Abstract
Background
Therapeutic hypothermia is commonly used in comatose survivors’ post-cardiopulmonary resuscitation (CPR). It is unknown whether outcome predictors perform accurately after hypothermia treatment.
Methods
Post-CPR comatose survivors were prospectively enrolled. Six outcome predictors [pupillary and corneal reflexes, motor response to pain, and somatosensory-evoked potentials (SSEP) >72 h; status myoclonus, and serum neuron-specific enolase (NSE) levels <72 h] were systematically recorded. Poor outcome was defined as death or vegetative state at 3 months. Patients were considered “sedated” if they received any sedative drugs ≤12 h prior the 72 h neurological assessment.
Results
Of 85 prospectively enrolled patients, 53 (62%) underwent hypothermia. Furthermore, 53 of the 85 patients (62%) had a poor outcome. Baseline characteristics did not differ between the hypothermia and normothermia groups. Sedative drugs at 72 h were used in 62 (73%) patients overall, and more frequently in hypothermia than in normothermia patients: 83 versus 60% (P = 0.02). Status myoclonus <72 h, absent cortical responses by SSEPs >72 h, and absent pupillary reflexes >72 h predicted poor outcome with a 100% specificity both in hypothermia and normothermia patients. In contrast, absent corneal reflexes >72 h, motor response extensor or absent >72 h, and peak NSE >33 ng/ml <72 h predicted poor outcome with 100% specificity only in non-sedated patients, irrespective of prior treatment with hypothermia.
Conclusions
Sedative medications are commonly used in proximity of the 72-h neurological examination in comatose CPR survivors and are an important prognostication confounder. Patients treated with hypothermia are more likely to receive sedation than those who are not treated with hypothermia.
Keywords: Coma, Hypothermia, Prognosis, Sedation, Cardiac arrest, Somatosensory-evoked potential, Neuron-specific enolase, Status myoclonus
Introduction
Cardiovascular disease is the leading cause of death in North America and Europe [1]. Sudden cardiac arrest accounts for almost half a million deaths per year in the United States and 47% of those occur outside hospitals [2]. Each year, there are 295,000 out-of-hospital cardiac arrests in the United States [3]. Even when patients are resuscitated in the hospital, fewer than 20% survive to discharge [4]. Most survivors remain comatose or in a vegetative state often leading to withdrawal of life support [5, 6].
It has been shown that therapeutic hypothermia ameliorates anoxic-ischemic brain injury after out-of-hospital ventricular fibrillation cardiac arrest and improves outcome [7, 8]. The 2005 American Heart Association guidelines recommend cooling comatose patients after out-of-hospital ventricular fibrillation arrest [9]. However, the use of therapeutic hypothermia has posed new challenges on prognostication of functional outcome in these patients. It has been postulated that clinical and electrophysiological outcome predictors may behave differently after hypothermia [10]. The use of sedating agents during therapeutic hypothermia and the hypothermia itself interfere with a proper bedside neurological examination. Hypothermia also reduces drug elimination and prolongs the effects of sedatives [11]. This raises the issue of whether exam prognosticators at 72 h after the arrest are still valid.
As the effect of therapeutic hypothermia protocols on conventional outcome predictors has been incompletely studied, we aimed to assess the prognostic accuracy of previously accepted outcome predictors in a prospective cohort of comatose post cardiopulmonary resuscitation survivors.
Methods
Patients
Consecutive in- and out-of-hospital post-cardiac arrest patients who remained comatose after successful resuscitation were prospectively enrolled over 4 years. Details on the study methodology have been described previously [12]. Patients were evaluated for study participation within 1 h after restoration of spontaneous circulation. Enrollment criteria included: age 18 years or older, successful cardiopulmonary resuscitation and persistent coma defined as: no eye opening to voice and inability to follow commands. Exclusion criteria were pre-existing “do not resuscitate” status, severe coexisting systemic disease with a limited life expectancy, and brain death. Furthermore, patients who died within 72 h of the initial cardiac arrest were excluded from this study. The study was approved by the institutional review board, and written informed consent from a legally authorized representative was obtained for study participation.
Clinical Examination, Laboratory Tests and Neurodiagnostic Studies
Clinical and neurophysiological studies were obtained in a prospective and standardized fashion at predefined time points. Neurologic exams were performed at 1, 24 + 12, 48 + 12, and 72 + 12 h after the arrest. Patients were considered to be under sedation if they received any sedative medication 12 h or less prior to the 72-h neurological exam. Status myoclonus was recorded when present at any time during this time period and was defined as spontaneous, repetitive, unrelenting, generalized, multifocal myoclonus involving the face, limbs, and axial musculature in the presence of coma [11]. The presence of unilateral or bilateral corneal reflexes, pupillary responses to light, and the motor response score to painful stimulation according to the Glasgow Coma Scale were systematically recorded at each neurological exam by a board-certified neurologist.
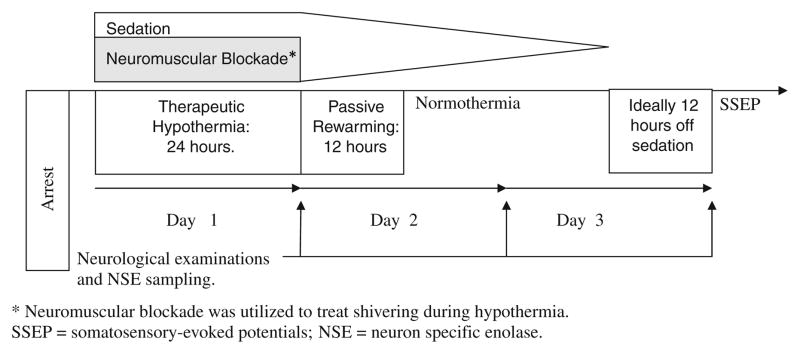
Neuron-specific enolase (NSE) levels were obtained at 24 ± 4, 48 ± 4, and 72 ± 4 h from the cardiac arrest and the highest measured value was used for the analyses. Cortical N20 responses of median nerve somatosensory-evoked potentials (SSEPs) were obtained after 72 h if the patient remained comatose. The chronology of therapeutic hypothermia and prognostication is outlined in Fig. 1.
Fig. 1.
Time course of the cooling phase and prognostication.
Hypothermia Protocol
A treatment protocol for the use of hypothermia in comatose post cardiac arrest survivors was adopted at our hospital while the study was ongoing. Hence, patients who were enrolled during the initial phase of the study were not cooled. Contraindications for cooling included severe coagulopathy or hemodynamic instability with a systolic blood pressure less than 90 mmHg despite intravenous pressor therapy. Eligible patients underwent hypothermia for 24 h with a target temperature of 33°C using either surface or catheter-based cooling technologies. All patients underwent analgesia and sedation with fentanyl (25–100 μg/h IV) and midazolam (2–6 mg/h IV) drips, respectively. Vecuronium (0.1 mg/kg bolus, followed by 0–5 mg/h to maintain 1–2 twitches using train of four) was used to achieve paralysis and avoid shivering during hypothermia. Paralytic agents, then sedatives and analgesics, were tapered off after rewarming to 36.5°C. Passive rewarming took place over 8–12 h. All patients had returned to normothermia at the time of the 72-h neurological exam.
After rewarming, sedation was used as needed for patient comfort, with efforts directed towards minimizing sedation. In our experience, a 70 kg subject typically received 50–100 μg/h of fentanyl, 2.5–5.0 mg/h of midazolam and 2.5 mg/h of vecuronium to achieve sedation and paralysis; therefore, over 24 h of therapeutic hypothermia and 12 h of passive rewarming the average patient received a total of 1800–3600 μg of fentanyl, 90–180 mg of midazolam and 90 mg of vecuronium. In addition, propofol (10–100 μg/kg/min) was used as an adjuvant sedative when deemed necessary by the treating team.
Outcome Measures
Glasgow Outcome Scale (GOS) scores (1–5 score, 1 is dead and 5 is good recovery) were recorded by follow-up clinic visit or by a standardized telephone interview at 3 months after the arrest. Poor outcome was defined as death or persistent vegetative state (GOS 1 and 2, respectively) at 3 months. Goals of care were addressed with patient’s next of kin on multiple occasions and the decision to withdraw life support was made in conjunction with them. Prediction of poor neurological outcome was typically based on multiple neurological assessments beyond 72 h with supportive evidence of poor outcome from neurophysiological testing.
Statistical Analysis
Student t test or Mann–Whitney U test were used to compare continuous variables and χ2 or Fisher’s exact tests to compare categorical data between the two groups. Statistical significance was defined at a P < 0.05. Statistical analyses were performed using SPSS 17.0 (SPSS, Chicago, IL, USA) and VassarStats [13].
Results
One hundred and thirty-seven comatose cardiac arrest survivors were evaluated at Stanford University Medical Center during a 4-year time period and 86 (63%) individuals were prospectively enrolled. Screen failures included: consent was not obtained (n = 13), pre-existing “do not resuscitate” status (n = 12), brain death (n = 6), death before the 72-h neurological assessment (n = 18), and patient was transferred to our hospital from an outside institution beyond 72 h of the arrest (n = 2). From the 86 patients who were enrolled, 85 were included in the analyses because one patient had a poorly documented 72-h neurological exam and had to be excluded. Fifty-three (62%) of the 85 patients underwent therapeutic hypothermia. Thirty-two patients (60%) who underwent therapeutic hypothermia had a poor outcome compared to 21 patients (65%) who did not undergo hypothermia.
Fifty-two patients (61%) died (GOS 1), one patient (1%) remained in vegetative state (GOS 2), and 32 had a good outcome at 3 months [GOS 3 (n = 8), GOS 4 (n = 7), GOS 5 (n = 17)]. The average period of time from arrest to death was 9 days. Forty-three patients were made comfort care due to poor prognosis, three patients progressed to brain death, and six patients died of medical complications despite full support. One patient remained in a vegetative state at 3 months (Fig. 2). Baseline characteristics did not differ between the group who underwent therapeutic hypothermia and the normothermia group (Table 1). When good and bad outcome groups were compared, patients with a shorter time of arrest had a better outcome compared to patients who were resuscitated longer (17 vs. 25 min, P = 0.02).
Fig. 2.
Poor outcome patients (N = 53). Fifty-two patients died: three patients progressed to brain death, six died of medical complications while on full support, and 43 patients were made comfort care. One patient remained in a vegetative state at 3 months
Table 1.
Clinical characteristics of 85 patients
| Normothermia (n = 32) | Hypothermia (n = 53) | P | Good Outcome (n = 32) | Poor Outcome (n = 53) | P | |
|---|---|---|---|---|---|---|
| Age, mean (range) | 55 (19–86) | 58 (19–84) | NS | 53 (19–84) | 59 (19–86) | NS |
| Male sex, n (%) | 17 (53) | 39 (73) | NS | 24 (75) | 32 (60) | NS |
| Medical history, n (%) | ||||||
| Coronary heart disease | 6 (19) | 10 (19) | NS | 4 (13) | 12 (23) | NS |
| Congestive heart failure | 4 (13) | 2 (4) | NS | 0 (0) | 6 (11) | NS |
| Hypertension | 12 (38) | 24 (45) | NS | 10 (31) | 26 (49) | NS |
| Diabetes | 6 (19) | 9 (17) | NS | 4 (13) | 11 (21) | NS |
| Pre-arrest mRS, median (IQR) | 0 (0–1) | 0 (0–1) | NS | 0 (0–1) | 0 (0–2) | NS |
| Arrest | ||||||
| Initial Rhythm | ||||||
| Ventricular fibrillation, n (%) | 11 (34) | 18 (34) | NS | 14 (44) | 15 (28) | NS |
| Ventricular tachycardia, n (%) | 1 (3) | 3 (6) | NS | 0 (0) | 4 (8) | NS |
| Pulseless electrical activity, n (%) | 12 (38) | 16 (30) | NS | 9 (28) | 19 (36) | NS |
| Asystole, n (%) | 5 (16) | 12 (23) | NS | 4 (13) | 13 (25) | NS |
| Unknown, n (%) | 3 (9) | 4 (8) | NS | 5 (16) | 2 (4) | NS |
| Arrest time (min), mean (range)a | 19 (3–45) | 24 (3–90) | NS | 17 (3–50) | 25 (5–90) | 0.02 |
| Cardiac origin of arrest, n (%)b | 15 (50) | 37 (70) | NS | 22 (69) | 30 (59) | NS |
| In-hospital cardiac arrest, n (%) | 12 (38) | 15 (28) | NS | 10 (31) | 17 (32) | NS |
| Therapeutic hypothermia (%) | 62 | 21 (66) | 32 (60) | NS | ||
| Surface-based cooling | – | 29 (55) | 12 (38) | 17 (32) | NS | |
| Catheter-based cooling | – | 22 (42) | 8 (25) | 14 (26) | NS | |
| Surface- and catheter-based cooling | – | 2 (4) | 1 (3) | 1 (2) | NS | |
| Sedation at 72-h assessment, n (%)b | 18 (60) | 44 (83) | 0.02 | 25 (78) | 37 (73) | NS |
Bold P-values are significant at alpha <0.05
Data not available for six patients in the normothermia group and eight patients in the hypothermia group; nine patients in the poor outcome group and five patients in the good outcome group
Data not available for two patients in the normothermia group; two patients in the poor outcome group mRS Modified Rankin Scale, IQR interquartile range
Sedative drugs were administered within 12 h of the 72 h examination in 62/85 (73%) of patients and included the following agents: propofol, fentanyl, midazolam, and lorazepam. In two patients, data was missing on whether the patient had or had not received any sedative drugs. Fentanyl was the most common agent used (n = 39, average dose of 80 μg/h); followed by midazolam (n = 25, average dose of 3.5 mg/h) and propofol (n = 11, average dose of 35 μg/kg/min).
Patients who underwent hypothermia were more likely to have received sedative medication within 12 h of the 72-h assessment when compared to patients in the normothermia group (83 vs. 60%, P = 0.02).
The presence of status myoclonus at any time within 72 h of the arrest, absence of pupillary responses to light at the 72 h exam, and absence of SSEPs (N20 response) after 72 h accurately predicted poor outcome (100% sensitivity) in both the hypothermia and normothermia groups. In contrast, absent or extensor motor responses at 72 h, unilateral or bilateral absent corneal reflexes at 72 h, and peak serum NSE levels >33 ng/ml did not accurately predict poor neurological outcome in either group (Table 2). The highest serum NSE level documented in a patient with good outcome was 85 ng/ml. The time required by the five patients with NSE levels >33 ng/ml and good outcome to follow commands was on average 7 days (range 1–14 days).
Table 2.
Three-month outcome prediction of death or vegetative state of the 85-patient cohort
| Patients (poor outcome/total) | Test | na | FP | FN | TP | TN | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|---|
| All Patients (53/85) | Absent SSEP | 46 | 0 | 13 | 23 | 10 | 64 (46–79) | 100 (66–100) | 100 (82–100) | 43 (24–65) |
| Status myoclonus | 85 | 0 | 44 | 9 | 32 | 17 (9–30) | 100 (87–100) | 100 (63–100) | 42 (31–54) | |
| No pupillary response | 83 | 0 | 37 | 15 | 31 | 29 (18–43) | 100 (86–100) | 100 (75–100) | 46 (34–58) | |
| No corneal reflexes | 85 | 2 | 33 | 20 | 30 | 38 (25–52) | 94 (78–99) | 91 (69–98) | 48 (35–60) | |
| Motor response ≤2 | 85 | 4 | 10 | 43 | 28 | 81 (68–90) | 88 (70–96) | 91 (79–97) | 74 (57–86) | |
| NSE >33 ng/ml | 69 | 5 | 10 | 33 | 21 | 77 (61–88) | 81 (60–93) | 87 (71–95) | 68 (49–83) | |
| All patients without sedation (14/21) | Absent SSEP | 10 | 0 | 1 | 7 | 2 | 88 (47–99) | 100 (20–100) | 100 (56–100) | 67 (13–98) |
| Status myoclonus | 21 | 0 | 10 | 4 | 7 | 36 (12–68) | 100 (66–100) | 100 (40–100) | 59 (33–81) | |
| No pupillary response | 21 | 0 | 8 | 6 | 7 | 43 (19–70) | 100 (56–100) | 100 (52–100) | 47 (22–73) | |
| No corneal reflexes | 21 | 0 | 8 | 6 | 7 | 43 (19–70) | 100 (56–100) | 100 (52–100) | 47 (22–73) | |
| Motor response ≤2 | 21 | 0 | 2 | 12 | 7 | 86 (56–98) | 100 (56–100) | 100 (70–100) | 78 (40–96) | |
| NSE >33 ng/ml | 15 | 0 | 1 | 10 | 4 | 91 (57–100) | 100 (40–100) | 100 (66–100) | 80 (30–99) | |
| Hypothermia with sedation (25/44) | Absent SSEP | 24 | 0 | 5 | 14 | 5 | 50 (20–80) | 100 (73–100) | 100 (46–100) | 74 (49–90) |
| Status myoclonus | 44 | 0 | 23 | 2 | 19 | 8 (1–27) | 100 (79–100) | 100 (20–100) | 45 (30–61) | |
| No pupillary response | 43 | 0 | 20 | 4 | 19 | 17 (5–38) | 100 (79–100) | 100 (40–100) | 49 (33–65) | |
| No corneal reflexes | 44 | 1 | 17 | 8 | 18 | 32 (16–54) | 95 (72–100) | 89 (50–100) | 51 (34–68) | |
| Motor response ≤2 | 44 | 2 | 3 | 22 | 17 | 88 (68–97) | 89 (65–98) | 92 (72–99) | 85 (61–96) | |
| NSE >33 ng/ml | 42 | 4 | 6 | 18 | 14 | 75 (53–89) | 78 (52–93) | 82 (59–94) | 70 (46–87) | |
| Normothermia with sedationb (12/18) | Absent SSEP | 11 | 0 | 6 | 2 | 3 | 25 (4–64) | 100 (31–100) | 100 (20–100) | 33 (9–69) |
| Status myoclonus | 18 | 0 | 10 | 2 | 6 | 17 (3–49) | 100 (52–100) | 100 (20–100) | 38 (16–64) | |
| No pupillary response | 17 | 0 | 7 | 5 | 5 | 42 (16–71) | 100 (46–100) | 100 (46–100) | 42 (16–71) | |
| No corneal reflexes | 18 | 1 | 7 | 5 | 5 | 42 (16–71) | 83 (36–99) | 83 (36–99) | 42 (16–71) | |
| Motor response ≤2 | 18 | 2 | 4 | 8 | 4 | 67 (35–89) | 67 (24–94) | 80 (44–96) | 50 (17–83) | |
| NSE >33 ng/ml | 12 | 1 | 3 | 5 | 3 | 63 (26–90) | 75 (22–99) | 83 (36–99) | 50 (14–86) |
95% Confidence interval in parentheses
Number of patients with available data in each subgroup (including both the poor and good outcome patients)
Information on the use of sedation not available for two patients
FP number of patients with a false-positive test result, FN number of patients with a false-negative test result, TP number of patients with a true-positive test result, TN number of patients with a true-negative test result, PPV positive predictive value, NPV negative predictive value, SSEP somatosensory-evoked potentials, NSE serum neuron-specific enolase
After excluding patients who received sedation within 12 h of the 72 h assessment (both in the hypothermia and normothermia groups), all prognosticators accurately predicted poor outcome with a specificity of 100% (Table 2). Of the 21 patients who were not sedated at 72 h, 14 (67%) had a poor outcome.
Of the predictors with 100% specificity regardless of sedation, status myoclonus had the lowest sensitivity to predict poor outcome (17%) and absent cortical responses by SSEPs the highest (64%). For all predictors, the sensitivity for poor outcome prediction was higher in patients who did not receive sedation within 12 h of the 72 h examination than in those who did; however, this difference was not statistically significant for any predictor.
Discussion
The 2006 American Academy of Neurology practice parameter for prediction of outcome in comatose survivors after cardiopulmonary resuscitation (CPR) is almost exclusively based on studies performed before the era of therapeutic hypothermia [11]. In fact, the authors call for reassessment of the generally accepted poor prognosticators in patients who undergo therapeutic hypothermia. They also urge to use caution in interpretation of various prognosticators in the presence of sedating agents. This study demonstrates that patients who undergo therapeutic hypothermia are more likely to receive sedative agents in proximity to the 72-h examination and that sedation is a confounder in the interpretation of corneal reflexes and best motor response to painful stimulation at that time point. Our study also shows that the previously proposed cut-off level for neuron-specific enolase of 33 ng/ml is not 100% specific for poor outcome.
In our cohort, three of the six commonly used prognosticators for poor outcome (absent corneal reflexes at 72 h, best motor response extensor posturing or worse at 72 h, and peak serum NSE levels >33 ng/ml at any time within 72 h) failed to accurately predict poor outcome both in patients treated with and without therapeutic hypothermia. This finding is concerning as patients may be taken off life support inappropriately and prematurely because of a presumed poor outcome based on any one of these three prognosticators. Potential confounders for the bedside neurologic exams are lingering sedation or neuromuscular blockade, differences in assessment of motor and corneal responses between examiners, and the presence of an underlying neuromuscular disorder.
Conversely, status myoclonus within 72 h, absence of pupillary responses to light at 72 h, and absence of recordable N20 SSEP peaks after 72 h accurately predicted poor outcome in all patients in our study irrespective of the use of hypothermia and sedating agents. Since the sensitivity of status myoclonus and absent pupillary reflexes for poor outcome was low (17 and 29%, respectively), the best performing and most useful prognosticator by far was the SSEP examination, which was 100% specific and 64% sensitive for poor outcome. This finding is consistent with a recently published pilot study demonstrating that absence of recordable N20 SSEP peaks during hypothermia accurately predicted poor outcome, but requires further study in larger patient cohorts [14].
The most important finding of this study is that sedation is commonly used in post cardiac arrest patients, even as late as 3 days after the ictus, and that it is a major confounder for accurate outcome prediction on the basis of the 72-h bedside neurological examination. We purposefully chose a low threshold to define “sedation” and included any sedating drug administered 12 h or less prior to the 72 h examination. We felt it was appropriate to choose a low threshold because critically ill patients commonly have altered hepatic and/or renal function impairing drug clearance [15], which may be even more pronounced after cardiac arrest and therapeutic hypothermia [16]. We found that we cannot reliably use the absence of corneal reflexes or a poor motor examination at 72 h as predictors of poor outcome in patients who have received sedative drugs within 12 h prior to the examination. Conversely, in patients who were not sedated all six prognosticators accurately predicted poor outcome. It is important to recognize that if the 72 h examinations are confounded by sedation, the 24 and 48 h examinations are even less reliable for prognostication since they are much more likely to be confounded by sedation use during and shortly after the hypothermia phase. Nevertheless, daily neurological examinations during this time period should still be performed as they may unveil overt seizure phenomena, status myoclonus, or other important neurological exam changes.
Most drugs used during therapeutic hypothermia, including sedatives, analgesics, and neuromuscular blocking agents, undergo significant pharmacokinetic and pharmacodynamic alterations [17, 18]. Fukuoka and collaborators reported a fivefold increase in midazolam plasma concentrations in traumatic brain-injured patients treated with hypothermia (32–34°C) compared with normothermic patients [19]. Propofol is also widely used for sedation in neurological patients; because of its short acting effect propofol facilitates neurological assessments. However, the plasmatic concentration of propofol can increase up to 30% in patients who are treated with hypothermia as compared with the concentration achieved in normothermic patients [18]. The clearance of fentanyl, the preferred analgesic used in our cohort, can increase up to 3.7 times during hypothermia, so high doses may produce significant delays in awakening after interruption of the infusion [20].
To our surprise, the use of sedative agents was extremely common in our patients with almost three-quarters of them having received a sedative agent within 12 h of the day 3 neurological assessment. Sedation practices vary widely between institutions and patients [21, 22]. The frequent use of sedatives in this study reflects the routine clinical practice in our medical and cardiac intensive care units, which do not routinely use sedation scale assessments to titrate drug. The potential confounding of sedative drugs on the bedside examination calls for the use of more objective outcome predictors in comatose post-cardiac arrest survivors such as biomarkers of neuronal injury, SSEPs, and brain imaging studies, which presumably are not affected by drug use or metabolic derangements. Studies reporting on the prognostic utility of SSEPs are very robust and MR imaging data is promising [12, 14].
We found that patients who underwent hypothermia more often received sedative drugs in proximity of the three day neurological assessment than those who did not. This is probably at least in part related to the routine use of sedation during the 24 h of induced hypothermia and the additional 8–12 h of re-warming. We were interested in assessing the effect of hypothermia on any of the six prognosticators independently of the use of sedation. However, since only nine patients in the hypothermia group did not receive any sedatives prior to the 72-h neurological examination our power to detect an independent effect of hypothermia on prognostic accuracy of any of the prognosticators is limited.
Contrary to what Zandbergen and collaborators found, peak serum NSE levels >33 ng/ml failed to reliably predict poor outcome in our study [23]; however, in the study of Zandbergen and colleagues overall mortality was exceedingly high (86%), which may have affected the accuracy of this cut-off. Five patients in our study with a peak NSE level >33 ng/ml (highest level of 85 ng/ml) regained consciousness. We carefully excluded hemolyzed serum samples from the analyses. The fact that serum NSE levels >33 ng/ml accurately predicted poor outcome in the non-sedated group and not in sedated patients is probably a result of chance, since it is unlikely that sedation affects serum NSE levels. Other studies have evaluated NSE after CPR and induced hypothermia and report different cut-offs for prediction of poor outcome [24–26] The heterogeneity of cut-off levels in published reports may be due to the use of different assays, different timing of serum sampling and the use of different outcome scales and criteria for withdrawing life support. Furthermore, since NSE is also present in platelets and red blood cells, hemolysis increases serum values and may yield false-positive test results. Some recent data suggest that serum NSE levels are different in patients treated with hypothermia [24]. Thus, cutoff levels will have to be redefined in large prospective cohorts of patients undergoing therapeutic hypothermia.
This study has several limitations. Since there were only nine patients who underwent hypothermia and who were not sedated at the 72 h assessment, the power to detect an independent effect of hypothermia on the accuracy of any one of the three prognosticators that was affected by sedation in our study is limited. In addition, some tests were not performed in all patients mainly because of logistic problems. This further widened the confidence intervals and reduced the precision of the estimated accuracy of each of the prognosticators.
Acknowledgments
The authors would like to thank Stephanie Kemp for administrative support for this study and Marion Buckwalter, Chitra Venkatasubramanian, Amie Hsia, Maarten Lansberg, Neil Schwartz, and Gregory Albers for their assistance with patient enrollment. Dr. Wijman has received funding from the following grants for this research: AHA Scientist Development Award, 043275N, and NIH RO1 HL089116-01A2.
Contributor Information
Edgar A. Samaniego, Email: edgarsama@gmail.com.
Michael Mlynash, Email: mmlynash@stanford.edu.
Anna Finley Caulfield, Email: afinley@stanford.edu.
Irina Eyngorn, Email: ieyngorn@stanford.edu.
Christine A. C. Wijman, Email: cwijman@stanford.edu.
References
- 1.Booth CM, et al. Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA. 2004;291(7):870–9. doi: 10.1001/jama.291.7.870. [DOI] [PubMed] [Google Scholar]
- 2.Zheng ZJ, et al. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104(18):2158–63. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 4.Peberdy MA, et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58(3):297–308. doi: 10.1016/s0300-9572(03)00215-6. [DOI] [PubMed] [Google Scholar]
- 5.Lim C, et al. The neurological and cognitive sequelae of cardiac arrest. Neurology. 2004;63(10):1774–8. doi: 10.1212/01.wnl.0000144189.83077.8e. [DOI] [PubMed] [Google Scholar]
- 6.Bates D, et al. A prospective study of nontraumatic coma: methods and results in 310 patients. Ann Neurol. 1977;2(3):211–20. doi: 10.1002/ana.410020306. [DOI] [PubMed] [Google Scholar]
- 7.Bernard SA, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 8.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–56. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 9.American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2005;112(24 Suppl):IV1–203. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]
- 10.Sunde K, et al. Determination of prognosis after cardiac arrest may be more difficult after introduction of therapeutic hypothermia. Resuscitation. 2006;69(1):29–32. doi: 10.1016/j.resuscitation.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Wijdicks EF, et al. Practice parameter: prediction of outcome in comatose survivors after cardiopulmonary resuscitation (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2006;67(2):203–10. doi: 10.1212/01.wnl.0000227183.21314.cd. [DOI] [PubMed] [Google Scholar]
- 12.Wijman CA, et al. Prognostic value of brain diffusion-weighted imaging after cardiac arrest. Ann Neurol. 2009;65(4):394–402. doi: 10.1002/ana.21632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowry R. VassarStats: web site for statistical computation. [cited 2009 November]. http://faculty.vassar.edu/lowry/VassarStats.html.
- 14.Bouwes A, et al. Somatosensory evoked potentials during mild hypothermia after cardiopulmonary resuscitation. Neurology. 2009;73(18):1457–61. doi: 10.1212/WNL.0b013e3181bf98f4. [DOI] [PubMed] [Google Scholar]
- 15.Bertz RJ, Granneman GR. Use of in vitro and in vivo data to estimate the likelihood of metabolic pharmacokinetic interactions. Clin Pharmacokinet. 1997;32(3):210–58. doi: 10.2165/00003088-199732030-00004. [DOI] [PubMed] [Google Scholar]
- 16.Tortorici MA, Kochanek PM, Poloyac SM. Effects of hypothermia on drug disposition, metabolism, and response: a focus of hypothermia-mediated alterations on the cytochrome P450 enzyme system. Crit Care Med. 2007;35(9):2196–204. doi: 10.1097/01.ccm.0000281517.97507.6e. [DOI] [PubMed] [Google Scholar]
- 17.Arpino PA, Greer DM. Practical pharmacologic aspects of therapeutic hypothermia after cardiac arrest. Pharmacotherapy. 2008;28(1):102–11. doi: 10.1592/phco.28.1.102. [DOI] [PubMed] [Google Scholar]
- 18.Sessler DI. Complications and treatment of mild hypothermia. Anesthesiology. 2001;95(2):531–43. doi: 10.1097/00000542-200108000-00040. [DOI] [PubMed] [Google Scholar]
- 19.Fukuoka N, et al. Biphasic concentration change during continuous midazolam administration in brain-injured patients undergoing therapeutic moderate hypothermia. Resuscitation. 2004;60(2):225–30. doi: 10.1016/j.resuscitation.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Fritz HG, et al. The effect of mild hypothermia on plasma fentanyl concentration and biotransformation in juvenile pigs. Anesth Analg. 2005;100(4):996–1002. doi: 10.1213/01.ANE.0000146517.17910.54. [DOI] [PubMed] [Google Scholar]
- 21.Kress JP, Pohlman AS, Hall JB. Sedation and analgesia in the intensive care unit. Am J Respir Crit Care Med. 2002;166(8):1024–8. doi: 10.1164/rccm.200204-270CC. [DOI] [PubMed] [Google Scholar]
- 22.Chamorro C, et al. Anesthesia and analgesia protocol during therapeutic hypothermia after cardiac arrest: a systematic review. Anesth Analg. 2010;110(5):1328–35. doi: 10.1213/ANE.0b013e3181d8cacf. [DOI] [PubMed] [Google Scholar]
- 23.Zandbergen EG, et al. Prediction of poor outcome within the first 3 days of postanoxic coma. Neurology. 2006;66(1):62–8. doi: 10.1212/01.wnl.0000191308.22233.88. [DOI] [PubMed] [Google Scholar]
- 24.Tiainen M, et al. Serum neuron-specific enolase and S-100B protein in cardiac arrest patients treated with hypothermia. Stroke. 2003;34(12):2881–6. doi: 10.1161/01.STR.0000103320.90706.35. [DOI] [PubMed] [Google Scholar]
- 25.Oksanen T, et al. Predictive power of serum NSE and OHCA score regarding 6-month neurologic outcome after out-of-hospital ventricular fibrillation and therapeutic hypothermia. Resuscitation. 2009;80(2):165–70. doi: 10.1016/j.resuscitation.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Rundgren M, et al. Neuron specific enolase and S-100B as predictors of outcome after cardiac arrest and induced hypothermia. Resuscitation. 2009;80(7):784–9. doi: 10.1016/j.resuscitation.2009.03.025. [DOI] [PubMed] [Google Scholar]