Abstract
Transforming growth factor β (TGF-β)/bone morphogenetic protein (BMP) signaling is crucial for regulating epithelial-mesenchymal interaction during organogenesis, and the canonical Smad pathway–mediated TGF-β/BMP signaling plays important roles during development and disease. During tooth development, dental epithelial cells, known as Hertwig's epithelial root sheath (HERS), participate in root formation following crown development. However, the functional significance of HERS in regulating root development remains unknown. In this study we investigated the signaling mechanism of Smad4, the common Smad for TGF-β/BMP signaling, in HERS in regulating root development. Tissue-specific inactivation of Smad4 in HERS results in abnormal enamel and dentin formation in K14-Cre;Smad4fl/fl mice. HERS enlarges but cannot elongate to guide root development without Smad4. At the molecular level, Smad4-mediated TGF-β/BMP signaling is required for Shh expression in HERS and Nfic (nuclear factor Ic) expression in the cranial neural crest (CNC)-derived dental mesenchyme. Nfic is crucial for root development, and loss of Nfic results in a CNC-derived dentin defect similar to the one of K14-Cre;Smad4fl/fl mice. Significantly, we show that ectopic Shh induces Nfic expression in dental mesenchyme and partially rescues root development in K14-Cre;Smad4fl/fl mice. Taken together, our study has revealed an important signaling mechanism in which TGF-β/BMP signaling relies on a Smad-dependent mechanism in regulating Nfic expression via Shh signaling to control root development. The interaction between HERS and the CNC-derived dental mesenchyme may guide the size, shape, and number of tooth roots. © 2010 American Society for Bone and Mineral Research.
Keywords: SMAD4, tooth root development, TGF-β/BMP, hers, shh, Nfic, mouse
Introduction
Mammalian ectodermal cells undergo complex reciprocal interactions with underlying mesenchymal cells to form a variety of appendages, including teeth, hair follicles, and mammary glands.(1–3) The tooth organ is an excellent model for studies of heterotypic tissue interactions and the formation of unique dental structures.(1,4–12) Tooth crown formation is controlled by many cytokines and transcription factors that regulate critical gene expression during tooth development.(4,13,14) Compared with the study of crown formation, we have limited information on the molecular regulatory mechanism of root development.
Following crown development, inner enamel epithelium (IEE) and outer enamel epithelium (OEE) form a bilayered epithelial structure termed Hertwig's epithelial root sheath (HERS) that migrates apically and participates in root formation and the completion of tooth organ development. Morphologically, HERS is a structural boundary between two dental mesenchymal tissues, the dental papilla and the follicle. Interestingly, HERS breaks up into epithelial rests and cords, allowing dental follicle cells to come in contact with the outer surface of the root during dentin and cementum formation. This strategic position suggests an important role for HERS during critical interactions between dental epithelium and mesenchyme.(15–17)
Previous studies have shown that many genes, including bone morphogenetic proteins (BMPs), fibroblast growth factors (FGFs), sonic hedgehog (Shh), Notch, Gli, Msx1, Msx2, and Nfic, are involved in tooth root development.(4,13,14,18,19) Nfic (nuclear factor Ic) may have a specific function as a key regulator of root formation because tooth root development is affected most prominently in Nfic−/− mice.(19,20) However, the mechanism of root formation and the interactions between the epithelial structure (HERS) and mesenchymal tissues (dental papilla and follicle) remain unclear.
The transforming growth factor β (TGF-β) superfamily of cytokines is comprised of TGF-βs, BMPs, activins, and related proteins. TGF-β signaling plays an important role in regulating a broad spectrum of biologic processes such as cell proliferation, differentiation, apoptosis, migration, and extracellular matrix remodeling.(21–23) The canonical TGF-β signaling pathway involves the TGF-β ligand binding to type II and type I receptors. The activated receptor complex phosphorylates Smad proteins (R-Smads), which form a complex with the common Smad (Smad4). The Smad complex then translocates into the nucleus to regulate the expression of an array of target genes.(24) Smad4 is a central mediator of the canonical TGF-β signaling pathway.
To date, few animal models have been identified to explore the function of the HERS directly. In this study we report a rootless animal model, K14-Cre;Smad4fl/fl mice. Following inactivation of Smad4 in dental epithelial cells, the development of molar roots is arrested, and the formation of enamel and dentin is also affected severely. Our data suggest that TGF-β/BMP signaling relies on a Smad4-dependent mechanism that regulates Nfic expression via Shh signaling. Thus Smad4 controls a genetic hierarchy involving Shh and Nfic that plays a crucial role in regulating epithelial-mesenchymal interaction during root development.
Materials and Methods
Generation of K14-Cre;Smad4fl/fl and Nfic−/− mice
Male mice carrying the K14-Cre allele(25) were crossed with Smad4fl/fl(26) females to generate K14-Cre;Smad4fl/+ mice. This transgene drives Cre expression in basal keratinocytes from E9.5.(27,28,32) The male K14-Cre;Smad4fl/+ mice were mated with Smad4fl/fl female mice to generate K14-Cre;Smad4fl/fl null alleles that were genotyped using polymerase chain reaction (PCR) primers as described previously.(33,34) Male and female heterozygous Nfic+/− mice were crossed to generate Nfic−/− progeny, and adult animals were determined by PCR primers as described previously.(19) All samples of K14-Cre;Smad4fl/fl and Nfic−/− mice were fixed in 4% paraformaldehyde and processed into paraffin-embedded serial sections using routine procedures. For general morphology, deparaffinized sections were stained with hematoxylin and eosin (H&E) using standard procedures.
Kidney capsule transplantation
Kidney capsule transplantation was carried out as described previously.(30,34) The mandibular process of the first branchial arch at E11.5 was dissected from embryos. The explants were cultured for 1 day during genotyping and then grafted under the kidney capsule according to standard procedures. Then 2 and 4 weeks after transplantation, the host mice were killed, and the grafts were processed for histologic analysis. For rescue experiment, Shh and BSA beads were applied to the grafts and transplanted into the capsule with the first branchial arches. Bead preparation was carried out as described previously.(30) Affi-Gel blue agarose beads (Bio-Rad, Hercules, CA, USA) were soaked in recombinant human Shh (100 µg/mL; R&D Systems, Minneapolis, MN, USA) or bovine serum albumin (BSA) (100 µg/mL) overnight at 4°C prior to use.
In vitro bead implantation
The molar tooth germs from wild-type and K14-Cre;Smad4fl/fl mandibles at the newborn stage were isolated in cold PBS and placed on filters in Trowell-type organ cultures. Protein-soaked beads were implanted onto the dental mesenchyme in BGJb culture medium (Gibco, Invitrogen, Carlsbad, CA, USA) supplemented with 10% ascorbic acid. Explants were harvested after 2 days in culture, fixed in 4% paraformaldehyde, and processed for whole-mount in situ hybridization and histologic analysis.
Immunohistochemistry
Tissues were fixed with 4% paraformaldehyde for immunohistochemistry. Paraffin blocks containing processed mouse tissue were sectioned coronally (6 µm in thickness) for immunohistochemical analysis. The slides were heated in a 60°C oven for 30 minutes and subsequently hydrated to water through a series of decreasing concentrations of ethanol. The immunohistochemical staining was performed by using the Zymed HistoStain SP kit (Invitrogen). The specific anti-Smad4 antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). It is noted that owing to the background issue, there are some limitations with data interpretation of anti-Smad4 staining. To address this issue, we had performed in situ hybridization analysis to verify the successful tissue-specific inactivation of Smad4 in neural crest and epithelial cells.(29,31)
In situ hybridization
Tissues were fixed with 4% paraformaldehyde in PBS, embedded in paraffin, serially sectioned, and mounted following standard procedures. DNA fragments of Amelogenin, dentin sialophosphoprotein (Dspp), Nfic, Shh, and Gli were subcloned in vector plasmids. Digoxigenin (DIG)–labeled sense and antisense cRNA riboprobes were synthesized using the DIG RNA Labeling Mix (Roche Molecular Biochemicals, Indianapolis, IN, USA). Paraffin-embedded sections were dewaxed and treated with proteinase K (20 µg/mL) and 0.2 M HCl, acetylated, and hybridized overnight with DIG- labeled probes as described previously.(31)
Results
Loss of Smad4 in the dental epithelium affects tooth root formation
We had previously generated mice with a conditional Smad4 knockout in the dental epithelial cells (K14-Cre;Smad4fl/fl)(23) to explore the functional significance of Smad4 in regulating the fate of the dental epithelium and tooth development. Unfortunately, K14-Cre;Smad4fl/fl mice die at birth, so we cannot investigate the role of Smad4 in regulating root development at postnatal stages. Therefore, we transplanted the mandible containing the lower first molars from E11.5 K14-Cre;Smad4fl/fl mice into a kidney capsule to observe root formation. At the newborn stage, the morphology of teeth in K14-Cre;Smad4fl/fl mice was affected (Fig. 1A, B), as described previously.(31) To confirm the loss of Smad4 in K14-Cre;Smad4fl/fl mice, we assayed the expression of Smad4 using immunohistochemistry. In wild-type teeth, Smad4 is strongly expressed in the dental epithelium and mesenchyme at the newborn stage and after 2 weeks in the kidney capsule (Fig. 1C, I) but is undetectable in the dental epithelium of K14-Cre;Smad4fl/fl mice (Fig. 1D, J). These data confirm that Smad4 is specifically inactivated in the dental epithelium of K14-Cre;Smad4fl/fl mice. After 2 weeks of cultivation in the kidney capsule, the mineralized teeth of the wild-type sample were well developed, with normal cusps and initiation of root formation (Fig. 1E, G). The HERS is formed by two layers of dental epithelial cells located in the apical part of the tooth (Fig. 1G, arrow). However, in K14-Cre;Smad4fl/fl samples, we did not observe root formation (Fig. 1F, H). Morphogenesis of the first mandible molar was severely disrupted. The HERS was enlarged, and HERS cells failed to grow out of the crown in K14-Cre;Smad4fl/fl samples (Fig. 1H, J, arrows). After 4 weeks of cultivation in the kidney capsule, wild-type teeth and roots developed well (Fig. 2A–D). We could clearly detect root dentin and cementum with H&E staining (Fig. 2I, J, cementocytes are indicated by arrows). In contrast, we still did not observe root formation in K14-Cre;Smad4fl/fl samples (Fig. 2E–H, K, L). The first mandible molar appeared translucent, and the enamel was not well-organized. Dentin formation appeared abnormal. Odontoblasts were embedded into the dentin, an abnormal condition not observed in wild-type samples (Fig. 2L, arrows).
Fig. 1.
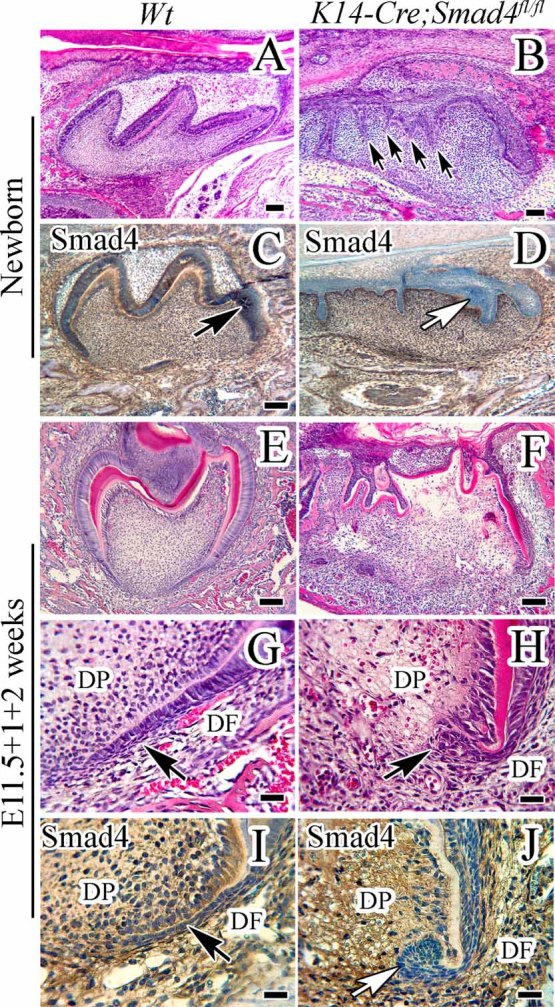
Tooth root development in mice lacking Smad4. (A–D) H&E staining (A, B) and immunohistochemistry of Smad4 (C, D) of sections of teeth from newborn wild-type (Wt) and K14-Cre;Smad4fl/fl mice. Compared with tooth development in wild type (A), K14-Cre;Smad4fl/fl teeth show more epithelium folding (B, arrows). Smad4 is detectable in both dental epithelium and mesenchyme in wild-type mice (C, arrow indicates the dental epithelium) but undetectable in the dental epithelium of K14-Cre;Smad4fl/fl mice (D, white arrow). (E–J) H&E staining (E–H) and immunohistochemistry of Smad4 (I, J) of E11.5 tooth germs cultured in kidney capsules. After 2 weeks of cultivation under the kidney capsule, the beginning of root formation and well-organized enamel and dentin are detectable in wild-type samples. Note that the HERS is elongated vertically (G, arrow). In K14-Cre;Smad4fl/fl mice, HERS is enlarged and does not grow out of the crown (H, J, arrows). Smad4, which is detectable in wild-type mice (I, arrow indicates the dental epithelium), is undetectable in the dental epithelium of K14-Cre;Smad4fl/fl mice (J, white arrow). Well-organized enamel and dentin are seldom found in K14-Cre;Smad4fl/fl mice (F). DF = dental follicle; DP = dental pulp. Scale bars (A–F) = 100 µm; scale bars (G–J) = 25 µm.
Fig. 2.
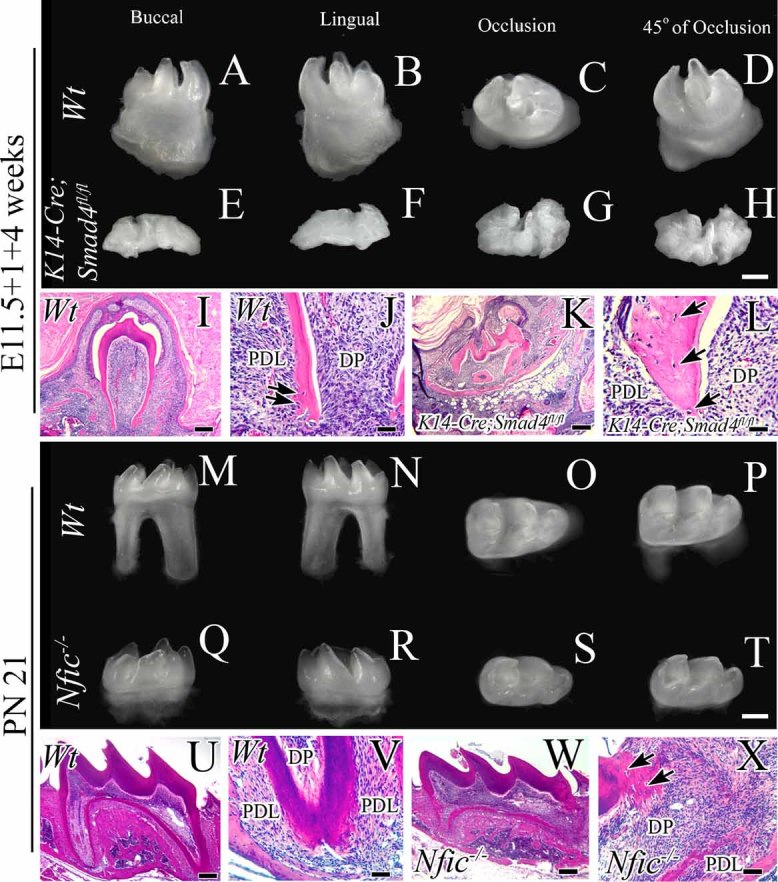
Root development in K14-Cre;Smad4fl/fl and Nfic−/− mice. (A–L) Whole-mount views and sections stained with H&E of tooth germs from wild-type (Wt) and K14-Cre;Smad4fl/fl mice cultured for 4 weeks in kidney capsules. After 4 weeks of cultivation in the kidney capsule, the mineralized teeth of wild-type mice are well developed, with normal cusps and root formation (A–D). Root dentin and cementum are also identifiable with H&E staining (I, J, arrows indicate the cementocytes). Root formation is not detectable in K14-Cre;Smad4fl/fl mice (E–H), and odontoblasts are embedded in the dentin to form cellular dentin (K, L, arrows indicate the cells in dentin). (M–X) Whole-mount views and sections stained with H&E of tooth germs from postnatal day 21 wild-type and Nfic−/− mice. Two roots are clearly identifiable in wild-type teeth (M–P, U, V). Nfic−/− mice do not develop a normal tooth root, and cellular dentin is detectable in root-like areas by H&E staining (Q–Y, W, X, arrows indicate the cells in dentin). The crown and cusps are normal in Nfic−/− teeth. DP = dental pulp; PDL = periodontal ligament. Scale bars (A–I, K, M–U, W) = 100 µm; scale bars (J, L, V, X) = 25 µm.
Abnormal enamel and dentin formation in K14-Cre;Smad4fl/fl mice
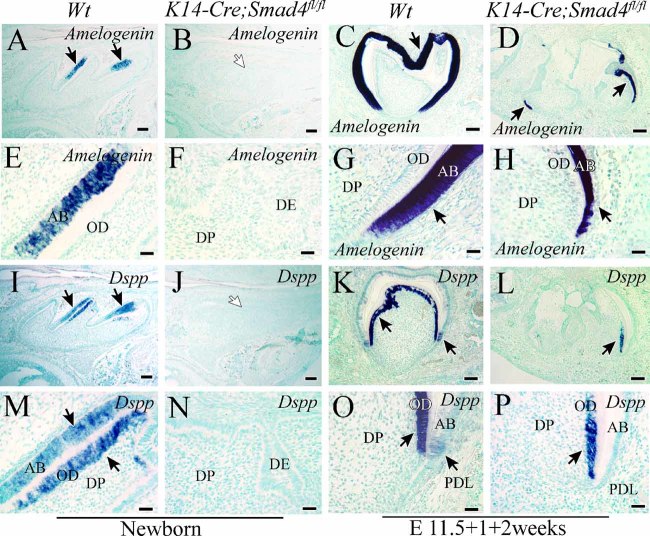
The enamel and dentin did not develop well in K14-Cre;Smad4fl/fl mice. After 2 weeks of cultivation under the kidney capsule, we observed well-organized enamel and dentin in wild-type samples (Fig. 1E) but not in K14-Cre;Smad4fl/fl samples (Fig. 1F). Amelogenin and Dspp have been used extensively as differentiation markers for ameloblasts and odontoblasts, respectively.(30) To examine the status of ameloblast and odontoblast differentiation in K14-Cre;Smad4fl/fl mice, we assayed the expression of Amelogenin and Dspp using in situ hybridization. In wild-type mice, we detected Amelogenin expression in all ameloblasts, and Dspp was present in the odontoblast layer and in some ameloblasts at the newborn stage in vivo (Fig. 3A, E, I, M) and on day 14 after kidney capsule transplantation (Fig. 3C, G, K, O). In K14-Cre;Smad4fl/fl mice, however, gene expression of Amelogenin and Dspp was not detectable at the newborn stage (Fig. 3B, F, J, N) and was dramatically reduced on day 14 after kidney capsule transplantation (Fig. 3D, H, L, P). Our data suggest that Smad4 is important for both enamel and dentin formation in the dental epithelium during tooth development but is not required for ameloblast and odontoblast differentiation.
Fig. 3.
Expression of Amelogenin and Dspp is delayed in K14-Cre;Smad4fl/fl teeth. In situ hybridization of Amelogenin (A–H) and Dspp (I–P) in wild-type (Wt) and K14-Cre;Smad4fl/fl teeth at the newborn stage (A, B, E, F, I, J, M, N) and 2 weeks after culture in kidney capsules (C, D, G, H, K, L, O, P). In wild-type mice, Amelogenin expression was detectable in all ameloblasts, and Dspp was detectable in the odontoblast layer and in some ameloblasts (A, B, E, F, I, J, M, N, arrows). In K14-Cre;Smad4fl/fl mice, gene expression of Amelogenin and Dspp could not be detected at the newborn stage (B, F, J, N, white arrows) and was dramatically reduced after 2 weeks in the kidney capsule (D, H, L, P, arrows). AB = ameloblast; DE = dental epithelium; DP = dental pulp; HERS = Hertwig's epithelial root sheath; OD = odontoblast. Scale bars (A–D, I–L) = 100 µm; scale bars (E–H, M–P) = 25 µm.
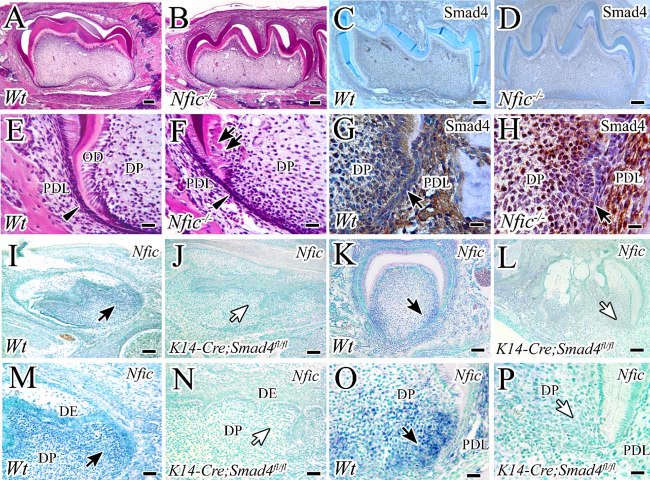
Smad4 is required in the dental epithelium for Nfic expression in the dental mesenchyme during root development
To investigate the signaling mechanisms of Smad4 in regulating epithelial–mesenchymal interactions during root development, we surveyed expression patterns of genes that are critical during early root formation. Because we observed the beginning of root formation after 2 weeks of cultivation under the kidney capsule (Fig. 1E, G), we compared gene expression patterns in K14-Cre;Smad4fl/fl and wild-type mice during that time period. The nuclear factor I (Nfi) family of transcription-replication factors is encoded by four genes in mammals and plays an important role in regulating the expression of many cellular genes. The four Nfi genes have unique functions and do not function redundantly.(32,33) Loss of Nfic results in major defects in postnatal murine tooth development and absence of molar root formation.(19) We confirmed that Nfic−/− mice fail to develop a normal tooth root (Fig. 2Q–T), which is present in wild-type mice on postnatal day 21, (Fig. 2M–P). We next examined root dentin formation and found abnormal cellular dentin in rootlike areas in Nfic−/− mice on postnatal day 7, although the dental crown and cusp are normal (Fig. 4A, B, E, F). Nfic is required for root development, especially root dentin formation, but not for crown formation. Furthermore, we found that defects of crown and root development in K14-Cre;Smad4fl/fl mice are more severe than those of Nfic−/− mice (Fig. 2E–H, Q–T). Therefore, we hypothesized that expression of Nfic in the dental mesenchyme may require Smad4 in the dental epithelium. The expression of Nfic, which was clearly detectable in preodontoblasts, odontoblasts, and dental pulp in wild-type mice (Fig. 4I, K, M, O), was not detectable in K14-Cre;Smad4fl/fl mice at the newborn stage and on day 14 after kidney capsule transplantation (Fig. 4J, L, N, P). In contrast, Smad4 expression could be identified in both wild-type and Nfic−/− mice (Fig. 4C, D, G, H). Our data suggest that Smad4 is required in the dental epithelium for Nfic expression in the dental mesenchyme.
Fig. 4.
Expression of Nfic is affected in K14-Cre;Smad4fl/fl teeth. (A–H) H&E staining (A, B, E, F) and immunohistochemistry of Smad4 (C, D, G, H) in postnatal day 7 wild-type (Wt) and Nfic−/− mice. HERS is detectable in wild-type and Nfic−/− mice (A, B, E, F, arrow heads). The crown and cusp appear unaffected, but the root does not develop well in Nfic−/− mice, and cellular dentin is detectable (B, F, arrows). Smad4 expression is indistinguishable in wild-type and Nfic−/− mice (C, D, G, H, arrows). (I–P) In situ hybridization of Nfic in wild-type and K14-Cre;Smad4fl/fl newborn teeth (I, J, M, N) and tooth germs cultured for 2 weeks in kidney capsules (K, L, O, P). Nfic expression is detectable in preodontoblasts, odontoblasts, and dental pulp in wild-type mice (I, M, K, O, black arrows) but undetectable in K14-Cre;Smad4fl/fl mice (J, L, N, P, white arrows). DP = dental pulp; PDL = periodontal ligament. Scale bars (A–D, I–L) = 100 µm; scale bars (M, N) = 50 µm; scale bars (E–H, O, P) = 25 µm.
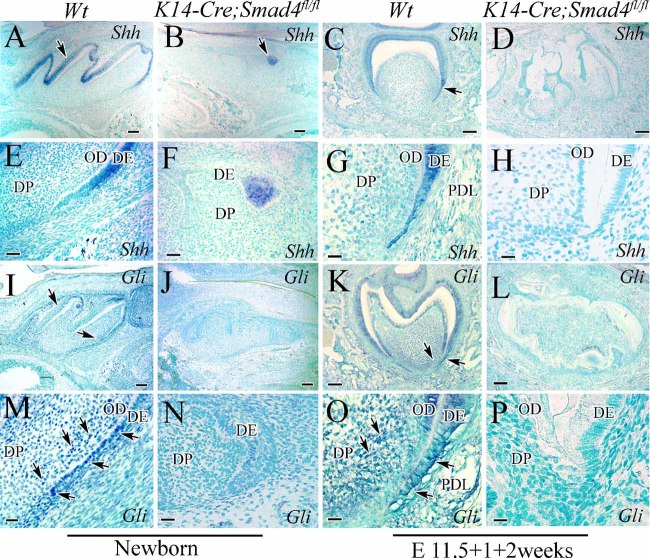
Smad4 is required for Shh expression during root development
Smad4 is an intracellular transcription factor, and its activity in the dental epithelium cannot directly affect Nfic expression in the dental mesenchyme. Therefore, we infer that some Smad4-dependent factor(s) must be released from the dental epithelium to control Nfic expression in the dental mesenchyme. Shh, a member of the hedgehog signaling family, is expressed in the dental epithelium and plays an essential role during tooth development.(34,35) Previous studies have suggested that Shh is induced by BMP signaling during tooth development.(36) We hypothesized that Shh might be one of the key factors controlled by Smad4 in the dental epithelium to affect gene expression in the dental mesenchyme. We found that Shh is expressed in ameloblasts and HERS cells in wild-type mice (Fig. 5A, C, E, G). Gli1, a transcription factor activated by Shh,(37) is also expressed in the dental epithelium and dental mesenchyme at the newborn stage and after 2 weeks of culture in a kidney capsule (Fig. 5I, K, M, O). In contrast, we could not detect Shh signaling in dental epithelial cells including HERS from K14-Cre;Smad4fl/fl mice after culture in a kidney capsule for 2 weeks (Fig. 5D, H). We found that Shh expression was dramatically reduced (Fig. 5B, F) and failed to detect Gli1 expression in newborn K14-Cre;Smad4fl/f mice (Fig. 5J, L, N, P). Our data suggest that Shh is a downstream target regulated by TGF-β/BMP signaling during root development.
Fig. 5.
Shh and Gli expression in wild-type and K14-Cre;Smad4fl/fl mice. In situ hybridization of Shh (A–H) and Gli (I–P) in newborn teeth (A, B, E, F, I, J, M, N) and tooth germs cultured 2 weeks in kidney capsules (C, D, G, H, K, L, O, P) from wild-type (Wt) and K14-Cre;Smad4fl/fl mice. In wild-type mice, Shh is expressed in dental epithelium at the newborn stage and in ameloblasts and HERS cells after 2 weeks of kidney capsule culture (A, E, C, G, arrows). In K14-Cre;Smad4fl/fl newborn mice, Shh expression is reduced dramatically (B, F) and is undetectable in dental epithelial cells, including HERS, after 2 weeks of kidney capsule culture (D, H). Gli is expressed in the dental epithelium and dental mesenchyme in wild-type mice (I, M, K, O, arrows) but is undetectable in the K14-Cre;Smad4fl/fl mice (J, N, L, P). AB = ameloblast; DE = dental epithelium; DP = dental pulp; OD = odontoblast, PDL = periodontal ligament. Scale bars (A–D, I–L) = 100 µm; scale bars (E–H, M–P) = 25 µm.
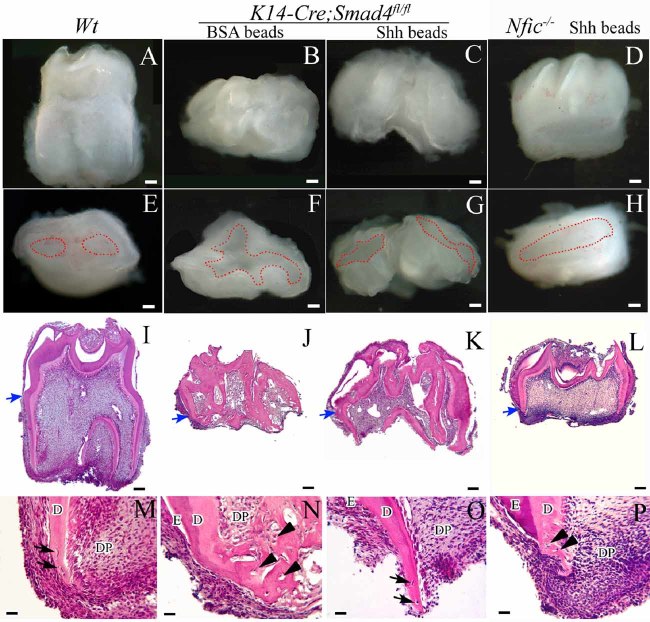
Partial rescue of root development in K14-Cre;Smad4fl/fl tooth germs by ectopic Shh protein
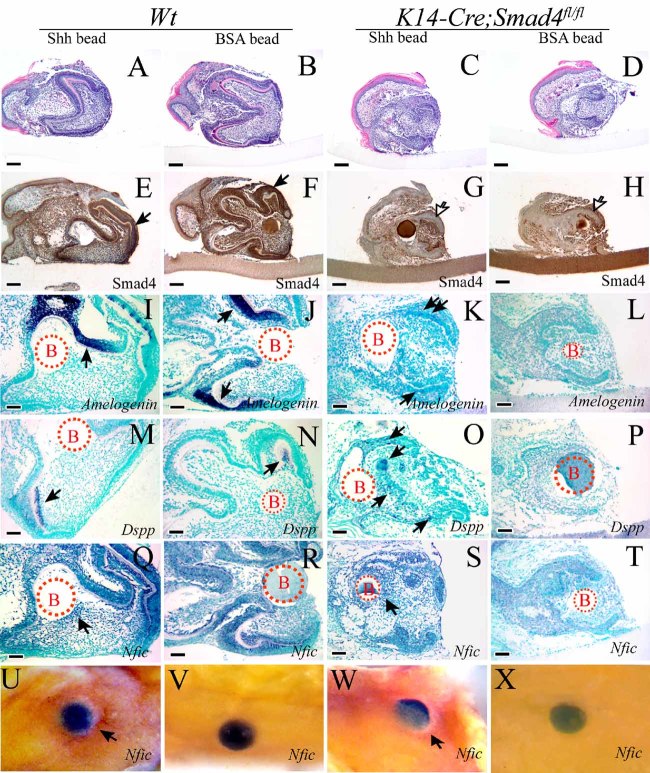
Because Shh is downregulated in the absence of Smad4, we tried to use ectopic Shh to rescue the root defect in K14-Cre;Smad4fl/fl tooth organs. After we added Shh beads into the kidney capsule for 4 weeks, we observed root formation in K14-Cre;Smad4fl/fl samples. Although the roots were not as long as in wild-type mice (Fig. 6A, E), we detected the development of two roots in K14-Cre;Smad4fl/fl samples with Shh beads (Fig. 6C, G). The cementum-enamel junction (CEJ), the boundary between the crown and the root, can be visualized with H&E staining after kidney capsule culture of wild-type samples (Fig. 6I–L, blue arrows). The CEJ and root structure are clearly identifiable in K14-Cre;Smad4fl/fl samples treated with Shh beads (Fig. 6G, K). Moreover, we detected normal dentin, but not cellular dentin, and cementum on the surface of the rescued root after addition of ectopic Shh (Fig. 6K, O, arrows indicate the cementocytes), as we did in wild-type samples (Fig. 6I, M, arrows indicate the cementocytes). We could not find root structures in K14-Cre;Smad4fl/fl samples treated with BSA beads (Fig. 6B, F, J, N, arrowheads indicate the cellular dentin). Shh could not rescue the root defect in Nfic−/− samples (Fig. 6D, H, L, P, arrowheads indicate the cellular dentin), suggesting that Nfic acts downstream of Shh signaling to control root formation. Our rescue experiments strongly suggest that Shh is a downstream target of TGF-β/BMP signaling that controls root development.
Fig. 6.
Ectopic Shh partially rescues the root defect in K14-Cre;Smad4fl/fl mice. Whole-mount views (A–H) and sections stained with H&E (I–P) of tooth germs from wild-type (Wt), K14-Cre;Smad4fl/fl, and Nfic−/− mice cultured for 4 weeks in kidney capsules with BSA or Shh beads. Red-dotted sections in panels E–H outline the roots. In wild-type samples, the root developed well; two roots, dentin, cementum, and the CEJ are clearly identifiable (A, E, I, M; blue arrow indicates the CEJ; black arrows indicate cementocytes). In K14-Cre;Smad4fl/fl samples treated with BSA beads, the root is undetectable, and abnormal cellular dentin is detectable (B, F, J, L; blue arrow indicates the CEJ; black arrowheads indicate the cellular dentin). In K14-Cre;Smad4fl/fl samples treated with Shh beads, root formation is detectable, but shorter than that in wild-type samples (C, G, K, O). In addition, root dentin is normal, cementum is detectable on the surface of root (O, black arrows indicate cementocytes), and the CEJ is clearly identifiable (K, blue arrow indicates the CEJ). In Nfic−/− samples treated with Shh beads, the root is hard to identify; abnormal cellular dentin is detectable (D, H, L, P, blue arrow indicates the CEJ; black arrowheads indicate the cellular dentin). E = enamel; D = dentin; DP = dental pulp. Scale bars (A–L) = 100 µm; scale bars (M–P) = 25 µm.
Shh induces Nfic expression during root formation
In order to explore the mechanism of Shh rescue of the root defect, we cultured newborn K14-Cre;Smad4fl/fl tooth germ with Shh beads in dental pulp. We used H&E staining (Fig. 7A–D) and immunohistochemistry of Smad4 (Fig. 7E–H) to confirm that the dental epithelium of the mutant samples was Smad4-negative. We detected Nfic expression around the Shh beads in the dental pulp of both wild-type and K14-Cre;Smad4fl/fl tooth germs by whole-mount and section in situ hybridization (Fig. 7Q, S, U, W, arrows), whereas we failed to detect Nfic expression in K14-Cre;Smad4fl/fl samples with BSA beads (Fig. 7T, X). Moreover, Amelogenin and Dspp are also expressed in K14-Cre;Smad4fl/fl samples treated with Shh beads (Fig. 7I, K, M, O) but undetectable with BSA beads (Fig. 7J, L, N, P). Our data suggest that Shh induces Nfic, Amelogenin, and Dspp expression during tooth and root formation.
Fig. 7.
Rescue of Nfic gene expression by ectopic Shh in K14-Cre;Smad4fl/fl mice. Analysis of gene expression in newborn wild-type (Wt) and K14-Cre;Smad4fl/fl tooth germs cultured with Shh and BSA beads in dental pulp for 2 days. (A–D) H&E staining. (E–H) Immunohistochemistry with Smad4 antibody. Smad4 expression is not detectable in the dental epithelium of K14-Cre;Smad4fl/fl tooth germs. (I–X) In situ hybridization of Amelogenin (I–L), Dspp (M-P), and Nfic (Q–X) of sections (I-T) and in whole mount (Nfic expression, U–X). Expression of Amelogenin and Dspp is clearly detectable in wild-type samples (I, J, M, N). Expression of Amelogenin and Dspp is not detectable in tooth germ of K14-Cre;Smad4fl/fl mice treated with BSA beads (L, P) but is modestly detectable following treatment with Shh beads (K, O, arrows indicate positive signal). Nfic expression is detectable around the Shh beads in the dental pulp of both wild-type and K14-Cre;Smad4fl/fl mice (Q, S, U, W, arrows indicate positive signal), whereas Nfic expression is undetectable in K14-Cre;Smad4fl/fl samples treated with BSA beads (T, X). Scale bars (A–H) = 100 µm; scale bars (I–T) = 50 µm.
Discussion
Organogenesis is the process by which the ectoderm, endoderm, and mesoderm interact with each other and develop into a complex functional unit. An understanding of organogenesis and its regulation is of paramount importance in developmental biology. Tooth organ development is a good model for studies of heterotypic tissue interactions, including crown and root formation. Tooth crown formation is a multistep process that is mediated through a series of epithelial-mesenchymal interactions.(1,22) Although BMP, Shh, Wnt, and FGF are expressed in the developing root, the molecular regulatory mechanism of root development remains unknown. During root development, the transition from dental cervical loop to HERS is generally regarded as the beginning of root formation.(38,39) However, the functional mechanism of HERS in guiding root development is still unclear.(38–41) In this study we investigated the functional significance of TGF-β/BMP signaling in regulating the fate of HERS cells and epithelial-mesenchymal interaction during root development.
TGF-β/BMP signaling has been shown to function in the initiation of tooth and root development.(4,13,14,18) Bmp2, -3, -4, and -7 are detected during root development, and inhibition of BMP signaling in K14-Noggin mice results in a severe root-development defect.(42,43) Smad4, the central mediator for the canonical TGF-β/BMP signaling pathway, plays a crucial role in regulating tooth development.(31) In order to investigate the functional significance of TGF-β/BMP signaling in dental epithelial cells during root development, we used K14-Cre;Smad4fl/fl mice specifically lacking Smad4 expression in dental epithelial cells. We found that root formation is arrested at the initiation stage in K14-Cre;Smad4fl/fl mice. The HERS fails to form its normal continuous bilayer structure and to grow apically. Instead, the cervical loop of the dental epithelium is enlarged in K14-Cre;Smad4fl/fl mice. TGF-β and BMP ligands are expressed in both dental epithelium and mesenchyme,(30,42,44) but without Smad4 in the dental epithelium, TGF-β/BMP signaling fails to induce root formation. Therefore, we conclude that the elongated HERS plays an essential role during root development and that Smad4 in dental epithelial cells is required for tooth root formation.
Morphogenesis of the tooth crown is governed by interactions between the oral ectoderm and neural crest–derived ectomesenchyme.(1,10,11,12,45) During root development, continuous HERS cells are located between the dental papilla and follicle, which are both derived from cranial neural crest. The special sandwich-like structure indicates a possible biologic significance of interaction between the dental epithelium and mesenchyme. In fact, HERS may function as an essential structure for the guidance of root formation.
In this study we compared two rootless animal models, Nfic−/− mice (Nfic is expressed primarily in dental mesenchyme) and K14-Cre;Smad4fl/fl mice, to investigate the tissue-tissue interaction and signaling-pathway cascade during root formation. We found that Smad4 is required in the HERS for Nfic expression in the dental mesenchyme. Therefore, we propose that interaction between HERS and dental mesenchymal cells controlled by TGF-β/BMP signaling is essential for root dentin formation.
We also found that the expression of Shh and its transcription factor, Gli1, is reduced dramatically in K14-Cre;Smad4fl/fl mice. Shh signaling is important for tooth development.(34,35,46–49) Previous studies also reported that Bmp signaling induces Shh expression(36) and that Smad4 is required for Shh expression in dental cusp epithelium during crown formation.(31) Loss of Ptc1, the receptor for Shh, results in a short molar root, which indicates the significance of Shh signaling during root development.(49) In this study we investigate the functional significance of Shh signaling in mediating epithelial and mesenchymal interaction during root development. After culturing tooth germs with ectopic Shh in a kidney capsule, we found that not only is the root defect in K14-Cre;Smad4fl/fl mice partially rescued but also Nfic expression and abnormal cellular dentin are restored to normal. However, ectopic Shh is unable to rescue the root defect in Nfic−/− mice. Taken together, we show that Smad4 in the dental epithelium is required for Shh expression. Shh released from the dental epithelium acts through Gli1 in the dental mesenchyme to induce Nfic expression to control root development (Fig. 8). Our model clearly demonstrates that the Smad4-Shh-Nfic signaling network plays a crucial role in mediating epithelial-mesenchymal interaction to control root development.
Fig. 8.
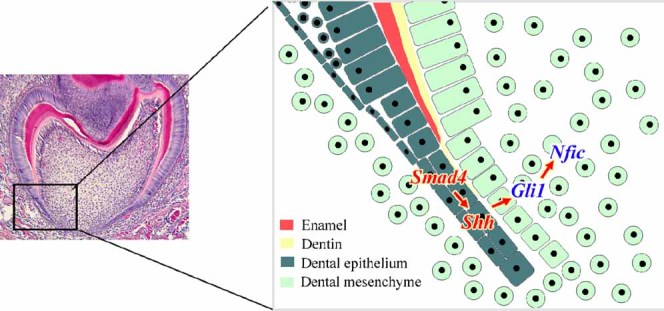
A Smad4-Shh-Gli1-Nfic signaling pathway mediates molar root development. Schematic diagram of our model for the Smad4 signaling pathway during tooth root development. Smad4 in the dental epithelium is required for Shh expression. Then Shh is released from the dental epithelium, acts through Gli1, and induces Nfic expression in the dental mesenchyme for normal root formation.
As an ectodermal appendage, tooth organ development involves crown and root formation. Crown pattern formation is almost completed by birth. Tooth root development begins following crown formation and lasts almost 3 weeks after birth in mice. To date, the transition from crown to root formation during development has remained undefined. Previous studies have shown that the regulatory mechanisms of crown and root development are not identical.(19,50) For example, Nfic is specifically required for root dentin development and root formation but is not involved in crown formation.(19) Intriguingly, however, HERS formation is not affected in Nfic−/− mice. Previous study shows that the disappearance of Fgf10 signaling leads to the transition from crown to root formation owing to a loss of the dental epithelial stem cell compartment.(51) Overexpression of Fgf10 results in enlargement of the HERS and failure to form root. We also observed enlarged HERS in K14-Cre;Smad4fl/fl mice, suggesting that TGF-β/BMP signaling in the dental epithelium is involved in HERS elongation. Smad4-mediated TGF-β/BMP signaling in the dental epithelium is required for Nfic expression in the dental mesenchyme. In parallel, our data show that TGF-β/BMP signaling in dental epithelial cells inhibits Fgf10 signaling in the dental mesenchyme (data not shown), suggesting that TGF-β/BMP signaling in the dental epithelium may play a key role in the initiation stage of root formation. Smad4 is the central mediator of the canonical TGF-β signaling pathway. TGF-β2, BMP-2, -4, -5, and -7, and activin are expressed in dental epithelium and/or mesenchyme during tooth and root development. The functional significance of these ligands in regulating root development awaits further investigation.
In summary, our study suggests that the elongation of dental epithelial cells, HERS, is essential for root development and that TGF-β/BMP signaling relies on a Smad4-dependent signaling mechanism to regulate Nfic expression via Shh signaling to control root formation. HERS cells may provide instructive signaling to control the size, shape, and number of roots. Root development involved dentin and cementum formation and serves as an ideal model for the investigation of epithelial-mesenchymal interaction in regulating postnatal organogenesis. From a clinical perspective, normal root development is critical for the function of dentition. A clear understanding of the regulatory mechanism of root development will provide a comprehensive knowledge of tooth morphogenesis. Furthermore, information generated from this study will lay the foundation for root regeneration, which can be used to support prosthetic restorations to restore dentition in order to provide a biologic solution for a biologic problem.
Acknowledgments
We would like to thank Sarah Millar for K14-Cre, Chuxia Deng for Smad4fl/fl, Richard Gronostajski for Nfic mutant mice, and Julie Mayo for critical reading of the manuscript. This study was supported by grants from the NIDCR and NIH (DE012711, DE017007 and DE014078) to YC.
Disclosures
All the authors state that they have no conflicts of interest.
References
- 1.Thesleff I, Sharpe PT. Signalling networks regulating dental development. Mech Dev. 1997;67:111–123. doi: 10.1016/s0925-4773(97)00115-9. [DOI] [PubMed] [Google Scholar]
- 2.Mikkola ML, Millar SE. The mammary bud as a skin appendage: unique and shared aspects of development. J Mam Gland Biol Neoplasia. 2006;11:187–203. doi: 10.1007/s10911-006-9029-x. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs E. Scratching the surface of skin development. Nature. 2007;22:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaahtokari A, Aberg T, Thesleff I. Apoptosis in the developing tooth: association with an embryonic signaling center and suppression by EGF and FGF-4. Development. 1996;122:121–129. doi: 10.1242/dev.122.1.121. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Bei M, Woo I, Satokata I, Maas R. Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development. 1996;122:3035–3044. doi: 10.1242/dev.122.10.3035. [DOI] [PubMed] [Google Scholar]
- 6.Kratochwil K, Dull M, Farinas I, Galceran J, Grosschedl R. Lef1 expression is activated by BMP-4 and regulates inductive tissue interactions in tooth and hair development. Genes Dev. 1996;10:1382–1394. doi: 10.1101/gad.10.11.1382. [DOI] [PubMed] [Google Scholar]
- 7.Vainio S, Karavanova I, Jowett A, Thesleff I. Identification of BMP-4 as a signal mediating secondary induction between epithelial and mesenchymal tissues during early tooth development. Cell. 1993;75:45–58. [PubMed] [Google Scholar]
- 8.Neubüser A, Peters H, Balling R, Martin GR. Antagonistic interactions between FGF and BMP signalling pathways: a mechanism for positioning the sites of tooth formation. Cell. 1997;90:247–255. doi: 10.1016/s0092-8674(00)80333-5. [DOI] [PubMed] [Google Scholar]
- 9.Jernvall J, Aberg T, Kettunen S, Keranen S, Thesleff I. The life history of an embryonic signalling center: BMP-4 induces p21 and is associated with apoptosis in the mouse tooth enamel knot. Development. 1998;125:161–169. doi: 10.1242/dev.125.2.161. [DOI] [PubMed] [Google Scholar]
- 10.Thesleff I, Vaahtokari A, Partanen AM. Regulation of organogenesis: common molecular mechanisms regulating the development of teeth and other organs. Int J Dev Biol. 1995;39:35–50. [PubMed] [Google Scholar]
- 11.Maas RL, Bei M. The genetic control of early tooth development. Crit Rev Oral Biol. 1997;8:4–31. doi: 10.1177/10454411970080010101. [DOI] [PubMed] [Google Scholar]
- 12.Chai Y, Maxson RE., Jr Recent advances in craniofacial morphogenesis. Dev Dyn. 2006;235:2353–2375. doi: 10.1002/dvdy.20833. [DOI] [PubMed] [Google Scholar]
- 13.Andl T, Ahn K, Kairo A, et al. Epithelial bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- 14.Liu W, Sun X, Braut A, et al. Distinct functions for bmp signaling in lip and palate fusion in mice. Development. 2005;132:1453–1461. doi: 10.1242/dev.01676. [DOI] [PubMed] [Google Scholar]
- 15.Owens PD. Ultrastructure of Hertwig's epithelial root sheath during early root development in premolar teeth in dogs. Arch Oral Biol. 1978;23:91–104. doi: 10.1016/0003-9969(78)90145-0. [DOI] [PubMed] [Google Scholar]
- 16.Diekwisch TG. The developmental biology of cementum. Int J Dev Biol. 2001;45:695–706. [PubMed] [Google Scholar]
- 17.Luan X, Ito Y, Diekwisch TG. Evolution and development of Hertwig's epithelial root sheath. Dev Dyn. 2006;235:1167–1180. doi: 10.1002/dvdy.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aberg T, Wozney J, Thesleff I. Expression patterns of bone morphogenetic proteins (BMPs) in the developing mouse tooth suggest roles in morphogenesis and cell differentiation. Dev Dyn. 1997;210:383–396. doi: 10.1002/(SICI)1097-0177(199712)210:4<383::AID-AJA3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 19.Steele-Perkins G, Butz KG, Lyons GE, et al. Essential role for NFI-C/CTF transcription-replication factor in tooth root development. Mol Cell Biol. 2003;23:1075–1084. doi: 10.1128/MCB.23.3.1075-1084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park JC, Herr Y, Kim HJ, Gronostajski RM, Cho MI. Nfic gene disruption inhibits differentiation of odontoblasts responsible for root formation and results in formation of short and abnormal roots in mice. J Periodontol. 2007;78:1795–1802. doi: 10.1902/jop.2007.060363. [DOI] [PubMed] [Google Scholar]
- 21.Massagué J. How cells read TGF-β signals. Nat. Rev, Mol Cell Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- 22.Chai Y, Slavkin HC. Prospects of tooth regeneration in the 21st century: a perspective. Micros Res Tech. 2003;60:469–479. doi: 10.1002/jemt.10287. [DOI] [PubMed] [Google Scholar]
- 23.Siegel PM, Massagué J. Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nat Rev Cancer. 2003;3:807–821. doi: 10.1038/nrc1208. [DOI] [PubMed] [Google Scholar]
- 24.Shi Y, Massagué J. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 25.Andl T, Ahn K, Kairo A, et al. Epithelial bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- 26.Yang X, Li C, Herrera PL, Deng CX. Generation of Smad4/Dpc4 conditional knockout mice. Genesis. 2002;32:80–81. doi: 10.1002/gene.10029. [DOI] [PubMed] [Google Scholar]
- 27.Indra AK, Warot X, Brocard J, et al. Temporally-controlled site-specific mutagenesis in the basal layer of the epidermis: comparison of the recombinase activity of the tamoxifen-inducible Cre-ER(T) and Cre-ER(T2) recombinases. Nucleic Acids Res. 1999;15:4324–4327. doi: 10.1093/nar/27.22.4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu X, Han J, Ito Y, Bringas P, Jr, Urata M, Chai Y. Cell autonomous requirement for Tgfbr2 in the disappearance of medial edge epithelium during palatal fusion. Dev Biol. 2006;297:238–248. doi: 10.1016/j.ydbio.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Ko SO, Chung IH, Xu X, et al. Smad4 is required to regulate the fate of cranial neural crest cells. Dev Biol. 2007;312:435–447. doi: 10.1016/j.ydbio.2007.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oka S, Oka K, Xu X, Sasaki T, Bringas P, Jr, Chai Y. Cell autonomous requirement for TGF-β signaling during odontoblast differentiation and dentin matrix formation. Mech Dev. 2007;124:409–415. doi: 10.1016/j.mod.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X, Han J, Ito Y, Bringas P, Jr, Deng C, Chai Y. Ectodermal Smad4 and p38 MAPK are functionally redundant in mediating TGF-β/BMP signaling during tooth and palate development. Dev Cell. 2008;15:322–329. doi: 10.1016/j.devcel.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groeneveld MC, Everts V, Beertsen W. Alkaline phosphatase activity in the periodontal ligament and gingiva of the rat molar: its relation to cementum formation. J Dent Res. 1995;74:1374–1381. doi: 10.1177/00220345950740070901. [DOI] [PubMed] [Google Scholar]
- 33.Kruse U, Qian F, Sippel AE. Identification of a fourth nuclear factor I gene in chicken by cDNA cloning: NFI-X. Nucleic Acids Res. 1991;11:6641. doi: 10.1093/nar/19.23.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hammerschmidt M, Brook A, McMahon AP. The world according to hedgehog. Trends Genet. 1997;13:14–1421. doi: 10.1016/s0168-9525(96)10051-2. [DOI] [PubMed] [Google Scholar]
- 35.Dassule HR, Lewis P, Bei M, Maas R, McMahon AP. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127:4775–4785. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Zhang Z, Zhao X, et al. A new function of BMP4: dual role for BMP4 in regulation of Sonic hedgehog expression in the mouse tooth germ. Development. 2000;127:1431–1443. doi: 10.1242/dev.127.7.1431. [DOI] [PubMed] [Google Scholar]
- 37.Hardcastle Z, Mo R, Hui CC, Sharpe PT. The Shh signalling pathway in tooth development: defects in Gli2 and Gli3 mutants. Development. 1998;125:2803–2811. doi: 10.1242/dev.125.15.2803. [DOI] [PubMed] [Google Scholar]
- 38.Thomas HF. Root formation. Int J Dev Biol. 1998;39:231–237. [PubMed] [Google Scholar]
- 39.Bosshardt DD, Schroeder HE. Cementogenesis reviewed: a comparison between human premolars and rodent molars. Anat Rec. 1996;245:267–292. doi: 10.1002/(SICI)1097-0185(199606)245:2<267::AID-AR12>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 40.Kagayama M, Sasano Y, Zhu J, Hirata M, Mizoguchi I, Kamakura S. Epithelial rests colocalize with cementoblasts forming acellular cementum but not with cementoblasts forming cellular cementum. Acta Anat. 1998;163:1–9. doi: 10.1159/000046440. [DOI] [PubMed] [Google Scholar]
- 41.Zeichner-David M, Oishi K, Su Z, et al. Role of Hertwig's epithelial root sheath cells in tooth root development. Dev Dyn. 2003;228:651–663. doi: 10.1002/dvdy.10404. [DOI] [PubMed] [Google Scholar]
- 42.Yamashiro T, Tummers M, Thesleff I. Expression of bone morphogenetic proteins and Msx genes during root formation. J Dent Res. 2003;82:172–176. doi: 10.1177/154405910308200305. [DOI] [PubMed] [Google Scholar]
- 43.Plikus MV, Zeichner-David M, Mayer JA, et al. Morphoregulation of teeth: modulating the number, size, shape and differentiation by tuning BMP activity. Evol Dev. 2005;7:440–457. doi: 10.1111/j.1525-142X.2005.05048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cassidy N, Fahey M, Prime SS, Smith AJ. Comparative analysis of transforming growth factor-beta isoforms 1–3 in human and rabbit dentine matrices. Arch Oral Biol. 1997;42:219–223. doi: 10.1016/S0003-9969(96)00115-X. [DOI] [PubMed] [Google Scholar]
- 45.Chai Y, Jiang X, Ito Y, et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- 46.Khan M, Seppala M, Zoupa M, Cobourne MT. Hedgehog pathway gene expression during early development of the molar tooth root in the mouse. Gene Expr Patterns. 2007;7:239–243. doi: 10.1016/j.modgep.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 47.Nakatomi M, Morita I, Eto K, Ota MS. Sonic hedgehog signaling is important in tooth root development. J Dent Res. 2006;85:427–431. doi: 10.1177/154405910608500506. [DOI] [PubMed] [Google Scholar]
- 48.Cobourne MT, Miletich I, Sharpe PT. Restriction of sonic hedgehog signaling during early tooth development. Development. 2004;131:2875–2885. doi: 10.1242/dev.01163. [DOI] [PubMed] [Google Scholar]
- 49.Gritli-Linde A, Bei M, Maas R, Zhang XM, Linde A, McMahon AP. Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization. Development. 2002;129:5323–5237. doi: 10.1242/dev.00100. [DOI] [PubMed] [Google Scholar]
- 50.Tummers M, Thesleff I. Root or crown: a developmental choice orchestrated by the differential regulation of the epithelial stem cell niche in the tooth of two rodent species. Development. 2003;130:1049–1057. doi: 10.1242/dev.00332. [DOI] [PubMed] [Google Scholar]
- 51.Yokohama TT, Ohshima H, et al. Cessation of Fgf10 signaling, resulting in a defective dental epithelial stem cell compartment, leads to the transition from crown to root formation. Development. 2006;133:1359–1136. doi: 10.1242/dev.02307. [DOI] [PubMed] [Google Scholar]