonidin chloride | 85.9 | >306.7 | 571.4 | 1076.9 | >1000.0 | >1111.1 |
| Piceatannol | >244.3 | >244.3 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| ß-Sitosterol | >414.7 | >414.7 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| Sorgoleone | >358.0 | >358.0 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| Stigmasterol | >412.7 | >412.7 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| Vitexin | >432.4 | >432.4 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| Wogonin | >284.2 | >284.2 | >2040.8 | >3846.2 | >1000.0 | >1111.1 |
| Zingiberone | >194.2 | 194.2 | >2040.8 | >3846.2 | 1000.0 | 1111.1 |
a 24-h 50% inhibition concentration; b Minimum inhibitory concentration; c Relative to drug control florfenicol; values close to “1.0” indicate stronger antibacterial activity; d Relative to drug control oxytetracycline; values close to “1.0” indicate stronger antibacterial activity.
Based upon 24-h IC50 and MIC results, the following compounds possess moderate to strong activity against the two genomovars of F. columnare (ALM-00-173 and BioMed): baicalein, chelerythrine chloride, cichoriin, ellagic acid, flavone, genistein, β-glycyrrhetinic acid, piceatannol, sorgoleone, and wogonin (Table 2 and Table 3). Embelin, a benzoquinone-derivative isolated from Embelia ribes Myrsinaceae, was also moderately toxic toward F. columnare ALM-00-173 based upon MIC results (2.9 mg/L), but not as toxic toward F. columnare BioMed (MIC = 29.4 mg/L). Conversely, apigenin, a flavonoid found in many fruits and vegetables, was more toxic against F. columnare BioMed than F. columnare ALM-00-173 based upon MIC results of 2.7 mg/L and 27.0 mg/L, respectively. The genomovar II isolate F. columnare ALM-00-173 has been identified to be more pathogenic for channel catfish than genomovar I isolate F. columnare BioMed [19,20].
Baicalein (5,6,7-trihydroxyflavone) had similar toxicity against both genomovars of F. columnare. For F. columnare ALM-00-173 and F. columnare BioMed, the 24-h IC50 results were 8.2 ± 1.1 mg/L and 8.2 ± 0.1 mg/L, respectively, while MIC values were 14.9 ± 12.2 mg/L and 15.0 ± 12.3 mg/L, respectively. Baicalein is a flavonoid originally isolated from the roots of Scutellaria baicalensis (Lamiaceae), and it has been previously identified as possessing antibacterial activity [21]. In addition, baicalein will enhance the toxic activity of certain antibiotics, such as tetracycline, against bacteria (e.g., methicillin-resistant Staphylococcus aureus) [22].
Based upon 24-h IC50 results, cichoriin and flavone had moderate toxicity towards both isolates of F. columnare. Cichoriin has been isolated from the flowers of the plant Cichorium intybus (Asteraceae), commonly known as chicory [23]. Previous research fount that extracts of C. intybus had antibacterial activity [24]. Flavone (2-phenylchromone) is a pigment ubiquitous in plants and serves as a precursor molecule for other flavones such as apigenin.
Ellagic acid was similarly toxic towards F. columnare ALM-00-173 (24-h IC50 of 9.7 ± 3.4 mg/L and MIC of 3.0 ± 0 mg/L (Table 2)), and F. columnare BioMed (24-h IC50 10.3 ± 2.6 mg/L and MIC of 16.6 ± 19.2 mg/L (Table 3)). For the different test concentrations used in this study, ellagic acid was only bactericidal towards F. columnare BioMed and only at the highest concentration tested (MBC of 302.2 mg/L). None of the other compounds evaluated in this study were bactericidal at the concentrations tested.
Table 2.
Bioassay results of the toxicities of various plant compounds toward Flavobacterium columnare (ALM-00-173).
| Compound | 24-h IC50a | MICb | 24-h IC50 | MIC | ||
|---|---|---|---|---|---|---|
| (mg/L) | (mg/L) | RDCFc | RDCOd | RDCFc | RDCOd | |
| Acacetin | >284.3 | >284.3 | >518.1 | >492.6 | >1000.0 | >1075.3 |
| Allyl disulfide | >146.3 | 146.3 | >518.1 | >492.6 | 1000.0 | 1075.3 |
| Apigenin | 22.8 | 27.0 | 43.8 | 41.6 | 100.0 | 107.5 |
| Apigenin 7-glucoside | 216.2 | >432.4 | 259.1 | 246.3 | >1000.0 | >1075.3 |
| Baicalein | 8.2 | 14.9 | 15.6 | 14.8 | 55.0 | 59.2 |
| Baicalin | 169.6 | 446.4 | 196.9 | 187.2 | 1000.0 | 1075.3 |
| Camphor | >152.2 | >154.2 | >518.1 | >492.6 | 1000.0 | 1075.3 |
| Chelerythrine chloride | 5.0 | 2.1 | 6.8 | 6.4 | 5.5 | 6.0 |
| Cichoriin | 12.4 | 17.8 | 36.0 | 34.3 | 100.0 | 107.5 |
| p-Coumaric acid | >164.2 | 164.2 | >518.1 | >492.6 | 1000.0 | 1075.3 |
| Cyanidin chloride | >322.7 | >322.7 | >518.1 | >492.6 | >1000.0 | >1075.3 |
| p-Cymene | >134.2 | >134.2 | >518.1 | >492.6 | >1000.0 | >1075.3 |
| Daidzein | >254.2 | >254.2 | >518.1 | >492.6 | >1000.0 | >1075.3 |
| Delphinidin chloride | 54.2 | >338.7 | 82.9 | 78.8 | >1000.0 | >1075.3 |
| Ellagic acid | 9.7 | 3.0 | 19.1 | 15.2 | 10.0 | 10.4 |
| Embelin | 13.0 | 2.9 | 22.8 | 21.7 | 100.0 | 10.8 |
| Emetine | 83.0 | 55.4 | 77.7 | 73.9 | 100.0 | 10.8 |
| Eucalyptol | >154.3 | >154.3 | >518.1 | >492.6 | >1000.0 | >1075.3 |
| Flavone | 6.7 | 22.2 | 15.5 | 14.8 | 100.0 | 107.5 |
| Gallocatechin | 55.1 | 30.6 | 93.3 | 88.7 | 100.0 | 10.8 |
| Genistein | 16.8 | 27.0 | 32.1 | 30.6 | 100.0 | 107.5 |
| Glycyrrhetinic acid (α) | >470.7 | >470.7 | >518.1 | >492.6 | >1000.0 | >1075.3 |
| Glycyrrhetinic acid (β) | 10.2 | 2.6 | 11.2 | 10.6 | 5.5 | 6.0 |
| Hyperforin | >5.4 | >5.4 | >518.2 | >4.9 | >10.0 | >10.8 |
| Inulin | >522.5 | 522.5 | >518.1 | >492.6 | 1000.0 | 1075.3 |
| Lavandulol | 29.3 | 15.4 | 98.5 | 93.6 | 100.0 | 107.5 |
| (S)-Limonene | >136.2 | >136.2 | >518.1 | >492.6 | >1000.0 | >1075.3 |
| Myrcene | >136.2 | >136.2 | >518.1 | >492.6 | >1000.0 | >1075.3 |
| Myricetin | 28.6 | 31.8 | 46.6 | 44.3 | 100.0 | 107.5 |
| Naringenin | 46.3 | 27.2 | 88.1 | 83.7 | 100.0 | 107.5 |
| Pelargonidin chloride | 52.1 | 30.7 | 88.1 | 83.7 | 100.0 | 107.5 |
| Piceatannol | 9.7 | 24.4 | 20.5 | 19.5 | 100.0 | 107.5 |
| ß-Sitosterol | >414.7 | >414.7 | >518.1 | >492.6 | >1000.0 | >1075.3 |
| Sorgoleone | 9.0 | 3.6 | 13.0 | 12.3 | 10.0 | 10.8 |
| Stigmasterol | >412.7 | 412.7 | >518.1 | >492.6 | 1000.0 | 1075.3 |
| Vitexin | >432.4 | >432.4 | >518.1 | >492.6 | >1000.0 | >1075.3 |
| Wogonin | 28.4 | 0.3 | 51.8 | 49.3 | 1.0 | 1.1 |
| Zingiberone | >194.2 | >194.2 | >518.1 | >492.6 | >1000.0 | >1075.3 |
a 24-h 50% inhibition concentration; b Minimum inhibitory concentration; c Relative to drug control florfenicol; values close to “1.0” indicate stronger antibacterial activity; d Relative to drug control oxytetracycline; values close to “1.0” indicate stronger antibacterial activity.
Table 3.
Bioassay results of the toxicities of various plant compounds toward Flavobacterium columnare (BioMed).
| Compound | 24-h IC50a | MICb | 24-h IC50 | MIC | ||
|---|---|---|---|---|---|---|
| (mg/L) | (mg/L) | RDCFc | RDCOd | RDCFc | RDCOd | |
| Acacetin | >284.3 | >284.3 | >423.7 | >588.2 | >1000.0 | >1075.3 |
| Allyl disulfide | >146.3 | 146.3 | >423.7 | >588.2 | 1000.0 | 1075.3 |
| Apigenin | 91.9 | 2.7 | 144.1 | 200.0 | 10.0 | 10.8 |
| Apigenin 7-glucoside | 255.1 | >432.4 | 250.0 | 347.0 | >1000.0 | >1075.3 |
| Baicalein | 8.2 | 15.0 | 12.7 | 17.7 | 55.0 | 59.2 |
| Baicalin | 116.1 | 446.4 | 110.2 | 152.9 | 1000.0 | 107.5 |
| Camphor | >152.2 | >152.2 | >423.7 | >588.2 | >1000.0 | >1075.3 |
| Chelerythrine chloride | 7.9 | 2.1 | 8.7 | 12.1 | 5.5 | 6.0 |
| Cichoriin | 7.5 | 17.8 | 17.8 | 24.8 | 100.0 | 107.5 |
| p-Coumaric acid | >164.2 | 164.2 | >423.7 | >588.2 | 1000.0 | 1075.3 |
| Cyanidin chloride | 242.0 | >322.7 | 317.8 | 441.2 | >1000.0 | >1075.3 |
| p-Cymene | >134.2 | >134.2 | >423.7 | >588.2 | >1000.0 | >1075.3 |
| Daidzein | >254.2 | >254.2 | >423.7 | >588.2 | >1000.0 | >1075.3 |
| Delphinidin chloride | 135.5 | 338.7 | 169.5 | 235.3 | 1000.0 | 1075.3 |
| Ellagic acid | 10.3 | 16.6 | 14.1 | 17.8 | 55.0 | 58.8 |
| Embelin | 53.0 | 29.4 | 76.3 | 105.9 | 100.0 | 107.5 |
| Emetine | 121.8 | 55.4 | 93.2 | 129.4 | 100.0 | 107.5 |
| Eucalyptol | >154.3 | >154.3 | >423.7 | >588.2 | >1000.0 | >1075.3 |
| Flavone | 7.8 | 22.2 | 14.8 | 20.6 | 100.0 | 107.5 |
| Gallocatechin | 147.0 | 306.3 | 203.4 | 282.4 | 1000.0 | 1075.3 |
| Genistein | 16.2 | 1.5 | 25.5 | 35.3 | 5.5 | 6.0 |
| Glycyrrhetinic acid (α) | >470.7 | >470.7 | >423.7 | >588.2 | >1000.0 | >1075.3 |
| Glycyrrhetinic acid (β) | 13.7 | 47.1 | 12.2 | 17.1 | 100.0 | 107.5 |
| Hyperforin | >5.4 | >5.4 | >4.2 | >5.9 | >10.0 | >10.8 |
| Inulin | >522.5 | >522.5 | >423.7 | >588.2 | >1000.0 | >1075.3 |
| Lavandulol | 29.3 | 15.4 | 80.5 | 111.8 | 100.0 | 107.5 |
| (S)-Limonene | >136.2 | >136.2 | >423.7 | >588.2 | >1000.0 | >1075.3 |
| Myrcene | >136.2 | >136.2 | >423.7 | >588.2 | >1000.0 | >1075.3 |
| Myricetin | >318.2 | 31.8 | >423.7 | >588.2 | 100.0 | 107.5 |
| Naringenin | 38.1 | 27.2 | 59.3 | 82.4 | 100.0 | 107.5 |
| Pelargonidin chloride | 116.5 | >306.7 | 161.0 | 223.5 | >1000.0 | >1075.3 |
| Piceatannol | 8.7 | 24.4 | 15.1 | 20.9 | 100.0 | 107.5 |
| ß-Sitosterol | >414.7 | >414.7 | >423.7 | >588.2 | >1000.0 | >1075.3 |
| Sorgoleone | 9.3 | 3.6 | 11.1 | 15.3 | 10.0 | 10.8 |
| Stigmasterol | >412.7 | 412.7 | >423.7 | >588.2 | 1000.0 | 1075.3 |
| Vitexin | >432.4 | >432.4 | >423.7 | >588.2 | >1000.0 | >1075.3 |
| Wogonin | 19.4 | 0.3 | 28.8 | 40.0 | 1.0 | 1.1 |
| Zingiberone | >194.2 | >194.2 | >423.7 | >588.2 | >1000.0 | >1075.3 |
a 24-h 50% inhibition concentration; b Minimum inhibitory concentration; c Relative to drug control florfenicol; values close to “1.0” indicate stronger antibacterial activity; d Relative to drug control oxytetracycline; values close to “1.0” indicate stronger antibacterial activity.
Genistein was more toxic against F. columnare BioMed than F. columnare ALM-00-173 based upon MIC-RDC values of RDCF = 5.5 ± 4.5 and RDCO = 6.0 ± 4.9 for F. columnare BioMed while RDCF = 100.0 ± 0 and RDCO = 107.5 ± 0 for F. columnare ALM-00-173. Genistein is an isoflavone that has been identified in various plants including the roots of kudzu vine (Pueraria lobata (Fabaceae)) and soybean (Glycine max (Fabaceae)) [25]. Previous research identified the differential antibacterial activity of genistein towards various test bacterial species [26]. Another study suggested genistein to possess bacteriostatic activity rather than bactericidal activity based upon cell survival [27].
Interestingly, β-glycyrrhetinic acid (Figure 1c) was toxic towards both F. columnare isolates, though slightly more toxic against F. columnare ALM-00-173, while α-glycyrrhetinic acid showed no toxicity towards both isolates at the highest test concentrations used in this study. β-Glycyrrhetinic acid yielded 24-h IC50 results of 10.2 ± 1.7 mg/L and 13.7 ± 0 mg/L for F. columnare ALM-00-173 and F. columnare BioMed, respectively. The MIC results also indicated greater toxicity of β-glycyrrhetinic acid toward F. columnare ALM-00-173, with a MIC of 2.6 ± 2.1 mg/L compared to a MIC of 47.1 ± 0 mg/L for F. columnare BioMed. The MIC-RDC values also indicated strong toxicity of β-glycyrrhetinic acid toward F. columnare ALM-00-173 (MIC-RDCF = 5.5 ± 4.5 and MIC-RDCO = 6.0 ± 4.9). Glycyrrhetinic acid can be obtained from licorice, a root extract from Glycyrrhiza glabra Fabaceae. Previous research found β-glycyrrhetinic acid to inhibit the growth of the Gram-positive bacteria Bacillus subtilis and Staphylococcus epidermidis, but not the Gram-negative bacteria Escherichia coli and Proteus vulgaris [28]. In the same study, β-glycyrrhetinic acid also inhibited DNA, RNA, and protein synthesis in the Gram-positive bacteria.
The hydroxystilbene piceatannol was moderately toxic towards both isolates of F. columnare based upon MIC results of 9.7 ± 0.9 mg/L and 8.7 ± 2.3 mg/L for F. columnare ALM-00-173 and F. columnare BioMed, respectively. Piceatannol has been isolated from the plant Mezoneuron cucullatum (Leguminosae) and exhibited anti-carcinogenic properties [29].
Sorgoleone (2-hydroxy-5-methoxy-3-((8’Z,11’Z)-8’,11’,14’-pentadecatriene)-p-benzoquinone) (Figure 1d), an allelochemical produced by sorghum (Sorghum bicolor (Poaceae)), was similar in toxicity to both genomovars of F. columnare based upon 24-h IC50 and MIC results. The 24-h IC50 of sorgoleone for F. columnare ALM-00-173 and F. columnare BioMed were 9.0 ± 0.4 mg/L and 9.3 ± 0.7 mg/L, respectively, while the MIC was 3.6 ± 0 mg/L for both isolates. Preliminary research has indicated that sorgoleone can inhibit the growth of certain microorganisms found in the soil while also being used as a carbon source by other soil microflora [30,31].
Wogonin (5,7-dihydroxy-8-methoxyflavone) (Figure 1e) has also been isolated from the roots of S. baicalensis [32]. However, wogonin is recognized more for its therapeutic and protective effects against certain toxins than antibacterial properties [33,34]. In the current study, wogonin possessed the strongest antibacterial activity against both genomovars of F. columnare based upon a MIC of 0.3 ± 0 mg/L. The MIC-RDC results for wogonin of 1.0 ± 0 and 1.1 ± 0 for RDCF and RDCO, respectively, indicate essentially the same degree of antibacterial activity as the antibiotics florfenicol and oxytetracycline.
Overall, chelerythrine chloride, ellagic acid, β-glycyrrhetinic acid, sorgoleone, and wogonin appear to be the most promising of the active compounds evaluated against the genomovars F. columnare ALM-00-173 and F. columnare BioMed when collectively considering 24-h IC50 and MIC results. Although the objective of this study was to identify plant compounds that might be useful as therapeutants, some of the active compounds have little or no solubility in water, and, therefore, it would be difficult to efficacy test such a compound as a therapeutant in an aquatic environment. Chemical modification of the structure might be one method to impart water solubility, though toxic activity could be compromised. A potential alternative might be incorporation of the compound into the fish feed in order to determine efficacy. For example, β-glycyrrhetinic acid is insoluble in water and could possibly be added to the feed to determine any benefits in reducing columnaris infection in channel catfish. However, palatability of the feed after incorporation of the test compound is critical under such circumstances.
3. Materials and Methods
A culture of E. ictaluri (isolate S02-1039) was obtained from Mr. Tim Santucci (College of Veterinary Medicine, Mississippi State University, Stoneville, Mississippi), and cultures of two genomovars of F. columnare (BioMed (genomovar I) and ALM-00-173 (genomovar II)) were obtained from Dr. Covadonga Arias (Department of Fisheries and Allied Aquacultures, Auburn University, Alabama). In order to assure purity, E. ictaluri was maintained on 3.8% Mueller-Hinton (MH) agar plates (pH 7.3) (Becton, Dickinson and Company, Sparks, Maryland) while cultures of F. columnare isolates were maintained on modified Shieh agar plates (pH 7.2–7.4) [35]. Prior to conducting the bioassay, single colonies of the test cultures were used to prepare the assay culture materials as follows: (1) for E. ictaluri, 45 mL of 3.8% MH at 0.5 McFarland standard [12]; and (2) for F. columnare, each genomovar isolate was cultured separately in 75 mL of modified Shieh broth (18 h for BioMed and 24 h for ALM-00-173) at 29 ± 1 at 150 rpm on a rotary shaker (model C24KC; New Brunswick Scientific, Edison, New Jersey).
Compounds were evaluated for antibacterial activity using a rapid 96-well microplate bioassay and following the procedures for the growth assay portion of the rapid bioassay developed by Schrader and Harries [12]. Florfenicol and oxytetracycline HCl, antibiotics that can be used in medicated feed for catfish, were included as positive drug controls for each assay. Also, control wells (no test compound added) were included in each assay. Technical grade solvents were used to dissolve the test compounds (see Table 4). Final concentrations of test compounds in the microplate wells were 1.0, 10.0, 100.0, and 1,000.0 µM. Three replications were used for each dilution of each test compound and controls. Final results were converted to units of mg/L to allow comparison with previous studies.
Table 4.
Compounds evaluated for toxicity towards Edwardsiella ictaluri and Flavobacterium columnare.
| Compound | Sourcea | Solvent | Purity (%) |
|---|---|---|---|
| Acacetin | 1 | Ethanol | 97 |
| Allyl disulfide | 1 | Methanol | 80 |
| Apigenin | 1 | Ethanol | 95 |
| Apigenin 7-glucoside | 1 | Water | 97 |
| Baicalein | 1 | Ethanol | 98 |
| Baicalin | 1 | Acetone | 95 |
| Camphor | 1 | Methanol | 98 |
| Chelerythrine chloride | 1 | Water | 95 |
| Cichoriin | 1 | Methanol | 98 |
| p-Coumaric acid | 1 | Ethanol | 100 |
| Cyanidin chloride | 2 | Methanol | 100 |
| p-Cymene | 1 | Methanol | 99 |
| Daidzein | 1 | Methanol | 98 |
| Delphinidin chloride | 1 | Methanol | 95 |
| Embelin | 1 | Methanol | 98 |
| Emetine | 1 | Methanol | 97 |
| Eucalyptol | 3 | Methanol | 99 |
| Flavone | 1 | Acetone | 100 |
| (-)-Gallocatechin | 1 | Methanol | 98 |
| Genistein | 2 | Ethanol | 99 |
| α-Glycyrrhetinic acid | 1 | Methanol | 98 |
| β-Glycyrrhetinic acid | 1 | Methanol | 97 |
| Hyperforin | 1 | Methanol | 85 |
| Inulin | 1 | Water | 100 |
| Lavandulol | 3 | Methanol | 90 |
| (S)-(-)-Limonene | 1 | Methanol | 96 |
| Myrcene | 3 | Dichloromethane | 90 |
| Myricetin | 2 | Ethanol | 100 |
| Naringenin | 1 | Ethanol | 100 |
| Pelargonidin chloride | 1 | Ethanol | 100 |
| Piceatannol | 4 | Ethanol | 98 |
| ß-Sitosterol | 1 | Methanol | 99 |
| Sorgoleone | 5 | Ethanol | 95 |
| Stigmasterol | 1 | Methanol | 99 |
| Vitexin | 1 | Methanol | 96 |
| Wogonin | 1 | Ethanol | 98 |
| Zingiberone | 1 | Ethanol | 96 |
a Sources are as follows: (1) Sigma-Aldrich, St. Louis, Missouri, USA; (2) Indofine Chemical Company, Hillsborough, New Jersey; (3) Fluka Analytical, Buchs, Switzerland; (4) A.G. Scientific, San Diego, California; (5) Dr. Franck Dayan, USDA-ARS-NPURU, University, Mississippi.
For determination of 24-h 50% inhibition concentration (IC50) and minimum inhibition concentration (MIC), sterile 96-well polystyrene microplates (Corning Costar Corp., Acton, Massachusetts) with flat-bottom wells were used to conduct the bioassay for compounds that were dissolved in 100% ethanol, 100% methanol, or double-deionized water. In order to prevent solvent interaction with the polystyrene, sterile 96-well quartz microplates (Hellma Cells, Inc., Forest Hills, New York) were used for compounds dissolved in acetone or dichloromethane. Dissolved test compounds were added to microplate wells (10 µL/well). Solvents were allowed to completely evaporate before standardized bacterial culture (0.5 MacFarland) was added to the microplate wells (200 µL/well). For compounds dissolved in water, 10 µL/well of dissolved compound were added, and 10 µL/well of double-deionized water were added to respective control wells. Microplates were incubated at 29 ± 1 °C (VWR model 2005 incubator; Sheldon Manufacturing, Inc., Cornelius, Oregon). A Packard model SpectraCount microplate photometer (Packard Instrument Company, Meriden, Connecticut) was used to measure the absorbance (630 nm) of the microplate wells at time 0 and 24 h.
In order to determine the minimum bactericidal concentration (MBC), microplates were inspected visually after the 24-h incubation period for the growth assay, and 5 µL of culture material was aseptically transferred from microplate treatment wells with observed growth inhibition onto 3.8% MH agar plates for E. ictaluri and onto modified Shieh agar plates for both genomovars of F. columnare. All agar plates were incubated at 29 ± 1 °C for 3–5 days. Plates were then inspected for growth, and the MBC was determined to be the lowest concentration for which no bacterial growth was observed on the agar surface.
The means and standard deviations of absorbance measurements were calculated, graphed, and compared to controls to help determine the 24-h IC50 and MIC for each test compound [12]. The 24-h IC50 and MIC results for each compound tested were divided by the respective 24-h IC50 and MIC results obtained for the positive controls florfenicol and oxytetracycline to determine the relative-to-drug-control florfenicol (RDCF) and relative-to-drug-control oxytetracycline (RDCO) values.
4. Conclusions
Organisms in the kingdom Plantae provide a vast number of compounds that have so far been relatively unexplored for antibacterial activity, especially against E. ictaluri and F. columnare. In this study, a rapid bioassay was used as the first step to identify plant compounds with potential for use as therapeutants against the common disease-producing bacteria responsible for causing ESC and columnaris in pond-raised channel catfish. Among the compounds evaluated, chelerythrine chloride and ellagic acid were the most toxic toward E. ictaluri. Chelerythrine chloride, ellagic acid, β-glycyrrhetinic acid, sorgoleone, and wogonin were the most toxic compounds toward the isolates F. columnare ALM-00-173 and F. columnare BioMed. Prior to conducting any efficacy studies, the water-solubility characteristics of these active compounds and the potential toxicity towards non-target organisms will need to be considered.
Acknowledgements
The technical assistance of Marcuslene Harries and Phaedra Page is greatly appreciated.
References
- 1.Hawke J.P., Durborow R.M., Thune R.L., Camus A.C. ESC: Enteric Septicemia of Catfish. Southern Regional Aquaculture Center; Stoneville, MS, USA: 1998. [Google Scholar]
- 2.Durborow R.M., Thune R.L., Hawke J.P., Camus A.C. Columnaris Disease: A Bacterial Infection Caused by Flavobacterium columnare. Southern Regional Aquaculture Center; Stoneville, MS, USA: 1998. [Google Scholar]
- 3.Plumb J.A. Health Maintenance and Principal Microbial Diseases of Cultured Fishes. Iowa State University Press; Ames, IA, USA: 1999. [Google Scholar]
- 4.Darwish A.M., Mitchell A.J., Straus D.L. Evaluation of potassium permanganate against an experimental subacute infection of Flavobacterium columnare in channel catfish, Ictalurus punctatus (Rafinesque) J. Fish Dis. 2009;32:193–199. doi: 10.1111/j.1365-2761.2008.01015.x. [DOI] [PubMed] [Google Scholar]
- 5.Boyd C.E., Tucker C.S. Pond Aquaculture Water Quality Management. Kluwer Academic Publishers; Boston, MA, USA: 1998. [Google Scholar]
- 6.Thomas-Jinu S., Goodwin A.E. Acute columnaris infection in channel catfish, Ictalurus punctatus (Rafinesque): Efficacy of practical treatments for warmwater aquaculture ponds. J. Fish Dis. 2004;27:23–28. doi: 10.1046/j.1365-2761.2003.00504.x. [DOI] [PubMed] [Google Scholar]
- 7.Darwish A.M., Mitchell A.J. Evaluation of diquat against an acute experimental infection of Flavobacterium columnare in channel catfish, Ictalurus punctatus (Rafinesque) J. Fish Dis. 2009;32:401–408. doi: 10.1111/j.1365-2761.2009.01024.x. [DOI] [PubMed] [Google Scholar]
- 8.Hawke J.P., Khoo L.H. Infectious diseases. In: Tucker C.S., Hargreaves J.A., editors. Biology and Culture of Channel Catfish. Elsevier; Amsterdam, The Netherlands: 2004. pp. 387–443. [Google Scholar]
- 9.Nascimento G.G.F., Locatelli J., Freitas P.C., Silva G.L. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz. J. Microbiol. 2000;31:247–256. [Google Scholar]
- 10.Pérez C., Anesini C. Antibacterial activity of alimentary plants against Staphylococcus aureus growth. Am. J. Chin. Med. 1994;22:169–174. doi: 10.1142/S0192415X94000206. [DOI] [PubMed] [Google Scholar]
- 11.Mansouri S. Inhibition of Staphylococcus aureus mediated by extracts of Iranian plants. Pharm. Biol. 1999;37:375–377. [Google Scholar]
- 12.Schrader K.K., Harries M.D. A rapid bioassay for bactericides against the catfish pathogens Edwardsiella ictaluri and Flavobacterium columnare. Aquacult. Res. 2006;37:928–937. [Google Scholar]
- 13.Schrader K.K. Compounds with inhibitory activity against the channel catfish pathogens Edwardsiella ictaluri and Flavobacterium columnare. N. Am. J. Aquacult. 2008;70:147–153. doi: 10.1577/A07-027.1. [DOI] [Google Scholar]
- 14.Gibbons S., Leimkugel J., Oluwatuyi M., Heinrich M. Activity of Zanthoxylum clava-herculis extracts against multi-drug resistant methicillin-resistant Staphylococcus aureus (mdr-MRSA) Phytother. Res. 2003;17:274–275. doi: 10.1002/ptr.1112. [DOI] [PubMed] [Google Scholar]
- 15.Zuo G.Y., Meng F.Y., Hao X.Y., Zhang Y.L., Wang G.C., Xu G.L. Antibacterial alkaloids from Chelidonium majus Linn (Papaveraceae) against clinical isolates of methicillin-resistant Staphylococcus aureus. J. Pharm. Pharmaceut. Sci. 2008;11:90–94. doi: 10.18433/j3d30q. [DOI] [PubMed] [Google Scholar]
- 16.Chung K.-T., Stevens S.E., Lin W.-F., Wei C.I. Growth inhibition of selected food-borne bacteria by tannic acid, propyl gallate and related compounds. Lett. Appl. Microbiol. 1993;17:29–32. [Google Scholar]
- 17.Cai L., Wu C.D. Compounds from Syzygium aromaticum possessing growth inhibitory activity against oral pathogens. J. Nat. Prod. 1996;59:987–990. doi: 10.1021/np960451q. [DOI] [PubMed] [Google Scholar]
- 18.Thiem B., Goślińska O. Antimicrobial activity of Rubus chamaemorus leaves. Fitoterapia. 2004;75:93–95. doi: 10.1016/j.fitote.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 19.Shoemaker C.A., Olivares-Fuster O., Arias C.R., Klesius P.H. Flavobacterium columnare genomovar influences mortality in channel catfish (Ictalurus punctatus) Vet. Microbiol. 2008;127:353–359. doi: 10.1016/j.vetmic.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Soto E., Mauel M.J., Karsi A., Lawerence M.L. Genetic and virulence characterization of Flavobacterium columnare from channel catfish (Ictalurus punctatus) J. Appl. Microbiol. 2008;104:1302–1310. doi: 10.1111/j.1365-2672.2007.03632.x. [DOI] [PubMed] [Google Scholar]
- 21.Wang X.K., Lee M.J. The extraction and analysis of antimicrobial components of Scutellaria baicalensis. Arch. Sichuan Medical College. 1959;4:97–98. [Google Scholar]
- 22.Fujita M., Shiota S., Kuroda T., Hatano T., Yoshida T., Mizushima T., Tsuchiya T. Remarkable synergies between baicalein and tetracycline, and baicalein and beta-lactams against methicillin-resistant Staphylococcus aureus. Microbiol. Immunol. 2005;49:391–396. doi: 10.1111/j.1348-0421.2005.tb03732.x. [DOI] [PubMed] [Google Scholar]
- 23.Saric M.R. Medicinal Plants of SR Serbia. Serbian Academy of Sciences and Arts; Belgrade, Yugoslavia: 1989. [Google Scholar]
- 24.Petrovic J., Stanojkovic A., Comic L., Curcic S. Antibacterial activity of Cichorium intybus. Fitoterapia. 2004;75:737–739. doi: 10.1016/j.fitote.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman P.B., Duke J.A., Brielmann H., Boik J., Hoyt J.E. A comparative survey of leguminous plants as sources of isoflavones, genistein and daidzein: Implications for human nutrition and health. J. Altern. Complement. Med. 1997;3:7–12. doi: 10.1089/acm.1997.3.7. [DOI] [PubMed] [Google Scholar]
- 26.Hong H., Landauer M.R., Foriska M.A., Ledney G.D. Antibacterial activity of the soy isoflavone genistein. J. Basic Microbiol. 2006;46:329–335. doi: 10.1002/jobm.200510073. [DOI] [PubMed] [Google Scholar]
- 27.Ulanowska K., Tkaczyk A., Konopa G., Węgrzyn G. Differential antibacterial activity of genistein arising from global inhibition of DNA, RNA and protein synthesis in some bacterial strains. Arch. Microbiol. 2006;184:271–278. doi: 10.1007/s00203-005-0063-7. [DOI] [PubMed] [Google Scholar]
- 28.Kim H.K., Park Y., Kim H.N., Choi B.H., Jeong H.G., Lee D.G., Hahm K.S. Antimicrobial mechanism of β-glycyrrhetinic acid isolated from licorice, Glycyrrhiza glabra. Biotechnol. Lett. 2002;24:1899–1902. [Google Scholar]
- 29.Lee S.K., Mbwambo Z.H., Chung H., Luyengi L., Gamez E.J., Mehta R.G., Kinghorn A.D., Pezzuto J.M. Evaluation of the antioxidant potential of natural products. Comb. Chem. High Throughput Screen. 1998;1:35–46. [PubMed] [Google Scholar]
- 30.Czarnota M.A., Paul R.N., Weston L.A., Duke S.O. Anatomy of sorgoleone-secreting root hairs of Sorghum species. Int. J. Plant Sci. 2003;164:861–866. [Google Scholar]
- 31.Gimsing A.L., Bælum J., Dayan F.E., Locke M., Sejerø L.H., Jacobsen C.S. Mineralization of the allelochemical sorgoleone in soil. Chemosphere. 2009;76:1041–1047. doi: 10.1016/j.chemosphere.2009.04.048. [DOI] [PubMed] [Google Scholar]
- 32.Hui K.M., Huen M.S.Y., Wang H.Y., Zheng H., Sigel E., Baur R., Ren H., Li Z.W., Wong J.T.-F., Xue H. Anxiolytic effect of wogonin, a benzodiazepine receptor ligand isolated from Scutellaria baicalensis Georgi. Biochem. Pharmacol. 2002;64:1415–1424. doi: 10.1016/s0006-2952(02)01347-3. [DOI] [PubMed] [Google Scholar]
- 33.Ueng Y.-F., Shyu C.-C., Liu T.-Y., Oda Y., Lin Y.-L., Liao J.-F., Chen C.-F. Protective effects of baicalein and wogonin against benzo[a]pyrene- and alflatoxin B1-induced genotoxicities. Biochem. Pharmacol. 2001;62:1653–1660. doi: 10.1016/s0006-2952(01)00816-4. [DOI] [PubMed] [Google Scholar]
- 34.Tai M.C., Tsang S.Y., Chang L.Y.F., Xue H. Therapeutic potential of wogonin: A naturally occurring flavonoid. CNS Drug Rev. 2005;11:141–150. doi: 10.1111/j.1527-3458.2005.tb00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Decostere A., Ducatelle R., Haesebrouck F. Flavobacterium columnare (Flexibacter columnaris) associated with severe gill necrosis in koi carp (Cyprinus carpio L) Vet. Res. 2002;150:694–705. doi: 10.1136/vr.150.22.694. [DOI] [PubMed] [Google Scholar]