Abstract
Treponema denticola is a major pathogen of chronic periodontitis. Analysis of the T. denticola genome revealed a gene orthologous with a gene encoding a cysteine protease from Streptococcus pyogenes (IdeS). IdeS interferes with IgG-dependent opsonophagocytosis by specific cleavage of IgG molecules. Analysis of this gene (termed ideT) revealed it to encode a two domain protein. The N-terminus of this protein is composed of a tandem, immunoglobulin-like domain, followed by a C-terminally located IdeS-like protease domain. We show here that during secretion the IdeT protein is processed into an N-terminal fragment which remains associated with the cell, and a C-terminal portion released into the medium. Although this secreted domain of IdeT, termed dentipain, shows only 25% identity with the IdeS protease, the putative catalytic cysteine and histidine residues are strongly conserved. Analysis of recombinant dentipain revealed that it cleaved the insulin β-chain, an activity which was inhibited by E-64, a diagnostic inhibitor of cysteine proteases. Apart from insulin no cleavage of other protein substrates was detected, suggesting that dentipain has oligopeptidase activity. A mutant strain was constructed bearing a modified ideT whose dentipain domain was deleted. This mutant was found to be significantly reduced in its abscess forming activity compared to the parental strain in a murine abscess model, suggesting that dentipain contributes to the virulence of T. denticola.
Keywords: Cysteine Proteases, Immunoglobulin-like protein, IgG-specific protease, Oligopeptidase, Periodontal Diseases
Introduction
Treponema denticola is frequently isolated from human chronic periodontal lesions, together with Porphyromonas gingivalis and Tannerella forsythia (Socransky et al., 1998), and has been strongly implicated in the development of this disease (Ishihara and Okuda, 1999a). Recently, these microorganisms have been detected in the atherosclerotic plaque of patients with cardiovascular disease (Ishihara et al., 2004), and also from Buerger’s disease lesions (Iwai et al., 2005). Several reports regarding colonization of the gingival crevice by T. denticola have suggested that this process is dependent on a number of virulence factors, including a major outer sheath protein, proteases and immunosuppressive activity (Ishihara and Okuda, 1999b). The proteases of T. denticola have been shown to hydrolyze cytokines (Miyamoto et al., 2006, Okuda et al., 2007), activate complement and generate iC3b (McDowell et al., 2009, Yamazaki et al., 2006). These activities are suggested to be involved in the obliteration of host defense mechanisms. In addition, T. denticola proteases degrade several other host proteins, contributing to bacterial migration through the basement membrane (Grenier et al., 1990, Ishihara et al., 1996, Uitto et al., 1988). Cumulatively, it is apparent that the proteolytic activity of T. denticola plays an important role in colonization, dissemination and induction of inflammation in periodontal tissues.
IdeS (also known as Mac) is an IgG-specific protease produced by Streptococcus pyogenes (Lei et al., 2003, von Pawel-Rammingen et al., 2002). The survival of S. pyogenes within the host depends on its ability to avoid innate and adaptive immunoresponses. IgGs play an important role in the defense against invading microorganisms by opsonizing bacteria and facilitating their phagocytosis by neutrophils. IdeS/Mac cleaves the hinge region of IgG molecules, dissecting the antigen recognition (Fab) and effector (Fc) domains of immunoglobulins. Due to its early and sustained expression during the growth of S. pyogenes, and its highly specific proteolytic activity, IdeS is thought to provide a very efficient defense against Fc-mediated phagocytic killing (Lei et al., 2002). Oral spirochetes, including T. denticola, are also resistant to phagocytosis (Boehringer et al., 1986), however the mechanism of this resistance is unknown. In addition, T. denticola activates neutrophils with the help of cell surface-attached extracellular proteases (Ding et al., 1996,Yamazaki et al., 2006). As such, the finding that this bacterium may secrete a cysteine protease proves inordinately interesting, since proteases of this catalytic class are essential for the pathogenicity of other orofacial microorganisms (Chen et al., 1992, Lukomski et al., 1997). In this study we have identified a protein ortholog of IdeS in the genome of T. denticola ATCC 35405, and demonstrated that it is indeed a functional protease which significantly contributes to the pathogenesis of T. denticola.
Results
Identification of a T. denticola IdeS homolog via in silico analysis
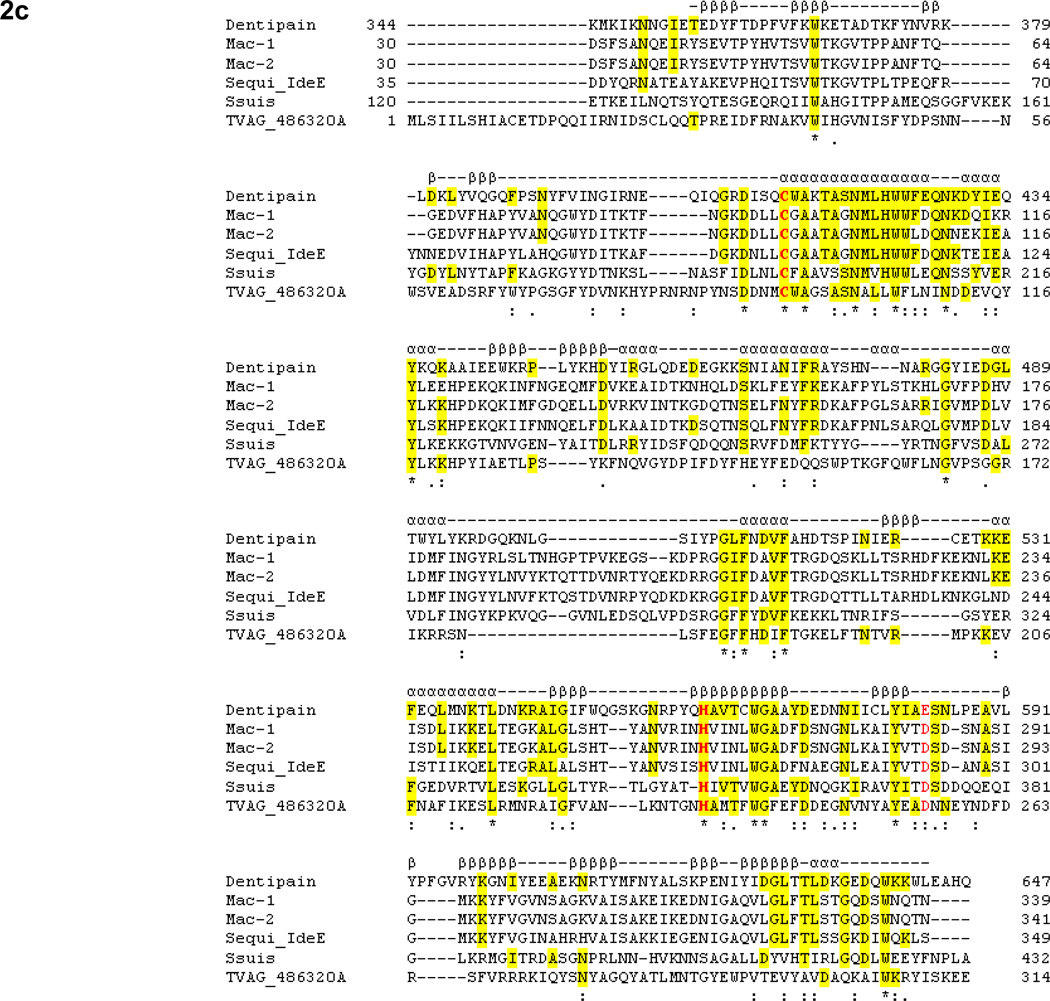
A homology search was performed using the amino acid sequence of the IdeS protease from S. pyogenes against the T. denticola ATCC 35405 genome sequence in the Oral Pathogen Sequence Databases (http://www.oralgen.lanl.gov/) at Los Alamos National Laboratories. A translated protein, encoded by a 2246-bp open reading frame annotated as TDE0362, showed significant homology with IdeS. We designated this protein and gene as IdeT and ideT, respectively. Figure 2A shows the full-length amino acid sequence of the IdeT protein. An analysis for the presence of functional and structural motifs indicated that IdeT is likely expressed as a multidomain protein. The composition of this protein appeared to consist of a cleavable signal peptide (Fig. 2a, lowercase letters); a tandem of bacterial, immunoglobulin-like domains (pfam02368) (Fig. 2a, bold) with similarity to the immunoglobulin-like protein of C. thermocellum (Fig. 2b); a unique segment with no significant similarity to any sequence in the database; and a C-terminally located IdeS-like domain (Fig. 2a, italic). We termed this putative proteolytic domain dentipain. Dentipain shares 25–27% identity with IdeS proteases from a number of different serotypes of S. pyogenes, and with IdeE from Streptococcus equi. Furthermore, it shows similar levels of identity to the IdeS-like domains present in the YSIRK Gram-positive signal peptide (EAP40144), otherwise known as methyl-accepting chemotaxis protein, of Streptococcus suis (accession no. ABP89511); and to a hypothetical protein (locus tag, TVAG_486320) from Trichomonas vaginalis strain G3 (Fig. 2c). Despite this low degree of homology, the residues that form the catalytic dyad, Cys and His, were strictly conserved, and the amino acids surrounding this region revealed a strong similarity to IdeS.
Fig. 2. Amino acid sequence and alignment analysis of IdeT.
A. The IdeT sequence is represented in lower case, bold and italic characters which indicate the cleavable signal peptide, the tandem repeats of bacterial immunoglobulin-like domains, and the C-terminal region with primary structure similarity to IdeS, respectively. The underlined portion of the protein was deleted in the IdeT-deficient mutant, while the highlighted part was expressed as a recombinant protein.
B. Comparison of residues 39–210 of the IdeT N-terminal region and the homologous domain of the immunoglobulin-like protein of C. thermocellum ATCC 27405 (accession no. ABN51297).
C. Alignment of the dentipain domain of IdeT (residues 334–647) with family I IdeS (Mac-1) from strain MGAS5005 (accession no. AAZ51286), family II IdeS (Mac-2) from strain MGAS10270 (accession no. YP_598336), IdeE of S. equi subsp. equi (accession no. ABF57910), the protease domain of the methyl-accepting chemotaxis protein from S. scui 05ZYH33 (accession no. ABP89511), and a hypothetical protein from T. vaginalis strain G3 (locus tag YVAG_486320). Residues identical in dentipain and the other protein(s) are highlighted. The catalytic Cys and His residues are in bold. Identical and similar residues conserved in all of the aligned proteins are indicated with asterix and dots beneath the sequence, respectively. Secondary structural elements of IdeS (Mac-1) (Agniswamy et al., 2006), such as β-strands (βββ) and α-helixes (ααα), are shown above the sequence.
Defining the presence and expression of ideT in multiple strains of T. denticola
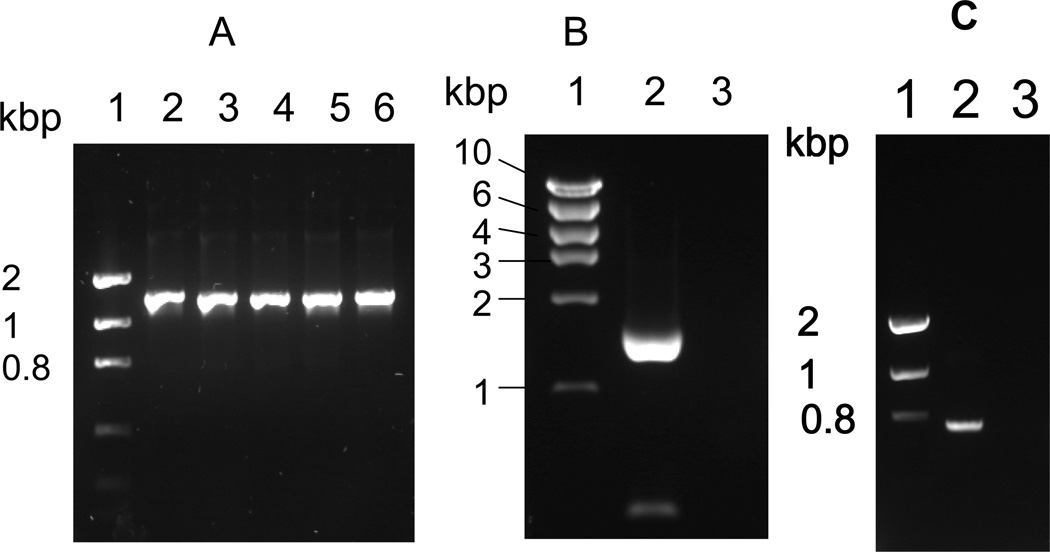
To verify the presence of ideT in strains of T. denticola other than ATCC 35405, genomic DNA was purified from strains ATCC 33520, 33521, 35404, 35405, and T. denticola strain GM1, and used as a template to amplify the gene using primer pair Ide1/Ide4 (Table 1). In all cases an amplicon of the expected 1372 bp size was obtained, revealing that ideT is apparently conserved amongst strains of T. denticola (Fig. 3A). Further to this, we subsequently confirmed the expression of ideT in growing cultures of T. denticola via RT-PCR analysis. To achieve this we conducted ideT-specific RT-PCR using mRNA from a late-exponential phase culture of T. denticola ATCC 35405, and primer pair IDEAF and IDEAR, which anneal at 171–197 bp and 1766–1792 bp of the ideT open reading frame. An approximately 1.6 kbp band was amplified (Fig. 3B, lane 2), which, along with the sequence of the open reading frame, indicates that the Ig-like domain and protease-domain are both expressed as a single protein. An amplified fragment of 699 bp (Fig. 3c), using primer pair ID-1/CATU was generated from late-exponential phase mRNA of T. denticola ATCC 35405, indicating that the ideT transcript, encompassing the dentipain domain, is expressed during growth of this organism.
Table 1.
Primer sequences used in this study.
| Name of primer | Sequence |
|---|---|
| OL31 | 5’-ATGGGATCCGAAGAAAAACAAGCTGTTATG-3’ |
| OL33 | 5’-GATGAATTCTTACTGATGTGCCTCAAGCCA-3’ |
| ID-1 | 5’-GGGGGGAATTCAATACGGAATTAGGTACGATGAAGG-3’ |
| ID-2 | 5’-GGGGGGGTACCGTCCGTAAAATAATCCTCTGTCTCA-3’ |
| ID-3 | 5’-GGGGGGGATCCTCCAATATGCTGCACTGGTGGTT-3’ |
| ID-4 | 5’-GGGGGGCATGCTTTCCACTGATCTTCGCCCTTGTC-3’ |
| IDEAF | 5’-CACGGCACAGACCATAACCGTGAAAAT-3’ |
| IDEAR | 5’-ATCTAACGCCGAATGGATAGAGGACAG-3’ |
| CATU | 5’-AGCGGTTTTTGCCCAGCATTGGCTAATATCT-3’ |
Fig. 3. Analysis of ideT transcription.
A. PCR amplification of the ideT locus using primers ID-1 and ID-4, which amplify the nucleotides encoding amino acid residues 172 to 640. Lane 1, Molecular size marker; lane 2, T. denticola ATCC 33520; lane 3, T. denticola 33521; lane 4, T. denticola 35404; lane 5, T. denticola 35405; lane 6, T. denticola GM1.
B. Amplification of ideT mRNA by RT-PCR using primers IDEAF and IDEAR, which amplify the sequence encoding amino acid residues 57 to 597. Lane 1, Molecular size markers; lane 2, T. denticola ATCC 35405; lane 3, T. denticola ATCC 35405 without reverse transcriptase.
C. Amplification of ideT mRNA by RT-PCR using primer ID-1 and CATU, which amplify the sequence coding for amino acid residues 172 to 417. Lane 1, Molecular size markers; lane 2, T. denticola ATCC 35405; lane 3, T. denticola ATCC 35405 without reverse transcriptase.
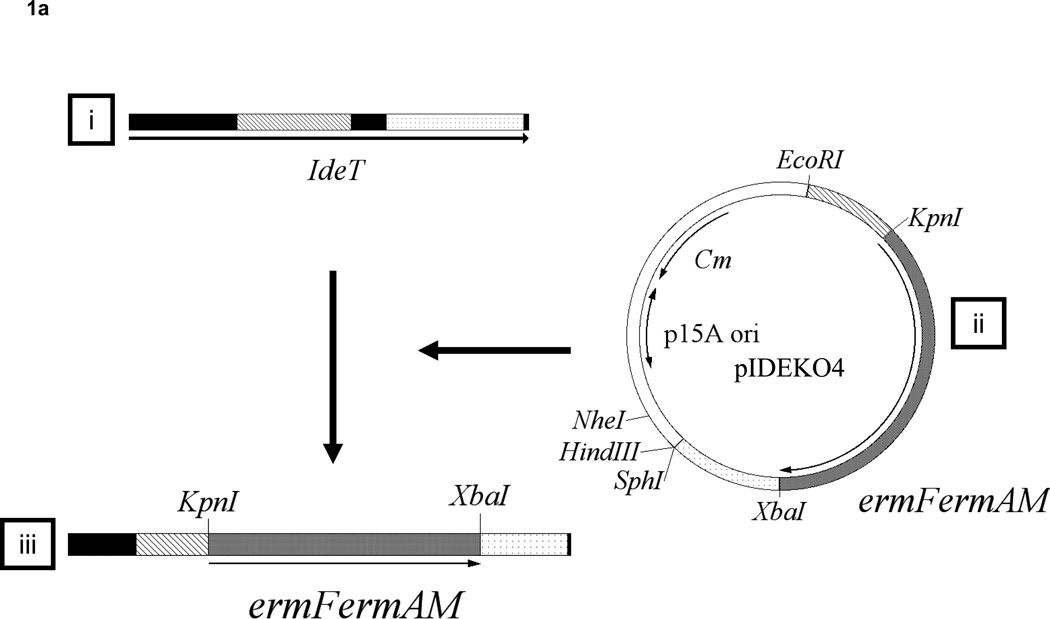
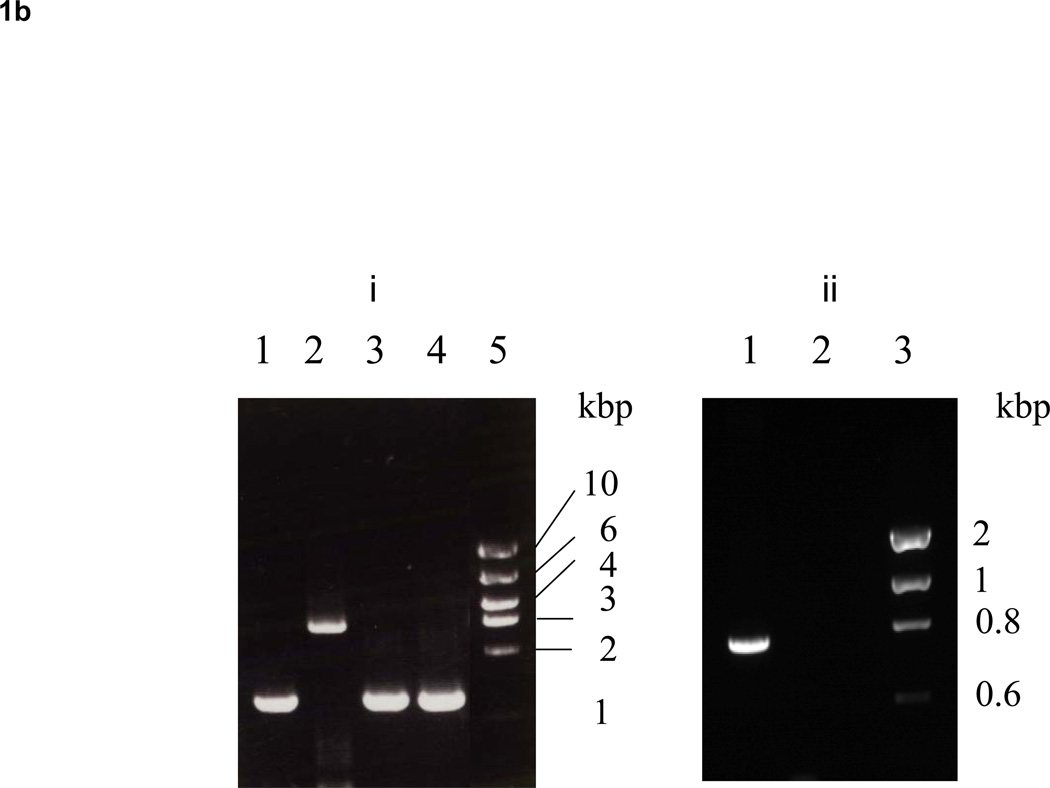
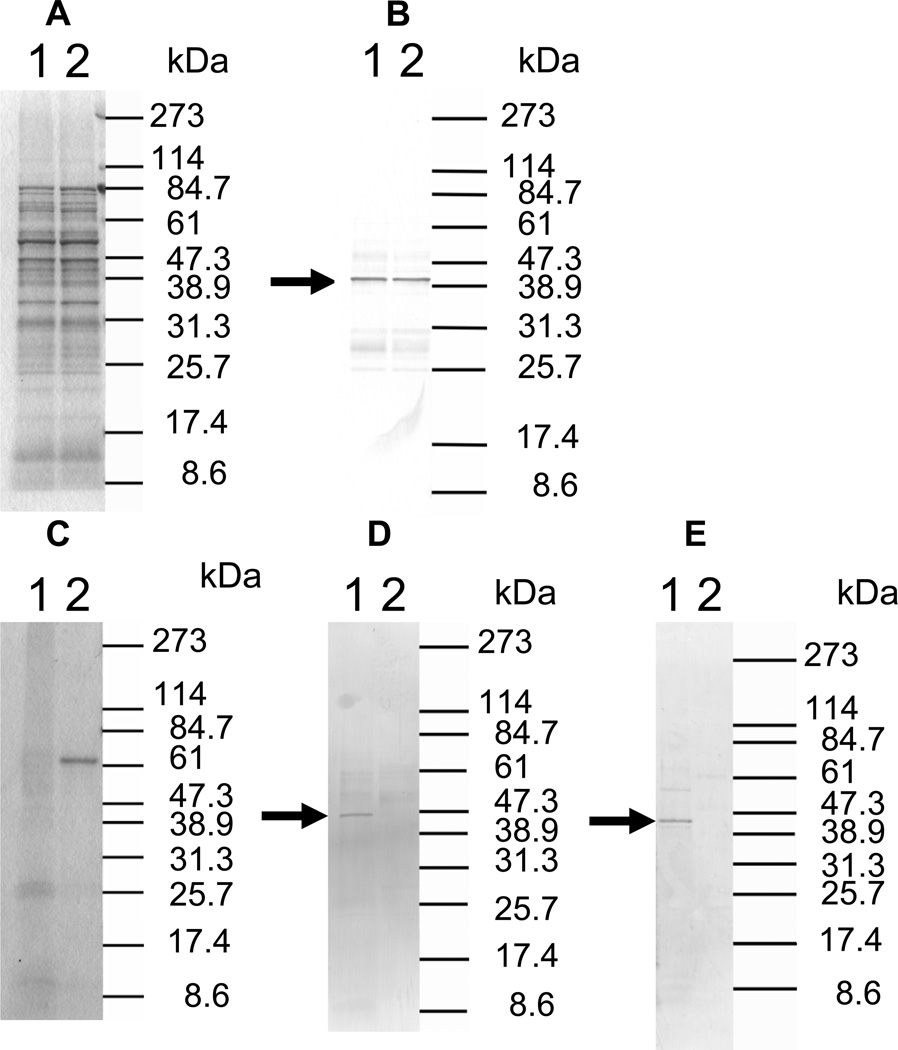
To analyze the effect of the dentipain domain, a deficient mutant for this region was constructed using primers listed in table 1, as shown Fig. 1A. Twelve clones (IDEKO1–12) obtained from these transformations were screened via PCR analysis to confirm that inactivation of the ideT gene had occurred. Specifically, the ideT sequences flanking the IdeS-homologous region were PCR amplified using primer pairs ID-1/ID-4 (Table 1). One such clone (IDEKO4) yielded an amplicon that corresponded to the predicted size of ideT with the inserted erythromycin cassette (Fig. 1B, lane 2). To confirm the lack of putative protease domain this clone was further analyzed by PCR, using primers located within (CATU) and outside (ID-1) of the region encoding the projected catalytic activity. Thus a band of approximately 700-bp was amplified from wild type genomic DNA, whilst no such band was observed in the dentipain-deficient mutant, IDEKO4 (Fig. 1B). Growth of the mutant IDEKO4 was at levels approximately the same level as the wild type strain. In order to relate the discerned transcriptional expression to protein synthesis, we performed Western Blot analysis using antibodies raised against the protease domain preceded by the profragment, and against a peptide epitope in the middle of the protease domain (residues 219–647 and 498–511 of the IdeT protein, respectively, see Fig. 2A). To this end sonicated extracts of washed cells from late post-exponential phase cultures of T. denticola were subjected to SDS-PAGE and Western blot analysis. This revealed a band of 43 kDa that was immunoreactive only with anti-protein antibodies (Fig. 4B); while no reaction was observed with anti-peptide antibodies (data not shown). Interestingly, this band was observed in cell extracts of both the wild-type strain and the dentipain deficient mutant. Apparently this represents the cell-associated N-terminal portion of IdeT, bearing the Ig-domains and a polypeptide (residues 23–360) preceding the protease domain (Fig. 2A). Clearly, the insertion of the antibiotic cassette disrupting the protease domain did not affect expression of the N-terminal fragment of IdeT.
Fig. 1. Construction of an IdeT protease-deficient mutant.
A. (i) The wild-type ideT locus detailing the four protein domains; (ii) pIDEKO4, constructed using pMCL191, two ideT PCR generated fragments flanking the proteolytic domain (hatched bar and dotted bar) and an erythromycin resistance cassette derived from pVA2198. The restriction sites introduced during PCR are also shown; (iii) the ideT mutant locus detailing the dentipain region replaced with an erythromycin resistance cassette by homologous recombination.
B. (i) PCR amplification of the ideT locus using primers ID-1 and ID-4, which amplify from amino acid residues 172 to 640. Lane 1, T. denticola ATCC 35405; lane 2, T. denticola IDEKO4; lane 3, T. denticola Ide5; lane 4, T. denticola Ide6; lane 5, Molecular size marker. (ii) PCR amplification of the ideT locus using primers ID-1 and CATU, which amplify from amino acid residues 172 to 417. Lane 1, T. denticola ATCC 35405; lane 2, T. denticola IDEKO4; lane 3, Molecular size marker.
Fig. 4. Analysis of IdeT translation.
A. SDS-PAGE of T. denticola sonicates. Lane 1, T. denticola ATCC 35405 sonicate; lane 2, T. denticola IDEKO4 sonicate.
B. Immunoblot analysis of IdeT from T. denticola cells. Lane 1, T. denticola ATCC 35405 sonicate; lane 2, T. denticola IDEKO4 sonicate. Antibody: rabbit serum anti-IdeT recombinant protein (219–647). The arrow indicates the band which reacted with this antibody.
C. SDS-PAGE of concentrated culture supernatant of T. denticola. Culture supernatant of T. denticola grown in E-TYGV medium was collected and concentrated. Lane 1, culture supernatant of T. denticola ATCC 35405; lane 2, culture supernatant of T. denticola IDEKO4.
D. Immunoblot analysis of IdeT in culture supernatants. Lane 1, culture supernatant of T. denticola ATCC 35405; lane 2, culture supernatant of T. denticola IDEKO4. Antibody: rabbit serum anti-IdeT recombinant protein (219–647). The arrow indicates the band which reacted with this antibody.
E. Immunoblot analysis of IdeT in culture supernatants. Lane 1, culture supernatant of T. denticola ATCC 35405; lane 2, culture supernatant of T. denticola IDEKO4. Antibody: rabbit serum anti-IdeT synthetic peptide (IdeT498–511). The arrow indicates the band which reacted with this antibody.
To determine if the protease domain, which is located at the C-terminus, is released into growth media, culture supernatants of the wild type and the dentipain deficient mutant grown in E-TYGV medium were analyzed by Western Blot. In this case both anti-protein and anti-peptide antibodies detected an immunoreactive protein of 45 kDa exclusively in the culture medium of the parental strain (Fig 4D&E).
Taken together these results confirm that the IdeT protein is produced by T. denticola. Moreover, our data indicates that after proteolytic cleavage of secreted IdeT, the N-terminal part of the protein is retained on the cell-surface, while the C-terminal IdeS-like domain is released into the media in soluble form.
The IdeS-like domain of dentipain possesses proteolytic activity
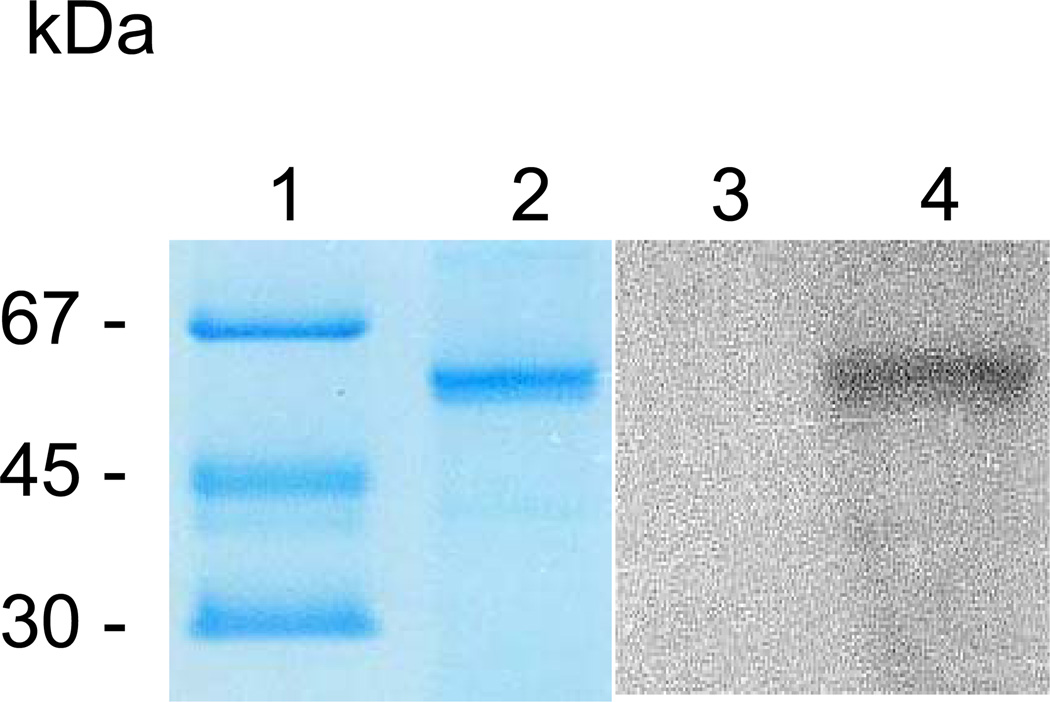
Conservation of the catalytic dyad residues in dentipain suggests that IdeT should possess proteolytic activity. Indeed, upon incubation of the recombinant dentipain with biotinylated E-64 (DCG-04, an irreversible probe specific for the active site of cysteine proteases) (Greenbaum et al., 2000), covalently labeled dentipain was generated (Fig. 5, lane 4). This biotinylation was abrogated by an excess of unlabeled E-64, confirming specific and covalent modification of the catalytic cysteine residue of dentipain by DCG-04 (Fig. 5, lane 3).
Fig. 5. SDS-PAGE analysis and active site probing of recombinant dentipain.
Lane 1, molecular mass standards; lane 2, recombinant dentipain (5 µg loaded); lane 3, dentipain (1 µg) preincubated first with 100 µM E-64 and then treated with 1 µM DCG-04; lane 4, dentipain directly treated with 1 µM DCG-04. Samples were resolved by SDS-PAGE and either stained with Coomassie blue R-250 (lanes 1 and 2), or electrotransferred onto PVDF membrane. Membranes were blocked using 2% BSA and probed with horseradish peroxidase conjugated streptavidine, before being developed with ECL reagents (lanes 3 and 4).
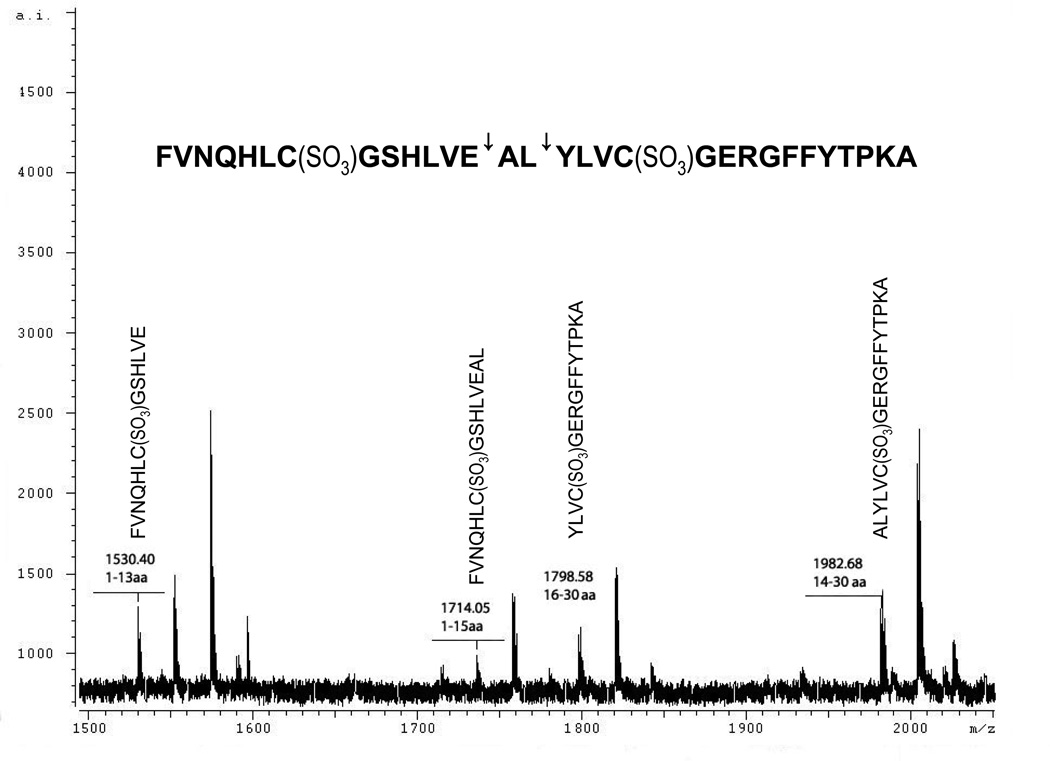
To confirm proteolytic activity and obtain information regarding substrate specificity, recombinant dentipain was incubated with oxidized bovine insulin β-chain, and the reaction products analyzed by MALDI-TOF MS. As shown in Fig. 5, insulin β-chain was hydrolyzed into several discrete peptides with molecular masses of 1530.4, 1714.05, 1798.58 and 1982.68 Da, which did not undergo any further degradation. These four peptides were identified as insulin β-chain fragments encompassing residues 1–13, 1–15, 14–30, and 16–30, respectively (Table 2). This indicates that dentipain cleaves insulin β-chain at two discrete sites; Leu-Val-Glu↓Ala14 and Glu-Ala-Leu↓Tyr16, where “↓” indicates the hydrolyzed peptide bond (Fig. 6, inset). However, despite degradation of insulin β-chain, dentipain did not show proteolytic activity towards IgG1, IgG2, or IgA, as well as general protease substrates such as azocoll and azocasein. Even after overnight incubation at high enzyme concentrations there was no significant cleavage of the immunoglobulins or digestion of other substrates (data not shown). This suggests that dentipain has oligopeptidase activity, and therefore does not cleave proteins.
Table 2.
Peptide mass fingerprinting for the hydrolysis of insulin by dentipain.
| Mass | DB mass | Δmass (Da) | Peptide | Position |
|---|---|---|---|---|
| 1530.400 | 1530.701 | 0.300 | FVNQHLCGSHLVE | 1–13 |
| 1714.050 | 1714.371 | 0.321 | FVNQHLCGSHLVEAL | 1–15 |
| 1798.580 | 1798.847 | 0.266 | YLVCGERGFFYTPKA | 16–30 |
| 1982.680 | 1982.968 | 0.288 | ALYLVCGERGFFYTPKA | 14–30 |
Fig. 6. MALDI-TOF analysis of insulin β-chain degradation by dentipain.
One µl samples were added to 1 µl of matrix solution (saturated α-cyano-4-hydroxycinnamic acid in acetonitril/water mixture 7:3 v/v) and transferred onto a SCOUT MALDI plate. Analysis was performed using a MALDI-MS Reflex IV spectrometer equipped with a λ = 331 nm laser and an ion Time of Flight (TOF) analyzer.
Dentipain is required for full virulence of T. denticola
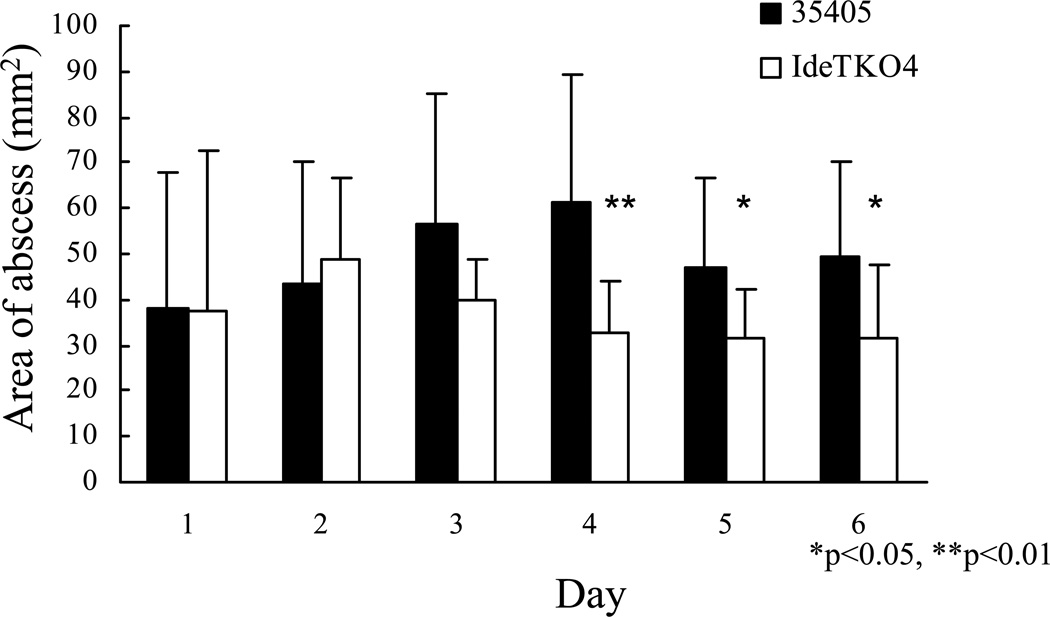
Bacterial proteases contribute significantly to virulence of pathogenic microorganisms (Potempa and Pike, 2009). Therefore, to determine the contribution of dentipain to the pathogenic nature of T. denticola, we analyzed the abscess-forming capacity of the isogenic ideT mutant strain, along with its parent. As can be clearly seen in Fig. 7, the size of abscesses formed by the mutant and wild-type strain were of similar sizes up to day 3, post-inoculation. However after this time there is an apparent difference between the sizes of abscesses formed by these two strains, with those caused by the ideT mutant being considerably smaller than those of the parent. Further analysis revealed this difference in lesion size during prolonged infection to be statistically significant, and demonstrates that the proteolytic activity of IdeT is of the utmost importance to the ability of T. denticola to cause infection.
Fig. 7.
Analysis of the role of IdeT proteolytic activity in the virulence of T. denticola. Bars represent the mean area of lesions at the infection sites following challenge with either T. denticola ATCC 35405 or T. denticola IDEKO4. Mice were injected with live T. denticola ATCC 35405 (black bars) or IDEKO4 (open bars), and lesion areas determined at the indicated times following infection. Bars indicate standard deviation. *P<0.05, **p<0.01 by Mann-Whitney U test
Discussion
What we have demonstrated here is the identification of a novel protease from T. denticola, which possess a discrete specificity, and is of considerable importance to the infectious capacity of this organism. In the IdeT protein, two well-defined domains, homologous to those found in proteins from Gram-positive bacteria, have been identified. The N-terminal tandem repeat of Ig-like domains shares homology with the C. thermocellum immunoglobulin-like protein. On the other hand, the C-terminal domain of IdeT shows significant sequence similarity to the IgG specific endopeptidases of S. pyogenes and S. equi (Lannergard and Guss, 2006, Vincents et al., 2004), and to a discrete domain present in a large secretory protein of S. suis described as methyl-accepting chemotaxis protein (Fig. 2c). In this way ideT may appear to belong to a group of several genes in T. denticola, including aspartate carbamoyl transferase and dentilisin, which were apparently acquired from Gram-positive bacteria (Ishihara et al., 1992, Ishihara et al., 1996). This contention is strengthened by the observation that the outer membrane lipids of T. denticola are far more similar to lipoteichoic acid than to lipopolysaccharide (Schultz et al., 1998). Together these results indicate that T. denticola is a unique and fascinating pathogen that shares a number of traits and components with Gram-positive microorganisms.
Our analysis has shown that ideT is not only expressed during growth by the T. denticola type strain, but that it is conserved across a number of other T. denticola strains. The calculated molecular mass of the secreted IdeT protein is 71.5 kDa, but no protein of this mass was found to be reactive with anti-IdeT antibodies in T. denticola cell extracts or culture media. Instead, 43 kDa and 45 kDa polypeptides were detected in cell sonicates and growth media, respectively, indicating proteolytic posttranslational processing of the nascent IdeT protein. Interestingly, immunoreactivity of the 43 kDa band to anti-protein, but not anti-peptide, antibodies, specific to an epitope within the protease domain, indicates that this fragment is derived from the N-terminal portion of the IdeT protein. Conversely, the IdeT portion released into culture media apparently contains the C-terminally located protease domain. Therefore it can be concluded that, as with other proteases secreted by periodontal pathogens (Karim et al., 2010, Mallorquí-Fernández et al., 2008, Mikolajczyk et al., 2003), dentipain is also subjected to proteolytic processing. In the case of the IdeT protein, proteolysis of the secreted protein leads to the N-terminal fragment containing the Ig-like domains being retained on the bacterial cell surface, and the C-terminal protease domain being released into the environment. The function of the N-terminal, bacteria associated Ig-like domains remains to be elucidated.
Active site labeling using biotinylated E-64, and the insulin degradation assay indicate that recombinant dentipain is a functional cysteine protease, adding to the list of other known proteolytic activities secreted by T. denticola (Mikx, 1991). Analysis of insulin cleavage sites indicates that, as with other members of the papain superfamily, dentipain specificity is dictated by recognition of the P2 residue (the penultimate residue upstream of the hydrolyzed peptide bond). However, in contrast to papain-like proteases, which favor large hydrophobic amino acids (Choe et al., 2006), dentipain prefers small aliphatic residues (valine and alanine) at the P2 position. Despite the relative abundance of these residues in proteins, dentipain does not show broad proteolytic activity, and does not appear to cleave proteins. This finding argues that as with the BANA-peptidase, now referred to as oligopeptidase B of T. denticola (Lee and Fenno, 2004), dentipain too possesses oligopeptidase activity. Conversely, it is possible that precise hydrolysis of the propeptide (the linker sequence) is required for dentipain to express full proteolytic activity.
It is clear that dentipain plays a significant role in the virulence of T. denticola, as demonstrated by the attenuation of the dentipain-deficient mutant in our murine abscess model. Importantly, this is despite the presence of the N-terminal portion of the IdeT protein. A significant difference was observed in areas of the abscesses in mice at 3 days after inoculation with IDEKO4 when compared to the wild-type strain. This is in keeping with results of from our previous study, showing that lesion sizes in mice injected with a mutant deficient in the surface protease dentilisin was also lower at 3 days after injection. Although lesions induced by T. denticola have been reported to be smaller than those induced by other periodontopathic bacteria, such as P. gingivalis and Aggregatibacter actinomycetemcomitans, T. denticola-induced lesions take considerably longer to resolve (Ebersole et al., 1995). Therefore, it is clear that dentipain plays a role in bacterial survival, and exerts a toxic effect on host tissue upon infection, which is consistent with the observation that all of the known bacterial IdeS orthologs are considered to be important virulence factors (Agniswamy et al., 2004, Chen et al., 2007, Lannergard and Guss, 2006). Taken together, these results suggest that the newly identified enzyme, dentipain, is a component of T. denticola proteolytic activity, and that this activity contributes to the pathogenicity of this organism. Further investigation is required to clarify how dentipain specifically functions in the physiology and pathogenesis of T. denticola; and in a broader context, what the role of the Ig-like domains of IdeT are, since these exert a variety of functions in bacterial proteins (Kataeva et al., 2005).
Materials and Methods
Bacterial strains
T. denticola ATCC 33520, 33521, 35404, 35405 and T. denticola strain GM1 were incubated in TYGVS medium under anaerobic conditions as described previously (Ishihara and Kuramitsu, 1995). To investigate the secretion of the IdeS ortholog into culture supernatants by immunoblotting, modified TYGVS medium (E-TYGV) was used, in which 1% EX-CYTE (Millipore) replaced 10% serum. For analysis, culture supernatants were collected after T. denticola had been allowed to grow in E-TYGV for three days.
Escherichia coli DH5α and BL21 (DE3) were cultured using LB and LB agar. Where appropriate antibiotics were added to LB media at the following concentrations: ampicillin 100 µg/ml and chloramphenicol 30 µg/mL.
In silico identification of the T. denticola IdeS homologue
Screening for the IdeS homologue was performed using the T. denticola ATCC 35405 genome sequence in the Oral Pathogen Sequence Database (http://www.oralgen.lanl.gov/) at Los Alamos National Laboratories. The specific analysis of derived sequences was performed using Genetics-Mac software ver. 14 (Genetyx, Tokyo Japan).
Cloning, overexpression and purification of recombinant IdeT (219–647)
The ideT coding sequence, specifying the C-terminal IdeS homologous domain (indicated in Fig. 2a, IdeT residues 219–647), was PCR amplified using primer pair OL31/OL33 (Table 1). The generated 942bp fragment was digested with BamHI and EcoRI and ligated into similarly digested pGEX5T (Pharmacia, Uppsala, Sweden). E. coli DH5α was used as a recipient for this ligation, with clones selected for using LB agar containing ampicillin. Plasmids were analyzed via restriction digest and DNA sequencing to confirm correct construction. The resultant plasmid (pLES167) was then purified and transformed into the E. coli expression host, BL21 (DE3). Five ml overnight cultures of E. coli BL21 (DE3) containing pLES167 were used to inoculate test cultures to an OD600 of 0.05, which were grown with shaking at 37°C until the optical density reached 0.5–0.8. Isopropyl-β-D-thio-galactopyranoside (IPTG) was then added to a final concentration of 1 mM. After 3 h of cultivation at room temperature, the cells were harvested, disrupted by sonication, and homogenates cleared by ultracentrifugation. The supernatant from this step was then loaded onto a Glutathion sepharose 4B purification affinity column. The matrix was washed thoroughly to remove non-binding proteins, and then incubated with thrombin to cleave GST from the GST-dentipain fusion protein immobilized on the matrix. The eluted dentipain was passed through Benzamidine-sepharose to remove residual thrombin, and was then concentrated via ultrafiltration. All recombinant protein purification and cleavage steps were performed according to the manufacturer’s (Amersham-Pharmacia) recommendations (GST Gene Fusion System Handbook).
Evaluation of the proteolytic activity of recombinant dentipain (IdeT 219–647)
To evaluate the proteolytic activity of the recombinant dentipain, 0.1 µg/µL of enzyme was preincubated at 37°C for 30 min in 20 mM Tris-HCl, pH 7.8, supplemented with 1 mM 2-mercapto ethanol and 5 mM EDTA. The activated enzyme (10 ng/mL final concentration) was then incubated with oxidized insulin β-chain (1 µg/mL) from bovine pancreas (Sigma) as a substrate. After 60 min of incubation at 37°C the reaction mixture was mixed with matrix solution and analyzed using a MALDI-MS Reflex IV spectrometer, equipped with a λ = 331 nm laser and an ion Time of Flight (TOF) analyzer (Bruker), at a 30 kV acceleration voltage in reflector mode. The molecular mass of the peptides observed in the spectra were aligned with the FINDPEPT tool available on the Expasy server (http://au.expasy.org/tools/findpept.html).
Insertional inactivation of the IdeT protease domain
To evaluate the contribution of ideT to the virulence of T. denticola, we constructed a knockout mutant by homologous recombination, as reported previously (Ishihara et al., 1998). Briefly, two 500bp DNA fragments flanking the IdeT protease domain (residues 361–417, see Figure 1 and 2) were PCR amplified using primer pairs ID-1/ID-2 and ID-3/ID-4 (Table 1). The amplified fragments were separately cloned into plasmid pMCL191 (Ishihara et al., 1998) to generate construct pISHI101. An erythromycin resistance cassette was then inserted at the confluence of these two fragments, in place of the proteolytic domain, generating pISHI102. pISHI102 was then linearized with EcoRI, and used to transform T. denticola ATCC 35405 by electroporation. The resulting transformants were isolated using TYGVS agar containing 40 µg/mL erythromycin.
Evaluation of dentipain expression levels via RT-PCR
Total RNA was isolated from T. denticola grown to late exponential phase using the Trizol (Gibco BRL, Grand Island, NY, USA) extraction method. The RNA was treated with RNase-free DNase (Qiagen, Valencia, CA, USA), followed by purification using an RNeasy kit (Qiagen). RT-PCR was performed using a one-step RNA PCR kit (TaKaRa Biomedicals, Otsu, Japan) and primer pair ID-1/CATU. Reverse transcription was carried out at 50°C for 30 min, followed by inactivation at 95 °C for 2 min. PCR reaction conditions were 30 cycles of denaturation at 94°C for 30s, annealing at 65°C for 30s and elongation at 72°C for 1.5 min.
Preparation of antibodies against dentipain
Recombinant protein encompassing residues from 219 to 647 of the IdeT protein in phosphate-buffered saline (PBS, pH 7.2) was mixed with Freund complete adjuvant (1 : 1 volume ratio) and emulsified. Rabbits were immunized with 0.2 mg recombinant protein on a weekly basis (6 injections), with blood collected 6 weeks after the initial immunization.
To prepare antibodies recognizing the C-terminal portion of the dentipain enzyme, a peptide QKNLGSIYPGLFC corresponding to residues 498–511 of IdeT located in the catalytic domain of the protein with an added C-terminal Cys residue was synthesized using F-moc chemistry. The peptide was conjugated to keyhole limpet hemocyanin using maleimidobenzoic acid N-hydroxysuccinimide ester. The conjugate (0.15 mg) was used to immunize rabbits (6 biweekly injections), with blood collected 12 weeks after the initial immunization. The immunization and bleeding protocols were approved by the Animal Use Committee of Tokyo Dental College.
Immunoblotting
Washed T. denticola cells were disrupted by ultrasonication at 100 W for 5 min (SONIFIER 250, Branson, Dunbery, CT), and unbroken cellular debris removed by centrifugation (10,000 × g for 15 min). T. denticola culture supernatants were collected and concentrated using Centriprep YM3 filter units (Milipore Corporation, Bedford, MA). Sonicates of culture supernatants were subjected to SDS-PAGE analysis, with the resolved proteins electrotransferred to PVDF membrane (Immobilon, Milipore Corporation) as described previously (Ishihara et al., 1998). Non-specific binding sites were blocked with 3% bovine serum albumin in PBS for 1 h. The membrane was washed with PBS containing 0.05% Tween 20 (PBST), and probed with anti-dentipain rabbit serum diluted 1000-fold. After washing with PBST, the membrane was incubated in a solution of peroxidase-conjugated goat anti-rabbit IgG, washed again, and developed with TMB Membrane Peroxidase Substrate (KPL, Gaithersburg, Maryland, MD).
Evaluation of the significance of dentipain in T. denticola pathogenicity
The abscess-forming ability of the T. denticola IdeT mutant and its parental strain was tested using a murine model of infection, and evaluated as described previously (Kesavalu et al., 1997). Briefly, 3-day cultures of T. denticola ATCC35404 or IDEKO4 were harvested and resuspended in PBS at a concentration of 1 × 109 cells per mL. Cell loads of 2 × 108 were subcutaneously inoculated into the dorsolateral surface of five Balb/c mice for each strain, and the size of abscesses measured daily for six days using a caliper gauge. The experiment was then repeated to confirm reproducibility, and statistical analysis was performed using the pooled data from both experiments.
Acknowledgement
This study was partially supported by a Grant HRC7 from the Oral Health Science Center of Tokyo Dental College, and a “High-Tech Research Center” Project for Private Universities: matching fund subsidy from MEXT, 2006–2010, JP acknowledges support from MNiSzW (Warsaw, Poland, Grant 1642/B/P01/2008/35), and the National Institutes of Health (Grant DE 09761). Finally, we are indebted to Matt Bogyo and Doron Greenbaum for kindly providing DCG-04, the irreversible probe specific for cysteine proteases used in this study. The Faculty of Biochemistry, Biophysics and Biotechnology of the Jagiellonian University is a beneficent of the structural funds from the European Union (grant No: POIG.02.01.00-12-064/08 – “Molecular biotechnology for health”).
References
- Agniswamy J, Lei B, Musser JM, Sun PD. Insight of host immune evasion mediated by two variants of group a Streptococcus Mac protein. J. Biol. Chem. 2004;279:52789–25796. doi: 10.1074/jbc.M410698200. [DOI] [PubMed] [Google Scholar]
- Agniswamy J, Nagiec MJ, Liu M, Schuck P, Musser JM, Sun PD. Crystal structure of group A streptococcus Mac-1: insight into dimer-mediated specificity for recognition of human IgG. Structure. 2006;14:225–235. doi: 10.1016/j.str.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Boehringer H, Berthold PH, Taichman NS. Studies on the interaction of human neutrophils with plaque spirochetes. J. Periodont. Res. 1986;21:195–209. doi: 10.1111/j.1600-0765.1986.tb01452.x. [DOI] [PubMed] [Google Scholar]
- Chen C, Tang J, Dong W, Wang C, Feng Y, Wang J, Zheng F, Pan X, Liu D, Li M, et al. A glimpse of streptococcal toxic shock syndrome from comparative genomics of S. suis 2 Chinese isolates. PLoS ONE. 2007;2:e315. doi: 10.1371/journal.pone.0000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Potempa J, Polanowski A, Wikstrom M, Travis J. Purification and characterization of a 50-kDa cysteine proteinase (gingipain) from Porphyromonas gingivalis. J. Biol. Chem. 1992;267:18896–18901. [PubMed] [Google Scholar]
- Choe Y, Leonetti F, Greenbaum DC, Lecaille F, Bogyo M, Bromme D, Ellman JA, Craik CS. Substrate profiling of cysteine proteases using a combinatorial peptide library identifies functionally unique specificities. J. Biol. Chem. 2006;281:12824–11232. doi: 10.1074/jbc.M513331200. [DOI] [PubMed] [Google Scholar]
- Ding Y, Uitto VJ, Haapasalo M, Lounatmaa K, Konttinen YT, Salo T, Grenier D, Sorsa T. Membrane components of Treponema denticola trigger proteinase release from human polymorphonuclear leukocytes. J. Dent. Res. 1996;75:1986–1993. doi: 10.1177/00220345960750121101. [DOI] [PubMed] [Google Scholar]
- Ebersole JL, Kesavalu L, Schneider SL, Machen RL, Holt SC. Comparative virulence of periodontopathogens in a mouse abscess model. Oral Dis. 1995;1:115–128. doi: 10.1111/j.1601-0825.1995.tb00174.x. [DOI] [PubMed] [Google Scholar]
- Greenbaum D, Medzihradszky KF, Burlingame A, Bogyo M. Epoxide electrophiles as activity-dependent cysteine protease profiling and discovery tools. Chem. Biol. 2000;7:569–581. doi: 10.1016/s1074-5521(00)00014-4. [DOI] [PubMed] [Google Scholar]
- Grenier D, Uitto VJ, McBride BC. Cellular location of a Treponema denticola chymotrypsinlike protease and importance of the protease in migration through the basement membrane. Infect. Immun. 1990;58:347–351. doi: 10.1128/iai.58.2.347-351.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K, Ishihara M, Takazoe I, Okuda K. Cloning and expression of the aspartate carbamoyltransferase gene from Treponema denticola. Appl. Environ. Microbiol. 1992;58:3399–3403. doi: 10.1128/aem.58.10.3399-3403.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K, Kuramitsu HK. Cloning and expression of a neutral phosphatase gene from Treponema denticola. Infect. Immun. 1995;63:1147–1152. doi: 10.1128/iai.63.4.1147-1152.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K, Kuramitsu HK, Miura T, Okuda K. Dentilisin activity affects the organization of the outer sheath of Treponema denticola. J. Bacteriol. 1998;180:3837–3844. doi: 10.1128/jb.180.15.3837-3844.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K, Miura T, Kuramitsu HK, Okuda K. Characterization of the Treponema denticola prtP gene encoding a prolyl-phenylalanine-specific protease (dentilisin) Infect. Immun. 1996;64:5178–5186. doi: 10.1128/iai.64.12.5178-5186.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K, Nabuchi A, Ito R, Miyachi K, Kuramitsu HK, Okuda K. Correlation between detection rates of periodontopathic bacterial DNA in carotid coronary stenotic artery plaque and in dental plaque samples. J. Clin. Microbiol. 2004;42:1313–1315. doi: 10.1128/JCM.42.3.1313-1315.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K, Okuda K. Molecular analysis for pathogenicity of oral treponemes. Microbiol. Immunol. 1999a;43:495–503. doi: 10.1111/j.1348-0421.1999.tb02434.x. [DOI] [PubMed] [Google Scholar]
- Ishihara K, Okuda K. Molecular pathogenesis of the cell surface proteins and lipids from Treponema denticola. FEMS Microbiol. Lett. 1999b;181:199–204. doi: 10.1111/j.1574-6968.1999.tb08844.x. [DOI] [PubMed] [Google Scholar]
- Iwai T, Inoue Y, Umeda M, Huang Y, Kurihara N, Koike M, Ishikawa I. Oral bacteria in the occluded arteries of patients with Buerger disease. J. Vasc. Surg. 2005;42:107–115. doi: 10.1016/j.jvs.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Karim AY, Kulczycka M, Kantyka T, Dubin G, Jabaiah A, Daugherty PS, Thogersen IB, Enghild JJ, Nguyen KA, Potempa J. A novel matrix metalloprotease-like enzyme (karilysin) of the periodontal pathogen Tannerella forsythia ATCC 43037. Biol. Chem. 2010;391:105–117. doi: 10.1515/BC.2010.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataeva IA, Brewer JM, Uversky VN, Ljungdahl LG. Domain coupling in a multimodular cellobiohydrolase CbhA from Clostridium thermocellum. FEBS Lett. 2005;579:4367–4373. doi: 10.1016/j.febslet.2005.06.074. [DOI] [PubMed] [Google Scholar]
- Kesavalu L, Walker SG, Holt SC, Crawley RR, Ebersole JL. Virulence characteristics of oral treponemes in a murine model. Infect. Immun. 1997;65:5096–5102. doi: 10.1128/iai.65.12.5096-5102.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannergard J, Guss B. IdeE, an IgG-endopeptidase of Streptococcus equi ssp. equi. FEMS Microbiol Lett. 2006;262:230–235. doi: 10.1111/j.1574-6968.2006.00404.x. [DOI] [PubMed] [Google Scholar]
- Lee SY, Fenno JC. Expression of Treponema denticola oligopeptidase B in Escherichia coli. Curr Microbiol. 2004;48:379–382. doi: 10.1007/s00284-003-4168-4. [DOI] [PubMed] [Google Scholar]
- Lei B, DeLeo FR, Reid SD, Voyich JM, Magoun L, Liu M, Braughton KR, Ricklefs S, Hoe NP, Cole RL, et al. Opsonophagocytosis-inhibiting mac protein of group a streptococcus: identification and characteristics of two genetic complexes. Infect. Immun. 2002;70:6880–6890. doi: 10.1128/IAI.70.12.6880-6890.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei B, Liu M, Meyers EG, Manning HM, Nagiec MJ, Musser JM. Histidine and aspartic acid residues important for immunoglobulin G endopeptidase activity of the group A Streptococcus opsonophagocytosis-inhibiting Mac protein. Infect. Immun. 2003;71:2881–2884. doi: 10.1128/IAI.71.5.2881-2884.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukomski S, Sreevatsan S, Amberg C, Reichardt W, Woischnik M, Podbielski A, Musser JM. Inactivation of Streptococcus pyogenes extracellular cysteine protease significantly decreases mouse lethality of serotype M3 and M49 strains. J. Clin. Invest. 1997;99:2574–2580. doi: 10.1172/JCI119445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallorquí-Fernández N, Manandhar SP, Mallorquí-Fernández G, Usón I, Wawrzonek K, Kantyka T, Sola M, Thøgersen IB, Enghild JJ, Potempa J, et al. A new autocatalytic activation mechanism for cysteine proteases revealed by Prevotella intermedia interpain A. J. Biol. Chem. 2008;283:2871–2882. doi: 10.1074/jbc.M708481200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell JV, Huang B, Fenno JC, Marconi RT. Analysis of a unique interaction between the complement regulatory protein factor H and the periodontal pathogen Treponema denticola. Infect. Immun. 2009;77:1417–1425. doi: 10.1128/IAI.01544-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikolajczyk J, Boatright KM, Stennicke HR, Nazif T, Potempa J, Bogyo M, Salvesen GS. Sequential autolytic processing activates the zymogen of Arg-gingipain. J. Biol. Chem. 2003;278:10458–11064. doi: 10.1074/jbc.M210564200. [DOI] [PubMed] [Google Scholar]
- Mikx FH. Comparison of peptidase, glycosidase and esterase activities of oral and non-oral Treponema species. J. Gen. Microbiol. 1991;137:63–68. doi: 10.1099/00221287-137-1-63. [DOI] [PubMed] [Google Scholar]
- Miyamoto M, Ishihara K, Okuda K. The Treponema denticola surface protease dentilisin degrades interleukin-1β (IL-1β), IL-6, and tumor necrosis factor alpha. Infect. Immun. 2006;74:2462–2467. doi: 10.1128/IAI.74.4.2462-2467.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T, Kimizuka R, Miyamoto M, Kato T, Yamada S, Okuda K, Ishihara K. Treponema denticola induces interleukin-8 and macrophage chemoattractant protein 1 production in human umbilical vein epithelial cells. Microb. Infect. 2007;9:907–913. doi: 10.1016/j.micinf.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Potempa J, Pike RN. Corruption of Innate Immunity by Bacterial Proteases. J. Innate. Immun. 2009;1:70–87. doi: 10.1159/000181144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz CP, Wolf V, Lange R, Mertens E, Wecke J, Naumann D, Zahringer U. Evidence for a new type of outer membrane lipid in oral spirochete Treponema denticola. Functioning permeation barrier without lipopolysaccharides. J. Biol. Chem. 1998;273:15661–15666. doi: 10.1074/jbc.273.25.15661. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RLJ. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Uitto V-J, Haapasalo M, Meldal M, Breddam K. Degradation of basement membrane collagen by proteases from some anaerobic oral microorganism. Oral Microbiol. Immunol. 1988;3:97–102. doi: 10.1111/j.1399-302x.1988.tb00092.x. [DOI] [PubMed] [Google Scholar]
- Vincents B, von Pawel-Rammingen U, Bjorck L, Abrahamson M. Enzymatic characterization of the streptococcal endopeptidase, IdeS, reveals that it is a cysteine protease with strict specificity for IgG cleavage due to exosite binding. Biochemistry. 2004;43:15540–15549. doi: 10.1021/bi048284d. [DOI] [PubMed] [Google Scholar]
- von Pawel-Rammingen U, Johansson BP, Bjorck L. IdeS, a novel streptococcal cysteine proteinase with unique specificity for immunoglobulin G. EMBO J. 2002;21:1607–1615. doi: 10.1093/emboj/21.7.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Miyamoto M, Yamada S, Okuda K, Ishihara K. Surface protease of Treponema denticola hydrolyzes C3 and influences function of polymorphonuclear leukocytes. Microbes. Infect. 2006;8:1758–1763. doi: 10.1016/j.micinf.2006.02.013. [DOI] [PubMed] [Google Scholar]