Abstract
The aging heart is characterized by morphological and structural changes that lead to its functional decline and are associated with diminished ability to meet increased demand. Extensive evidence, derived from both clinical and experimental studies suggests that the aging heart undergoes fibrotic remodeling. Age-dependent accumulation of collagen in the heart leads to progressive increase in ventricular stiffness and impaired diastolic function. Increased mechanical load, due to reduced arterial compliance, and direct senescence-associated fibrogenic actions appear to be implicated in the pathogenesis of cardiac fibrosis in the elderly. Evolving evidence suggests that activation of several distinct molecular pathways may contribute to age-related fibrotic cardiac remodeling. Reactive oxygen species, chemokine-mediated recruitment of mononuclear cells and fibroblast progenitors, transforming growth factor (TGF)-β activation, endothelin-1 and angiotensin II signaling mediate interstitial and perivascular fibrosis in the senescent heart. Reduced collagen degradation may be more important than increased de novo synthesis in the pathogenesis of aging-associated fibrosis. In contrast to the baseline activation of fibrogenic pathways in the senescent heart, aging is associated with an impaired reparative response to cardiac injury and defective activation of reparative fibroblasts in response to growth factors. Because these reparative defects result in defective scar formation, senescent hearts are prone to adverse dilative remodeling following myocardial infarction. Understanding the pathogenesis of interstitial fibrosis in the aging heart and dissecting the mechanisms responsible for age-associated healing defects following cardiac injury are critical in order to design new strategies for prevention of adverse remodeling and heart failure in elderly patients.
Keywords: Aging, cardiac fibrosis, chemokine, MCP-1, TGF-β, angiotensin II, cardiac remodeling
Heart failure is the most common cause of hospitalization for patients older than 65 years. As the number of people over age 65 in North America is expected to double over the next 25 years, the burden of heart failure in the elderly will markedly increase. The increased incidence of heart failure in the elderly is only in part explained by the aging-associated, increase in the prevalence of coronary disease, hypertension, and diabetes, resulting in the development of ischemic, hypertensive or diabetic cardiomyopathy. Evolving evidence suggests that direct effects of cardiac senescence on myocardial structure and function may contribute to the development of heart failure in the elderly. Cardiac aging is associated with left ventricular hypertrophy and fibrosis leading to diastolic dysfunction and heart failure with preserved systolic function [1, 2]. Age-related diastolic dysfunction has a significant impact on the healthy elderly. Left ventricular filling is impaired with normal aging, limiting the exercise tolerance and reducing the quality of life [3]. Moreover, direct effects of senescence on the reparative mechanisms following cardiac injury may be responsible for worse heart failure, accentuated adverse remodeling and increased dysfunction in elderly individuals with myocardial infarction.
Progressive fibrosis is a hallmark of aging in various organs such as kidney [4, 5], liver [6, 7], pancreas [8] and lung [9]. In the cardiovascular system a progressive age-related deposition of collagen in the vascular wall and in the cardiac interstitial and perivascular space leads to reduction of myocardial and arterial compliance [1]. Animal model experiments have demonstrated increased collagen deposition in the aging heart [10] and studies involving human subjects have documented an age-related increase in cardiac fibrosis [11, 12]. Increased fibrosis is a major determinant of increased myocardial stiffness, which together with impaired relaxation create the basis for development of diastolic dysfunction [13]. This review manuscript deals with the mechanisms responsible for the development of fibrosis in the aging heart. In addition, we discuss the potential involvement of impaired reparative fibroblast function in mediating the development of age-associated adverse remodeling following myocardial infarction.
Fibroblasts and the extracellular matrix in the normal heart
The adult mammalian myocardium is composed of cellular and extracellular compartments. In the normal heart cardiac myocytes occupy approximately 75% of the myocardial tissue volume, but account only for 30–40% of the total number of cells [14]. Endothelial cells, fibroblasts and pericytes are abundant in the myocardium; small numbers of macrophages, mast cells and dendritic cells are also noted in the perivascular and interstitial space [15]. Despite the absence of hard evidence using flow cytometry or cell sorting to quantitatively assess the numbers of various cell types in the myocardium, fibroblasts are generally considered the predominant non-cardiomyocyte cell type in the adult mammalian heart. Cardiac fibroblasts are multifunctional cells that contribute to cardiac homeostasis by maintaining the matrix network. Moreover, fibroblasts are critically involved in cardiac repair following myocardial infarction and in the pathogenesis of cardiac fibrosis [16].
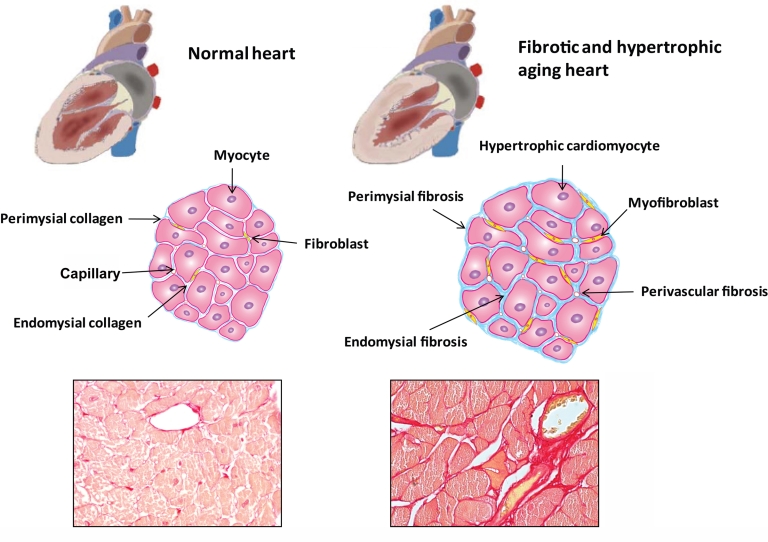
The cellular elements are enmeshed in a complex network of extracellular matrix. In the normal myocardium the extracellular matrix provides a scaffold for cellular elements and blood vessels and serves to maintain tissue architecture and the geometry of the heart. The cardiac matrix network is subdivided into three constituents: the epi-, peri- and endomysium [17]. The epimysium envelops the entire cardiac muscle and is located on the endocardial and epicardial surfaces. The perimysium arises from the epimysium and surrounds groups of muscle fibers. The endomysium is the final arborization of the perimysium and enwraps individual cardiomyocytes (Fig. 1). Endomysial struts tether muscle fibers together and to their nutrient microvasculature and function as the sites for connections to cardiomyocyte cytoskeletal proteins across the plasma membrane [18, 19]. The matrix directly influences ventricular pump function by transmitting cardiomyocyte-generated force and electrically separates the atria and the ventricles to facilitate proper cardiac contraction [18]. Moreover, matrix proteins transduce important cell survival signals in cardiomyocytes and shield fibroblasts from mechanical stress promoting a quiescent phenotype. The homeostatic effects of the matrix on myocardial cells are mediated through interactions between matrix proteins and cellular receptors (such as dystroglycans and integrins); these actions are essential for contractile synchrony and cardiomyocyte function.
Figure 1.
Fibrosis of the aging heart. Cardiac aging is associated with significant alterations in cardiac structure and function. Elderly patients often present with left ventricular hypertrophy and diastolic dysfunction while the systolic function is usually preserved. Age-dependent remodeling of the heart is associated with cardiomyocyte hypertrophy and interstitial fibrosis. In the normal heart, thin layers of perimysium and endomysium surround myocardial bundles and individual myocytes, respectively. The walls of the blood vessels also contain adventitial fibroblasts that contribute to the endomysial collagen network. In the senescent heart, there is hypertrophy of cardiomyocytes, transition of fibroblasts to myofibroblasts and accumulation of extracellular matrix proteins in the interstitium. These alterations lead to perivascular, endomysial and perimysial fibrosis. The histopathologic images show fibrotic remodeling of the heart in aging wild type mice. Picrosirius red staining identifies the collagen network in the myocardium of 2 mo and 24 mo C57BL/6 mice. Senescent hearts (S) display markedly increased collagen content compared to young hearts (Y).
Reactive and reparative cardiac fibrosis
Cardiac fibrosis is a hallmark of heart disease and is associated with increased deposition of matrix proteins in the myocardium. The term “reactive interstitial fibrosis” is used to describe expansion of the cardiac interstitial space in the absence of significant cardiomyocyte loss; in contrast “reparative fibrosis” refers to the formation of a scar in response to myocardial infarction [17, 20]. In the fibrotic heart, increased deposition of perimysial and endomysial collagen results in interstitial fibrosis, while perivascular fibrosis may also develop leading to collagen deposition in the adventitia of intramural coronary arterioles (Fig. 1). In animal models of left ventricular pressure overload, reactive interstitial fibrosis is observed first and may initially progress without loss of cardiomyocytes. This initial reactive perivascular and interstitial fibrosis is accompanied by cardiomyocyte hypertrophy and is a part of an adaptive response aimed at preserving cardiac output while normalizing wall stress. Eventually, however, reparative fibrosis is noted as cardiomyocytes undergo necrosis and apoptosis [21]. One possible mechanism is that the thickening of the extracellular matrix around hypertrophied cardiomyocytes may result in a mismatch between supply and demand of nutrients, leading to cell death. Interstitial fibroblasts respond to signals released by dying cardiomyocytes by synthesizing new matrix aimed at replacing the damaged cells. On the other hand, in models of acute myocardial infarction, sudden death of a large number of cardiomyocytes triggers an intense inflammatory reaction that is followed by replacement fibrosis [22].
Fibrotic remodeling of the aging heart
Even in apparently healthy individuals, aging is associated with progressive changes in cardiac anatomy and physiology [23]. Although left ventricular ejection fraction and contractility are usually preserved in elderly subjects, myocardial compliance is compromised [24]. Despite a reduction in the total number of cardiomyocytes, the aging heart exhibits a progressive increase in left ventricular mass [25]. The progressive hypertrophy of the aging heart is a consequence of peripheral vascular stiffening. Loss of aortic elasticity plays an important role in the hemodynamic alterations noted in elderly individuals. As the aorta becomes less compliant, an increased pulse pressure and a lower diastolic pressure are noted. Age-related arterial stiffening increases hemodynamic load, contributing to the development of cardiomyocyte hypertrophy [26] and leading to enhanced collagen deposition in the interstitial and perivascular space.
Animal model studies provide consistent evidence of aging-related cardiomyocyte hypertrophy accompanied by an increase in myocardial collagen content. Histological analysis of the non-hypertensive aging heart reveals progressive loss of cardiomyocytes due to necrotic and apoptotic cell death [27]. While the absolute number of myocytes decreases in aging hearts, the remaining cardiomyocytes undergo hypertrophy [28]. Eghbali and coworkers demonstrated that collagen content in the left ventricle increased from 5.5% of total protein in young Fischer 344 rats to approximately 12% in senescent animals [29]. In addition, collagen content is significantly increased in old normocholesterolemic rabbits [30] and in senescent mice [31]. The relative proportion of types I and III collagen also changes with time. Mays and colleagues observed a progressive increase in the proportion of type III collagen in the myocardium of male Lewis rats [32].
Similar findings were observed in human hearts. Collagen content increases with age in the normal human heart [33]; myocardium from senescent individuals exhibited increased collagen deposition and thicker endomysial and perimysial collagen fibers [34]. Autopsy studies of cardiac tissue from human subjects free of pathologic conditions showed that collagen content increases by almost 50% between the third and seventh decade of life [33].
Collagen turnover in the aging myocardium
Resident fibroblasts regulate extracellular matrix protein turnover in the myocardium by modulating the balance between synthesis and degradation. When stimulated by fibrogenic growth factors, such as transforming growth factor (TGF)-β, cardiac fibroblasts synthesize matrix proteins and express protease inhibitors (such as the tissue inhibitors of metalloproteinases/TIMPs). In contrast, pro-inflammatory mediators such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β stimulate matrix metalloproteinases (MMPs) expression by cardiac fibroblasts activating matrix degrading pathways [35]. Collagen levels in the heart are determined by the balance between matrix-preserving and matrix-degrading signals. Evidence suggests that, in contrast to hypertensive heart disease where collagen expression is markedly elevated, increased collagen synthesis may not be the main culprit of fibrosis in the senescent myocardium. By measuring the incorporation of radiolabeled proline Mays et al. estimated that in the heart of 1-month old rat about 20% of collagen is newly synthesized per day. Synthesis remains high in rats aged to 15 months and drops significantly to 2% per day in 24-month old animals [36]. Similarly mRNA expression of collagens I and III is reduced in aging rat myocardium [37, 38]. Robert et al. described the differential regulation of MMPs associated with aging and hypertension in the rat heart [39]. Hypertension was induced by continuous infusion of aldosterone for 2 months, and was associated with development of perivascular fibrosis. At the molecular level, aldosterone-induced fibrosis was associated with a 40% increase in MMP-2 expression and activity. Aged non-hypertensive rats showed significant interstitial fibrosis when compared with young animals. However, in contrast to the observations made in the model of aldosterone infusion, aging was associated with reduced MMP-2 and MMP-1 expression and activity [39]. A similar phenomenon has been described in the liver of the aging rat [40]. Interstitial collagen (especially collagen III) accumulated significantly in the oldest animals, mainly in the periportal area. Conversely, type III collagen gene transcription decreased during the animal’s lifespan (2–19-months), whereas collagen I and TGF-β1 mRNA expression remained unchanged. Importantly in the aged rats, hepatic MMP-1 and MMP-2 decreased significantly [40]. These findings suggest that in normal aging, fibrosis may be primarily due to a reduction in the proteolytic activity of matrix MMPs; increased expression of TIMP-1 may be a possible regulatory factor. The mechanisms leading to cardiac fibrosis in hypertension and aging are therefore different: increased collagen synthesis explains the accumulation of collagen in models of hypertension, whereas attenuation of matrix-degrading pathways may account for excessive collagen deposition in the aging heart.
Collagen cross-linking in the aging myocardium
Production of mature collagen requires complex post-translational events [41]. Procollagen is synthesized by fibroblasts and secreted into the pericellular space, where it forms collagen fibrils that assemble into fibers. Covalent cross-linking of collagen by the enzyme lysyl oxidase stabilizes fibrillar collagen, increasing its tensile strength and limiting its degradation. Accumulation of cross-linked collagen has been proposed as a major mechanism in the pathogenesis of increased stiffness in the aging heart. The degree of collagen cross-linking may be measured in tissue by assaying hydroxylysylpyridinoline (HP) residues after hydrolysis [10]. The concentration of ventricular HP increases approximately fivefold in senescent Fischer 344 rats compared to sedentary young animals [42]. Interestingly collagen cross-linking is significantly lower in old trained rats, compared with their sedentary counterparts [42–44]. Glucose can react nonenzymatically with myocardial collagens and link them together, producing advanced glycation endproducts (AGEs) [45]. Protein cross-linking through AGEs may be important in the pathogenesis of diastolic dysfunction in the aging heart. However, experimental studies examining this concept have produced contradictory results. Treatment with the AGE breaker ALT-711 attenuated age-related left ventricular stiffness [46] in normal aged dogs, suggesting a significant role for accumulation of AGE cross-links in promoting the decreased cardiovascular compliance of aging. In contrast, a more recent study showed no effects of the same AGE breaker on diastolic ventricular function in elderly hypertensive canines and suggested that AGE accumulation and AGE cross-link breaker effects were confined to the vasculature without evidence of myocardial accumulation [47].
Functional consequences of cardiac fibrosis in elderly patients
Aging-associated cardiac fibrosis may be involved in the pathogenesis of diastolic dysfunction in the elderly. The Framingham Heart Study [48] and the Baltimore Longitudinal Study on Aging [26] have demonstrated that, in healthy populations, there is an age-dependent increase in the prevalence of left ventricular hypertrophy accompanied by a decline in diastolic function. Systolic function at rest is relatively preserved; however, exercise capacity is reduced. Diastolic dysfunction plays a dominant role in the pathogenesis of heart failure and impaired exercise tolerance in elderly individuals [3, 49]. Because senescent subjects exhibit significant alterations in cardiomyocyte function, the contribution of fibrosis to the aging-associated diastolic dysfunction remains unknown.
Although fibrosis in senescent hearts is primarily associated with a stiffer ventricle and diastolic dysfunction, fibrotic remodeling may also induce impaired systolic function. Activation of matrix-degrading pathways leads to the development of ventricular dilation and systolic failure [50]. Although systolic hypocontractility is not observed in healthy aging hearts, aging-associated fibrotic remodeling of the ventricle may contribute to the pathogenesis of systolic dysfunction in the presence of other conditions, such as hypertensive or diabetic cardiomyopathy. Disturbance of the collagen network in the fibrotic heart may cause systolic dysfunction through several distinct mechanisms. First, fibrosis may result in impairment of systolic function through disruption of the coordination of myocardial excitation–contraction coupling [51]. Second, loss of fibrillar collagen may impair transduction of cardiomyocyte contraction into myocardial force development, resulting in uncoordinated contraction of cardiomyocyte bundles [52]. Third, interactions between endomysial components (such as laminin and collagen) and their receptors may play an important role in cardiomyocyte homeostasis [53]. Finally, fibrotic remodeling of the cardiac interstitium is often associated with MMPs activation and enhanced matrix degradation, resulting in sliding displacement (slippage) of cardiomyocytes and leading to a decrease in the number of muscular layers in the ventricular wall and subsequent left ventricular dilation [54].
In addition, the fibrotic process has profound effects on the cardiac conduction system. An autopsy study of 230 non-cardiac patients demonstrated increased fibrosis and fat within the cardiac conduction system of elderly patients [11]. Fibrotic ventricular remodeling may also promote arrhythmogenesis through impaired anisotropic conduction and subsequent generation of reentry circuits [55].
Mechanisms of fibrosis in the aging heart
Cellular effectors of cardiac fibrosis
Collagen turnover in tissues is primarily regulated by fibroblasts. Under certain conditions, fibroblasts are activated and undergo phenotypic transition into “myofibroblasts”, the key effector cells in fibrotic states. The myofibroblast phenotype is characterized by the expression of contractile proteins, such as α-smooth muscle actin (α-SMA). In healing wounds myofibroblasts are required for tissue repair; however in pathologic conditions activated myofibroblasts become the cellular effectors of the fibrotic process.
Transition of fibroblasts to myofibroblasts and myofibroblast-mediated collagen turnover are regulated by autocrine and paracrine factors generated within the myocardium and by endocrine hormones derived from the circulation. The main regulators involve angiotensin (ANG) II, endothelin (ET)-1, and TGF-β1. TGF-β1 is a crucial factor because it stimulates both fibroblast to myofibroblast transdifferentiation and collagen synthesis.
The origin of participating fibroblasts in fibrotic tissues remains controversial. The traditional view is that activated myofibroblasts in fibrotic hearts derive from resident fibroblasts through proliferation and activation. However, investigations that tracked proliferating cell populations during cardiac hypertrophy and reactive interstitial fibrosis showed proliferating fibroblast-like cells only in the neighborhood of blood vessels [56, 57]. These data suggest that proliferating profibrotic cells may be recruited from other cellular sources during reactive interstitial fibrosis such as endothelial cells, pericytes [58], bone marrow progenitor cells [59, 60], circulating monocytes [61] and fibrocytes [62]. Cardiac fibroblasts are derived from epithelial and endothelial cells during embryonic development of the heart in a process called epithelial–mesenchymal transition (EMT) and endothelial–mesenchymal transition (EndMT) respectively [16]. Acquisition of a fibroblast phenotype is stimulated by various cytokines and growth factors, such as platelet-derived growth factor (PDGF), fibroblast growth factors (FGFs) and TGF-β [63]. Fate mapping studies have demonstrated that, while there is no significant endothelial contribution to the fibroblast population in the normal adult heart [64–66], up to 30% of fibroblasts in damaged myocardium may be of endothelial origin [67] suggesting that EndMT play a significant role in cardiac fibrosis. A recent study has suggested that EndMT may contribute to profibrotic responses during myocardial fibrosis in aged mouse hearts and that this process may involve constitutive TGF-β signaling [68]. In this study Ghosh et al. used a murine model of spontaneous age-dependent cardiac fibrosis that develops in the absence of the plasminogen activator inhibitor-1 (PAI-1) gene. PAI-1 plays a significant role in regulation of fibrosis by inhibiting the tissue collagenolytic activities and by protecting matrix proteins from proteolytic degradation [69]. Paradoxically, mice lacking PAI-1 develop age-dependent cardiac fibrosis; the mechanism responsible for this phenomenon is not understood [70]. Ghosh et al. showed that aged hearts from PAI-1–deficient mice displayed increased inflammation, elevated levels of TGF-β and induction of TGF-β-mediated profibrotic responses. Moreover, PAI-1-deficient endothelial cells are more susceptible to EndMT in response to TGF-β via induction of both Smad and ERK1/2 MAPK pathways. These findings suggest that physiologic PAI-1 levels help to protect the heart from age-dependent fibrogenesis [68]. Bone marrow-derived progenitor cells may be an additional potential source of fibroblasts in the fibrotic heart. However, their role in aging-associated fibrosis remains unknown.
Molecular signals involved in aging-associated fibrosis
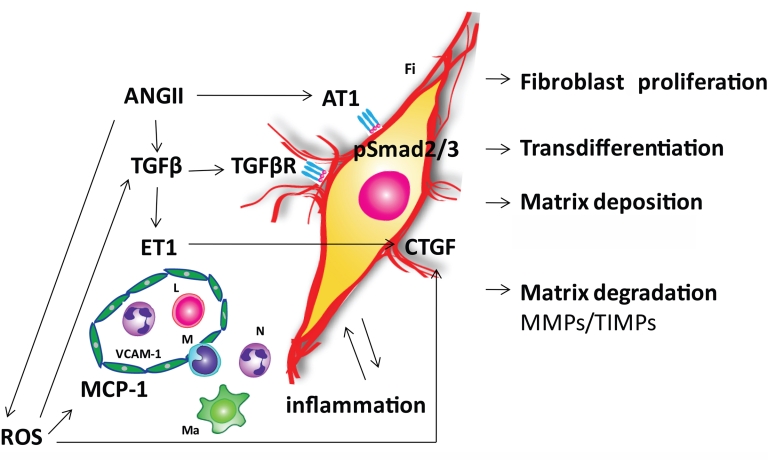
Several distinct molecular signals have been proposed to contribute to age-related fibrotic cardiac remodeling (Fig. 2).
Figure 2.
Pathways involved in the pathogenesis of cardiac fibrosis in the senescent heart. Angiotensin II (ANG II), reactive oxygen species (ROS), transforming growth factor β (TGF-β) and endothelin-1 (ET-1) signaling appear to play an important role in mediating fibrotic remodeling of the aging heart. ANG II exerts its effects directly through the ANG II type 1 receptor (AT1) and indirectly through induction of TGF-β. Smad 2/3 seems to be a common pathway for these two mechanisms. Age-dependent mitochondrial dysfunction is the major source of ROS in the myocardium. ANG II induces NADPH oxidase dependent generation of ROS. ROS activate TGF-β and upregulate its downstream fibrogenic effector connective tissue growth factor (CTGF). ROS, ANG II and ET-1 induce adhesion molecules in the microvascular endothelium and pro-inflammatory mediator expression. Inflammatory cytokines may induce and activate matrix metalloproteinases (MMPs) enhancing matrix degradation. TGF-β/Smad2/3 signaling promotes fibroblast proliferation, phenotypic conversion to myofibroblasts and the production of extracellular matrix components including fibrillar collagen, fibronectin, and proteoglycans. Changes in the extracellular matrix occur in part due to an imbalance of MMPs and their inhibitors (TIMPs). The altered matrix modifies the pro-survival signals that cardiac myocytes receive from their scaffolding environment, leading to cardiomyocyte loss due to apoptosis or necrosis. Symbols: E, endothelial cell; Fi, fibroblast; L, lymphocyte, Ma, macrophage; M, monocyte; N, neutrophil; TGF-βR, TGF-β receptor; VCAM-1, vascular cell adhesion molecule-1; MCP-1, monocyte chemoattractant protein-1
The role of inflammatory mediators
In several fibrotic processes the role of inflammation has been clearly demonstrated. Most types of tissue injury trigger a local inflammatory reaction. Inflammatory cells release fibrogenic cytokines and growth factors as important components of the reparative process [71, 72]. The close association between the inflammatory and fibrosis is well-established in the reparative fibrotic process observed following myocardial infarction. However, the potential involvement of the inflammatory response in aging-associated fibrosis is not well-established. Recent studies suggested that interstitial fibrosis in senescent mice may arise from age-dependent immunoinflammatory dysregulation [73]. In C57BL/6 mice, cardiac fibrosis and diastolic dysfunction were present at the end of the first year of life and became progressively worse as the mice aged up to 30 months. These morphological and functional changes were associated with increased myocardial expression of monocyte chemoattractant protein (MCP)-1/CCL2, interleukins 4 and 13 (IL-4 and IL-13) and with accumulation of CD45+ myeloid-derived fibroblasts that correlated temporally and quantitatively with the degree of fibrosis and the development of diastolic dysfunction. MCP-1 may induce fibrosis through recruitment of monocytes with fibrogenic properties or through chemoattraction of fibroblast progenitors. Although both animal and human studies have suggested a significant role for MCP-1/CCL2 in ischemic cardiac fibrosis [36, 37], its involvement in aging-associated fibrotic cardiac remodeling has not been investigated. The potential role of other chemokines with fibrogenic and anti-fibrotic properties [74, 75] in regulation of aging-related fibrosis remains unknown.
IL-13 is known to exert profibrotic actions in vivo [76, 77]; however its role in the aging heart has not been examined. Elevation of IL-4 and IL-13 expression in the aging mouse heart suggests a shift to a Th2 phenotype. Age-associated changes in immune function characterized by a shift from Th1 (IL-12, IFN-γ) to Th2 (IL-13, IL-4) cytokines have been shown in animal studies [78] and in human aging [79, 80] and may be important in senescence-associated fibrosis.
The role of the renin–angiotensin-aldosteron system
A large body of evidence indicates that the activation of renin–angiotensin-aldosteron system (RAAS) might play a central role in cardiac aging and in age-associated fibrotic remodeling. Even in the absence of overt hypertension, arterial vascular walls become less compliant with age, resulting in some degree of pressure overload. ANG II concentrations increase significantly in aged rodent hearts [81, 82], probably due to an increase tissue levels of angiotensin II converting enzyme (ACE) [83]. ANG II promotes cardiomyocyte hypertrophy [84] and stimulates fibroblast proliferation and expression of extracellular matrix proteins [85]. ANG II exerts its effects directly through the ANG II type 1 receptor (AT1) and indirectly through induction of TGF-β1 [86]. Long-term inhibition with angiotensin receptor blockers, or AT1 disruption, reduce age-dependent cardiac pathology and prolong rat [87] and mouse [88] survival. In contrast, knock-in mice with a gain-of-function mutation of AT1A develop progressive cardiac fibrosis with increased expression of collagen [89]. Stein et al. showed that chronic RAAS inhibition resulted in the reduction of both interstitial and patchy fibrosis in the senescent mouse heart. Interestingly, a significant reduction in the susceptibility to arrhythmias was observed after RAAS inhibition, and was directly correlated to the reduction of patchy fibrosis [90].
The role of β-adrenergic signaling
Activation of β-adrenergic signaling increases heart rate, contractility and afterload, enhancing cardiac metabolic demand. Chronic activation of β-adrenergic signaling is deleterious to the heart; several clinical trials have demonstrated that inhibition of β-adrenergic signaling by β-blockers provides survival benefit in patients with heart failure. Mice with disruption of adenylate cyclase type 5 (AC5), a major mediator of β-adrenergic signaling in the heart, had prolonged life span and were protected from cardiac aging, exhibiting reduced age-dependent cardiac hypertrophy and attenuated fibrosis [91].
The role of reactive oxygen species (ROS)
Experimental studies have demonstrated increased generation of ROS in the aging heart. Within the cells, ROS are produced in multiple compartments; however, mitochondria contribute to the majority of ROS generation as a byproduct of electron transfer during oxidative phosphorylation. Mitochondrial DNA (mtDNA), lipids, and proteins are therefore at the highest risk from free radical-induced damage and dysfunction. Several studies have documented an age-dependent impairment of mitochondrial function associated with increased production of ROS. The heart, with its high metabolic demand is rich in mitochondria and is especially vulnerable to mitochondrial oxidative damage. Impairment of mitochondrial function has been widely documented in heart failure in both human patients and mouse models [92]. Moreover a significant increase in superoxide radical production was seen in mitochondria prepared from aging rat hearts [93]. Direct evidence for the critical role of mitochondrial ROS in cardiac aging was reinforced by studies on mice overexpressing catalase targeted to the mitochondria (mCAT). First, overexpression of mCAT prolonged murine median lifespan by 17% to 21% [94]. Second in senescent mice, overexpression of mCAT reduced cardiomyocyte hypertrophy, diminished cardiac fibrosis, and attenuated diastolic dysfunction [81]. Third mCAT overexpression attenuated age-dependent mitochondrial oxidative damage, as displayed by significant reductions of mtDNA mutation and deletion frequencies, better protection of ultrastructure of mitochondrial cristae and attenuation of age-dependent activation of mitochondrial biogenesis [81]. Another recent study focused on the role of mitochondrial NAD-dependent deacetylase sirtuin-3 (SIRT3) in cardiac senescence. Overexpression of SIRT3 in cultured cells increases respiration and decreases the production of reactive oxygen species. SIRT3 knockout mice show accelerated signs of aging in the heart exhibiting cardiac hypertrophy and fibrosis at 13 months of age. SIRT3 knockout mice are also hypersensitive to cardiac stress induced by transverse aortic constriction (TAC), as evidenced by cardiac hypertrophy, fibrosis, and increased mortality. Together, these data show that SIRT3 activity is necessary to prevent mitochondrial dysfunction and cardiac hypertrophy during aging [95]. These data suggest an important role for ROS in the pathogenesis of aging-associated fibrotic cardiac remodeling.
Which pathways are responsible for ROS-induced fibrosis in aging hearts? ROS may exert fibrogenic actions both through direct effects on cardiac fibroblasts and through modulation of cytokine signaling. Oxidative stress regulates the quantity and quality of extracellular matrix by modulating both collagen synthesis and metabolism [96]. In addition, ROS mediate cytokine and ANG II-induced effects on fibroblasts [97]. On the other hand, ROS are capable of inducing expression of inflammatory and fibrogenic mediators that may play an essential role in aging-associated fibrosis. ROS-mediated upregulation of CC chemokines (such as MCP-1/CCL2), accompanied by induction of adhesion molecules in the microvascular endothelium [30], may promote recruitment of mononuclear cells and fibroblast progenitors in the aging myocardium creating a fibrogenic milieu [98, 99].
The role of TGF-β
TGF-β may play an essential role in aging-associated cardiac fibrosis by inducing myofibroblast transdifferentiation [100] and by enhancing matrix protein synthesis by cardiac fibroblasts [101]. In addition, TGF-β may exert potent matrix-preserving actions by suppressing the activity of MMPs and by inducing synthesis of protease inhibitors, such as PAI-1 and TIMPs [102, 103]. Beyond its effects on mesenchymal cells, TGF-β also induces hypertrophic effects on cardiomyocytes [104] and modulates the function of inflammatory cells [105–107].
The canonical signaling pathway for TGF-β involves the Smad family of transcriptional activators [108, 109]. The receptor-associated R-Smads, Smad2, and Smad3 are phosphorylated directly by the TGF-β type 1 receptor kinase, after which they heterooligomerize with Smad4, translocate to the nucleus, and together with their binding partners, activate or repress their target genes. Several studies have shown that TGF-β can also signal in a Smad-independent fashion, activating extracellular signal-regulated kinase (ERK), c-Abl or TAK-1 pathways [110].
TGF-β1-overexpressing mice exhibited enhanced β-adrenergic signaling and significant cardiac hypertrophy accompanied by interstitial fibrosis [111]. On the other hand, loss of one TGF-β1 allele in TGF-β1 heterozygous mice appears to ameliorate age-associated myocardial fibrosis and improve left ventricular compliance [112]. Both ROS and ANG II may activate TGF-β signaling pathways in the senescent heart. ROS activate TGF-β and upregulate its downstream fibrogenic effector [113], connective tissue growth factor (CTGF) [114]. In addition, ANG II markedly upregulates TGF-β1 synthesis by cardiac fibroblasts and myofibroblasts [115, 116]. ANG II-induced TGF-β upregulation is followed by the development of cardiac fibrosis [45]; however, the dependence of the pro-fibrotic actions of ANG II on TGF-β has not been established [85].
The antifibrotic role of C-type natriuretic peptide
C-type natriuretic peptide (CNP) is an endothelial cell-derived peptide that shares key biological actions with the cardiac natriuretic peptides such as atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) [117, 118]. In vitro studies showed that CNP exerted potent antifibrotic and antiproliferative properties in rat cardiac fibroblasts [119]. Recent work by Sangaralingham et al. showed a progressive decline in circulating CNP that characterizes natural aging and is strongly associated with a reciprocal increase in cardiac fibrosis that precedes impairment of diastolic and systolic function [120]. Whether CNP plays a direct role in regulation of fibrosis in vivo has not been examined.
Age-related defects in the reparative response following cardiac injury
The average age for a first myocardial infarction (MI) is 66 years in men and 70 years in women [121]. Patients aged 65 years and over account for about 50% of hospital admissions and 80% of deaths from acute MI [122]. The incidence of post-MI heart failure is increased; adverse ventricular remodeling is more common in elderly patients. The age-related increase in post-MI mortality and morbidity cannot be explained by larger infarcts [123]. Thus, distinct responses of the senescent heart to cardiac injury may play a role in aging-associated heart failure. Optimal healing of the infarcted heart is critical to repair structural damage and preserve left ventricular function. Aging may modulate post-MI reparative responses and may promote adverse remodeling, reducing survival and increasing the likelihood for development of heart failure [122].
Healing after MI is dependent on an inflammatory reaction that ultimately results in formation of a scar [124]. Inflammatory signals regulate key reparative pathways in the infarcted heart, modulating deposition, and metabolism of extracellular matrix proteins in the wound [75, 125]. These actions ultimately determine the mechanical properties and geometric characteristics of the infarcted ventricle by affecting the tensile strength of the scar.
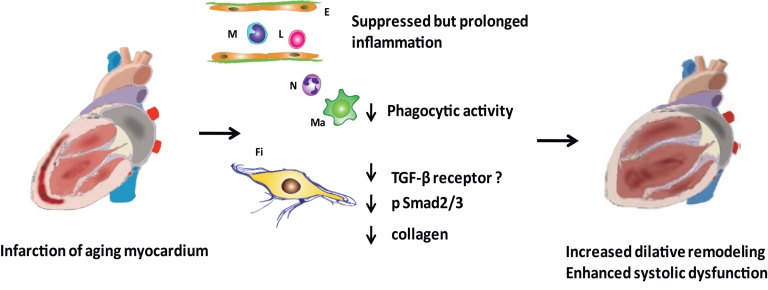
We have recently tested the hypothesis that aging-related changes in inflammatory mediator expression and impaired responsiveness of senescent cells to growth factors may be responsible for defective infarct healing and adverse remodeling of infarcted heart. Using a mouse model of reperfused infarction, we compared the inflammatory and fibrotic response between young (3–4 month old) and old mice (>24-month old) as well as the response of isolated cardiac fibroblasts to TGF-β stimulation [126]. We found that aging was associated with suppressed post-infarction inflammation, decreased and delayed neutrophil and macrophage infiltration, reduced cytokine and chemokine expression, and impaired phagocytosis of dead cardiomyocytes. Temporally, despite similar infarct size, reperfused MI in young mice induced intense inflammation after 24 h and replacement with granulation tissue within 72 h, whereas healing in the older mice was delayed beyond 7 days. Decreased phagocytotic activity [127] and diminished oxidative response to activating signals [128] displayed by senescent macrophages and neutrophils may explain the impaired clearance of dead cardiomyocytes in the infarcted myocardium. The suppressed inflammatory reaction was followed by decreased myofibroblast infiltration and markedly attenuated collagen and matricellular protein deposition in senescent mouse infarcts, resulting in formation of a scar containing loose connective tissue. The reduction in scar collagen content in old animals was associated with increased dilative remodeling and markedly enhanced systolic dysfunction following infarction.
Because of the critical role of the Smad2/3 pathway in mediating fibrogenic TGF-β responses [129, 300], we hypothesized that defective fibrous tissue deposition in senescent infarcted hearts may be due to impaired responses of aged mouse fibroblasts to growth factor stimulation. Young mouse cardiac fibroblasts exhibited a robust increase in Smad2 phosphorylation after stimulation with TGF-β1. In contrast, fibroblasts isolated from senescent hearts showed a blunted response to TGF-β stimulation [126], suggesting that aging results in impaired fibroblast responses to growth factors. The blunted response of senescent fibroblasts to fibrogenic mediators is not limited to TGF-β stimulation. The stimulatory effect of ANG II on matrix synthesis is reduced in rat fibroblasts isolated from senescent hearts in comparison with fibroblasts harvested from young hearts [131].
Therefore, the enhanced baseline activation of fibrogenic pathways and increased collagen deposition in senescent hearts may be associated with an impaired reparative reserve, due to blunted responses of mesenchymal cells to stimulatory signals. Defective scar formation may play an essential role in the pathogenesis of adverse remodeling and heart failure in senescent subjects (Fig. 3).
Figure 3.
Age-related defects in the inflammatory and reparative response lead to enhanced adverse remodeling following myocardial infarction. Although aging is associated with enhanced baseline inflammation and fibrosis, acute infarction results in suppressed, but prolonged, inflammatory reaction, impaired cardiomyocyte phagocytosis, and markedly diminished collagen deposition in the scar. Evolving evidence suggests that the alterations in post-infarction cardiac repair may be due to impaired responsiveness of senescent fibroblasts to growth factors, such as TGF-β. Whether this is due to an aging-related reduction of TGF-β receptor (TGF-βR) expression by fibroblasts, or reflects impaired TGF-β/Smad2/3 signaling in senescent cells, remains unknown. Diminished collagen deposition may lead to a marked reduction in tensile strength of the scar, resulting in accentuated dilation of the infarcted ventricle. Symbols: E, endothelial cell; Fi, fibroblast; L, lymphocyte, Ma, macrophage; M, monocyte; N, neutrophil
Therapeutic targets to attenuate fibrotic remodeling in senescent hearts
Our expanding knowledge on the molecular signals involved in aging-associated fibrosis suggests several potential therapeutic targets. MCP-1 inhibition, targeting of the TGF-β cascade, attenuation of ROS signaling and AGE breakers may be reasonable therapeutic approaches to prevent progression of cardiac fibrosis in the elderly. However, several important concerns dampen enthusiasm about the potential usefulness of these strategies. First, whether cardiac fibrosis can be reversed remains controversial. It has been suggested that established fibrotic changes may no longer be reversible due to the absence of cellular mediators that could produce proteases to degrade the collagen-rich tissue [71]. In addition, the formation of cross-linked matrix proteins in advanced lesions of senescent hearts may prevent reversal of the fibrotic process. Thus, effective inhibition of age-related cardiac fibrosis may require early and prolonged treatment, exposing patients to the consequences of therapeutic agents that interfere with tissue repair. Second, blockade of fibrogenic pathways may in many cases also inhibit adaptive processes with protective effects on the aging heart. For example, chronic MCP-1 inhibition may not only exert anti-fibrotic actions, but may also reduce arteriogenesis, interfering with collateral vessel formation. TGF-β inhibition, on the other hand, may interfere with important actions on immune responses [132]. Third, the clinical significance of age-associated cardiac fibrosis in patients without concomitant conditions (such as diabetes, hypertension, or coronary atherosclerotic disease) is unclear. Whether age-associated fibrotic remodeling significantly contributes to diastolic dysfunction in elderly patients remains unknown. Attenuation of the modest fibrosis noted in healthy elderly individuals may not confer clinically significant benefits. Thus, it may be more reasonable to focus on specific subpopulations of senescent patients who are at a high risk for development of fibrotic remodeling and diastolic dysfunction due to the presence of hypertensive, diabetic, or ischemic heart disease. Beyond, the established beneficial effects of ACE inhibitors in patients with hypertension that may be due, at least in part, to attenuation of cardiac fibrosis, other anti-fibrotic strategies (such as AGE breakers, anti-MCP-1 strategies, or TGF-β inhibitors) may exert beneficial actions in high-risk elderly patients with diastolic heart failure.
A much more realistic goal would be to target specific age-associated healing defects in senescent patients with cardiac injury, in order to prevent adverse remodeling and to protect from the development of heart failure [133, 134]. In senescent mice, a suppressed post-infarction inflammatory response results in delayed replacement of dead cardiomyocytes with granulation tissue [126], while blunted responses of senescent fibroblasts to fibrogenic growth factors markedly decrease collagen deposition in the scar, resulting in decreased tensile strength and enhanced ventricular dilation. These findings suggest that age-associated adverse remodeling of the infarcted ventricle is not due to enhanced inflammatory injury, or increased fibrosis, but rather results from a defective fibroblast response and impaired formation of the reparative matrix network, necessary to mechanically support the infarcted heart. Thus, strategies aiming at enhancing reparative responses following cardiac injury through the cautious administration of growth factors along with injection of smart biomaterials [135] may represent new therapeutic opportunities for preventing the development of heart failure in elderly patients with acute myocardial infarction.
Acknowledgments
Our laboratory is supported by NIH grants R01 HL-76246 and R01 HL-85440 and by the Wilf family Cardiovascular Research Institute.
REFERENCES
- [1].Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–54. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- [2].Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group Cardiovascular Health Study. Am J Cardiol. 2001;87:413–9. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- [3].Vanoverschelde JJ, Essamri B, Vanbutsele R, d’Hondt A, Cosyns JR, Detry JR, Melin JA. Contribution of left ventricular diastolic function to exercise capacity in normal subjects. J Appl Physiol. 1993;74:2225–33. doi: 10.1152/jappl.1993.74.5.2225. [DOI] [PubMed] [Google Scholar]
- [4].Gagliano N, Arosio B, Santambrogio D, Balestrieri MR, Padoani G, Tagliabue J, Masson S, Vergani C, Annoni G. Age-dependent expression of fibrosis-related genes and collagen deposition in rat kidney cortex. J Gerontol A Biol Sci Med Sci. 2000;55:B365–72. doi: 10.1093/gerona/55.8.b365. [DOI] [PubMed] [Google Scholar]
- [5].Abrass CK, Adcox MJ, Raugi GJ. Aging-associated changes in renal extracellular matrix. Am J Pathol. 1995;146:742–52. [PMC free article] [PubMed] [Google Scholar]
- [6].Hinton DE, Williams WL. Hepatic fibrosis associated with aging in four stocks of mice. J Gerontol. 1968;23:205–11. doi: 10.1093/geronj/23.2.205. [DOI] [PubMed] [Google Scholar]
- [7].Gagliano N, Grizzi F, Annoni G. Mechanisms of aging and liver functions. Dig Dis. 2007;25:118–23. doi: 10.1159/000099475. [DOI] [PubMed] [Google Scholar]
- [8].Glaser J, Stienecker K. Pancreas and aging: a study using ultrasonography. Gerontology. 2000;46:93–6. doi: 10.1159/000022141. [DOI] [PubMed] [Google Scholar]
- [9].Calabresi C, Arosio B, Galimberti L, Scanziani E, Bergottini R, Annoni G, Vergani C. Natural aging, expression of fibrosis-related genes and collagen deposition in rat lung. Exp Gerontol. 2007;42:1003–11. doi: 10.1016/j.exger.2007.06.016. [DOI] [PubMed] [Google Scholar]
- [10].Mukherjee D, Sen S. Collagen phenotypes during development and regression of myocardial hypertrophy in spontaneously hypertensive rats. Circ Res. 1990;67:1474–80. doi: 10.1161/01.res.67.6.1474. [DOI] [PubMed] [Google Scholar]
- [11].Song Y, Yao Q, Zhu J, Luo B, Liang S. Age-related variation in the interstitial tissues of the cardiac conduction system; and autopsy study of 230 Han Chinese. Forensic Sci Int. 1999;104:133–42. doi: 10.1016/s0379-0738(99)00103-6. [DOI] [PubMed] [Google Scholar]
- [12].Burkauskiene A, Mackiewicz Z, Virtanen I, Konttinen YT. Age-related changes in myocardial nerve and collagen networks of the auricle of the right atrium. Acta Cardiol. 2006;61:513–8. doi: 10.2143/AC.61.5.2017765. [DOI] [PubMed] [Google Scholar]
- [13].Burlew BS. Diastolic dysfunction in the elderly--the interstitial issue. Am J Geriatr Cardiol. 2004;13:29–38. doi: 10.1111/j.1076-7460.2004.00059.x. [DOI] [PubMed] [Google Scholar]
- [14].Nag AC. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios. 1980;28:41–61. [PubMed] [Google Scholar]
- [15].Gersch C, Dewald O, Zoerlein M, Michael LH, Entman ML, Frangogiannis NG. Mast cells and macrophages in normal C57/BL/6 mice. Histochem Cell Biol. 2002;118:41–9. doi: 10.1007/s00418-002-0425-z. [DOI] [PubMed] [Google Scholar]
- [16].Zeisberg EM, Kalluri R. Origins of cardiac fibroblasts. Circ Res. 2010;107:1304–12. doi: 10.1161/CIRCRESAHA.110.231910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Weber KT. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol. 1989;13:1637–52. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- [18].Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117:568–75. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shirwany A, Weber KT. Extracellular matrix remodeling in hypertensive heart disease. J Am Coll Cardiol. 2006;48:97–8. doi: 10.1016/j.jacc.2006.04.004. [DOI] [PubMed] [Google Scholar]
- [20].Anderson KR, Sutton MG, Lie JT. Histopathological types of cardiac fibrosis in myocardial disease. J Pathol. 1979;128:79–85. doi: 10.1002/path.1711280205. [DOI] [PubMed] [Google Scholar]
- [21].Isoyama S, Nitta-Komatsubara Y. Acute and chronic adaptation to hemodynamic overload and ischemia in the aged heart. Heart Fail Rev. 2002;7:63–9. doi: 10.1023/a:1013701923065. [DOI] [PubMed] [Google Scholar]
- [22].Hasenfuss G. Animal models of human cardiovascular disease, heart failure and hypertrophy. Cardiovasc Res. 1998;39:60–76. doi: 10.1016/s0008-6363(98)00110-2. [DOI] [PubMed] [Google Scholar]
- [23].Pugh KG, Wei JY. Clinical implications of physiological changes in the aging heart. Drugs Aging. 2001;18:263–76. doi: 10.2165/00002512-200118040-00004. [DOI] [PubMed] [Google Scholar]
- [24].Chen MA. Heart failure with preserved ejection fraction in older adults. Am J Med. 2009;122:713–23. doi: 10.1016/j.amjmed.2009.01.038. [DOI] [PubMed] [Google Scholar]
- [25].Olivetti G, Melissari M, Capasso JM, Anversa P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68:1560–8. doi: 10.1161/01.res.68.6.1560. [DOI] [PubMed] [Google Scholar]
- [26].Lakatta EG. Age-associated cardiovascular changes in health: impact on cardiovascular disease in older persons. Heart Fail Rev. 2002;7:29–49. doi: 10.1023/a:1013797722156. [DOI] [PubMed] [Google Scholar]
- [27].Kajstura J, Cheng W, Sarangarajan R, Li P, Li B, Nitahara JA, Chapnick S, Reiss K, Olivetti G, Anversa P. Necrotic and apoptotic myocyte cell death in the aging heart of Fischer 344 rats. Am J Physiol. 1996;271:H1215–28. doi: 10.1152/ajpheart.1996.271.3.H1215. [DOI] [PubMed] [Google Scholar]
- [28].Anversa P, Palackal T, Sonnenblick EH, Olivetti G, Meggs LG, Capasso JM. Myocyte cell loss and myocyte cellular hyperplasia in the hypertrophied aging rat heart. Circ Res. 1990;67:871–85. doi: 10.1161/01.res.67.4.871. [DOI] [PubMed] [Google Scholar]
- [29].Eghbali M, Robinson TF, Seifter S, Blumenfeld OO. Collagen accumulation in heart ventricles as a function of growth and aging. Cardiovasc Res. 1989;23:723–9. doi: 10.1093/cvr/23.8.723. [DOI] [PubMed] [Google Scholar]
- [30].Orlandi A, Francesconi A, Marcellini M, Ferlosio A, Spagnoli LG. Role of ageing and coronary atherosclerosis in the development of cardiac fibrosis in the rabbit. Cardiovasc Res. 2004;64:544–52. doi: 10.1016/j.cardiores.2004.07.024. [DOI] [PubMed] [Google Scholar]
- [31].Lin J, Lopez EF, Jin Y, Van Remmen H, Bauch T, Han HC, Lindsey ML. Age-related cardiac muscle sarcopenia: Combining experimental and mathematical modeling to identify mechanisms. Exp Gerontol. 2008;43:296–306. doi: 10.1016/j.exger.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mays PK, Bishop JE, Laurent GJ. Age-related changes in the proportion of types I and III collagen. Mech Ageing Dev. 1988;45:203–12. doi: 10.1016/0047-6374(88)90002-4. [DOI] [PubMed] [Google Scholar]
- [33].Gazoti Debessa CR, Mesiano Maifrino LB, Rodrigues de Souza R. Age related changes of the collagen network of the human heart. Mech Ageing Dev. 2001;122:1049–58. doi: 10.1016/s0047-6374(01)00238-x. [DOI] [PubMed] [Google Scholar]
- [34].de Souza RR. Aging of myocardial collagen. Biogerontology. 2002;3:325–35. doi: 10.1023/a:1021312027486. [DOI] [PubMed] [Google Scholar]
- [35].Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, Reddy A, Frangogiannis NG. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008;173:57–67. doi: 10.2353/ajpath.2008.070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Mays PK, McAnulty RJ, Campa JS, Laurent GJ. Age-related changes in collagen synthesis and degradation in rat tissues. Importance of degradation of newly synthesized collagen in regulating collagen production. Biochem J. 1991;276(Pt 2):307–13. doi: 10.1042/bj2760307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Besse S, Robert V, Assayag P, Delcayre C, Swynghedauw B. Nonsynchronous changes in myocardial collagen mRNA and protein during aging: effect of DOCA-salt hypertension. Am J Physiol. 1994;267:H2237–44. doi: 10.1152/ajpheart.1994.267.6.H2237. [DOI] [PubMed] [Google Scholar]
- [38].Annoni G, Luvara G, Arosio B, Gagliano N, Fiordaliso F, Santambrogio D, Jeremic G, Mircoli L, Latini R, Vergani C, Masson S. Age-dependent expression of fibrosis-related genes and collagen deposition in the rat myocardium. Mech Ageing Dev. 1998;101:57–72. doi: 10.1016/s0047-6374(97)00165-6. [DOI] [PubMed] [Google Scholar]
- [39].Robert V, Besse S, Sabri A, Silvestre JS, Assayag P, Nguyen VT, Swynghedauw B, Delcayre C. Differential regulation of matrix metalloproteinases associated with aging and hypertension in the rat heart. Lab Invest. 1997;76:729–38. [PubMed] [Google Scholar]
- [40].Gagliano N, Arosio B, Grizzi F, Masson S, Tagliabue J, Dioguardi N, Vergani C, Annoni G. Reduced collagenolytic activity of matrix metalloproteinases and development of liver fibrosis in the aging rat. Mech Ageing Dev. 2002;123:413–25. doi: 10.1016/s0047-6374(01)00398-0. [DOI] [PubMed] [Google Scholar]
- [41].van der Rest M, Garrone R. Collagen family of proteins. FASEB J. 1991;5:2814–23. [PubMed] [Google Scholar]
- [42].Thomas DP, Cotter TA, Li X, McCormick RJ, Gosselin LE. Exercise training attenuates aging-associated increases in collagen and collagen crosslinking of the left but not the right ventricle in the rat. Eur J Appl Physiol. 2001;85:164–9. doi: 10.1007/s004210100447. [DOI] [PubMed] [Google Scholar]
- [43].Choi SY, Chang HJ, Choi SI, Kim KI, Cho YS, Youn TJ, Chung WY, Chae IH, Choi DJ, Kim HS, Kim CH, Oh BH, Kim MH. Long-term exercise training attenuates age-related diastolic dysfunction: association of myocardial collagen cross-linking. J Korean Med Sci. 2009;24:32–9. doi: 10.3346/jkms.2009.24.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Thomas DP, Zimmerman SD, Hansen TR, Martin DT, McCormick RJ. Collagen gene expression in rat left ventricle: interactive effect of age and exercise training. J Appl Physiol. 2000;89:1462–8. doi: 10.1152/jappl.2000.89.4.1462. [DOI] [PubMed] [Google Scholar]
- [45].Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens. 2003;21:3–12. doi: 10.1097/00004872-200301000-00002. [DOI] [PubMed] [Google Scholar]
- [46].Asif M, Egan J, Vasan S, Jyothirmayi GN, Masurekar MR, Lopez S, Williams C, Torres RL, Wagle D, Ulrich P, Cerami A, Brines M, Regan TJ. An advanced glycation endproduct cross-link breaker can reverse age-related increases in myocardial stiffness. Proc Natl Acad Sci U S A. 2000;97:2809–13. doi: 10.1073/pnas.040558497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Shapiro BP, Owan TE, Mohammed SF, Meyer DM, Mills LD, Schalkwijk CG, Redfield MM. Advanced glycation end products accumulate in vascular smooth muscle and modify vascular but not ventricular properties in elderly hypertensive canines. Circulation. 2008;118:1002–10. doi: 10.1161/CIRCULATIONAHA.108.777326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dannenberg AL, Levy D, Garrison RJ. Impact of age on echocardiographic left ventricular mass in a healthy population (the Framingham Study) Am J Cardiol. 1989;64:1066–8. doi: 10.1016/0002-9149(89)90816-3. [DOI] [PubMed] [Google Scholar]
- [49].Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002;105:1387–93. doi: 10.1161/hc1102.105289. [DOI] [PubMed] [Google Scholar]
- [50].Iwanaga Y, Aoyama T, Kihara Y, Onozawa Y, Yoneda T, Sasayama S. Excessive activation of matrix metalloproteinases coincides with left ventricular remodeling during transition from hypertrophy to heart failure in hypertensive rats. J Am Coll Cardiol. 2002;39:1384–91. doi: 10.1016/s0735-1097(02)01756-4. [DOI] [PubMed] [Google Scholar]
- [51].Janicki JS, Brower GL. The role of myocardial fibrillar collagen in ventricular remodeling and function. J Card Fail. 2002;8:S319–25. doi: 10.1054/jcaf.2002.129260. [DOI] [PubMed] [Google Scholar]
- [52].Baicu CF, Stroud JD, Livesay VA, Hapke E, Holder J, Spinale FG, Zile MR. Changes in extracellular collagen matrix alter myocardial systolic performance. Am J Physiol Heart Circ Physiol. 2003;284:H122–32. doi: 10.1152/ajpheart.00233.2002. [DOI] [PubMed] [Google Scholar]
- [53].Wang J, Hoshijima M, Lam J, Zhou Z, Jokiel A, Dalton ND, Hultenby K, Ruiz-Lozano P, Ross J, Jr, Tryggvason K, Chien KR. Cardiomyopathy associated with microcirculation dysfunction in laminin alpha4 chain-deficient mice. J Biol Chem. 2006;281:213–20. doi: 10.1074/jbc.M505061200. [DOI] [PubMed] [Google Scholar]
- [54].Beltrami CA, Finato N, Rocco M, Feruglio GA, Puricelli C, Cigola E, Quaini F, Sonnenblick EH, Olivetti G, Anversa P. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation. 1994;89:151–63. doi: 10.1161/01.cir.89.1.151. [DOI] [PubMed] [Google Scholar]
- [55].Khan R, Sheppard R. Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology. 2006;118:10–24. doi: 10.1111/j.1365-2567.2006.02336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ljungqvist A, Unge G. The proliferative activity of the myocardial tissue in various forms of experimental cardiac hypertrophy. Acta Pathol Microbiol Scand A. 1973;81:233–40. doi: 10.1111/j.1699-0463.1973.tb03530.x. [DOI] [PubMed] [Google Scholar]
- [57].Mandache E, Unge G, Appelgren LE, Ljungqvist A. The proliferative activity of the heart tissues in various forms of experimental cardiac hypertrophy studied by electron microscope autoradiography. Virchows Arch B Cell Pathol. 1973;12:112–22. doi: 10.1007/BF02893991. [DOI] [PubMed] [Google Scholar]
- [58].Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].van Amerongen MJ, Bou-Gharios G, Popa E, van Ark J, Petersen AH, van Dam GM, van Luyn MJ, Harmsen MC. Bone marrow-derived myofibroblasts contribute functionally to scar formation after myocardial infarction. J Pathol. 2008;214:377–86. doi: 10.1002/path.2281. [DOI] [PubMed] [Google Scholar]
- [60].Kania G, Blyszczuk P, Stein S, Valaperti A, Germano D, Dirnhofer S, Hunziker L, Matter CM, Eriksson U. Heart-infiltrating prominin-1+/CD133+ progenitor cells represent the cellular source of transforming growth factor beta-mediated cardiac fibrosis in experimental autoimmune myocarditis. Circ Res. 2009;105:462–70. doi: 10.1161/CIRCRESAHA.109.196287. [DOI] [PubMed] [Google Scholar]
- [61].Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, Pilling D, Gomer RH, Trial J, Frangogiannis NG, Entman ML. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci U S A. 2006;103:18284–9. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–62. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- [63].Olivey HE, Mundell NA, Austin AF, Barnett JV. Transforming growth factor-beta stimulates epithelial-mesenchymal transformation in the proepicardium. Dev Dyn. 2006;235:50–9. doi: 10.1002/dvdy.20593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–42. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- [65].Alva JA, Zovein AC, Monvoisin A, Murphy T, Salazar A, Harvey NL, Carmeliet P, Iruela-Arispe ML. VE-Cadherin-Cre-recombinase transgenic mouse: a tool for lineage analysis and gene deletion in endothelial cells. Dev Dyn. 2006;235:759–67. doi: 10.1002/dvdy.20643. [DOI] [PubMed] [Google Scholar]
- [66].Lugus JJ, Park C, Ma YD, Choi K. Both primitive and definitive blood cells are derived from Flk-1+ mesoderm. Blood. 2009;113:563–6. doi: 10.1182/blood-2008-06-162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–61. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- [68].Ghosh AK, Bradham WS, Gleaves LA, De Taeye B, Murphy SB, Covington JW, Vaughan DE. Genetic deficiency of plasminogen activator inhibitor-1 promotes cardiac fibrosis in aged mice: involvement of constitutive transforming growth factor-beta signaling and endothelial-to-mesenchymal transition. Circulation. 2010;122:1200–9. doi: 10.1161/CIRCULATIONAHA.110.955245. [DOI] [PubMed] [Google Scholar]
- [69].Jankun J, Skrzypczak-Jankun E. Yin and yang of the plasminogen activator inhibitor. Pol Arch Med Wewn. 2009;119:410–7. [PubMed] [Google Scholar]
- [70].Moriwaki H, Stempien-Otero A, Kremen M, Cozen AE, Dichek DA. Overexpression of urokinase by macrophages or deficiency of plasminogen activator inhibitor type 1 causes cardiac fibrosis in mice. Circ Res. 2004;95:637–44. doi: 10.1161/01.RES.0000141427.61023.f4. [DOI] [PubMed] [Google Scholar]
- [71].Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Frangogiannis NG. Targeting the inflammatory response in healing myocardial infarcts. Curr Med Chem. 2006;13:1877–93. doi: 10.2174/092986706777585086. [DOI] [PubMed] [Google Scholar]
- [73].Cieslik KA, Taffet GE, Carlson S, Hermosillo J, Trial J, Entman ML. Immune-inflammatory dysregulation modulates the incidence of progressive fibrosis and diastolic stiffness in the aging heart. J Mol Cell Cardiol. 2011;50:248–56. doi: 10.1016/j.yjmcc.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Dobaczewski M, Frangogiannis NG. Chemokines and cardiac fibrosis. Front Biosci (Schol Ed) 2009;1:391–405. doi: 10.2741/s33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Bujak M, Dobaczewski M, Gonzalez-Quesada C, Xia Y, Leucker T, Zymek P, Veeranna V, Tager AM, Luster AD, Frangogiannis NG. Induction of the CXC chemokine interferon-gamma-inducible protein 10 regulates the reparative response following myocardial infarction. Circ Res. 2009;105:973–83. doi: 10.1161/CIRCRESAHA.109.199471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Fallon PG, Richardson EJ, McKenzie GJ, McKenzie AN. Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL-4 and IL-13: IL-13 is a profibrotic agent. J Immunol. 2000;164:2585–91. doi: 10.4049/jimmunol.164.5.2585. [DOI] [PubMed] [Google Scholar]
- [77].Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- [78].Shearer GM. Th1/Th2 changes in aging. Mech Ageing Dev. 1997;94:1–5. doi: 10.1016/s0047-6374(96)01849-0. [DOI] [PubMed] [Google Scholar]
- [79].Deng Y, Jing Y, Campbell AE, Gravenstein S. Age-related impaired type 1 T cell responses to influenza: reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J Immunol. 2004;172:3437–46. doi: 10.4049/jimmunol.172.6.3437. [DOI] [PubMed] [Google Scholar]
- [80].Jing Y, Gravenstein S, Chaganty NR, Chen N, Lyerly KH, Joyce S, Deng Y. Aging is associated with a rapid decline in frequency, alterations in subset composition, and enhanced Th2 response in CD1d-restricted NKT cells from human peripheral blood. Exp Gerontol. 2007;42:719–32. doi: 10.1016/j.exger.2007.01.009. [DOI] [PubMed] [Google Scholar]
- [81].Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–97. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Groban L, Pailes NA, Bennett CD, Carter CS, Chappell MC, Kitzman DW, Sonntag WE. Growth hormone replacement attenuates diastolic dysfunction and cardiac angiotensin II expression in senescent rats. J Gerontol A Biol Sci Med Sci. 2006;61:28–35. doi: 10.1093/gerona/61.1.28. [DOI] [PubMed] [Google Scholar]
- [83].Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–7. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- [84].Sadoshima J, Izumo S. Molecular characterization of angiotensin II--induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res. 1993;73:413–23. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- [85].Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res. 2004;63:423–32. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- [86].Weber KT, Swamynathan SK, Guntaka RV, Sun Y. Angiotensin II and extracellular matrix homeostasis. Int J Biochem Cell Biol. 1999;31:395–403. doi: 10.1016/s1357-2725(98)00125-3. [DOI] [PubMed] [Google Scholar]
- [87].Basso N, Cini R, Pietrelli A, Ferder L, Terragno NA, Inserra F. Protective effect of long-term angiotensin II inhibition. Am J Physiol Heart Circ Physiol. 2007;293:H1351–8. doi: 10.1152/ajpheart.00393.2007. [DOI] [PubMed] [Google Scholar]
- [88].Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, Conti S, Rottoli D, Longaretti L, Cassis P, Morigi M, Coffman TM, Remuzzi G. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524–30. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Billet S, Bardin S, Verp S, Baudrie V, Michaud A, Conchon S, Muffat-Joly M, Escoubet B, Souil E, Hamard G, Bernstein KE, Gasc JM, Elghozi JL, Corvol P, Clauser E. Gain-of-function mutant of angiotensin II receptor, type 1A, causes hypertension and cardiovascular fibrosis in mice. J Clin Invest. 2007;117:1914–25. doi: 10.1172/JCI28764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Stein M, Boulaksil M, Jansen JA, Herold E, Noorman M, Joles JA, van Veen TA, Houtman MJ, Engelen MA, Hauer RN, de Bakker JM, van Rijen HV. Reduction of fibrosis-related arrhythmias by chronic renin-angiotensin-aldosterone system inhibitors in an aged mouse model. Am J Physiol Heart Circ Physiol. 2010;299:H310–21. doi: 10.1152/ajpheart.01137.2009. [DOI] [PubMed] [Google Scholar]
- [91].Yan L, Vatner DE, O’Connor JP, Ivessa A, Ge H, Chen W, Hirotani S, Ishikawa Y, Sadoshima J, Vatner SF. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007;130:247–58. doi: 10.1016/j.cell.2007.05.038. [DOI] [PubMed] [Google Scholar]
- [92].Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res. 2008;79:208–17. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- [93].Sawada M, Carlson JC. Changes in superoxide radical and lipid peroxide formation in the brain, heart and liver during the lifetime of the rat. Mech Ageing Dev. 1987;41:125–37. doi: 10.1016/0047-6374(87)90057-1. [DOI] [PubMed] [Google Scholar]
- [94].Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–11. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- [95].Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY) 2010;2:914–23. doi: 10.18632/aging.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Siwik DA, Pagano PJ, Colucci WS. Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol. 2001;280:C53–60. doi: 10.1152/ajpcell.2001.280.1.C53. [DOI] [PubMed] [Google Scholar]
- [97].Cheng TH, Cheng PY, Shih NL, Chen IB, Wang DL, Chen JJ. Involvement of reactive oxygen species in angiotensin II-induced endothelin-1 gene expression in rat cardiac fibroblasts. J Am Coll Cardiol. 2003;42:1845–54. doi: 10.1016/j.jacc.2003.06.010. [DOI] [PubMed] [Google Scholar]
- [98].Frangogiannis NG. Chemokines in the ischemic myocardium: from inflammation to fibrosis. Inflamm Res. 2004;53:585–95. doi: 10.1007/s00011-004-1298-5. [DOI] [PubMed] [Google Scholar]
- [99].Frangogiannis NG. Chemokines in ischemia and reperfusion. Thromb Haemost. 2007;97:738–47. [PubMed] [Google Scholar]
- [100].Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol. 1993;122:103–11. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–95. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Schiller M, Javelaud D, Mauviel A. TGF-beta-induced SMAD signaling and gene regulation: consequences for extracellular matrix remodeling and wound healing. J Dermatol Sci. 2004;35:83–92. doi: 10.1016/j.jdermsci.2003.12.006. [DOI] [PubMed] [Google Scholar]
- [103].Mauviel A. Transforming growth factor-beta: a key mediator of fibrosis. Methods Mol Med. 2005;117:69–80. doi: 10.1385/1-59259-940-0:069. [DOI] [PubMed] [Google Scholar]
- [104].Parker TG, Packer SE, Schneider MD. Peptide growth factors can provoke “fetal” contractile protein gene expression in rat cardiac myocytes. J Clin Invest. 1990;85:507–14. doi: 10.1172/JCI114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Kehrl JH, Wakefield LM, Roberts AB, Jakowlew S, Alvarez-Mon M, Derynck R, Sporn MB, Fauci AS. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986;163:1037–50. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Lee G, Ellingsworth LR, Gillis S, Wall R, Kincade PW. Beta transforming growth factors are potential regulators of B lymphopoiesis. J Exp Med. 1987;166:1290–9. doi: 10.1084/jem.166.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Fan K, Ruan Q, Sensenbrenner L, Chen B. Transforming growth factor-beta 1 bifunctionally regulates murine macrophage proliferation. Blood. 1992;79:1679–85. [PubMed] [Google Scholar]
- [108].Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- [109].Miyazawa K, Shinozaki M, Hara T, Furuya T, Miyazono K. Two major Smad pathways in TGF-beta superfamily signalling. Genes Cells. 2002;7:1191–204. doi: 10.1046/j.1365-2443.2002.00599.x. [DOI] [PubMed] [Google Scholar]
- [110].Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- [111].Rosenkranz S, Flesch M, Amann K, Haeuseler C, Kilter H, Seeland U, Schluter KD, Bohm M. Alterations of beta-adrenergic signaling and cardiac hypertrophy in transgenic mice overexpressing TGF-beta(1) Am J Physiol Heart Circ Physiol. 2002;283:H1253–62. doi: 10.1152/ajpheart.00578.2001. [DOI] [PubMed] [Google Scholar]
- [112].Brooks WW, Conrad CH. Myocardial fibrosis in transforming growth factor beta(1)heterozygous mice. J Mol Cell Cardiol. 2000;32:187–95. doi: 10.1006/jmcc.1999.1065. [DOI] [PubMed] [Google Scholar]
- [113].Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol. 1996;10:1077–83. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]
- [114].Park SK, Kim J, Seomun Y, Choi J, Kim DH, Han IO, Lee EH, Chung SK, Joo CK. Hydrogen peroxide is a novel inducer of connective tissue growth factor. Biochem Biophys Res Commun. 2001;284:966–71. doi: 10.1006/bbrc.2001.5058. [DOI] [PubMed] [Google Scholar]
- [115].Lee AA, Dillmann WH, McCulloch AD, Villarreal FJ. Angiotensin II stimulates the autocrine production of transforming growth factor-beta 1 in adult rat cardiac fibroblasts. J Mol Cell Cardiol. 1995;27:2347–57. doi: 10.1016/s0022-2828(95)91983-x. [DOI] [PubMed] [Google Scholar]
- [116].Campbell SE, Katwa LC. Angiotensin II stimulated expression of transforming growth factor-beta1 in cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol. 1997;29:1947–58. doi: 10.1006/jmcc.1997.0435. [DOI] [PubMed] [Google Scholar]
- [117].D’Souza SP, Davis M, Baxter GF. Autocrine and paracrine actions of natriuretic peptides in the heart. Pharmacol Ther. 2004;101:113–29. doi: 10.1016/j.pharmthera.2003.11.001. [DOI] [PubMed] [Google Scholar]
- [118].Suga S, Nakao K, Itoh H, Komatsu Y, Ogawa Y, Hama N, Imura H. Endothelial production of C-type natriuretic peptide and its marked augmentation by transforming growth factor-beta. Possible existence of “vascular natriuretic peptide system”. J Clin Invest. 1992;90:1145–9. doi: 10.1172/JCI115933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Horio T, Tokudome T, Maki T, Yoshihara F, Suga S, Nishikimi T, Kojima M, Kawano Y, Kangawa K. Gene expression, secretion, and autocrine action of C-type natriuretic peptide in cultured adult rat cardiac fibroblasts. Endocrinology. 2003;144:2279–84. doi: 10.1210/en.2003-0128. [DOI] [PubMed] [Google Scholar]
- [120].Sangaralingham SJ, Huntley BK, Martin FL, McKie PM, Bellavia D, Ichiki T, Harders GE, Chen HH, Burnett JC., Jr The aging heart, myocardial fibrosis, and its relationship to circulating C-type natriuretic Peptide. Hypertension. 2011;57:201–7. doi: 10.1161/HYPERTENSIONAHA.110.160796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O’Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- [122].Ertl G, Frantz S. Healing after myocardial infarction. Cardiovasc Res. 2005;66:22–32. doi: 10.1016/j.cardiores.2005.01.011. [DOI] [PubMed] [Google Scholar]
- [123].Maggioni AP, Maseri A, Fresco C, Franzosi MG, Mauri F, Santoro E, Tognoni G. Age-related increase in mortality among patients with first myocardial infarctions treated with thrombolysis. The Investigators of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI-2) N Engl J Med. 1993;329:1442–8. doi: 10.1056/NEJM199311113292002. [DOI] [PubMed] [Google Scholar]
- [124].Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48:504–11. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Bujak M, Kweon HJ, Chatila K, Li N, Taffet G, Frangogiannis NG. Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J Am Coll Cardiol. 2008;51:1384–92. doi: 10.1016/j.jacc.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Swift ME, Burns AL, Gray KL, DiPietro LA. Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol. 2001;117:1027–35. doi: 10.1046/j.0022-202x.2001.01539.x. [DOI] [PubMed] [Google Scholar]
- [128].Ding A, Hwang S, Schwab R. Effect of aging on murine macrophages. Diminished response to IFN-gamma for enhanced oxidative metabolism. J Immunol. 1994;153:2146–52. [PubMed] [Google Scholar]
- [129].Flanders KC. Smad3 as a mediator of the fibrotic response. Int J Exp Pathol. 2004;85:47–64. doi: 10.1111/j.0959-9673.2004.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, Wang XF, Frangogiannis NG. Essential role of Smad3 in infarct healing and in the pathogenesis of cardiac remodeling. Circulation. 2007;116:2127–38. doi: 10.1161/CIRCULATIONAHA.107.704197. [DOI] [PubMed] [Google Scholar]
- [131].Shivakumar K, Dostal DE, Boheler K, Baker KM, Lakatta EG. Differential response of cardiac fibroblasts from young adult and senescent rats to ANG II. Am J Physiol Heart Circ Physiol. 2003;284:H1454–9. doi: 10.1152/ajpheart.00766.2002. [DOI] [PubMed] [Google Scholar]
- [132].Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol. 2010 doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Jugdutt BI, Jelani A. Aging and defective healing, adverse remodeling, and blunted postconditioning in the reperfused wounded heart. J Am Coll Cardiol. 2008;51:1399–403. doi: 10.1016/j.jacc.2007.12.027. [DOI] [PubMed] [Google Scholar]
- [134].Jugdutt BI. Pleiotropic effects of cardiac drugs on healing post-MI. The good, bad, and ugly. Heart Fail Rev. 2008;13:439–52. doi: 10.1007/s10741-008-9090-1. [DOI] [PubMed] [Google Scholar]
- [135].Davis ME, Hsieh PC, Grodzinsky AJ, Lee RT. Custom design of the cardiac microenvironment with biomaterials. Circ Res. 2005;97:8–15. doi: 10.1161/01.RES.0000173376.39447.01. [DOI] [PMC free article] [PubMed] [Google Scholar]