Abstract
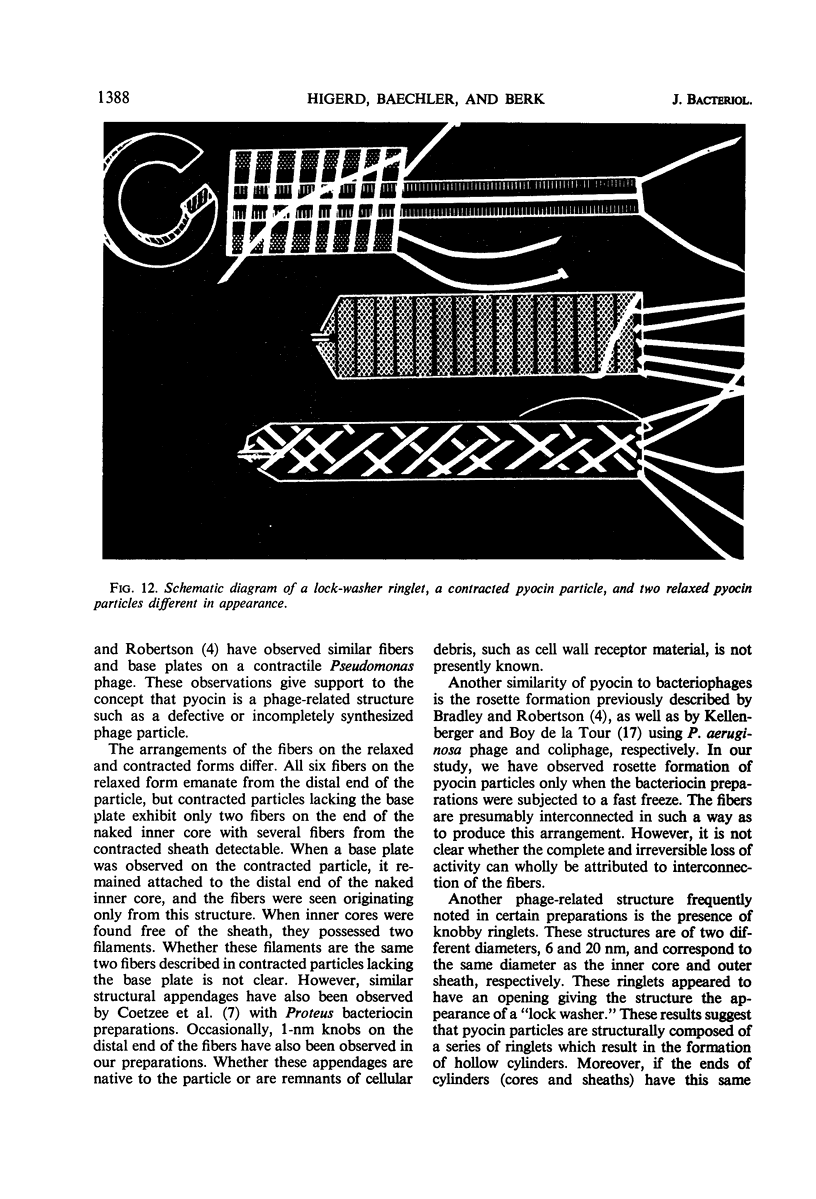
The bacteriocin from Pseudomonas aeruginosa, pyocin, consists of a contractile sheath and inner core reminiscent of T-even coliphage tails. Contraction of the outer sheath was found to be promoted by 0.5 m magnesium chloride, 1% Formalin, low pH, sonic treatment, and freezing or thawing or both. The contraction caused by 0.5 m magnesium chloride, however, was found to be reversible and occurred upon reduction of the salt concentration from 0.5 to 0.02 m. In addition, direct assay showed that pyocin activity was nearly proportional to the percentage of only uncontracted forms. Initial studies suggested that the adsorption of purified pyocin onto cell wall fragments from the sensitive indicator strain of P. aeruginosa occurs with the relaxed particle only and not with the contracted form. However, after adsorption, contraction occurred. Various morphological structures, such as tail fibers and base-platelike appendages, were also observed. Upon contraction, six tail fibers were observed on many particles, four of which appeared to originate from the sheath and two from the inner core. Polysheaths and polycores several hundred nanometers in length were also occasionally observed.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRENNER S., HORNE R. W. A negative staining method for high resolution electron microscopy of viruses. Biochim Biophys Acta. 1959 Jul;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- Bradley D. E., Robertson D. The structure and infective process of a contractile Pseudomonas aeruginosa bacteriophage. J Gen Virol. 1968 Sep;3(2):247–254. doi: 10.1099/0022-1317-3-2-247. [DOI] [PubMed] [Google Scholar]
- Coetzee H. L., De Klerk H. C., Coetzee J. N., Smit J. A. Bacteriophage-tail-like particles associated with intra-species killing of Proteus vulgaris. J Gen Virol. 1968 Jan;2(1):29–36. doi: 10.1099/0022-1317-2-1-29. [DOI] [PubMed] [Google Scholar]
- Goto S., Homma J. Y., Mudd S. Electron microscopy of the combination of antibodies with flagellar antigen and with a pyocine. J Bacteriol. 1967 Sep;94(3):751–758. doi: 10.1128/jb.94.3.751-758.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumpert J., Taubeneck U. "Mikrotubuli" bei Proteus mirabilis als Produkte defekter Lysogenie. Z Allg Mikrobiol. 1968;8(2):101–105. [PubMed] [Google Scholar]
- HALL C. E. Electron densitometry of stained virus particles. J Biophys Biochem Cytol. 1955 Jan;1(1):1–12. doi: 10.1083/jcb.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higerd T. B., Baechler C. A., Berk R. S. In vitro and in vivo characterization of pyocin. J Bacteriol. 1967 Jun;93(6):1976–1986. doi: 10.1128/jb.93.6.1976-1986.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma J. Y., Goto S., Shionoya H. Relationship between pyocine and temperate phage of Pseudomonas aeruginosa. II. Isolation of pyocines from strain P 1-III and their characteristics. Jpn J Exp Med. 1967 Oct;37(5):373–393. [PubMed] [Google Scholar]
- Homma J. Y., Shionoya H. Relationship between pyocine and temperate phage of Pseudomonas aeruginosa. 3. Serological relationship between pyocines and temperate phages. Jpn J Exp Med. 1967 Oct;37(5):395–421. [PubMed] [Google Scholar]
- Ishii S. I., Nishi Y., Egami F. The fine structure of a pyocin. J Mol Biol. 1965 Sep;13(2):428–431. doi: 10.1016/s0022-2836(65)80107-3. [DOI] [PubMed] [Google Scholar]
- JACOB F. Biosynthèse induite et mode d'action d'une pyocine, antibiotique de Pseudomonas pyocyanea. Ann Inst Pasteur (Paris) 1954 Feb;86(2):149–160. [PubMed] [Google Scholar]
- KAGEYAMA M. STUDIES OF A PYOCIN. I. PHYSICAL AND CHEMICAL PROPERTIES. J Biochem. 1964 Jan;55:49–53. doi: 10.1093/oxfordjournals.jbchem.a127839. [DOI] [PubMed] [Google Scholar]
- KELLENBERGER E., DELATOUR E. B. ON THE FINE STRUCTURE OF NORMAL AND "POLYMERIZED" TAIL SHEATH OF PHAGE T4. J Ultrastruct Res. 1964 Dec;11:545–563. doi: 10.1016/s0022-5320(64)80081-2. [DOI] [PubMed] [Google Scholar]
- Krimm S., Anderson T. F. Structure of normal and contracted tail sheaths of T4 bacteriophage. J Mol Biol. 1967 Jul 28;27(2):197–202. doi: 10.1016/0022-2836(67)90015-0. [DOI] [PubMed] [Google Scholar]
- Moody M. F. Structure of the sheath of bacteriophage T4. I. Structure of the contracted sheath and polysheath. J Mol Biol. 1967 Apr 28;25(2):167–200. doi: 10.1016/0022-2836(67)90136-2. [DOI] [PubMed] [Google Scholar]
- Moody M. F. Structure of the sheath of bacteriophage T4. II. Rearrangement of the sheath subunits during contraction. J Mol Biol. 1967 Apr 28;25(2):201–208. doi: 10.1016/0022-2836(67)90137-4. [DOI] [PubMed] [Google Scholar]
- Reichle R. E., Lewin R. A. Purification and structure of rhapidosomes. Can J Microbiol. 1968 Mar;14(3):211–213. doi: 10.1139/m68-036. [DOI] [PubMed] [Google Scholar]