Abstract
Given their immune modulating capacity, regulatory T cells (Treg) cells may be important players in the induction of the protective T cell response (Th1) to genital chlamydial infection. Recent work has demonstrated that plasmacytoid dendritic cells (pDC) respond to genital chlamydial infection, and that pDC may be uniquely positioned for the induction of Treg cells during this infection. Here we present the first data demonstrating that Treg influx into the draining lymph node and site of infection during genital chlamydial infection. We found that pDC depletion altered the numbers of Treg and non-protective inflammatory cells (IFNγ producing CD8+ T and IFNγ producing NKT cells) in the spleens of mice genitally infected with C. muridarum. Furthermore, pDC depletion did not alter Th1 cell numbers, indicating that pDC modulate cells which could inhibit and promote non-protective inflammation during genital chlamydial infection. Finally, we demonstrate that depletion of pDC results in less severe dilation and collagen deposition in the oviduct following resolution of infection, implicating pDC activity in the formation of sequelae following genital C. muridarum infection.
Keywords: Chlamydia muridarum, T regulatory cell, FoxP3, Th1 cell
Introduction
Genital infection by C. trachomatis is the most prevalent bacterial sexually transmitted disease worldwide (WHO, 2004). Sequelae of these infections include the related pathologies of pelvic inflammatory disease, ectopic pregnancy, and infertility. As a result of the prevalence and sequelae of C. trachomatis infection, recent efforts have focused on the development of a vaccine to curtail spread of this pathogen (Brunham & Rey-Ladino, 2005). These efforts have yet to result in an efficacious vaccine due, in part, to the lack of understanding of the immunological factors required to elicit a protective and non-pathological response to genital infection by C. trachomatis. T cells are central in the eradication of chlamydial infection, but are also implicated in exacerbating the sequelae associated with genital chlamydial infection (Brunham & Rekart, 2008). Therefore, recent efforts to develop a vaccine against C. trachomatis genital infection have focused on the induction of T cells which do not promote immune mediated pathology. As dendritic cells (DC) are central in the induction of T cell responses, recent work has focused on the involvement of DC during genital chlamydial infection; (Rey-Ladino, et al., 2005, Rey-Ladino, et al., 2007, Moniz, et al., 2009).
The capacity of DC to elicit varying immune responses is due, in part, to the existence of multiple DC subsets. The examination of DC subsets responding to genital chlamydial infection revealed that two DC subsets respond differentially to this infection. These include a CD11b+ conventional DC (cDC) and a plasmacytoid DC (pDC) subset (Moniz, et al., 2009). The cDC subset seems most well poised for the induction of a protective Th1 response whereas pDC do not appear capable of directly interacting with T cells and inducing this response during chlamydial genital infection (Moniz, et al., 2009). Alternatively, pDC activity during genital chlamydial infection indicates that pDC may be poised to promote tolerance during chlamydial infection through the induction of regulatory T cells (Treg). The capacity of pDC to induce Treg has been shown in several disease models (de Heer, et al., 2004, Ochando, et al., 2006, Goubier, et al., 2008), and has particular significance towards chlamydial infection. In particular, the immunomudulatory potential of Treg may prove to be significant given the implication of immune mediated pathology during genital chlamydial infection. In this work we examined the role that pDC may have in regulating various T cell subsets in the hope of providing further insight towards the formation of protective and pathological responses during genital C. trachomatis infection.
Materials and Methods
C. muridarum propagation and infection
C. muridarum (Nigg strain) was grown, purified, and titrated in McCoy cells as previously described (Maxion, et al., 2004). Elementary bodies (EB) and reticulate bodies were isolated from McCoy cells and frozen in sucrose-phosphate buffered saline (SPS) at −80°C until use. Female BALB/c mice, aged 5–6 weeks, were treated with progesterone (Depo-Provera, 2.5 mg in 100 μl sterile PBS) one week prior to infection to hormonally synchronize mice. Following anesthetization, mice were inoculated with 1×105 infection forming units (IFU) intra-vaginally. For determination of bacterial load, mice were swabbed vaginally every three days after infection. Swabs were placed in 200 μl SPS and frozen at −80°C prior to determination of bacterial load. Bacterial load (IFU) was determined in McCoy cells, as previously described (Maxion, et al., 2004). Mice were housed and treated in accordance with the AmericanAssociation of Accreditation of Laboratory Animal Care guidelines. Experimental procedures were approved by the UCLA InstitutionalAnimal Care and Use Committee.
Isolation and analysis of T cell subsets
T cells were isolated from individual mice on the indicated day after infection. For analysis of T cells from the genital tract (GT), tissues were excised from individual mice, diced, and cultured for one hour at 37°C in the presence of collagenase (1mg/ml, Type I, Sigma) and DNAse (0.4 mg/ml, Sigma) in Hank’s Balanced Salt solution (HBS) with Ca++ and Mg++. Spleens and iliac nodes (ILN) were diced and kept on ice. Single cell suspensions were obtained by mincing tissue through a 70 μm filter (Falcon) in HBS without Ca++ and Mg++, 5mM EDTA, and 0.5% BSA. Cells were washed twice and maintained in solution containing 5mM EDTA to minimize cell adhesion. Cell suspensions from the GT and spleen were applied to lympholyte M gradients (Cedarlane Labs) and spun at 1200xg for 20 minutes at room temperature. Low density cells at the interface were collected by aspiration and washed twice.
Identification of T cell subsets was done by FACS as previously described for DC subsets (Moniz, et al., 2009), with some modification. Briefly, lymphocytes obtained above were cultured at a concentration of 2×106 cells/ml in complete medium (RPMI 1640 with 10 mM HEPES, 10 mM sodium pyruvate, 2 mM L-glutamine, 10 mM non-essential amino acids, 10% v/v FCS, and 100 μg/ml penicillin and streptomycin) in the presence of brefeldin A (BFA, 10 μg/ml), phorbol myristate acetate (PMA, 5 ng/ml), and ionomycin (500 ng/ml, all from Sigma) at 37°C in a tissue culture incubator. After four hours, DNAse was added to a final concentration of 200 μg/ml and incubated at room temperature for fifteen minutes on an orbital rotator. The suspension was washed and then stained for FACS analysis. T cell subsets were defined as live CD3ε+ (145-2C11) CD4+ (RM4-5) cells expressing either IFNγ (Th1, XMG1.2) or FOXP3 (Treg, FJK-16s). Analysis of Th1 and Treg cells was done simultaneously. Evaluation of additional T cell subsets was done separately by analysis of live CD3ε+ CD8α+ (CD8+ T cells, 53-6.7) cells or CD3ε+ CD49b+ (NKT cells, DX5) cells. FACS acquisition was done on a FACSCaliber (Becton Dickinson) at the UCLA Jonsson Comprehensive Cancer Center and Center for AIDS Research Flow Cytometry Core Facility. FACS analysis was performed using FCS Express (DeNovo Software). For each indicated time point, 10 mice were examined individually from two independent experiments.
pDC depletion
Mice were treated as described above. At one day prior to infection, mice were injected with 500 μg anti-BST2 (mAb 927) antibody or 500 μg isotype control (IgG2b, Biolegend) to deplete pDC (de Heer, et al., 2004, Blasius, et al., 2006). Subsequent injections were done every three days thereafter until day 8 post infection (PI) where mice were either sacrificed on day 9 PI to examine pDC depletion levels or at 7 weeks PI to examine the course and sequelae of infection. pDC depletion efficiency was determined by analysis of pDC numbers in the ILN, GT, and spleen of individual mice by FACS as previously described (Moniz, et al., 2009). Briefly, this examination consisted of isolation of DC using density centrifugation (15% metrizamide) and subsequent FACS analysis of the low density fraction. pDC were examined by gating on CD11c+ CD3- CD19- CD45R(B220)+ cells. Percent depletion of pDC was determined by comparison of total pDC per tissue versus isotype control injections, as shown in Figure 2A.
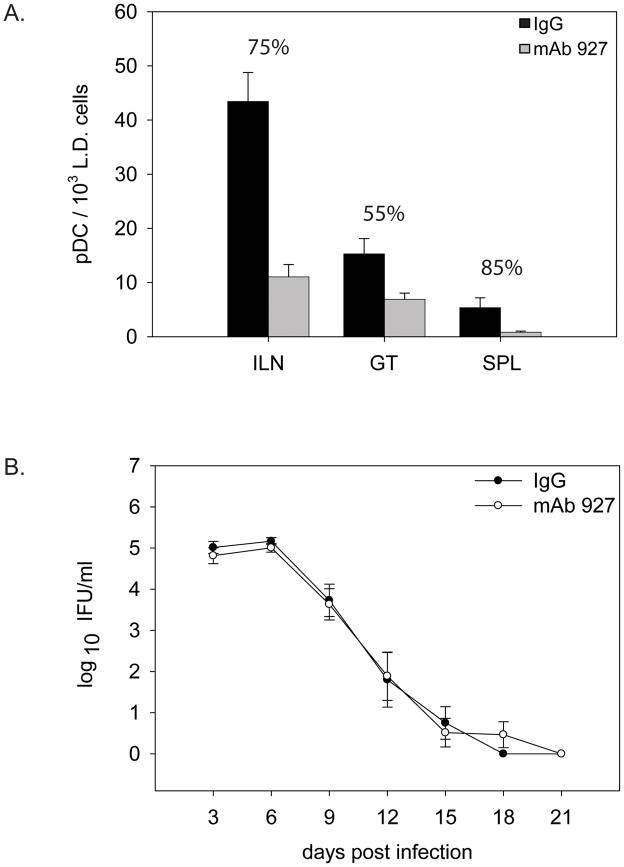
Figure 2.
pDC depletion does not alter the course of infection. A. Mean numbers of pDC in the indicated tissue from a low-density enrichment fraction. Percentages indicate the mean percent depletion of pDC in mice treated with mAb 927 antibody versus mice treated with non-specific antibody (IgG) +/− SEM. Data represent the mean depletion of two independent experiments on day 9 PI. B. Bacterial load of mice treated as in A and described in Materials and Methods. Error bars indicate SEM, with no significant difference found between treated groups by two-way repeated measures ANOVA.
Histological Analysis
Tissue was paraffin-embedded and sectioned, and evaluated by an experienced veterinary research pathologist using blinded samples. Sections stained with hematoxylin and eosin were evaluated for oviduct dilatation by an established semiquantitative scoring system from 1–5 (1+ = ≤1.0; 2+ = ≤2.0; 3+ = ≤3.0; 4+ = ≤4.0 and 5+ = >4.0). Sections stained with trichrome were evaluated for fibrosis using digital color microscopy on an Aperio instrument (Vista, CA) using semi quantitative scoring from 1–4 (1+ = <25% light blue oviducts; 2+ = >25% light blue oviducts; 3+ = <25% dark blue oviducts and 4+ = >25% dark blue oviducts)(Dahab, et al., 2004, Kaori Shimazaki, 2009, Krajewska, et al., 2009).
Statistical analysis
Kinetic changes in the frequency of Treg or Th1 cells was determined by two-way analysis of variance (ANOVA) with n=10 (Figure 1B–D). Comparison of the numbers of Treg or Th1 cell numbers throughout the course of infection in the ILN, GT and spleen were performed by two way ANOVA and Dunn’s post-hoc test. Statistical comparison of Treg versus Th1 cells on individual days were compared by t-test (Figure 1B–D). Bacterial loads in vivo were measured for mice depleted of pDC or treated with control antibody and the mean IFU/ml for each treatment was compared by two way repeated measures ANOVA, with n=12 (Figure 2B). The effect of pDC depletion of ratio of the number of Treg and Th1 cells was performed by t-test on day 9 post infection, with n=10 (Figure 3A–B). Oviduct histology scoring of individual control or pDC depleted mice was compared by non-parametric Mann-Whitney Rank Sum Test, with n=12 and representing two independent experiments (Figure 4B–C). The above statistical tests were suggested by and performed using SigmaStat software based on the distribution of the data and sample size (Jandel Scientific, San Rafael, CA). Groups were considered statistically different at p<0.05.
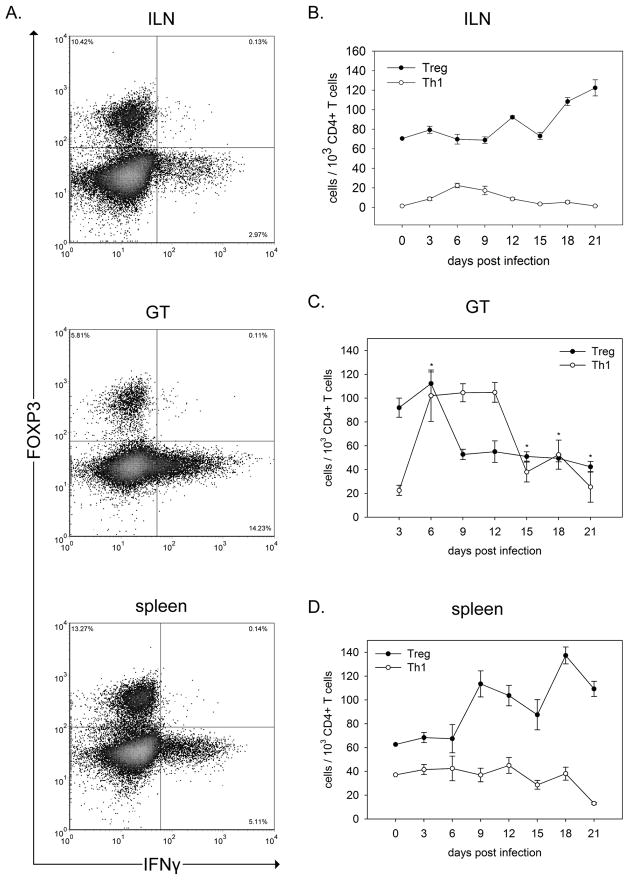
Figure 1.
Treg and Th1 cells show differential responses to genital infection by C. muridarum. A. Representative FACS plots of T cell subsets from the indicated tissue, gated on live CD3+CD4+ cells on day 9 PI. B–D. Kinetic analysis of Treg (CD3+CD4+FOXP3+) and Th1 (CD3+CD4+IFNγ+) cells in the indicated tissue. Points represent the mean number of cells in individual mice +/− SEM with n=10 for each time point. The comparison between the T cell subset numbers revealed a significant difference in the ILN and spleen but not the GT by two way ANOVA, p<0.001. Likewise, post-hoc t-test analysis showed differences between Treg and Th1 numbers at all time points analyzed for the ILN and spleen. The number of Treg and Th1 cells at individual time points was analyzed by t-test. The asterisk (*) indicates no significant difference on that day by t-test in the GT. All other values in the GT were significantly different by t-test. The number of Tregs diminished over the course of infection by one way ANOVA, p<0.001 followed by Dunn’s post-hoc test p<0.05.
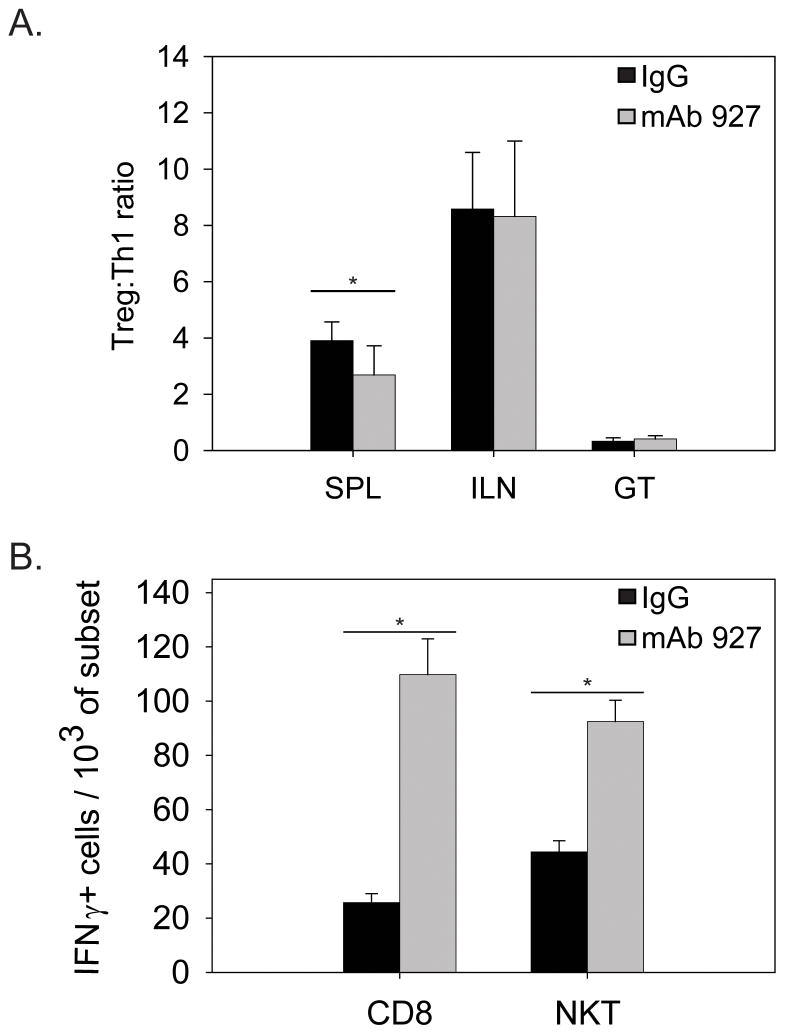
Figure 3.
pDC depletion affects distinct T cell populations in the spleen. T cell subsets were isolated from infected mice depleted of pDC (mAb 927) or treated with control antibody (IgG) and analyzed as described in materials and methods. Data represent the mean number of cells in individual mice on day 9 PI, with n=10 for A (Treg) and B (Th1) and n=5 for C (CD8 & NKT) +/− SEM. ** p<0.05 by paired t-test. *p<0.001 by t-test.
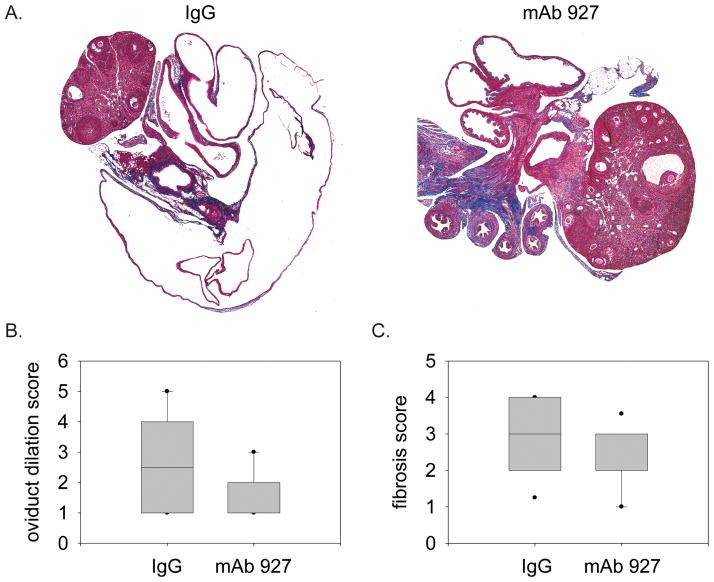
Figure 4.
pDC contribute to the sequelae of genital C. muridarum infection. GT were harvested 7 weeks after infection following treatment with control antibody (IgG) or anti-BST2 (mAb 927) as described in materials and methods. Trichrome slides were prepared as described in materials and methods, and scanned with Aperio ScansScope. A. Representative trichrome staining of oviducts at 2× using the ScanScope software. B. Oviduct dilation scoring measured by oviduct area using the ScanScope software, with n=12 for each group. C. Fibrosis score as measured by collagen deposition scoring in the oviduct, with n=12 for each group.
Results
Th1 and Treg cells respond differentially following genital chlamydial infection
The role of Treg during genital chlamydial infection is not well defined. Given the immune-modulating potential of these cells, Treg activity would seem immediately relevant to the formation of the protective, CD4+ IFNγ-producing T cell response (Th1). Therefore, we examined the induction of Treg as in relation to Th1 cells to establish the balance between these T cell subsets during the course of chlamydial infection. To this end, we utilized intracellular FACS analysis to characterize CD3+CD4+ lymphocytes from infected tissues of individual mice for Treg (FOXP3) and Th1 (IFNγ) cells (Figure 1A). Our examination included the entire course of infection through to approximately one week after bacteria was undetectable by vaginal swabs (Figure 2B). In the draining ILN where T cell responses are initiated, Treg were dominant throughout the course of infection and continued to increase in number after resolution of infection (Figure 1B). Alternatively, Th1 cells increased in the ILN only during time points in which bacterial loads were detectable by vaginal swabs, and returned to levels comparable to naïve mice following clearance of infection. A similar distribution of Treg and Th1 cells was found in the spleen, though increases in Th1 cells were less than seen in the ILN (Figure 1D). Together, these data demonstrate that the number of Treg (FOXP3+) increase in peripheral lymphoid tissue as a result of genital infection, and remain at elevated numbers in these tissues beyond clearance of infection. Alternatively, Th1 numbers in these tissues closely follows the course of infection.
In the GT tissue, the ratio of Treg to Th1 cells differed from that seen in the secondary lymphoid organs. At early time points following infection, we found Treg to be present in the GT in greater numbers than Th1 (Figure 1C). Coincident with an increase in Th1 numbers in the ILN (Figure 1B), these cells significantly outnumbered Treg in the GT and remain in greater numbers than Treg throughout the course of infection. Following clearance of infection (as measured by undetectable bacterial load in the GT, Figure 2B), Th1 cell numbers diminish in the GT. Surprisingly, Treg numbers also diminished during the course of infection compared to pre-infected levels which suggests that Treg cells were either leaving GT possibly to secondary lymphoid tissues or dying. Thus, similar to the ILN and spleen, the presence of Th1 cells in the GT closely mimics the course of infection, indicating that Th1 retention in the GT is dependent on a productive infection. Alternatively, Treg numbers diminish early and then remain constant throughout and beyond the course of infection.
pDC depletion does not alter the course of infection or Th1 numbers, but does alter Treg numbers in the spleen
Based on our previous work and that of others, a possible role for pDC during chlamydial infection may be to regulate Treg cell numbers. To examine this possibility, we devised a pDC depletion strategy starting before infection and continuing through day 10 after infection. We depleted pDC using a monoclonal antibody (m927) against the BST2 antigen expressed on pDC (see materials and methods). The antibody has been characterized for in vivo depletion (Blasius, et al., 2006). This strategy was employed to deplete pDC throughout early infection when DC and T cells interact to generate T cell subsets as shown in Figure 1B and previous studies (Moniz, et al., 2009). We found this method to be effective in depleting pDC in all tissues examined, with the most marked depletion of 85% occurring in the spleen (Figure 2A). Vaginal swabs revealed that in mice depleted of pDC, there was no change in the bacterial load at any time point during the course of infection when compared to mice treated with isotype control antibody (Figure 2B). This indicates that although the methodology was sufficient in depleting pDC, there was no effect of this depletion on the course of infection.
Although this level of pDC depletion in vivo did not alter the overall chlamydial burden in the GT we reasoned fewer pDC may alter a non-Th1 subset. We determined the ratio of the number of Tregs (CD3+CD4+FoxP3+) in relation to Th1 cells (CD3+CD4+IFNγ+) cells in the ILN, GT, and spleens of individual mice on day 9 PI of control and pDC depleted mice. In the ILN and GT we found no significant difference in the ratio of the numbers of Treg:Th1 cells (Figure 3A). This is consistent with the observation that mice depleted of pDC showed no change in the course of infection and indicates that pDC do not directly modulate Th1 cells as suggested by our previous study (Moniz et al., 2009). However, our analysis found that pDC depletion resulted in a significant decrease in the ratio of the number of Treg to Th1 cells in only the spleen (Figure 3A). Examination of the individual number of Tregs and Th1 cells showed that the number of Tregs significantly decreased while that of Th1 cells remained similar. This indicates that during genital chlamydial infection, pDC modulate the numbers of Treg but not Th1 cells in the spleen.
pDC depletion alters the numbers of non-protective T cells
As pDC may also affect the numbers and activity of T cells other than Treg, we next examined the effect of pDC depletion T cell subsets shown to be activated during chlamydial infection. Our efforts focused on T cells which have been shown or have the capacity to produce IFNγ during chlamydial infection, CD8+ T cells and NKT cells, respectively. In the ILN and GT, we found no difference in the numbers of CD8+IFNγ producing T cells of mice depleted of pDC versus mice treated with control antibody (data not shown). In the spleen, however, there were significantly elevated numbers of CD8+IFNγ producing T cells in mice depleted of pDC versus control mice (Figure 3B). Likewise, NKT cells were not detectable in the ILN or GT on day 9 PI (data not shown) but were detectable in the spleen, where depletion of pDC resulted in a significant increase in the number of IFNγ producing NKT cells (Figure 3B). This data demonstrates that pDC depletion increases the number of IFNγ producing CD8+ T cells and NKT cells in the spleen during genital chlamydial infection.
pDC depletion results in decreased sequelae of genital chlamydial infection
pDC have been shown to have a broad tolerogenic potential that includes limiting inflammation. As prolonged inflammation is implicated in the sequelae of genital chlamydial infection (Brunham & Rekart, 2008), we examined the formation of sequelae in mice depleted of pDC. To this end, we examined the GT of mice for prototypical signs of sequelae formation, oviduct dilation and collagen deposition (Cohen & Brunham, 1999). Seven weeks after clearance of infection, we found mice depleted of pDC exhibited significant reduction in oviduct dilation (Figure 4A & B) as well as collagen deposition in the oviduct (Figure 4B & C) compared to that seen in control mice. These data indicate that depletion of pDC results in less severe sequelae following genital C. muridarum infection.
Discussion
Recent evidence in the literature suggests that pDC are unique amongst DC subsets, particular in their inability to induce effector responses against microbial insult. This activity of pDC seemingly stems from the unique expression of surface molecules for antigen acquisition, a limited repertoire of receptors for microbial recognition (i.e., toll like receptors), and capacity to present antigen on the cell surface that is markedly less efficient than other DC subsets (Villadangos & Young, 2008). Our recent work suggested that pDC were not capable of inducing the protective Th1 response to genital chlamydial infection. The work here is in agreement with that supposition as well as recent findings in the literature demonstrating that pDC do not affect induction of effector responses to genital viral infection (Lund, et al., 2006). That pDC depletion altered neither the induction of a protective Th1 response or the clearance of infection indicates that pDC do not influence the induction of protective T cell responses to genital infection by C. muridarum.
Tolerogenic responses during genital chlamydial infection are not well defined. Given our recent demonstration of the induction of pDC during genital chlamydial infection (Moniz, et al., 2009), and recent evidence demonstrating pDC as central factors in the maintenance of tolerance (Ochando, et al., 2006, Goubier, et al., 2008), we sought to determine whether pDC modulate tolerogenic responses during genital chlamydial infection. Treg appear to be central in effector mediated maintenance of tolerance (Tang & Bluestone, 2008), yet the role of Treg during genital chlamydial infection has not been examined. In this work, we demonstrate a marked Treg response to genital chlamydial infection (Figure 1), and further examined whether pDC played a role in the maintenance of this response by depletion of pDC during the course of genital chlamydial infection. Depletion of pDC resulted in a decrease in the number of Treg in the spleen, but not the site (GT) or draining lymph node (ILN) of infection. These observations indicate that modulation of Treg numbers by pDC is limited to the spleen during chlamydial infection. However, our work utilized antibody mediated depletion of pDC which does not completely ablate pDC. It is possible that in the total absence of pDC, Treg numbers may be affected in the draining lymph nodes and site of chlamydial infection. Thus further efforts are warranted to determine the role pDC play in modulation of Treg responses during chlamydial infection using more absolute means of pDC ablation, such as that recently shown through constitutive and conditional deletion of the transcription factor E2-2 (Cisse, et al., 2008).
The role of T cell subsets such as IFNγ-producing NKT and CD8 cells during genital chlamydial infection is not well described. In models of chlamydial infection of the lung, NKT have been clearly shown to be detrimental to the host by prolonging bacterial burden and exacerbating the sequelae of infection (Bilenki, et al., 2005). This activity was shown, in part, to be due to the promotion of Th2 responses by NKT cells. Such divergence from the protective Th1 response is thought to result in the sequelae of genital chlamydial infection (Brunham & Rey-Ladino, 2005), and as pDC have been shown to interact with NKT cells in other models of infection (Diana, et al., 2009), we examined the effect of pDC on NKT cells during genital chlamydial infection. Interestingly, we found that NKT cells were not detectable in either the ILN or GT later in a primary infection (data not shown) and that their presence in the spleen during infection coincided with production of IFNγ and not IL-4 (Figure 3B, data not shown). Furthermore, we show that pDC depletion results in an increase in the numbers of IFNγ-producing NKT cells in the spleen. This suggests that pDC modulate the production of IFNγ by NKT cells, the consequence of which is unclear and warrants both the further examination of NKT cell activity during genital chlamydial infection as well as the consequence of this activity.
In a similar fashion to NKT cells, we found that pDC depletion resulted in higher numbers of IFNγ producing CD8 T cells in the spleen. It is clear that CD8 T cells are not central in clearance of genital chlamydial infection, but instead appear involved in promotion of sequelae following chlamydial infection (Igietseme, et al., 2009). In mice, the sequelae of genital chlamydial infection is most apparent in the formation of hydrosalpinx and fibrosis in the oviducts. The cause of these pathologies is not well understood, but is associated with excessive inflammation (Cohen & Brunham, 1999, Brunham & Rekart, 2008) which is thought to promote the fibrosis implicated in the sequelae of infection. As the tolerogenic potential of pDC may include regulation of inflammation, we anticipated pDC depletion to result in increased pathology. Surprisingly, depletion of pDC resulted in a decrease in the sequelae following genital chlamydial infection (Figure 4), indicating that pDC contribute to the formation of pathology following chlamydial infection. Furthermore, the decrease in sequelae following pDC depletion is inconsistent with our finding that pDC increase the activity of CD8 T cells which are implicated in promoting pathology. The possible reason for this discrepancy is that our method of pDC depletion was incomplete in the GT (Figure 2A), and that this incomplete depletion resulted in the lack of an effect on CD8 T cells in the GT following pDC depletion (data not shown). Again, these findings warrant further analysis of the role pDC play during genital chlamydial infection in a model of complete pDC ablation (Cisse, et al., 2008).
Our finding that pDC depletion results in less severe pathology was unexpected, and raises the question as to how pDC promote formation of sequelae. A recent study showed that pDC were associated with mucopurulent cervicitis and inflammatory factors in women with a chlamydial genital infection (Agrawal, et al., 2008). The mechanism of this contribution is unclear, but might include pDC-mediated modulation of either Treg or non-protective T cell subsets (CD8+ or NKT) as demonstrated in this work. Another possibility is that depletion of pDC altered the specific antigens to which T cell responses are induced. As we saw no change in course of infection, it would seem that such a scenario does not include responses to chlamydial antigens central to clearance of infection (protective responses). Instead, pDC may modulate non-protective but pathologically relevant responses as we show with CD8 and NKT cells. Before such a possibility can be addressed, however, a more clear definition of the roles that both Treg and non-protective T cells play during chlamydial infection is required. Following these analyses, a more thorough examination of the role pDC play in the modulation of Treg and non-protective T cell responses may then lead to a clearer understanding of the role that pDC play during genital chlamydial infection.
In summary, we demonstrate that pDC are not central in the induction of protective Th1 responses during genital C. muridarum infection, but rather affect the numbers of Treg and non-protective T cells in the spleen. We also provide evidence that pDC contribute to the sequelae of genital chlamydial infection, which may be the result of pDC modulation of Treg and non-protective T cells. Together, these findings indicate that pDC play an unexpected role in the formation of sequelae following genital chlamydial infection that is independent of the clearance of infection.
Acknowledgments
We would like to thank M. Colonna and M. Arditi for the generous gift of the mAb 927 antibody. This work was supported by NIH grants RO1 IA-026328 (KAK & RJM), T32 AI107126-28 (RJM) and T32 AI52031 (RJM).
References
- 1.Agrawal T, Vats V, Wallace PK, Singh A, Salhan S, Mittal A. Recruitment of myeloid and plasmacytoid dendritic cells in cervical mucosa during Chlamydia trachomatis infection. Clin Microbiol Infect. 2008 doi: 10.1111/j.1469-0691.2008.02113.x. [DOI] [PubMed] [Google Scholar]
- 2.Bilenki L, Wang S, Yang J, Fan Y, Joyee AG, Yang X. NK T cell activation promotes Chlamydia trachomatis infection in vivo. J Immunol. 2005;175:3197–3206. doi: 10.4049/jimmunol.175.5.3197. [DOI] [PubMed] [Google Scholar]
- 3.Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J Immunol. 2006;177:3260–3265. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- 4.Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone Marrow Stromal Cell Antigen 2 Is a Specific Marker of Type I IFN-Producing Cells in the Naive Mouse, but a Promiscuous Cell Surface Antigen following IFN Stimulation. J Immunol. 2006;177:3260–3265. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- 5.Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005;5:149–161. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 6.Brunham RC, Rekart ML. The arrested immunity hypothesis and the epidemiology of chlamydia control. Sex Transm Dis. 2008;35:53–54. doi: 10.1097/OLQ.0b013e31815e41a3. [DOI] [PubMed] [Google Scholar]
- 7.Cisse B, Caton ML, Lehner M, et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen CR, Brunham RC. Pathogenesis of Chlamydia induced pelvic inflammatory disease. Sex Transm Infect. 1999;75:21–24. doi: 10.1136/sti.75.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahab GM, Kheriza MM, El-Beltagi HM, Fouda AM, El-Din OA. Digital quantification of fibrosis in liver biopsy sections: descriptions of a new method by Photoshop software. J Gastroenterol Hepatol. 2004;19:78–85. doi: 10.1111/j.1440-1746.2004.03183.x. [DOI] [PubMed] [Google Scholar]
- 10.de Heer HJ, Hammad H, Soullie T, et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Heer HJ, Hammad H, Soullie T, et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diana J, Griseri T, Lagaye S, et al. NKT cell-plasmacytoid dendritic cell cooperation via OX40 controls viral infection in a tissue-specific manner. Immunity. 2009;30:289–299. doi: 10.1016/j.immuni.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Goubier A, Dubois B, Gheit H, et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Igietseme JU, He Q, Joseph K, et al. Role of T lymphocytes in the pathogenesis of Chlamydia disease. J Infect Dis. 2009;200:926–934. doi: 10.1086/605411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaori Shimazaki AMCRJMMWANCPCSMEMLAMWKAKJB. Blockade of epithelial membrane protein 2 (EMP2) abrogates infection of Chlamydia muridarum murine genital infection model. FEMS Immunology & Medical Microbiology. 2009;55:240–249. doi: 10.1111/j.1574-695X.2008.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krajewska M, Smith LH, Rong J, et al. Image Analysis Algorithms for Immunohistochemical Assessment of Cell Death Events and Fibrosis in Tissue Sections. J Histochem Cytochem. 2009 doi: 10.1369/jhc.2009.952812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lund JM, Linehan MM, Iijima N, Iwasaki A. Cutting Edge: Plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J Immunol. 2006;177:7510–7514. doi: 10.4049/jimmunol.177.11.7510. [DOI] [PubMed] [Google Scholar]
- 18.Maxion HK, Liu W, Chang MH, Kelly KA. The infecting dose of Chlamydia muridarum modulates the innate immune response and ascending infection. Infect Immun. 2004;72:6330–6340. doi: 10.1128/IAI.72.11.6330-6340.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moniz RJ, Chan AM, Kelly KA. Identification of dendritic cell subsets responding to genital infection by Chlamydia muridarum. FEMS Immunology & Medical Microbiology. 2009;55:226–236. doi: 10.1111/j.1574-695X.2008.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moniz RJ, Chan AM, Kelly KA. Identification of dendritic cell subsets responding to genital infection by Chlamydia muridarum. FEMS Immunol Med Microbiol. 2009;55:226–236. doi: 10.1111/j.1574-695X.2008.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ochando JC, Homma C, Yang Y, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 22.Ochando JC, Homma C, Yang Y, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 23.Rey-Ladino J, Koochesfahani KM, Zaharik ML, Shen C, Brunham RC. A Live and Inactivated Chlamydia trachomatis Mouse Pneumonitis Strain Induces the Maturation of Dendritic Cells That Are Phenotypically and Immunologically Distinct. Infect Immun. 2005;73:1568–1577. doi: 10.1128/IAI.73.3.1568-1577.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rey-Ladino J, Jiang X, Gabel BR, Shen C, Brunham RC. Survival of Chlamydia muridarum within Dendritic Cells. Infect Immun. 2007;75:3707–3714. doi: 10.1128/IAI.01618-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29:352–361. doi: 10.1016/j.immuni.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 27.WHO. Global Prevalence and Incidence of Selected Curable Sexually Transmitted Infections Oveview and Estimates. 2004 http://www.who.int/hiv/pub/sti/pub7/en/index.html. [PubMed]