Abstract
Resveratrol (RES) is a potent anti-cancer agent. We have previously reported that RES arrests the growth of invasive human A431 squamous cell carcinoma cells. In this study, we show that oral administration of RES to highly tumor-susceptible p53+/−/SKH-1 mice markedly delayed ultraviolet B (UVB)-induced skin tumorigenesis and reduced the malignant conversion of benign papillomas to SCCs. Tumor growth factor β2 (TGF-β2) was predominantly overexpressed in UVB-induced SCCs and its expression was diminished in RES-treated SCCs/skin. In addition to inhibition of TGF-β2 expression, RES increased the level of E-cadherin. This RES-mediated TGF-β2 downregulation led to the inhibition of both TGF-β2/Smad-dependent and -independent pathways and suppressed the invasiveness of A431 cells. Addition of TGF-β2, but not TGF-β1, rescued the RES-mediated downregulation of p-ERK1/2, p-Smad3, and α-SMA. The Akt substrate cAMP response-binding protein (pCREB) transcription factor is known to regulate TGF-β2 expression, and RES treatment decreased phosphorylation of Akt and pCREB. Expression of constitutively active Akt blocked RES inhibition of CREB and TGF-β2, and rescued RES inhibition of cellular invasiveness. Our data indicate that RES suppresses UVB-induced malignant tumor progression in p53+/−/SKH-1 mice and that RES-inhibited invasiveness of human A431 SCC cells appears to occur, in part, through the Akt-mediated downregulation of TGF-β2.
INTRODUCTION
Transforming growth factor-β(TGF-β) is known to drive tumor progression in epithelial tumors. Its active form is a 25 kDa dimer bound together by a disulfide bond and hydrophobic interactions (Rahimi and Leof, 2007). It is overexpressed in both malignant human tumors and various experimental animal cancers, including squamous cell carcinomas (SCCs). Overexpression of TGF-β at the early stages of carcinogenesis appears to be primarily tumor-suppressive, mainly through growth inhibition. However, during the later stages of carcinogenesis, TGF-β overexpression drives epithelial-mesenchymal transition (EMT), tumor progression, and metastasis. Multiple explanations for this procarcinogenic effect of TGF-β have been proposed including loss of adhesion molecules, increased angiogenesis, protease activation, and immunosuppression (Han et al., 2005). It is known that TGF-β signaling pathways can be Smad-dependent or -independent. The Smad-independent pathway involves proteins such as Tak1, ERK/MAPK and RhoA (Choi et al., 2007; Davies et al., 2005; Derynck and Zhang, 2003; Yue and Mulder, 2001). Additionally, crosstalk occurs between distinct TGF-β-activated signaling cascades, such as the MAPK and Smad pathways. For example, ERK1/2, JNK, and p38 MAPK can modulate Smad signaling by altering the phosphorylation state of Smad2 or Smad3 (Derynck and Zhang, 2003; Leivonen et al., 2005). Given the growing evidence supporting the role of TGF-β in driving tumorigenesis and metastasis, efforts to target its inhibition could be useful both for understanding its biological functions and verifying it as a potential target for the prevention/treatment of invasive cancer.
Resveratrol (RES) is a polyphenolic phytoalexin found in grapes and other fruits. Numerous studies have verified its inhibitory effects on multiple cellular and molecular events linked to tumorigenesis (Athar et al., 2007; Baur and Sinclair, 2006). These potentially chemopreventive and chemotherapeutic effects have been demonstrated in all three stages of carcinogenesis (initiation, promotion, and progression) in UVB-and chemically-induced skin tumor growth in mice (Aziz et al., 2005 and references therein) and numerous animal models of human cancers (Athar et al., 2007). Because TGF-β signaling may cooperate with other pathways important to tumor development, we sought to determine the effects of RES on TGF-β signaling during UVB-induced skin carcinogenesis. Our data demonstrate that RES suppressed UVB-induced SCC tumorigenesis by modulating the TGF-β2 pathway in p53+/−/SKH-1 mice, and further show that RES inhibits human SCC cell invasiveness by downregulating Akt-mediated TGF-β2 expression.
RESULTS
Resveratrol suppresses and delays onset of UVB-induced SCCs in p53+/−/SKH-1 murine skin
Chronic UVB irradiation is a well-defined skin carcinogen and has been shown to cause signature mutations in the p53 tumor suppressor gene, and the mutational inactivation of p53 is thought to contribute to SCCs in both humans and mice (Benjamin et al., 2008). We have previously shown that RES, which displays both p53-dependent and –independent effects, inhibits the growth of A431 human epidermoid SCC cells lacking functional p53 (Kim et al., 2006). To determine whether RES can suppress SCC growth in vivo in a p53-independent manner, we generated p53+/−/SKH-1 mice, which are known to develop skin tumors at an accelerated rate compared to wild-type animals. Many of the tumors in these mice grow more aggressively and exhibit a poorly differentiated phenotype following chronic UV irradiation (van Kranen et al., 2005). Importantly, p53+/−/SKH-1 mice do not die prematurely due to spontaneous development of internal malignancies.
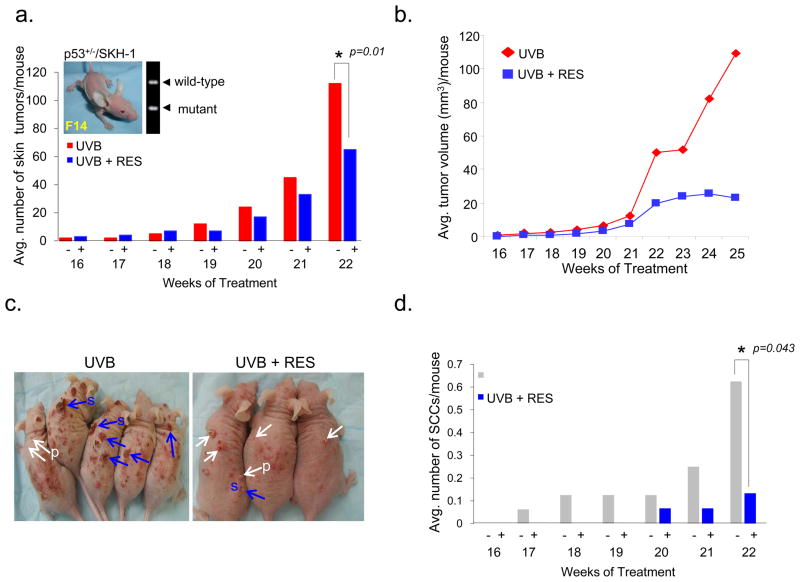
Thirty-one p53+/−/SKH-1 mice were divided into two groups (group-1, n = 16; group-2, n = 15) and irradiated with 180 mJ/cm2 UVB twice a week for 25 weeks. Two weeks prior to the first UVB irradiation, RES was administered to group-2 mice by oral gavage (200 mg/kg body weight in 0.5% methylcellulose) three times per week. Control mice (group-1) were treated with 0.5% methylcellulose suspension alone and served as age-matched, positive controls. RES treatment reduced the average number of skin tumors by 43% at week 22 and the average tumor volume was diminished by 79%, compared with control mice (Fig. 1a–1c). SCCs were detectable as early as week 17 in the UVB-irradiated/untreated control animals, whereas carcinoma development was delayed until week 20 in the RES-treated animals (Fig. 1d). Histological assessment revealed that the number of SCCs in RES-treated mice was 80% less than in the UVB-only control group at week 22 (Fig. 1d). Direct sequencing analyses identified p53 mutations in SCCs from both the RES-treated and RES-untreated groups (data not shown), suggesting that the anti-tumor effects of RES are p53-independent.
Figure 1. Resveratrol treatment suppresses the growth of UVB-induced SCCs in the skin of p53+/−/SKH-1 mice.
Average skin tumor number (a) and volume (b) per mouse in each group during UVB-induced skin carcinogenesis. Group 1, UVB-irradiated (180 mJ/cm2, twice a week, n = 16); Group 2, UVB-irradiated/RES-treated (200 mg/kg body weight, by gavage, three times a week, n = 15). p53+/−/SKH-1 (F14) mouse is shown in inset in (a). (c) Representative pictures of UVB-irradiated mice not-treated (UVB) and treated (UVB+RES) with RES at week 22. Tumors were categorized as benign papillomas (P, white arrows) and SCCs (S, blue arrows) based on histological evaluation. (d) Average number of SCCs per mouse.
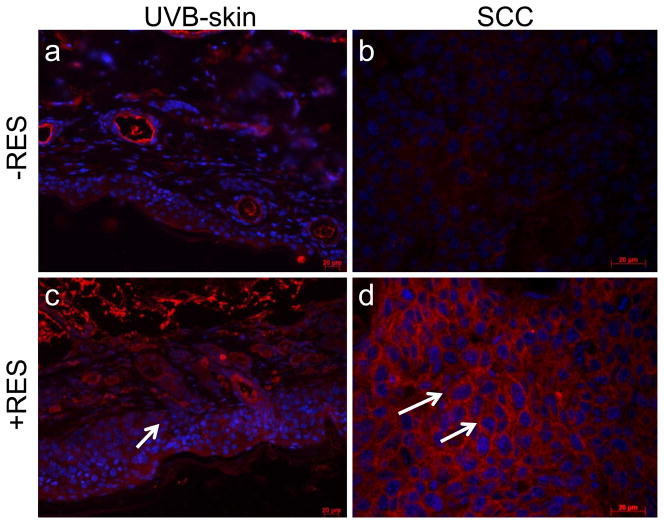
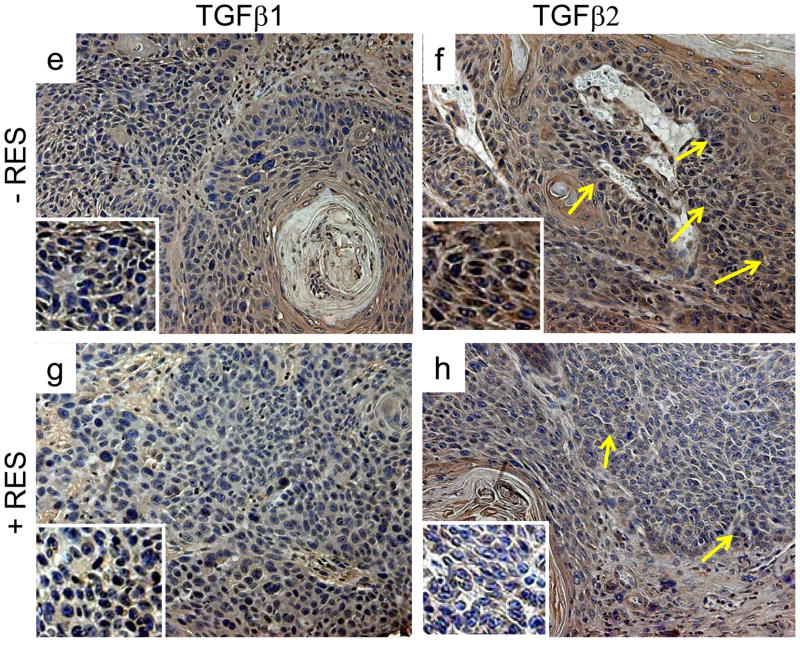
Resveratrol decreases TGFβ-2 expression in SCCs and skin of p53+/−/SKH-1 mice following chronic UVB irradiation
Because EMT is critical to tumor progression and metastases, the delayed growth of SCCs in the RES-treated animals led us to ask whether this could be explained by interference with modulators of EMT. A central event in EMT is the loss of expression of epithelial cadherin (E-cadherin), a surface receptor that plays an essential role in the formation of adherence junctions and it is often mutated or lost in cancer cells (Thiery, 2003; Thiery and Chopin, 1999). SCCs excised from the RES-treated mice showed increased levels of E-cadherin by immunofluorescence (Fig. 2d). E-cadherin staining was barely detectable in UVB-induced SCCs harvested from non-treated control animals (Fig. 2b). Furthermore similar to human SCCs, both TGF-β1 and TGF-β2 were overexpressed in UVB-induced SCCs in the p53+/−/SKH-1 mice (Fig. 2e, 2f). However, RES treatment substantially decreased TGF-β2 expression in marina SCCs (Fig. 2h), whereas the effect on TGF-β1 expression was substantially less (Fig. 2g). These data indicate that both TGF-β1 and TGF-β2 are overexpressed in UVB-induced murine SCCs and human SCCs, and that treatment with RES may inhibit tumorigenicity and malignant tumor progression inp53+/−/SKH-1 mice by downregulating TGF-β2 and/or TGF-β2-mediated regulatory proteins.
Figure 2. Resveratrol decreases UVB-induced TGF-β2 expression and increases E- cadherin.
Immunofluorescent distribution of E-cadherin (a–d) and TGF-β1 and TGF-β2 (e–h) in UVB-induced SCCs harvested from RES-treated or non-treated animals. Magnification 200x, for immunohistochemical staining with enlargement of staining in the insets.
Resveratrol downregulates TGF-β2/Smad-dependent and independent signaling pathways in A431 cells
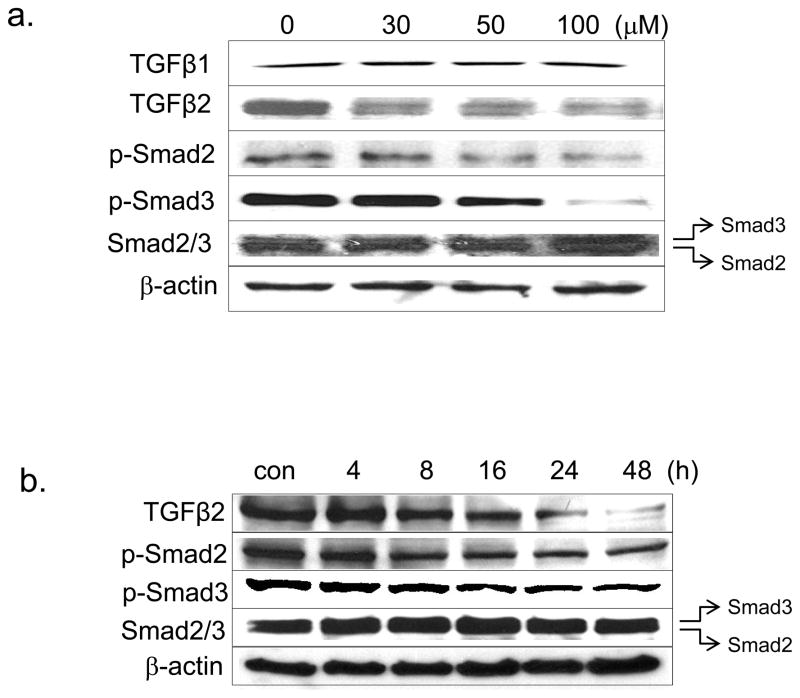
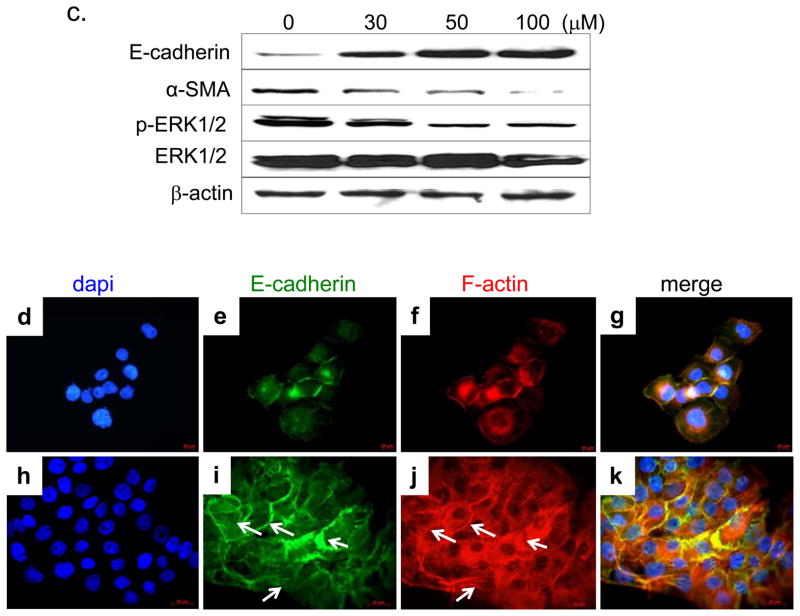
We further investigated the effects of RES on TGF-β2 and its downstream signaling pathways in human A431 SCC cells. RES decreased TGF-β2 levels in a time-dependent and concentration-dependent manner (Fig. 3a and 3b), whereas TGF-β1 was unaffected (Fig. 3a). In RES-treated cells, the levels of phospho-Smad2/3 (signal transducers that are downstream targets of TGF-β receptor-I) were decreased dose-dependently, completely abolishing Smad2/3 phosphorylation at a dose of 100 μM (Fig. 3a). TGF-β1–mediated EMT has been attributed to the relocalization and downregulation of cell contact proteins and cytoskeleton reorganization (stress fiber assembly), and upregulation of α-SMA. RES treatment substantially reduced α-SMA and increased E-cadherin expression (Fig. 3c). E-cadherin–deficient SCC cells reportedly manifest circumferential cortical actin reorganization and actin stress fiber loss (Alt-Holland et al., 2008). We used fluorescent-tagged phalloidin, which binds specifically to F-actin, to visualize the actin skeleton in RES-treated A431 cells. RES-treated A431 cells displayed a complex network of stress fibers with perimarginal axially aligned actin bundles (Fig. 3j, arrows). In contrast, untreated A431 cells showed no apparent stress fibers, but displayed a cortical actin network (Fig. 3f). Interestingly, the cellular distribution of E-cadherin coincided with that of F-actin. E-cadherin was present cortically in untreated cells (Fig. 3e), whereas after RES treatment its distribution resembled that of the actin stress fiber network (Fig. 3i).
Figure 3. Resveratrol inhibits both TGF-β-mediated Smad-dependent and -independent ERK1/2 pathways.
(a, c) Western blot of protein extracts prepared from A431 cells treated with the indicated concentrations of RES for 24 h. (b) A431 cells treated with 50 μM RES at the indicated time periods. β-actin (internal loading control); 80 μg/lane. Cellular distribution of E-cadherin (e, i) and actin stress fibers (f, j) following RES treatment (50 μM for 24 h), assessed by indirect immunofluorescent and phalloidin staining, respectively.
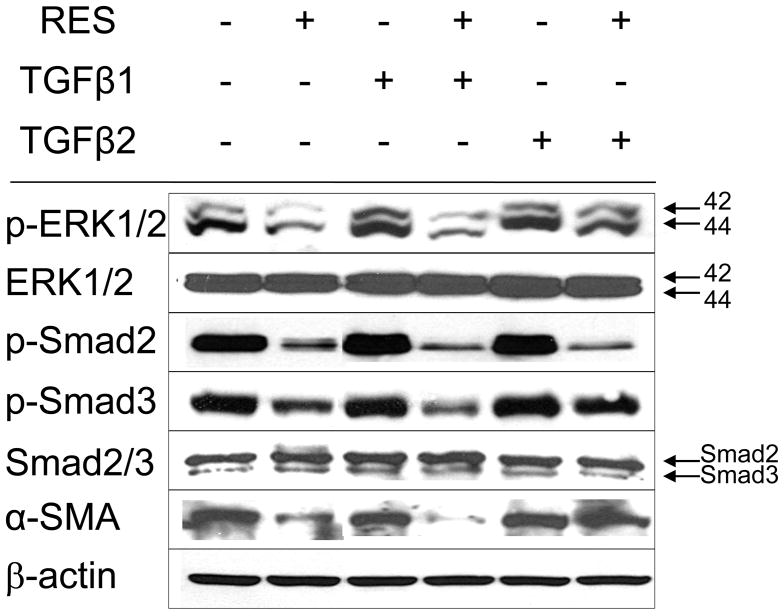
TGF-β is also known to activate non-Smad signaling pathways such as mitogen- and stress-activated kinase (MAPK). The ERK signaling pathway activation is required for TGF-β1–mediated EMT in vitro (Xie et al., 2004). Consistent with this, we also observed phospho-ERK1/2 downregulation (Fig. 3c), indicating that RES likely suppressed both TGF-β/Smad-dependent and -independent pathways. The addition of TGF-β2 (2 ng/mL), but not TGF-β1, restored the levels of p-ERK1/2, p-Smad3, and α-SMA (Fig. 4). In contrast, p-Smad2 was not restored by the addition of TGF-β2 (Fig. 4), suggesting that additional mechanisms regulate Smad2 phosphorylation.
Figure 4. TGF-β2 activates ERK-1 and Smad3.
Western blot of TGF-β2 pathway components in A431 cells following RES treatment in the presence or absence of porcine TGF-β1, or TGF-β2 at 2 ng/mL. β-actin (internal loading control); 80 μg/lane per lane.
Resveratrol reduces Akt/CREB-mediated TGF-β2 expression
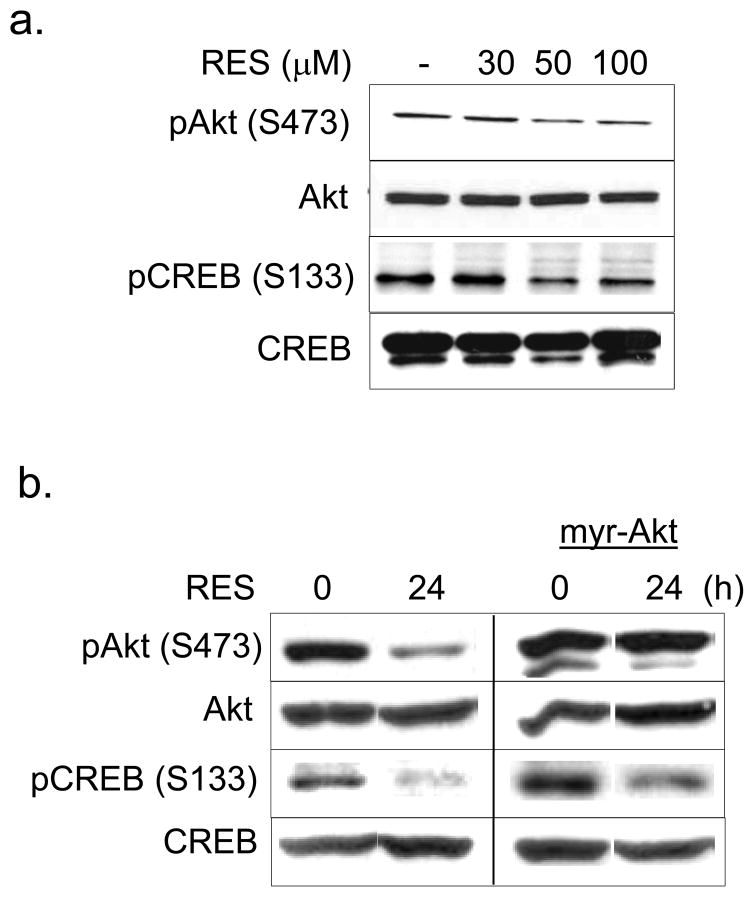
TGF-β2 gene transcription is regulated by CREB and activating transcription factor-1 (ATF-1) (Kingsley-Kallesen et al., 1999). Specific phosphorylation of CREB at serine 133 induces a stable interaction with the co-activators p300 and the CREB-binding protein CBP, leading to target gene transactivation (Chrivia et al., 1993). Akt is an anti-apoptotic serine-threonine kinase that regulates numerous critical cellular pathways, including those that drive cellular proliferation and inhibition of apoptosis (Garcia et al., 2006). Upon activation, Akt phosphorylates various transcription factors such as CREB (Du and Montminy, 1998). Because RES decreased the TGF-β2 transcript level in A431 cells (Fig. 5d), we hypothesized that the mechanistic basis of RES-mediated TGF-β2 downregulation may be through modulation of Akt- CREB signaling. In RES-treated A431 cells, the levels of both phospho-Akt (S473) and phospho-CREB (ser 133) were decreased dose-dependently, whereas Akt and CREB were unaffected (Fig. 5a). To further assess the effects of RES on CREB phosphorylation in A431 cells, we stably expressed constitutively active myristoylated Akt, which bypasses the requirement for pleckstrin homology domain–mediated membrane recruitment (Kumar et al., 2003). Akt overexpression only partially rescued RES-mediated inhibition of CREB phosphorylation (Fig. 5b), suggesting that RES-mediated inhibition of CREB phosphorylation involves both Akt-dependent and -independent mechanisms.
Figure 5. Resveratrol suppresses Akt/CREB activation and in vitro invasiveness in A431 cells.
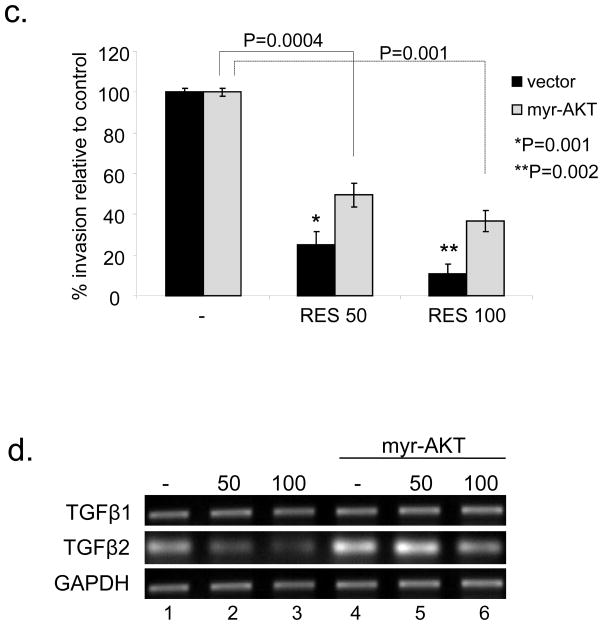
(a) RES inhibits Akt/CREB phosphorylation. Total extracts prepared from A431 cells treated with the indicated concentrations of RES for 24 h were analyzed by Western blotting. Constitutively active Akt suppresses RES inhibition of CREB phosphorylation (b) and in vitro invasiveness of A431 cells (c). A431 cells or A431 cells expressing myr-Akt were treated with RES at 50 μM for 24 h (b) or at 50–100 μM for 24 h (c). Cell invasiveness was assessed in Matrigel-coated chambers. Results from three independent experiments performed in triplicate are expressed as percent of control. (d) Cells treated as in (c) were assessed for TGF-β1 and 2 expression by RT-PCR. GAPDH served as an internal control.
Resveratrol-mediated suppression of in vitro invasiveness is rescued by Akt-dependent TGF-β2 upregulation
Loss of E-cadherin is known to be associated with an increase in the invasive potential of cancer cells. Therefore, we assessed the invasive capacity of RES-treated A431 cells employing cell invasion assays using a Matrigel Matrix. RES at 50–100 μm diminished the invasiveness of A431 cells by 75–90% (p < 0.002) compared to untreated cells (Fig. 5c), accompanied by downregulation of TGF-β2 but not TGF-β1 (Fig. 5d). The invasive capacity of RES-treated cells increased 2–3 fold in the presence of constitutively active Akt (Fig. 5c). Furthermore, overexpression of active Akt alone induced TGF-β2 expression in the absence of RES (Fig. 5d, lane 4), suggesting a direct link between Akt and TGFβ2. Active Akt was able to overcome the RES-mediated inhibition of TGF-β2 expression (Fig. 5d, lanes 5 and 6). These results indicate that the growth inhibitory effects of RES are in part mediated by downregulation of Akt-TGF-β2 signaling in A431 cells.
DISCUSSION
A major challenge in current cancer research is to identify novel approaches to chemoprevention using non-toxic natural substances. RES is a natural phytoalexin found in various dietary constituents including the skin of red grapes. We previously showed that RES can inhibit cell cycle progression A431 cells (G1/S phase) lacking functional p53 (Kim et al., 2006). In this study, we show that oral administration of RES to p53+/−/SKH-1 transgenic mice results in p53-independent suppression of UVB-induced skin carcinogenesis. Specifically, UVB-induced SCCs appeared later in the RES-treated mice, and the malignant conversion of benign papillomas to SCCs was substantially inhibited. RES treatment resulted in increased expression of E-cadherin and decreased TGF-β2 expression in SCCs.
In probing the mechanism(s) underlying TGF-β2 downregulation, we found that RES inhibited both TGF-β2/Smad-dependent and -independent ERK signaling pathways and suppressed the in vitro invasiveness of A431 cells. RES inhibited TGF-β2 expression, which is known to be regulated by CREB transcriptional factors. The CREB/ATF family of transcription factors responds to various signals that drive cell proliferation, differentiation, and adaptive responses (Shaywitz and Greenberg, 1999). In addition to cAMP-dependent protein kinase A (PKA), CREB is a target of various kinases, including Akt1 and mitogen- and stress-activated kinase 1, a kinase activated in the p38 MAPK- and ERK-dependent cascades (Caravatta et al., 2008; Deak et al., 1998; Xing et al., 1996). RES-mediated Akt1 inhibition occurred concomitant with decreased CREB phosphorylation. Furthermore, by constitutively activating Akt it was possible to partially rescue RES inhibition of CREB phosphorylation and TGF-β2 expression, indicating that RES suppression of TGF-β2 involves downregulation of the Akt/CREB pathway.
TGF-β is important in modulating tumor progression (Cui et al., 1996; Han et al., 2005; Valcourt et al., 2005). At least three TGF-β isoforms have been identified (TGF-β1, TGF-β2, and TGF-β3). Among these, TGF-β2 overexpression has been observed in more advanced stages of gastric carcinoma and is thought to correlate with a poor prognosis (Vagenas et al., 2007). It has been reported that specific inhibition of TGF-β2 using an antisense oligodeoxynucleotide prolongs the survival time of patients suffering from recurrent or refractory malignant gliomas, including two patients in whom long-lasting complete tumor remission occurred (Hau et al., 2007). These observations suggest that identifying effective methods for TGF-β2 inhibition/downregulation may be therapeutically efficacious in later stages of tumor progression. In skin, it is possible that each TGF-β isoform could have differential effects during various stages of carcinogenesis. TGF-β1 expression appears to be associated with a more differentiated tumor phenotype, whereas TGF-β2 expression is more typical of highly malignant and invasive phenotypes. TGF-β3 expression is enhanced in the stroma of malignant tumors in subcutaneously growing HaCat (immortalized human keratinocytes)-ras tumors in nude mice (Gold et al., 2000). Our results are consistent with this pattern in that TGF-β2 was less strongly overexpressed in UVB-induced benign precursors (papillomas) of SCCs than in UVB-induced SCCs, implying that TGF-β2 may drive the latter stages of UVB-induced skin carcinogenesis. RES reduced TGF-β2 expression both in vitro and in vivo. It should be pointed out that in contrast to our data; RES was previously shown to increase the transcription of the TGF-β2 gene and the TGF-β2 protein expression, and to activate Smad signaling in an autocrine manner in the A549 human lung epithelial cell line (Suenaga et al., 2008). However, lower doses (3–10 μM) of RES were used this study, and the effect of these dosages on TGF-β2 induction was not correlated to cell viability or other cellular functions. In another study RES (10μM) inhibited TGF-β2 in breast cancer cells (Serrero and Lu, 2001). These differential effects may be due to differences in the RES concentrations used or to differential effects in various tissue types. In our in vitro study, RES downregulated both TGF-β/Smad and Smad-independent ERK activation. Our data indicate that RES-mediated inhibition of ERK activation is likely mediated through TGF-β2, since exogenous addition of TGF-β2 but not TGF-β1 rescued ERK activation. Interestingly, Smad-2 inhibition was not rescued by the addition of TGF-β2 or -β1, suggesting TGF-β-independent mechanisms for the RES-mediated suppression of Smad2 activation. Although TGF-β signaling is known to promote EMT via both Smad and non-Smad dependent pathways (Zavadil and Bottinger, 2005), mice with a keratinocyte-specific Smad2 deletion reportedly have accelerated formation and malignant progression of chemically induced skin tumors, and Smad2-deficient tumors exhibit EMT (Hoot et al., 2008). In our studies in A431 cells, RES had no effect on Smad2. It is not clear in the present study whether Smad2 deficiency and inhibition of Smad2 phosphorylation have different functions.
EMT blockade offers a potentially novel and effective approach for the prevention/treatment of cancer, particularly in lesions with a propensity for metastasis. However, accelerated EMT may be only a partial determinant of malignant progression that may require other oncogenic events to promote tumor invasion. Other pathways commonly activated in late-stage skin carcinogenesis, such as Akt and NF-κB, are known to enhance EMT (Dong et al., 2007; Hoot et al., 2008; Lester et al., 2007). Here we have shown that RES inhibits Akt-mediated TGF-β2 functional cooperation between cellular growth signaling and TGF-β pathway regulators, which may enhance EMT. Whether there is a spatial correlation (i.e., expression in the invasion front) between TGF-β2/Akt expression and tumor progression, or whether and how RES specifically targets these cells in vivo requires further investigation. In summary, our data indicate that RES is a potent inhibitor of tumor cell growth and may modulate tumor progression by altering the EMT process by downregulating TGF-β2.
MATERIALS AND METHODS
Cell lines and reagents
A431 human epidermoid carcinoma cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). They were maintained in Dulbecco’s modified Eagle medium (DMEM), supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin, and kept in an atmosphere of 95% air/5% CO2 in a 37 °C humidified incubator, in accordance with ATCC guidelines. Resveratrol was purchased from LKT Laboratories Inc (St. Paul, MN) and porcine TGF-β1, 2 from R&D systems (Minneapolis, MN). pUSEamp-myrAKT1 was obtained from millipore (Billerica, MA). A431 cells cultured in 100 mm plate were transfected with pUSEamp-myrAKT1 at 10 μg using lipofectamine 2000 (Invitrogen, Carlsbed, CA) and stable colonies were selected in the presence of G418 (Sigma-aldrich, St Louis, MO).
Total RNA preparation and RT-PCR
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA). About 2 μg of total RNA and SuperScript™ III First-Strand Synthesis System (Invitrogen, Carlsbad, CA) were used for the cDNA synthesis reaction according to the manufacturer’s instructions. PCRs were carried out with the TGF-β1 primer set with forward primer 5′-GGGACTATCCACCTGCAAGA-3′ and reverse primer 5′-CAACTCCGGTGACATCAAAA-3′, and the TGF-β2 primer set with forward primer 5′-CCGGAGGTGATTTCCATCTA-3′ and reverse primer 5′-TGCAGCAGGGACAGTGTAAG-3′. Human GAPDH cDNA was amplified with hGAPDH forward primer 5′-TCATTGACCTCAACTACATGGTTTAC-3′ and hGAPDH reverse primer 5′-GGCATGGA-CTGTGGTCATGAGTC-3′ to determine the integrity of the RNA and for use as an internal control.
Preparation of cell lysates and Western blotting analysis
The preparation of total cell lysates and Western blotting analysis were performed as previously described (Kim et al., 2002). Antibodies against TGF-β1 and 2, and Smad2/3 were from Santa Cruz Biotechnology (Santa Cruz, CA), p-CREB (S133), p-Smad2 (S465/467) and p- Smad3(S423/425), p-ERK1/2(T202/Y204), p-Akt(S473), and Akt from Cell Signaling Technology (Danvers, MA), α-SMA from Sigma-Aldrich (St. Louis, MO) and ERK1/2 from Upstate (Lake Placid, NY).
Generation of p53+/−/SKH-1 hairless mice
Eight-week-old female non-agouti p53−/− mice (B6/C57BL/6J-Trp53−/−) were obtained from Jackson Laboratories (Bar Harbor, ME). These mice were crossed with SKH-1 males (Charles River Laboratories, Wilmington, MA) to generate littermates heterozygous for both p53 and hairless alleles. p53+/− hairless SKH-1 mice (F2) were selected from inter-generation crosses among F1 littermates of SKH-1 and p53−/−/C57BL/6 genetic background. These mice were then backcrossed for 11 generations onto SKH-1 hairless mice to minimize the C57BL/6 genetic background, resulting in a colony of p53+/− hairless SKH-1 mice (F14). Mice were genotyped by PCR for both wild-type and the knock-out gene using specific primers for p53, which are 5′-CTTGGGTGGAGAGGCTATTC, AGGTGAGATGACAGGAGATC, 5′-ATAGGTCGGCGGTTCAT, and 5′-CCCGAGTATCTGGAAGACAG amplifying a 600 bp fragment for the wild-type or a 280 bp fragment for the mutant allele. (The sequences of PCR primers to detect p53 +/+, p53 +/−, and p53 −/− were obtained from The Jackson Labs, Bar Harbor, ME.).
UV light source
A UV Irradiation Unit (Daavlin Co., Bryan, OH) equipped with an electronic controller to regulate dosage was used. The UVB source consisted of eight FS72T12-UVB-HO lamps that emit UVB (290–320 nm, 75–80% of total energy) and UVA (320–380 nm, 20–25% of total energy). We employed a Kodacel cellulose film (Kodacel TA401/407) to eliminate UVC radiation. A UVC sensor (Oriel’s Goldilux UVC Probe) was routinely used during each exposure to confirm lack of UVC emission. The dose of UVB was quantified with a UVB Spectrum 305 Dosimeter obtained from the Daavlin Co. (Bryan, OH). The radiation was further calibrated with an IL1700 Research Radiometer/Photometer from International Light Inc. (Newburyport, MA). The distance between the radiation source and targets was maintained at 30 cm. The irradiation assembly was kept in an air-conditioned room and a fan was kept inside the exposure chamber to minimize temperature fluctuations during irradiation.
Tumor study in p53+/−/SKH-1 mice
Animal experiments were performed in accordance with guidelines of our approved Columbia University Institutional Animal Care and Use Committee (IACUC) protocol. Thirty-one p53+/−/SKH-1 mice were divided into group 1 (n=16) and group 2 (n=15) and irradiated with 180 mJ/cm2 UVB twice a week for 25 weeks. Two weeks prior to the beginning of UVB irradiation, resveratrol was administered to mice in group 2 by oral gavage, 200 mg/kg body weight in 0.5% methylcellulose (Sigma-Aldrich, St. Louis, MO), three times per week. Control mice (group 1) were treated with 0.5 % methylcellulose suspension alone and served as age-matched, untreated controls. Mice were sacrificed at week 25. Their dorsal skin was removed and tumors were harvested and collected for histological and immunohistochemical studies. Immunohistochemical staining was performed as previously described (LeBel et al., 1992).
Cell invasion assay
The cell invasion assays were performed using BD Matrigel Matrix which provides a biologically active basement membrane model for in vitro invasion assay. Briefly, A431 cells stably transfected with pUSEamp-myrAKT1 or vector were cultures in the presence of resveratrol in a BD BioCoat™ Matrigel™ Invasion Chamber (BD Bioscience, San Jose, CA). In this assay, cells transmigrate from an upper well through a porous filter coated with Matrigel toward a chemoattractant in the lower chamber. A431 cell suspensions were prepared in a culture medium containing 5×104 cells/mL in 24-well chambers. After 22 hours of incubation in a humidified tissue culture incubator at 37 °C in a 5% CO2 atmosphere, cells that invaded the lower compartment of the membranes were stained with Toluidine blue and counted with a Coulter counter. Three independent experiments were performed in triplicate and the percentage of invasive cells was determined in at least three fields per experiment.
Statistical analyses
Statistical analyses were performed using the Student’s t test (two-tailed): p < 0.05 was considered statistically significant.
Acknowledgments
This work was partially supported by the NIH Grants, R01-ES-015323 to MA, N01-CN-43300 to MA and DRB, Irving Scholar Award and R03 CA125855-02 to ALK, and R01 CA-097249-01 to DRB.
Abbreviation
- Akt
protein kinase B
- CREB
cAMP response-binding protein
- EMT
epithelial-mesenchymal transition
- Erk1/2
extracellular signal-regulated kinases
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- MAPK
Mitogen-activated protein kinases
- RES
resveratrol
- SCC
squamous cell carcinoma
- TGF-β
transforming growth factor-β
- UV
ultraviolet
Footnotes
The work performance site: New York, New York, USA
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Alt-Holland A, Shamis Y, Riley KN, DesRochers TM, Fusenig NE, Herman IM, et al. E-cadherin suppression directs cytoskeletal rearrangement and intraepithelial tumor cell migration in 3D human skin equivalents. The Journal of investigative dermatology. 2008;128:2498–2507. doi: 10.1038/jid.2008.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, et al. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224:274–283. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz MH, Reagan-Shaw S, Wu J, Longley BJ, Ahmad N. Chemoprevention of skin cancer by grape constituent resveratrol: relevance to human disease? Faseb J. 2005;19:1193–1195. doi: 10.1096/fj.04-3582fje. [DOI] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Benjamin CL, Ullrich SE, Kripke ML, Ananthaswamy HN. p53 tumor suppressor gene: a critical molecular target for UV induction and prevention of skin cancer. Photochemistry and photobiology. 2008;84:55–62. doi: 10.1111/j.1751-1097.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- Caravatta L, Sancilio S, di Giacomo V, Rana R, Cataldi A, Di Pietro R. PI3-K/Akt-dependent activation of cAMP-response element-binding (CREB) protein in Jurkat T leukemia cells treated with TRAIL. J Cell Physiol. 2008;214:192–200. doi: 10.1002/jcp.21186. [DOI] [PubMed] [Google Scholar]
- Choi J, Park SY, Joo CK. Transforming growth factor-beta1 represses E-cadherin production via slug expression in lens epithelial cells. Invest Ophthalmol Vis Sci. 2007;48:2708–2718. doi: 10.1167/iovs.06-0639. [DOI] [PubMed] [Google Scholar]
- Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- Cui W, Fowlis DJ, Bryson S, Duffie E, Ireland H, Balmain A, et al. TGFbeta1 inhibits the formation of benign skin tumors, but enhances progression to invasive spindle carcinomas in transgenic mice. Cell. 1996;86:531–542. doi: 10.1016/s0092-8674(00)80127-0. [DOI] [PubMed] [Google Scholar]
- Davies M, Robinson M, Smith E, Huntley S, Prime S, Paterson I. Induction of an epithelial to mesenchymal transition in human immortal and malignant keratinocytes by TGF-beta1 involves MAPK, Smad and AP-1 signalling pathways. J Cell Biochem. 2005;95:918–931. doi: 10.1002/jcb.20458. [DOI] [PubMed] [Google Scholar]
- Deak M, Clifton AD, Lucocq LM, Alessi DR. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 1998;17:4426–4441. doi: 10.1093/emboj/17.15.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Dong R, Wang Q, He XL, Chu YK, Lu JG, Ma QJ. Role of nuclear factor kappa B and reactive oxygen species in the tumor necrosis factor-alpha-induced epithelial-mesenchymal transition of MCF-7 cells. Braz J Med Biol Res. 2007;40:1071–1078. doi: 10.1590/s0100-879x2007000800007. [DOI] [PubMed] [Google Scholar]
- Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- Garcia GE, Nicole A, Bhaskaran S, Gupta A, Kyprianou N, Kumar AP. Akt-and CREB-mediated prostate cancer cell proliferation inhibition by Nexrutine, a Phellodendron amurense extract. Neoplasia. 2006;8:523–533. doi: 10.1593/neo.05745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold LI, Jussila T, Fusenig NE, Stenback F. TGF-beta isoforms are differentially expressed in increasing malignant grades of HaCaT keratinocytes, suggesting separate roles in skin carcinogenesis. J Pathol. 2000;190:579–588. doi: 10.1002/(SICI)1096-9896(200004)190:5<579::AID-PATH548>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Han G, Lu SL, Li AG, He W, Corless CL, Kulesz-Martin M, et al. Distinct mechanisms of TGF-beta1-mediated epithelial-to-mesenchymal transition and metastasis during skin carcinogenesis. J Clin Invest. 2005;115:1714–1723. doi: 10.1172/JCI24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hau P, Jachimczak P, Schlingensiepen R, Schulmeyer F, Jauch T, Steinbrecher A, et al. Inhibition of TGF-beta2 with AP 12009 in recurrent malignant gliomas: from preclinical to phase I/II studies. Oligonucleotides. 2007;17:201–212. doi: 10.1089/oli.2006.0053. [DOI] [PubMed] [Google Scholar]
- Hoot KE, Lighthall J, Han G, Lu SL, Li A, Ju W, et al. Keratinocyte-specific Smad2 ablation results in increased epithelial-mesenchymal transition during skin cancer formation and progression. J Clin Invest. 2008;118:2722–2732. doi: 10.1172/JCI33713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AL, Athar M, Bickers DR, Gautier J. Ultraviolet-B-induced G1 arrest is mediated by downregulation of cyclin-dependent kinase 4 in transformed keratinocytes lacking functional p53. The Journal of investigative dermatology. 2002;118:818–824. doi: 10.1046/j.1523-1747.2002.01734.x. [DOI] [PubMed] [Google Scholar]
- Kim AL, Zhu Y, Zhu H, Han L, Kopelovich L, Bickers DR, et al. Resveratrol inhibits proliferation of human epidermoid carcinoma A431 cells by modulating MEK1 and AP-1 signalling pathways. Exp Dermatol. 2006;15:538–546. doi: 10.1111/j.1600-0625.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- Kingsley-Kallesen ML, Kelly D, Rizzino A. Transcriptional regulation of the transforming growth factor-beta2 promoter by cAMP-responsive element-binding protein (CREB) and activating transcription factor-1 (ATF-1) is modulated by protein kinases and the coactivators p300 and CREB-binding protein. The Journal of biological chemistry. 1999;274:34020–34028. doi: 10.1074/jbc.274.48.34020. [DOI] [PubMed] [Google Scholar]
- Kumar AP, Garcia GE, Ghosh R, Rajnarayanan RV, Alworth WL, Slaga TJ. 4-Hydroxy-3-methoxybenzoic acid methyl ester: a curcumin derivative targets Akt/NF kappa B cell survival signaling pathway: potential for prostate cancer management. Neoplasia. 2003;5:255–266. doi: 10.1016/S1476-5586(03)80057-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- Leivonen SK, Hakkinen L, Liu D, Kahari VM. Smad3 and extracellular signal-regulated kinase 1/2 coordinately mediate transforming growth factor-beta-induced expression of connective tissue growth factor in human fibroblasts. J Invest Dermatol. 2005;124:1162–1169. doi: 10.1111/j.0022-202X.2005.23750.x. [DOI] [PubMed] [Google Scholar]
- Lester RD, Jo M, Montel V, Takimoto S, Gonias SL. uPAR induces epithelial-mesenchymal transition in hypoxic breast cancer cells. J Cell Biol. 2007;178:425–436. doi: 10.1083/jcb.200701092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi RA, Leof EB. TGF-beta signaling: a tale of two responses. J Cell Biochem. 2007;102:593–608. doi: 10.1002/jcb.21501. [DOI] [PubMed] [Google Scholar]
- Serrero G, Lu R. Effect of resveratrol on the expression of autocrine growth modulators in human breast cancer cells. Antioxid Redox Signal. 2001;3:969–979. doi: 10.1089/152308601317203512. [DOI] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem. 1999;68:821–861. doi: 10.1146/annurev.biochem.68.1.821. [DOI] [PubMed] [Google Scholar]
- Suenaga F, Hatsushika K, Takano S, Ando T, Ohnuma Y, Ogawa H, et al. A possible link between resveratrol and TGF-beta: resveratrol induction of TGF-beta expression and signaling. FEBS Lett. 2008;582:586–590. doi: 10.1016/j.febslet.2008.01.024. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Current opinion in cell biology. 2003;15:740–746. doi: 10.1016/j.ceb.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Chopin D. Epithelial cell plasticity in development and tumor progression. Cancer Metastasis Rev. 1999;18:31–42. doi: 10.1023/a:1006256219004. [DOI] [PubMed] [Google Scholar]
- Vagenas K, Spyropoulos C, Gavala V, Tsamandas AC. TGFbeta1, TGFbeta2, and TGFbeta3 protein expression in gastric carcinomas: correlation with prognostics factors and patient survival. The Journal of surgical research. 2007;139:182–188. doi: 10.1016/j.jss.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Valcourt U, Kowanetz M, Niimi H, Heldin CH, Moustakas A. TGF-beta and the Smad signaling pathway support transcriptomic reprogramming during epithelial-mesenchymal cell transition. Mol Biol Cell. 2005;16:1987–2002. doi: 10.1091/mbc.E04-08-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kranen HJ, Westerman A, Berg RJ, Kram N, van Kreijl CF, Wester PW, et al. Dose-dependent effects of UVB-induced skin carcinogenesis in hairless p53 knockout mice. Mutation research. 2005;571:81–90. doi: 10.1016/j.mrfmmm.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Xie L, Law BK, Chytil AM, Brown KA, Aakre ME, Moses HL. Activation of the Erk pathway is required for TGF-beta1-induced EMT in vitro. Neoplasia (New York, NY. 2004;6:603–610. doi: 10.1593/neo.04241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Ginty DD, Greenberg ME. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science (New York, NY. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- Yue J, Mulder KM. Transforming growth factor-beta signal transduction in epithelial cells. Pharmacol Ther. 2001;91:1–34. doi: 10.1016/s0163-7258(01)00143-7. [DOI] [PubMed] [Google Scholar]
- Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24:5764–5774. doi: 10.1038/sj.onc.1208927. [DOI] [PubMed] [Google Scholar]