Abstract
The human chromosomal 15q11–15q13 region is subject to both maternal and paternal genomic imprinting. Absence of paternal gene expression from this region results in Prader–Willi syndrome (PWS), while absence of maternal gene expression leads to Angelman syndrome. Transcription of paternally expressed genes in the region depends upon an imprinting center termed the PWS-IC. Imprinting defects in PWS can be caused by microdeletions and the smallest commonly deleted region indicates that the PWS-IC lies within a region of 4.3 kb. The function and location of the PWS-IC is evolutionarily conserved, but delineation of the PWS-IC in mouse has proven difficult. The first targeted mutation of the PWS-IC, a deletion of 35 kb spanning Snrpn exon 1, exhibited a complete PWS-IC deletion phenotype. Pups inheriting this mutation paternally showed a complete loss of paternal gene expression and died neonatally. A reported deletion of 4.8 kb showed only a reduction in paternal gene expression and incomplete penetrance of neonatal lethality, suggesting that some PWS-IC function had been retained. Here, we report that a 6 kb deletion spanning Snrpn exon 1 exhibits a complete PWS-IC deletion phenotype. Pups inheriting this mutation paternally lack detectable expression of all PWS genes and paternal silencing of Ube3a, exhibit maternal DNA methylation imprints at Ndn and Mkrn3 and suffer failure to thrive leading to a fully penetrant neonatal lethality.
INTRODUCTION
A small percentage of mammalian genes are subject to genomic imprinting, an epigenetic mechanism causing unequal expression of parental alleles. Imprinted genes tend to be organized in clusters regulated by one or more imprinting centers (ICs). The IC controls both gene expression and epigenotype within the domain. An imprinted region located at 15q11–q13 is responsible for both Prader–Willi syndrome (PWS) and Angelman syndrome (AS), two neurobehavioral disorders arising from reciprocal patterns of imprinted gene expression (1). Both gene order and allelic patterns of gene expression are conserved at the syntenic region on mouse chromosome 7.
PWS patients lack the paternal-only expression of a number of genes, including NDN, MAGEL2, MKRN3, C15ORF2, SNRPN (a bicistronic transcript of SNURF and SNRPN), UBE3A-AS and several small nucleolar RNAs (snoRNAs) (1). In some regions of the brain, UBE3A expression is restricted to the maternal allele and its function is disrupted in AS patients (2–4). Although most cases of PWS or AS result from a 5–7 mb deletion that removes the entire imprinted domain, some patients harbor microdeletions which disrupt imprinted gene expression (5). The smallest regions of overlap shared by these microdeletions define a bipartite IC comprised of the AS-IC and the PWS-IC (6). Gene expression patterns in both PWS individuals and mouse mutants support a model in which the PWS-IC functions as a positive regulator of transcription of paternal-only genes at the locus. The AS-IC functions in the maternal germline to epigenetically inactivate the PWS-IC so that paternal-only genes are silenced on the future maternal allele. AS-IC mediated silencing of a large transcript encoding SNURF/SNRPN, several snoRNAs and the UBE3A-antisense transcript (UBE3A-AS) on the maternal allele allows for UBE3A expression by an unknown mechanism (7).
Conservation of gene order and imprinting patterns suggests that mouse mutants can provide faithful models of imprinting mechanisms at the PWS/AS locus. The smallest region of overlap of microdeletions defining the human PWS-IC currently stands at 4.3 kb including the SNRPN promoter and exon 1, and includes a differentially DNA methylated region (DMR) characterized by DNA hypermethylation of the maternal allele (8). A differentially methylated enhancer associated with an evolutionarily conserved sequence located just outside of the minimal PWS-IC in the first intron of SNRPN, termed the conserved activator sequence (CAS), may also contribute to PWS-IC function (9).
The murine PWS-IC remains poorly characterized. A 35 kb deletion removing the first six exons of Snrpn and 16 kb of 5′ flanking sequence exhibits a complete PWS-IC imprinting defect, indicating that the entire murine PWS-IC is contained within this deletion. Paternal inheritance of this deletion is characterized by a highly penetrant neonatal lethality and absent expression of paternal-only genes (10). To date, smaller deletions within the boundaries of the 35 kb deletion have not yielded a similar complete PWS-IC phenotype. Paternal transmission of a 0.9 kb deletion removing Snrpn exon1 led to normal expression of paternal-only genes and appropriate DNA methylation at the remaining portion of the Snrpn DMR (11). A 4.8 kb deletion, revealed to be 5.07 kb by complete DNA sequencing of the region, that extended further into the DMR yielded partial neonatal lethality with residual expression of the paternal-only genes Mkrn3 and Ndn (11). More recently, we reported a mutant in which a 6.9 kb fragment containing the entire human PWS-IC replaced 6.0 kb of mouse sequence with the same 3′ breakpoint as the 4.8 kb PWS-IC deletion. Following paternal transmission of this PWS-ICHs allele, both Mkrn3 and Ndn were silenced and acquired a maternal DNA methylation pattern (12). Together, these results suggest that the 6.0 kb region replaced in the mutant contains the entire PWS-IC. We have now tested this idea by creating a targeted deletion of this 6.0 kb interval. Paternal transmission of this deletion leads to undetectable expression of paternal-only genes at the locus and a highly penetrant neonatal lethality. We conclude that all elements of the murine PWS-IC are contained within the boundaries of this deletion.
RESULTS
Generation of a 6 kb deletion at the PWS-IC
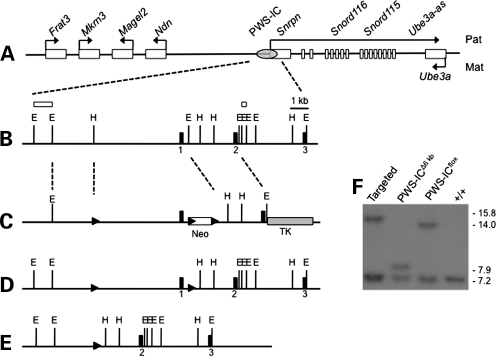
The imprinting defects characteristic of the PWS-ICHs allele suggest that the entire PWS-IC is located within a 6 kb region centered around Snrpn exon (12). An ES cell clone containing a loxP site at –3.7 kb, with reference to the 5′ end of Snrpn exon 1, and a floxed PGK-neo cassette at +2.3 kb was generated by gene targeting (Fig. 1C). Following transfection of a Cre-expressing plasmid, G418-sensitive clones lacking the PGK-neo cassette but retaining the floxed Snrpn allele were identified by polymerase chain reaction (PCR) and southern blot (Fig. 1D). A clone with the 6 kb region flanked by loxP sites was successfully transmitted to the germline. These mice were mated with the germline Cre-expressing line, 129-Alpltm1(cre)Nagy/J (13), to create the 6 kb deletion allele termed PWS-ICΔ6kb (Fig. 1E and F).
Figure 1.
Generation of mice with a targeted 6 kb deletion of the PWS-IC. (A) A map of the murine PWS/AS locus, not drawn to scale. Genes listed above the line are normally expressed from the paternal allele. Ube3a is normally expressed from the maternal allele. The PWS-IC is represented by a grey oval. Arrows indicate the direction of transcription. (B) Restriction map of the PWS-IC region. The first three exons of Snrpn are shown as black rectangles. Selected EcoRI (E) and HpaI (H) sites are indicated. Open rectangles indicate the positions of probes used in determining recombination. Dashed lines illustrate the region of the locus shown in this panel. (C) The gene targeting construct. loxP sites are shown as black arrowheads. The open box shows the positive PGK-neo selection cassette and the grey box indicates the negative HSV-tk selection cassette. Dashed lines represent regions for homologous recombination. Open rectangles above the map indicate the position of probes used in southern analysis of recombinants. (D) The recombinant allele created by homologous recombination within the arms of homology. After transient transfection with a Cre expressing plasmid, the conditional floxed allele was identified. These ES cells were utilized for blastocyst injection. (E) The PWS-ICΔ6kb allele was generated by deletion of the PWS-IC in the germline. (F) Southern blot analysis of various ES cell and mouse DNAs. Genomic DNA was digested with HpaI and EcoRV and hybridized with the 5′ probe shown in (B). The wild-type allele generates a 7.2 kb fragment present in all samples. Correct homologous recombination with the targeting vector yields a 15.8 kb allele arising from the disruption of the HpaI site by the upstream loxP site and insertion of the floxed PGK-neo cassette. Removal of the PGK-neo cassette reduces this band to 14 kb. Deletion of the PWS-IC yields a 7.9 kb band.
Paternal inheritance of the 6 kb PWS-IC deletion leads to reduced birth weight and survival
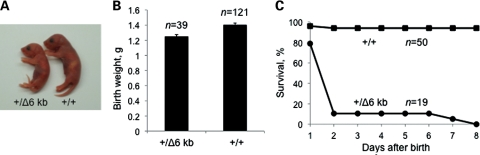
Paternal inheritance of a 35 kb deletion removing the entire murine PWS-IC is associated with low birth weight, failure to thrive, fully penetrant neonatal lethality and loss of expression of paternal-only genes from the region. At birth, mice inheriting the PWS-ICΔ6kb allele paternally (PWS-IC+/Δ6kb) are smaller than wild-type littermates and often lack milk spots. PWS-IC+/Δ6kb neonates weigh significantly less than their wild-type littermates and rarely survive beyond postnatal day (P) 2, although we did observe one pup surviving to P7 (Fig. 2). Similar to the previously reported PWS-ICΔ35kb mutation, we observed no overt phenotypes following maternal inheritance of the PWS-ICΔ6kb allele.
Figure 2.
Physical characteristics of PWS-IC+/Δ6kb pups. (A) Newborn PWS-IC+/Δ6kb pups are distinguishable from wild-type littermates by their small size and absence of a milk spot. At P2, this PWS-IC+/Δ6kb pup weighed 1.4 g and the wild-type littermate weighed 1.9 g. (B) At birth, PWS-IC+/Δ6kb pups typically weigh less than their littermates. The mean birth weight for PWS-IC+/Δ6kb pups is 1.25 g (N = 39) and for PWS-IC+/+ pups is 1.40 g (N = 121) (P-value < 0.0001). (C) PWS-IC+/Δ6kb pups have reduced survival compared with wild-type littermates with most PWS-IC+/Δ6kb pups dying within 2 days and none surviving past P7 (N = 19 PWS-IC+/Δ6kb pups, N = 50 PWS-IC+/+ pups).
PWS gene expression is undetectable in PWS-IC+/Δ6kb pups
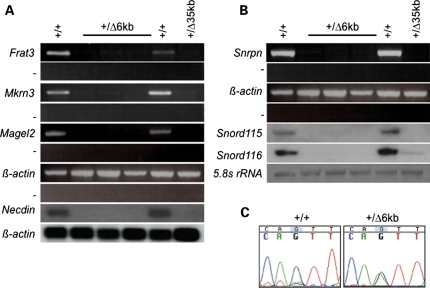
We investigated the molecular consequences of paternal inheritance of the 6 kb PWS-IC deletion by analyzing gene expression in newborn brain. Paternally expressed genes at the PWS/AS locus are arranged in two clusters. The centromeric cluster of genes comprising Snrpn, several snoRNAs and Ube3a-as are likely to be processed from a common primary transcript (14–18). Snrpn RNA and the snoRNAs Snord115 and Snord116 were not detected in the newborn PWS-IC+/Δ6kb brain (Fig. 3B). A low level of Snord116 RNA was detected in the 35 kb PWS-IC deletion control. As previously noted, this low level of expression may be a consequence of continued maintenance of the mouse line or result from the backcross onto the C57BL/6 background (19). We also examined expression of the telomeric cluster of genes composed of Frat3, Magel2, Mkrn3 and Ndn. Again, expression of these genes was not detectable in brains of pups with a paternal PWS-IC 6 kb deletion allele (Fig. 3A).
Figure 3.
Gene expression patterns in PWS-IC+/Δ6kb newborn brain. Total brain RNA was prepared from wild-type (+/+), PWS-IC+/Δ35kb (+/Δ35 kb) and PWS-IC+/Δ6kb (+/Δ6 kb) neonates and analyzed either by RT–PCR or northern blot. (A) Analysis of the upstream group of genes. The minus sign in RT–PCR analyses indicates control samples in which reverse transcriptase was omitted during cDNA synthesis. (B) Analysis of the downstream cluster of genes. (C) Allelic analysis of Ube3a expression. P1 progeny from a cross between a B6.CAST.c7 homozygous female and a PWS-ICΔ6kb/+ male were analyzed for allelic expression of Ube3a in the brain. The maternal castaneus allele has a guanine residue at the analyzed single nucleotide polymorphism, while the paternal domesticus allele has an adenine residue. Wild-type mice exhibit predominantly maternal Ube3a expression, while biallelic expression is evident in PWS-IC+/Δ6kb mice.
Ube3a expression is increased in PWS-IC+/Δ6kb mice
Ube3a is normally paternally silenced by a mechanism speculated to depend upon Ube3a-as, a transcript antisense to Ube3a. Consistent with this model, paternal inheritance of the 35 kb deletion of the PWS-IC results in increased paternal expression of Ube3a (20). To determine the imprinted status of Ube3a in PWS-IC+/Δ6kb mice, we took advantage of the B6.CAST.c7 strain in which the PWS/AS region is congenic for Mus musculus castaneus on a C57BL/6 background. DNA sequencing of a region containing a single nucleotide polymorphism demonstrated biparental expression of Ube3a in PWS-IC+/Δ6kb mice, while the wild-type littermates exhibited predominantly maternal expression (Fig. 3C). Thus, similar to the 35 kb deletion, the 6 kb PWS-IC deletion allele lacks elements necessary for silencing paternal Ube3a.
DNA methylation analysis of Mkrn3 and Ndn in PWS-IC+/Δ6kb mice
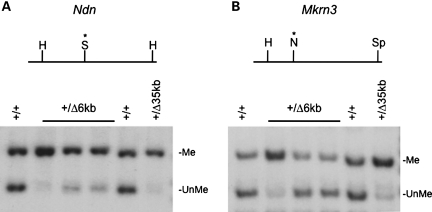
The paternally expressed genes Ndn and Mkrn3 both contain DMRs characterized by DNA hypermethylation of the normally silent maternal allele. Disruption of the PWS-IC leads to paternal hypermethylation in both individuals with PWS and mice lacking PWS-IC function (21–23). We used DNA methylation sensitive restriction endonucleases to investigate the role of the 6 kb PWS-IC deletion on DNA methylation status of these two loci. Paternal inheritance of the 6 kb PWS-IC deletion resulted in increased DNA methylation at both the Mkrn3 and Ndn loci, although to varying extent (Fig. 4). Increased DNA methylation appeared more extensive and reproducible at Ndn than at Mkrn3. The same DNA preparations were analyzed at both loci, suggesting that DNA methylation defects in the absence of the PWS-IC may be more penetrant at the Ndn locus. Partial hypermethylation of these paternal alleles was evident following paternal inheritance of both the 4.8 and 35 kb PWS-IC deletions (11,22).
Figure 4.
DNA methylation analysis of the Ndn and Mkrn3 DMRs. DNA from brains of wild-type (+/+), PWS-IC+/Δ35kb (+/Δ35 kb) and PWS-IC+/Δ6kb (+/Δ6 kb) newborns was digested with the indicated restriction endonucleases, southern blotted and probed. (A) The upper part of the panel shows a restriction map of the Ndn locus. The DNA methylation sensitive endonuclease SacII (S) is located between two HindIII (H) sites.The Ndn probe detects a 3.3 kb fragmet (Me) resulting from DNA methylation as the SacII site and a 1.8 kb fragment obtained from unmethylated DNA (UnMe). (B) A DNA methylation sensitive NotI is located between HindIII and SpeI (Sp) sites at the Mkrn3 locus. The probe detects methylated and unmethylated fragments of 6.8 and 5.0 kb, respectively. The same DNA preparations were analyzed in both (A) and (B).
DISCUSSION
ICs are DNA sequences that regulate both epigenotype and allelic gene expression (12). At the PWS/AS locus, overlapping microdeletions define a bipartite IC controlling both paternal- and maternal-only gene expression. A previously described 4.8 kb deletion at the murine PWS-IC exhibited a partial imprinting phenotype. Here, we have extended this deletion 1 kb further upstream of Snrpn exon 1. Paternal transmission results in fully penetrant neonatal lethality, undetectable expression of paternal-only genes and increased DNA methylation at Mkrn3 and Ndn. These traits are identical to a 35 kb PWS-IC deletion and indicate that all PWS-IC elements are located within the 6 kb deletion boundaries.
This 6 kb PWS-IC deletes most of the DMR, the Snrpn promoter region and the murine CAS. Both the promoter and the CAS are associated with paternal-specific DNase I hypersensitive (DH) sites at the human SNRPN gene in lymphoblasts and have been proposed to be involved in PWS-IC function (9). However, the human CAS is located in SNRPN intron 1, just outside of the minimally defined human PWS-IC. The inclusion of the transcription activating CAS in the 6 kb murine PWS-IC is consistent with the postulated function of the PWS-IC as a positive regulator of paternally expressed genes in the PWS/AS domain (7). The partial imprinting defect characteristic of the previously described murine 4.8 kb PWS-IC mutation indicates that this deletion contains some but not all elements required for a fully functional PWS-IC. Both the murine Snrpn promoter region and the CAS element are included in the 4.8 kb deletion, suggesting DNA sequence elements associated with these regions may contribute to PWS-IC activity. However, the partial imprinting defect of the 4.8 kb deletion indicates that one or more elements outside of this deletion are additionally required for full PWS-IC activity. Because the 4.8 kb deletion and the 6 kb deletion described here share the same 3′ boundary, we postulate that functional elements that confer full PWS-IC activity must be present within this differential 1.0 kb interval. A DH site has been detected within this 1.0 kb interval (S. Rodriguez-Jato, J.R. Khadake, T.P. Yang, unpublished data). We are currently refining its location and determining its parent of origin. If present only on the paternal allele, this would be consistent with the hypothesis that the PWS-IC is comprised of multiple functional elements that contribute to PWS-IC activity by creating a chromatin holocomplex, similar to an active chromatin hub, specifically on the paternal chromosome (9).
Snrpn, the snoRNAs and Ube3a-as are all processed from a common transcript. While the majority of transcription initiates within the PWS-IC, some transcripts initiate at several alternative upstream exons and splice into Snrpn exon 2, or less commonly, further downstream (24). Although the splice acceptor site at Snrpn exon 2 is intact, these transcripts are not detected in mice with a paternal 6 kb PWS-IC deletion, indicating that these upstream transcription initiation sites are themselves subject to regulation by the PWS-IC.
MATERIALS AND METHODS
Gene targeting
A targeting vector was constructed from a phage library of fragments generated from BAC 397F16 (Research Genetics). Homology arms consisted of a 3′ 3.5 kb EcoRI and a 5′ 8.6 kb HindIII/EcoRI fragment. Oligonucleotides containing a loxP site were inserted into the HpaI site of the 8.6 kb HindIII/EcoRI fragment, splitting it into a 2.6 kb 5′ homologous arm and a 6 kb PWS-IC region. A PGK-neo gene flanked by loxP sites was placed between the 3′ homology arm and the PWS-IC region, and a PGK-tk cassette was placed between the 3′ arm and the pBluescript KS + vector backbone.
Homologous recombination was performed in CJ.7 cells (129S1/Sv strain) as previously described (12). Clones with the appropriate homologous recombination were identified by southern blot. Homologous recombination at the 5′ end was identified by SpeI digest and at the 3′ end by HpaI/EcoRV digest.
The floxed neomycin cassette was removed by transient transfection of the ES cells with a Cre expressing plasmid, pCAGcre (25). Colonies sensitive to G418 were analyzed by PCR and southern blot. An ES cell line with a floxed 6 kb region of the PWS-IC was selected for injection into C57BL/6 blastocysts and chimeric mice identified. The PWS-ICΔ6kb mutation was obtained by breeding the PWS-ICflox6kb allele with 129-Alpltm1(cre)Nagy/J mice (Jax stock 008569) that express Cre in primordial germ cells and subsequently breeding progeny that inherited both the Cre transgene and the PWS-ICflox6kb allele to wild-type C57BL/6 mice. All genotyping was done by PCR. Primer sequences are available upon request. All animal procedures were previously approved by the University of Florida Institutional Animal Care and Use Committee.
Southern blot analysis
For southern blot analysis, ES cell or newborn brain DNA was digested with restriction endonucleases as indicated, electrophoresed through 0.8% TAE agarose gels and transferred to a positively charged nylon membrane (Hybond, GE Healthcare). Membranes were hybridized to 32P labeled probe and exposed to Kodak XAR film. The probes used for identifying ES clones with homologous recombination were outside of the targeting vector's arms of homology. At the 5′ end, a 800 bp EcoRI fragment was used while at the 3′ end, a 358 bp EcoRI fragment was used, both from a phage library of BAC 397F16 (Research Genetics) fragments.
Gene expression analysis
Analysis of paternal-only gene expression by either RT–PCR or northern blot was as previously described (12). Ube3a allelic expression was determined in newborn brains obtained from matings of B6.CAST.c7 females with PWS-IC+/Δ6kb males. B6.CAST.c7 are C57BL/6 congenic for the Mus castaneous PWS/AS region (26). RT–PCR for Ube3a was performed across a polymorphism between Mus castaneus and Mus musculus domesticus using primers Ube3a 5F, 5′-CACATATGATGAAGCTACGA-3′ and Ube3a 6R, 5′-CACACTCCCTTCATATTCC-3′ (20). The RT–PCR product was sequenced by the UF Center for Epigenetics.
FUNDING
This work was supported by grants from the Foundation for Prader-Willi Research and the National Institutes of Health (R01 HD 037872). E.Y.S. was supported by a UF Alumni Fellowship.
ACKNOWLEDGEMENTS
The authors thank Ryan Fiske of the UF Mouse Models Core.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Nicholls R.D., Knepper J.L. Genome organization, function, and imprinting in Prader-Willi and Angelman syndromes. Annu. Rev. Genomics Hum. Genet. 2001;2:153–175. doi: 10.1146/annurev.genom.2.1.153. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht U., Sutcliffe J.S., Cattanach B.M., Beechey C.V., Armstrong D., Eichele G., Beaudet A.L. Imprinted expression of the murine Angelman syndrome gene, Ube3a, in hippocampal and Purkinje neurons. Nat. Genet. 1997;17:75–78. doi: 10.1038/ng0997-75. [DOI] [PubMed] [Google Scholar]
- 3.Matsuura T., Sutcliffe J.S., Fang P., Galjaard R.-J., Jiang Y.-H., Benton C.S., Rommens J.M., Beaudet A.L. De novo truncating mutations in E6-AP ubiquitin-protein ligase gene (UBE3A) in Angelman syndrome. Nat. Genet. 1997;15:74–77. doi: 10.1038/ng0197-74. [DOI] [PubMed] [Google Scholar]
- 4.Kishino T., Lalande M., Wagstaff J. UBE3A/E6-AP mutations cause Angelman syndrome. Nat. Genet. 1997;15:70–73. doi: 10.1038/ng0197-70. [DOI] [PubMed] [Google Scholar]
- 5.Buiting K. Prader-Willi syndrome and Angelman syndrome. Am. J. Med. Genet. C Semin. Med. Genet. 2010;154C:365–376. doi: 10.1002/ajmg.c.30273. [DOI] [PubMed] [Google Scholar]
- 6.Horsthemke B., Wagstaff J. Mechanisms of imprinting of the Prader-Willi/Angelman region. Am. J. Med. Genet. A. 2008;146A:2041–2052. doi: 10.1002/ajmg.a.32364. [DOI] [PubMed] [Google Scholar]
- 7.Brannan C.I., Bartolomei M.S. Mechanisms of genomic imprinting. Curr. Opin. Genet. Dev. 1999;9:164–170. doi: 10.1016/S0959-437X(99)80025-2. [DOI] [PubMed] [Google Scholar]
- 8.Ohta T., Gray T.A., Rogan P.K., Buiting K., Gabriel J.M., Saitoh S., Muralidhar B., Bilienska B., Krajewska-Walasek M., Driscoll D.J., et al. Imprinting-mutation mechanisms in Prader-Willi syndrome. Am. J. Hum. Genet. 1999;64:397–413. doi: 10.1086/302233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodriguez-Jato S., Nicholls R.D., Driscoll D.J., Yang T.P. Characterization of cis- and trans-acting elements in the imprinted human SNURF-SNRPN locus. Nucleic Acids Res. 2005;33:4740–4753. doi: 10.1093/nar/gki786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang T., Adamson T.E., Resnick J.L., Leff S., Wevrick R., Francke U., Jenkins N.A., Copeland N.G., Brannan C.I. A mouse model for Prader-Willi syndrome imprinting-centre mutations. Nat. Genet. 1998;19:25–31. doi: 10.1038/ng0598-25. [DOI] [PubMed] [Google Scholar]
- 11.Bressler J., Tsai T.F., Wu M.Y., Tsai S.F., Ramirez M.A., Armstrong D., Beaudet A.L. The SNRPN promoter is not required for genomic imprinting of the Prader-Willi/Angelman domain in mice. Nat. Genet. 2001;28:232–240. doi: 10.1038/90067. [DOI] [PubMed] [Google Scholar]
- 12.Johnstone K.A., DuBose A.J., Futtner C.R., Elmore M.D., Brannan C.I., Resnick J.L. A human imprinting centre demonstrates conserved acquisition but diverged maintenance of imprinting in a mouse model for Angelman syndrome imprinting defects. Hum. Mol. Genet. 2006;15:393–404. doi: 10.1093/hmg/ddi456. [DOI] [PubMed] [Google Scholar]
- 13.Lomeli H., Ramos-Mejia V., Gertsenstein M., Lobe C.G., Nagy A. Targeted insertion of Cre recombinase into the TNAP gene: excision in primordial germ cells. Genesis. 2000;26:116–117. [PubMed] [Google Scholar]
- 14.Wevrick R., Kerns J.A., Francke U. Identification of a novel paternally expressed gene in the Prader-Willi-syndrome region. Hum. Mol. Genet. 1994;3:1877–1882. doi: 10.1093/hmg/3.10.1877. [DOI] [PubMed] [Google Scholar]
- 15.de los Santos T., Schweizer J., Rees C.A., Francke U. Small evolutionarily conserved RNA, resembling C/D box small nucleolar RNA, is transcribed from the PWCR1, a novel imprinted gene in the Prader-Willi deleted region, which is highly expressed in the brain. Am. J. Hum. Genet. 2000;67:1067–1082. doi: 10.1086/303106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavaillé J., Buiting K., Kiefmann M., Lalande M., Brannan C.I., Horsthemke B., Bachellerie J.P., Brosius J., Huttenhofer A. Identification of brain-specific and imprinted small nucleolar RNA genes exhibiting an unusual genomic organization. Proc. Natl Acad. Sci. USA. 2000;97:14311–14316. doi: 10.1073/pnas.250426397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Runte M., Hüttenhofer A., Gross S., Kiefmann M., Horsthemke B., Buiting K. The IC-SNURF-SNRPN transcript serves as a host for multiple small nucleolar RNA species and as an antisense RNA for UBE3A. Hum. Mol. Genet. 2001;10:2687–2700. doi: 10.1093/hmg/10.23.2687. [DOI] [PubMed] [Google Scholar]
- 18.Le Meur E., Watrin F., Landers M., Sturny R., Lalande M., Muscatelli F. Dynamic developmental regulation of the large non-coding RNA associated with the mouse 7C imprinted chromosomal region. Dev. Biol. 2005;286:587–600. doi: 10.1016/j.ydbio.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 19.Chamberlain S.J., Johnstone K.A., Dubose A.J., Simon T.A., Bartolomei M.S., Resnick J.L., Brannan C.I. Evidence for genetic modifiers of postnatal lethality in PWS-IC deletion mice. Hum. Mol. Genet. 2004;13:2971–2977. doi: 10.1093/hmg/ddh314. [DOI] [PubMed] [Google Scholar]
- 20.Chamberlain S.J., Brannan C.I. The Prader-Willi syndrome imprinting center activates the paternally expressed murine Ube3a antisense transcript but represses paternal Ube3a. Genomics. 2001;73:316–322. doi: 10.1006/geno.2001.6543. [DOI] [PubMed] [Google Scholar]
- 21.Buiting K., Saitoh S., Gross S., Dittrich B., Schwartz S., Nicholls R.D., Horsthemke B. Inherited microdeletions in the Angelman and Prader-Willi syndromes define an imprinting centre on human chromosome 15. Nat. Genet. 1995;9:395–400. doi: 10.1038/ng0495-395. [DOI] [PubMed] [Google Scholar]
- 22.Bielinska B., Blaydes S.M., Buiting K., Yang T., Krajewska-Walasek M., Horsthemke B., Brannan C.I. De novo deletions of SNRPN exon 1 in early human and mouse embryos result in a paternal to maternal imprint switch. Nat. Genet. 2000;25:74–78. doi: 10.1038/75629. [DOI] [PubMed] [Google Scholar]
- 23.El-Maarri O., Buiting K., Peery E.G., Kroisel P.M., Balaban B., Wagner K., Urman B., Heyd J., Lich C., Brannan C.I., Walter J., Horsthemke B. Maternal methylation imprints on human chromosome 15 are established during or after fertilization. Nat. Genet. 2001;27:341–344. doi: 10.1038/85927. [DOI] [PubMed] [Google Scholar]
- 24.Landers M., Bancescu D.L., Le Meur E., Rougeulle C., Glatt-Deeley H., Brannan C., Muscatelli F., Lalande M. Regulation of the large (∼1000 kb) imprinted murine Ube3a antisense transcript by alternative exons upstream of Snurf/Snrpn. Nucleic Acids Res. 2004;32:3480–3492. doi: 10.1093/nar/gkh670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith A.J., Xian J., Richardson M., Johnstone K.A., Rabbitts P.H. Cre-loxP chromosome engineering of a targeted deletion in the mouse corresponding to the 3p21.3 region of homozygous loss in human tumours. Oncogene. 2002;21:4521–4529. doi: 10.1038/sj.onc.1205530. [DOI] [PubMed] [Google Scholar]
- 26.Wakeland E.K., Morel L., Achey K., Yui M., Longmate J. Speed congenics: a classic technique in the fast lane (relatively speaking) Immunol. Today. 1997;18:472–477. doi: 10.1016/s0167-5699(97)01126-2. [DOI] [PubMed] [Google Scholar]